Abstract
The genus Berberis includes about 500 different species and commonly grown in Europe, the United States, South Asia, and some northern areas of Iran and Pakistan. Leaves and fruits can be prepared as food flavorings, juices, and teas. Phytochemical analysis of these species has reported alkaloids, tannins, phenolic compounds and oleanolic acid, among others. Moreover, p-cymene, limonene and ocimene as major compounds in essential oils were found by gas chromatography. Berberis is an important group of the plants having enormous potential in the food and pharmaceutical industry, since they possess several properties, including antioxidant, antimicrobial, anticancer activities. Here we would like to review the biological properties of the phytoconstituents of this genus. We emphasize the cultivation control in order to obtain the main bioactive compounds, the antioxidant and antimicrobial properties in order to apply them for food preservation and for treating several diseases, such as cancer, diabetes or Alzheimer. However, further study is needed to confirm the biological efficacy as well as, the toxicity.
Keywords: Berberis, food preservative, alkaloid, antioxidant, human health
1. Introduction
Berberis spp. are shrubs in the family Berberidaceae, native to central and southern Europe, western Asia, as well as northwest Africa [1]. About 500 species of these plants are found in most areas of central and southern Europe, the north-eastern region of United States, and Asia (including the northern area of Pakistan [2] and Iran [3]). The genus Berberis consists of spiny deciduous evergreen shrubs which are characterized by yellow wood and flowers [2], dimorphic long and short shoots (1–2 mm). Some Berberis fruits are small oblong berries 7–10 mm long and 3–5 mm broad and turn blue or red upon ripening during the late summer or autumn [1].
Berberis species are mainly consumed fresh, dried and used in juice production [4]. The fruits are very popular, known as zereshk in Iran where they are commonly used for cooking and in jam production, thus, encouraging the production of fresh edible seedless barberries fruits reaching about 22,000 tons per annum [5]. The fruits are also processed into beverages, drinks, syrups, candy and other confectionary products which are popular Iran. Furthermore, the leaves and fruits have also found applications in the production of food flavorings and teas. Berberis are popular due to their nutritional importance; however, they have found most usefulness in folk and traditional medicine where various parts, including roots, bark, leaves and fruits serve as major ingredients of herbal remedies in Ayurvedic, Iranian and Chinese medicine dating back at least 3000 years [6]. Currently, this species flower is popularly used amongst Tibetan speaking population in areas, such as Litang, China [7].
The effect of cold-pressed filtered oil of Berberis spp. seeds in delaying soybean oil oxidation in comparison to commercial antioxidants were carried out, and the study reported that Berberis oil contributed to oxidative stability of soybean oil comparably to commercial antioxidants [8]. Antioxidant and antibacterial activity of water extract of barberry has suggested their possible application as preservatives in food industries [9].
Isoquinoline alkaloids are the major bioactive constituents in Berberis [10]. Protoberberines and bisbenzyl-isoquinoline alkaloids, such as berbamine, tetrandrine and chondocurine, which have been known for their anti-inflammatory and immunosuppressive properties, have been detected by phytochemical analysis of the root and stem back extracts of B. vulgaris. Berberine (an isoquinoline alkaloid) and berbamine are the most abundant phytochemicals of Berberis species [2]. The fruits contain a high amount of alkaloids, tannins, phenolic compounds and oleanolic acid [3,11], gum, pectin, oleoresins, organic acids, anthocyanins and carotenoids. In addition, palmitine [10], stigmasterol and its glycoside [12] have all been detected in various species of the Berberis plant.
Some Berberis fruits have been employed in the treatment of guts [13] kidney stones [14] and liver [15] and gall bladder [10] conditions. The root bark and stem of the Berberis have found usage as a diuretic, febrifuge, cathartic and antiseptic. Furthermore, preparations of the stem and root bark have been used to treat mouth and stomach ulcers [16]. Several parts of the plant have been reported to possess astringent and antiseptic properties, while the stem bark and flowers were found to be anti-rheumatic [17]. The alkaloid rich root bark of the plant has also been used as purgative and treatment for both diarrhea and rheumatism [18]. The berberine-rich rhizomes of Berberis species possess marked antibacterial and antitumor properties, with reported efficacies in treatment of various eye conditions [10,19]. Furthermore, the anti-inflammatory activity of berberine has been extensively studied amongst other pharmacological actions [10,20].
Berberine sulphate which is an alkaloid extracted from the roots and bark of various Berberis spp. Have been reported to possess antibacterial, antifungal and antiprotozoal activities. Reported the bacteriostatic activity of berberine against streptococci, and that the sub-minimum inhibitory concentrations (MICs) of the compound blocked the adherence of streptococci to host cells, immobilized fibronectin, and hexadecane in epithelial cells [21]. Furthermore, blood glucose and lipid regulatory properties of Berberis have been demonstrated [3,22,23,24]; and this was due to berberine-induced improvement in insulin sensitivity through regulation of adipokine secretion [25,26,27]. Effectiveness of Berberis species in the maintenance of heart health has been demonstrated in their ability to improve hypertension, ischemic heart disease, cardiac arrhythmias and cardiomyopathy [2,28].
The health-promoting effect of Berberis spp. cannot be overemphasized, as well as its popularity; however, this is restricted to central and southern Europe, western Asia, as well as northwest Africa. Hence, efforts should be geared towards making the Berberis plant also available to other regions of the world. Furthermore, most studies on Berberis spp. have been on berberine; therefore, efforts should be made towards researching possible therapeutic benefits of all other important phytoconstituents of the plant. Furthermore, the synergistic or additive effect of these phytoconstituents should be studied so as to elucidate the complex molecular interaction amongst various phytochemicals leading to the observed therapeutic properties. In addition, the modulatory effect of the plant/plant materials on gene expression should be prioritized.
The aim of this review is to provide a detailed overview to the cultivation of Berberis species, in-depth insight on the biological properties of the phytoconstituents of this genus, regarding its food preservative applications, antimicrobial, antioxidant and anticancer effects, and lastly, special emphasis to its clinical effectiveness in humans. The present work was performed by consulting the database of PubMed, Web of Science, Embase, and Google Scholar (as a search engine) to retrieve the most updated articles on the topic under investigation (phytochemicals and biological activities of Berberis species). The strategy of the search included the use of the following keywords: “Berberis” or “barberry” and “cultivation” or “essential oil” or “antimicrobial” or “food preservative” or “antioxidant” or “anticancer”. Authors carefully examined articles and for the review, prioritizing the articles published from 2013 to 2018 [29]. Only English articles having full text were considered.
2. Cultivation of Berberis Plants
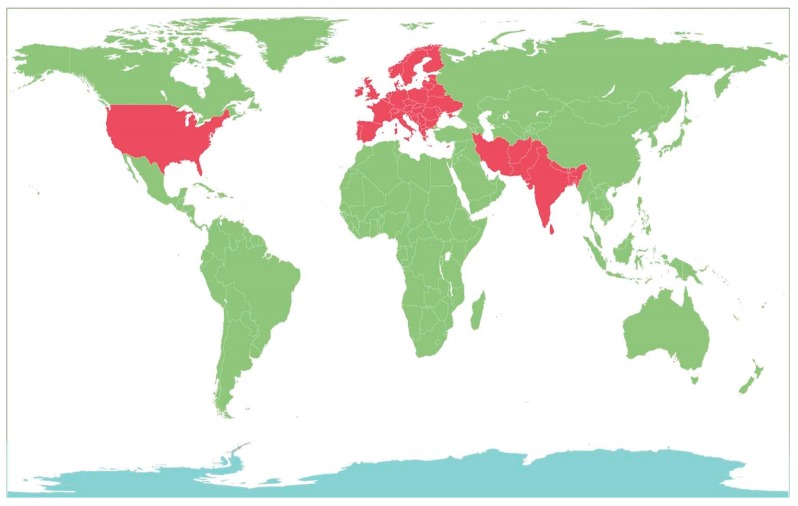
The genus Berberis include about 500 different species and commonly grown in Europe, United States, South Asia and some northern areas of Iran and Pakistan (Figure 1) [11,30]. In Pakistan, majority of Berberis species are found in high mountains (1400 m–3500 m above sea level). In Iran, five Berberis species are present, including two important species, i.e., B. orthobotrys and B. khorassanica, which are grown in eastern, northern and southern regions of Iran [31]. Other important local species zereshk is also widely cultivated in the South-Khorasan province of Iran [32]. In Iran, 11,000 hectares of area is under cultivation of common barberry (B. vulgaris) species, and one of the most leading producers of barberry fruit in the world. Annually, Iran produces more than 10,000 tons of dried barberry fruits, while maximum production comes from the Southern Khorasan. More than 97% of the area located near Ghaenat County and Southern Khorasan province is cultivated with common barberry that produces 95% of fruit in Iran [33]. B. vulgaris is gathered from the wild in Eastern Europe in countries like Poland [34,35]. In addition, it is known as K’otsakhuri in Georgia, where it is grown and collected from the southeast forests of the country [36]. The yield-related important traits of many Berberis species are significantly affected by environmental factors, biotic stresses, seasonal variations, the climatic condition of an area, planting and harvesting date or methods, irrigation source, fruit processing and storage methods, etc.
Figure 1.
The areas where the Berberis plants are commonly grown (shown in red).
The fruit remains a vital part, producing many important secondary metabolites and used in pharmaceutical and food industries. In this section, we have highlighted the cultivation of different Berberis species, its status and various factors affecting its cultivation. Common barberry is a native plant in Asia’s western and middle mountains and non-native to North America [37]. The hybrid species Berberis × ottawaensis are widely cultivated in Europe and North America [38]. The European settlers introduced common barberry to New England, and used it as a source of medicine, food, and for other aesthetic purposes [37,39]. The colonists of New England determined its cultivation spread Puccinia graminis fungus, causing wheat rust and important reduction of its crop [40].
The seedless type (B. vulgaris var. asperma) is commonly cultivated in the southern parts of the Khorasan province of Iran for domestic purposes [41]. B. lyceum represents a native species of Nepal and distributed in the temperate and subtropical regions of the world, including some part of Australia. It is distributed from Kashmir to Uttaranchal North-western Himalayas [42]. Sixteen species, and some varieties of barberry, were found in the Boaxing country, situated on the Eastern side of Hengduan Mountains in Sichuan Province, Southwest China [43]. The Japanese barberry is invasive in twenty different states of the world and five other provinces of Canada [44]. According to Reference [45] there are 21 Berberis species in Nepal, including two new species Berberis pendryi Bh. Adhikari and Berberis karnaliensis Bh. Adhikari. Berberis crataegina DC. is commonly grown in Turkey, Asia and European regions. The fruit are locally known as “karamuk” and “kadıntuzluğu” in Turkey and used as traditional medicine [46].
In Pakistan, twenty species and six subspecies of Berberis have been reported, and the majority of these is growing in northern parts of the country [47]. The other dominant species of Berberis is B. aristata, grown in Nepal, Pakistan and India. In Pakistan, it mainly cultivated in the Hazara division of Khyber Pakhtunkhwa (KP), and Azad Jamu and Kashmir (AJK) regions. In AJK it is locally named sumbal and commonly known as daruharidra [48]. B. lyceum is another key species, highly distributed in different Asian countries, including, Afghanistan, Pakistan, Nepal, India and Bangladesh. In Pakistan, it grows in different areas of KP, Punjab and Baluchistan [49]. The fruit part locally called kashmal, is used as a source of food, and for preparing the sauce in Himalayan regions of Jammu and Kashmir, and Himachal Pradesh [50,51].
The yield of barberry plants depends on various factors like managing operations, size and age of shrub and date and method of harvesting [52,53]. Proper harvesting method at a suitable time is one of the key steps in berry yield production because the shrubs include maximum spines in shoot part and also the fruit peel is so thin. The harvesting date plays a vital role to gain maximum yield with high quality. The local farmers set some useful sensory parameters for starting the harvest of the crop. These parameters include change of fruit color from bright red to dark red, tissue softening, the concentration of contents, and reducing sourness in fruit, etc. [11]. The optimum time for barberry harvesting in the autumn cold season, when the berries ripen. In this stage, the fruit gains dark red color due to the presence of high anthocyanin content, sweetness increases, while the berberine and sourness are reduced [53,54,55,56,57]. In different regions of North America and Western Europe, the common Barberry ripens in the month of August or September [58,59]. While the seeds can be mature in October [60]. The berries of common barberry remain with the stem through winter [61]. However, the delay in harvesting from 10 September to 13 November increases the anthocyanin content about 2.5 times [53]. It may also increase the yield and quality, but too much delaying may lead to early autumn chilling injury to plant. For Iranian seedless barberry 170 days after the flowering is an optimum date for harvesting [56]. Fruit maturation and development vary in different geographic regions. So, it is important to optimize a suitable harvesting date for each region.
3. Berberis Plants Essential Oils and Phytochemical Composition
Essential oils (EO) are volatile, complex natural compounds, which formed in aromatic plants as secondary metabolites. They are used in pharmaceutical, agricultural, and food industries, as well as are associated with antibacterial, anti-inflammatory, antioxidant, and insecticidal potential [62,63,64].
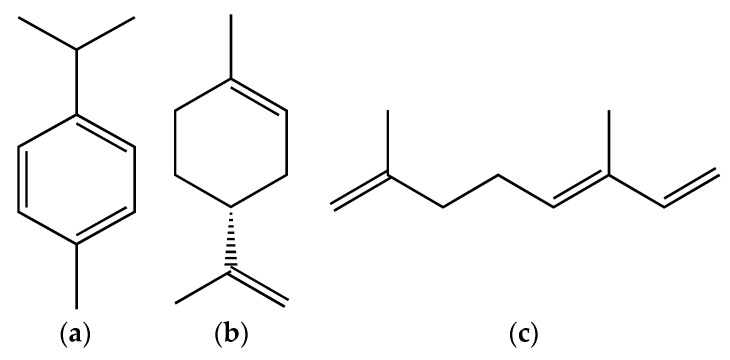
The gas chromatography coupled to mass spectrometry (GC-MS) analysis of various parts of B. vulgaris revealed that benzaldehyde, benzyl alcohol, 1-hexanol and I-2-hexenal [65] were major compounds of the EOs from fruit, while p-cymene, limonene and ocimene were identified as major compounds of the EOs (Figure 2) from leaves and flowers [66].
Figure 2.
Major compounds of the essential oils (EOs) of Berberis vulgaris leaves and flowers. (a) p-cymene; (b) limonene; (c) ocimene.
Turkish B. crataegina fruit berry has 22 volatile compounds which are aldehydes had the highest concentration (5382 μg/kg), followed by alcohols (2487 μg/kg) and lactone (2422 μg/kg).
Major volatile compounds of the B. crataegina fruit are γ-butyrolactone, 3-hexanal and 2,6-dimethylphenol. Moreover, the olfactometric analysis of dry B. crataegina resulted eight aroma active compounds [67].
EOs of the roots of B. integerrima were analyzed by using modified microwave-assisted hydrodistillation (MAHD). Chemical diversity of 10 and 18 compounds were obtained from MAHD, MAHD with modified anyl, and with modified phenyl magnetic nanoparticles, the yields of the EOs were 0.16, 0.61 and 0.71 w/w %, respectively. Hexadecanoic acid was identified as a major compound for MAHD and modified MAHD methods [68].
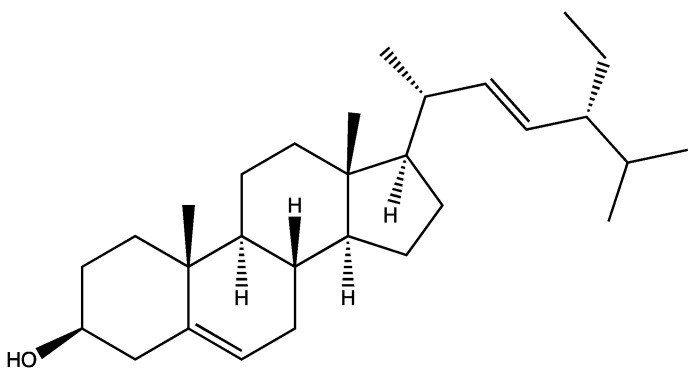
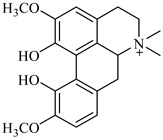
Moreover, the GC/MS study on hexane extracts of the B. aetnensis and B. libanotica roots was showed that B. aetnensis have twenty-six and B. libanotica have thirty-seven non-polar compounds. Stigmasterol (Figure 3) is the major compound of both species [69].
Figure 3.
Stigmasterol.
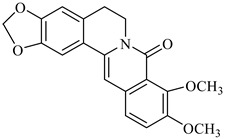
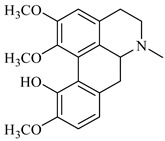
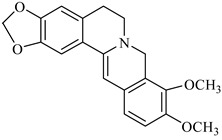
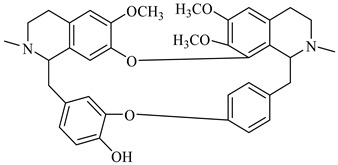
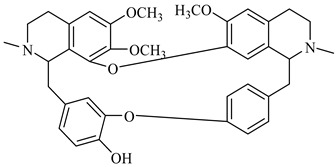
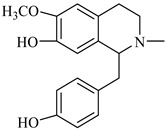
On the other hand, alkaloids (Table 1) represent the main compounds in Berberis species, and many of them have been identified by different spectroscopic techniques previously mentioned. The most known are berberine, berbamine, palmitine, jatrorrhizine, and isotetrandrine. They are located mainly in the cortical tissues of the roots and stems and have important biological activities. In fact, in vitro and in vivo anti-proliferative and anti-metastatic effects on various types of cancers have been reported for different alkaloids. These compounds, such as vinblastine, have already used as anticancer drugs [3].
Table 1.
Alkaloids from Berberis species.
| Chemical structure | Name | Plant |
|---|---|---|
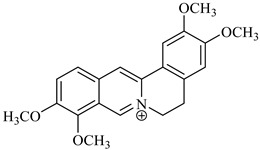
|
palmatine | B. vulgaris |
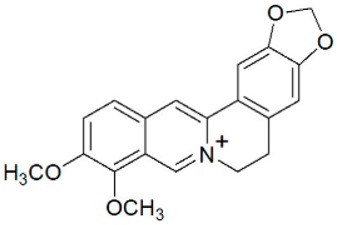
|
berberine | B. vulgaris |
|
oxyberberine | B. vulgaris |
|
isocoridine | B. vulgaris |
|
lambertine | B. vulgaris |
|
magniflorine | B. vulgaris |
|
oxycanthine | B. vulgaris |
|
berbamine | B. aristata |
|
(+)-N-methylcoclaurine | B. montana |
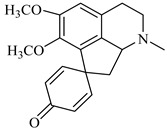
|
(−)-pronuciferine | B. montana |
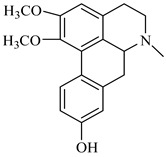
|
(+)-9-hydroxynuciferine | B. montana |
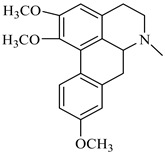
|
(+)-orientine | B. montana |
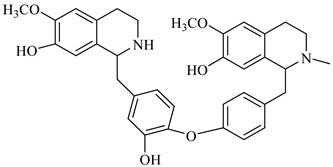
|
2-norberbamunine | B. stoloniferais |
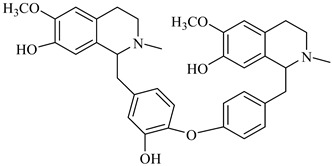
|
berbamunine | B. stoloniferais |
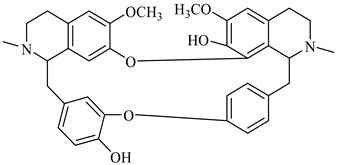
|
aromoline | B. stoloniferais |
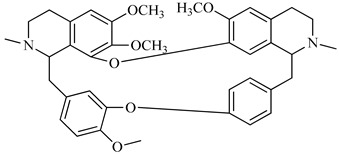
|
isotetrandrine | B. stoloniferais |
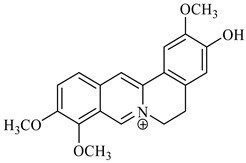
|
jatrorrhizine | B. umbellate |
4. Food Preservative Applications of Berberis Plants
Food preservation is the most vital issue in food industries to ensure food safety for a longer period. Basically, the process of food preservation depends on the growth inhibition of undesirable microorganisms. Use of chemical agents with antimicrobial activity is commonly used a traditional method for food preservation [70]. However, antimicrobial agents also gain momentum, due to their fewer side effects and compatibility with the human body. Further, synthetic antimicrobials and their toxicological safety as food additives needed to be ensured by regulatory authorities. Moreover, processed foods with natural preservatives have great demand and considered safer and beneficial for public health [71]. The naturally occurring compounds demonstrated antimicrobial activity in foods as natural ingredients and can be used as additives to other foods.
Berberis is an important plants having enormous potential in the food industry. However, only a few reports are available on the direct application of these plants in food products. For example, seed oil and fruit extracts of B. crataegina were supplementing into chitosan matrix for preparation of a chitosan-based edible film. The films produced have been analyzed for the physiochemical and biological activities. Results showed that chitosan-fruit extract film exhibited higher thermal stability, antimicrobial, antioxidant, and anti-quorum sensing activity as compared to other films. Furthermore, the addition of B. crataegina seed oil and fruit extract into the chitosan film create a mark reduction in the UV-vis transmittance but improve the tensile strength. Likewise, hydrophobicity of the chitosan-seed oil film was found to be higher than chitosan-control film, while chitosan-fruit extract film displayed slightly lower hydrophobicity than chitosan film. These results indicated that chitosan-fruit extract film of B. crataegina fruit extract could be used as an effective ingredient for the production of the edible film with increased physicochemical and biological properties [72].
A list of the antimicrobial potential of the Berberis species evaluated across the globe is provided which support the use of Berberis species in food preservation (Table 2).
Table 2.
A list of the antimicrobial potential of the Berberis species evaluated across the globe is provided which support the use of Berberis species in food preservation.
| S. No. | Species | Part | Country | Extract/Model/Compound | Tested Micro-Organism | Results | Reference |
|---|---|---|---|---|---|---|---|
| 1 | B. aristata | Stem and leaves | Nepal | Hexane, Ethyl acetate, Methanol | Staphylococcus aureus, Kleibsella pneumoniae, Salmonella typhimurium | Against S. aureus: methanol significant zone of inhibition (21 mm), ethyl acetate extracts moderate activity, hexane extract of stem slightly active. | [73] |
| 2 | B. aristata, and B. ligulata | Bark stem Leaves | Nepal | Ethanol | Bacillus subtilis, Escherichia coli, Pseudomona aeruginosa, Salmonella. typhi, Salmonella dyjenteriae, Salmonella cholerae | Ethanol extract of B. aristata: largest zone of inhibition (21 mm) against B. subtilis and the smallest MBC value (90 mg/mL) for S. aureus. Gram positive bacteria more susceptible to the ethanol extract. B. aristata relatively broad-spectrum antibacterial activity. | [74] |
| 3 | B. vulgaris | Stem | Iran | Ethanol | P. aeruginosa, Acinetobacter baumannii, E. coli and Salmonella enteritidis | MIC determination: stem extracts inhibit the growth of all the studied bacteria (3900 to 37,500 μg/mL) by synergistic effects with ciprofloxacin. | [75] |
| 4 | B. asiatica | Leaves | Uttarakhand, India | Methanol | E. coli, Enterobacter aerogenes, Proteus vulgaris, P. aeruginosa, K. pneumoniae, B. subtilis, S. aureus | Methanol extracts of leaves: high inhibitory potential on S. aureus, K. pneumoniae, E. coli, B. subtilis and P. vulgaris in all concentration. | [76] |
| 5 | B. aristata, B. asiatica, B. lycium | Stem | Bangalore, India | Methanol | Nocardia sp., S. aureus, S. pneumonia, P. aeruginosa, Streptococcus viridians, E. coli | Sensitivity to Nocardia sp., S. pneumonia and E. coli. | [77] |
| 6 | B. glaucocarpa | Root wood | Pakistan | Ethanol | SMRSA, EMRSA, Mycobacterium marinum, E. coli, Trypanosoma brucei | Berberine (MIC = 12.5 and 25 μg/mL), berberine chloroform (MIC = 25 and 12.5 μg/mL) and syringaresinol (12.5 μg/mL): very active against SMRSA, M. marinum and T. brucei. | [78] |
| 7 | B. vulgaris | Stem bark | Romania | Ethanol | Botrytis cinerea | B. vulgaris bark extract, berberine, and fluconazole significantly inhibited growth of B. cinerea. | [79] |
| 8 | B. vulgaris | Ethanol | S. aureus, Staphylococcus epidermidis, K. pneumoniae, B. subtilis, E. coli, Aspergillus niger, Trichoderma, Alternaria solanai | 20 mm zone of inhibition against E. coli. Good activity against B. Subtilis, moderate against Trichoderma, insignificant against other stains. | [80] | ||
| 9 | B. vulgaris and its active constituent, berberine | Root | Egypt | Ethanolic extract | Candida albicans, E. coli | Berberis ethanolic extract and berberine standard can inhibit C. albicans and E. coli growth. | [81] |
| 10 | B. vulgaris | Fruit | Pakistan | Distilled water | S. aureus, Proteus, S. typhi, Salmonella paratyphi A, Salmonella paratyphi B, K. pneumoniae, E. coli, P. aeruginosa | Antibacterial activity against all tested pathogens. | [82] |
| 11 | B. thunbergii | Fruit | Hungary | Juice; water extract and -methanol extract | B. subtilis, Bacillus cereus var. mycoides, E. coli, Serratia marcescens | Juice, water extract and methanol extract showed activity against all bacteria. | [83] |
| 12 | B. calliobotrys | Stems and branches | Pakistan | Methanol | B. subtilis, P. aeruginosa, S. aureus fungal strains namely C. albicans, Penicillium notatum | The methanol extract, ethyl acetate and n-butanol fractions: maximum zone of inhibition against all bacterial strains especially S. aureus and antifungal effects. | [84] |
| 13 | B. lycium | Roots | Libya | Distilled water, ethanol, isopropanol and methanol | Pseudomonas sp., E. coli, Streptococcus sp., Staphylococcus sp. | Methanolic displayed maximum inhibitory zone (16 mm), isopropanol extract (13 mm) and ethanol extract (12 mm). The aqueous extract exhibited the least inhibitory zone (10 mm). The methanolic extract: maximum inhibitory zone (12 mm), Pseudomonas (11 mm) and Staphylococcus (10 mm). | [85] |
| 14 | B. hispanica | Root Bark | Marocco | Ethanolic extract | Mycobactérium smegmatis, Mycobacterium aurum | The ethanolic extract from root bark displayed an important antimycobacterial activity. The inhibition zones for M. aurum A+ were significantly larger than those for M. smegmatis MC2. | [86] |
| 15 | B. ruscifolia | - | Argentina | Acetone, chloroform-methanol (1:1) and methanol | E. coli, P. aeruginosa, Listeria monocytogenes, S. aureus | All extracts exhibited antibacterial activity with MIC varying from 16 to 2 mg/mL. The highest inhibition with acetonic and chloroform-methanolic extracts of species against S. aureus (MIC = 2 mg/mL). Methanolic extracts B. ruscifolia showed no antibacterial activity against all tested bacteria. | [87] |
| 16 | B. aristata | Stem bark | India | Ethanol and aqueous extracts | Shigella flexneri, Shigella sonnei, Shigella dysenteriae, Shigella boydii | Extracts of B. aristata: antibacterial activity against four strains of Shigella (8 and 23 mm). | [88] |
| 17 | B. aristata, B. asiatica, B. chitria and B. lycium | Root and stem | India | Ethanol | Micrococcus luteus, B. subtilis, B. cereus, Enterobacter aerogenus, E. coli, K. pneumoniae, Proteus mirabilis, P. aeruginosa, S. aureus, S. typhimurium, Streptococcus pneumonia, Fungal strains Aspergillus nidulans, C. albicans, Aspergillus terreus, Trichophyton rubrum, Cistus albidus, Aspergillus flavus, A. niger | B. lycium, B. aristata and B. asiatica root extract showed significant antifungal activity against A. terreus and A. flavus. B. aristata root and B. lycium (stem) extracts gave very low MIC values (0.31 μg/mL) as compared to other tested species. | [89] |
| 18 | B. Lycium | Root | Pakistan | Ethanol, petroleum ether | S. aureus, S. epidermidis, B. subtilis, S. typhi, E. coli, C. albicans | The ethanolic and aqueous crud root extract: most effective antifungal and antibacterial agents. | [90] |
| 19 | B. integerrima Syn: B. densiflora | Roots | Iran | Methanol | Brucella abortus | MIC and MBC results, jatrorhizine exhibited higher antibacterial activity with MIC (0.78 μg/mL) and MBC (1.56 μg/mL) compared with the standard (streptomycin, 10 μg/mL). | [91] |
| 20 | B. lycium | Roots | Pakistan | Hydric extract | E. coli, Pseudomonas, Staphylococcus, Proteus | Significant activity against E. coli and Proteus (80 to 100%), while it demonstrated a good activity against Pseudomonas and Staphylococcus (60 to 70%). | [92] |
| 21 | B. aristata | Bark and leaves | India | Methanol, ethanol and hexane | B. subtilis, Agrobacterium tumefaciens, E. coli, Xanthomonas. Phaseoli, Erwinia chrysanthemi | All the extracts of tested plants showed variable activity against all the tested bacterial strains. Methanol extract revealed highest antibacterial activity (11 mm) recorded against E. chrysanthemi. Hexane extract: totally inactive against all the tested strains. | [93] |
| 22 | B. aristata | Roots | India | Aqueous and alcohol extracts | S. aureus, B. subtilis, E. coli, S. typhimurium | Alcoholic and aqueous extract showed antimicrobial activity against four tested bacteria. B. aristata exhibited highest zone of inhibition for B. subtilis followed by S. aureus, E. coli and S. typhimurium. | [94] |
| 23 | B. microphylla | Leaves, stems and roots | Chile | Methanol | E. coli, S. typhimurium, L. monocytogenes, E. aerogenes, S. aureus, B. cereus, S. epidermidis and B. subtilis | All extract possesses significant antibacterial activity against Gram-positive bacteria but not against Gram-negative bacteria. | [95] |
| 24 | B. lycium | Root bark | Pakistan | E. coli, K. pneumoniae, P. aeruginosa, S. aureus, B. subtilis | Silver nanoparticles were very active against Gram-negative and Gram-positive bacteria Aqueous bark extract (10 μg/mL) possess highest activity against E. coli and P. aeruginosa. | [96] | |
| 25 | B. vulgaris | Fruit | Iran | L. monocytogenes | Average diagonal of growing area in disk diffusion test for species: 12 mm and MIC was 125 μg/mL and MBC of B. vulgaris was 500 μg/mL. | [97] | |
| 26 | B. aristata | Stem bark | Alcohol | In vivo in an animal model using Sprague Dawley rats | Carbapenem-resistant E. coli | An aquo-alcoholic extract of the species: effectively manage peritonitis induced by Carbapenem-resistant E. coli in a rat model at a single post-exposure prophylactic dose of 0.5 mg/kg body weight. | [98] |
| 27 | B. aristata | Roots | India | Aqueous and alcoholic extract of fresh roots, as well as aqueous extract of dried roots | S. aureus, S. epidermidis, Streptococcus pyogenes, Streptococcus viridans, Enterococcus faecalis, B. subtilis, B. cereus, E. coli, K. pneumoniae, P. aeruginosa, P. vulgaris, P. mirabilis, S. typhi, S. paratyphi A, S. typhimurium, S. dysenteriae type 1, Vibrio cholerae | All three extracts displayed wide antibacterial activity against Gram-positive bacteria. Among the Gram-negative bacteria tested, the antibacterial activity was limited to E. coli, S. typhimurium, S. dysenteriae type 1 and V. cholerae. All extracts also possess antifungal activity against the fungal species tested, except Candida krusei. | [99] |
| 28 | B. aristata | Root Stem Leaf | Pakistan | E. coli, S. typhi, S. aureus, Shigella, Citrobacter, P. vulgaris,Enterobacter, Streptococcus pyrogenes, V. cholera, Klebsiella spp., A. niger, Cladosporium, Rhizoctonia, Alternaria, Trichoderma, Penicillium, Curvularia, Paecilomyces and Rhizopus | The extracts significantly inhibited the growth of the studied microbes, except A. niger, Curvularia, Paecilomyces and Rhizopus. | [100] | |
| 29 | B. aristata | India | V. cholerae, S. aureus | All the strains of V. cholerae are susceptible. All the Salmonella sp., Pseudomonas sp., and some of the E. coli strains are highly resistant, except some strains of E. coli as AL26, and Shigella sp. are susceptible. All Xanthomonas sp. were highly susceptible. Berberine sulfate showed antifungal action against C. albicans, Candida tropicalis, Trichophyton mentagrophytes, Microsporum gypseum, Cryptococcus neoformans and Sporothrix schenkii, Mycobacterium tuberculosis var. hominis H37RV and Entamoeba histolytica. | [101] | ||
| 30 | B. heterophylla | Leaves, stems and roots berberine | Argentina | S. aureus, E. faecali, P.aeruginosa, E. coli, C. albicans, Candida glabrata, Candida haemulonii, Candida lusitaniae, C. krusei, Candida parapsilosis | The aqueous extracts of B. heterophylla do not possess significant antimicrobial activity. Berberine displayed a significant antibacterial and antifungal activity against S. aureus and different Candida spp., some of them obtained from the clinical isolated. | [102] | |
| 31 | B. amurensis | Branches and leaves | Korea | Bacillus atrophaeus, Kocuria rhizophila, M. luteus, S. epidermidis, B. subtilis subsp. Spizizenii, K. pneumoniae, Enterobacter cloacae, Salmonella enterica subsp. enterica, P. aeruginosa | No significant activity against gram-negative bacteria. | [103] | |
| 32 | B. croatica and B. vulgaris | Roots, leaves, and twigs | Croatia | Ethanol | B. subtilis, S. aureus, E. coli, P. aeruginosa, C. albicans | Extracts of both species: significant antibacterial activity against the Gram-positive bacteria. Root extracts of B. croatica: activity against P. aeruginosa, and leaf extracts against B. subtilis. Neither species possessed antifungal activity. Leaf extracts of B. croatica: antibacterial activity against B. subtilis. Likewise, neither of the species extracts showed activity against E. coli and C. albicans, except when were diluted. Ethanolic extracts of twigs of both species: inactive against B. subtilis and against S. aureus, with the exception of B. croatica twig from Kiza locality. | [104] |
| 33 | B. lycium | Roots | India | Hexane extract, Methanolic extract, aqueous extract and berberine | K. pneumonia, E. coli, P. aeuroginosa, S. aureus, B. subtilis, C. albicans, A. niger, Aspergillus fumigates | Methanolic extract of species was highly effective against E. coli, S. aureus, B. subtilis, C. albicans, A. fumigates. Pure berberine was effective against E. coli and C. albicans. | [105] |
| 34 | B. aetnensis | Roots | Italy | Ethanol ether and chloroform | S. aureus, B. subtilis, E. faecalis, E. coli, P. aeruginosa, Stenotrophomonas maltophilia, against 14 strains of nosocomial origin: two strains of S. aureus (1 Met-S, 1 Met-R); four strains of S. epidermidis (2 Met-S, 2 Met-R); three strains of E. coli; four strains of P. aeruginosa, Hafnia alvei and C. albicans, C. parapsilosis, C. krusei | The root and leaf extracts showed a greater activity against Gram-positive bacteria and yeasts than against Gram-negative bacteria, except for P. aeruginosa. The chloroform extract of leaves was more active than the ethanol. | [106] |
| 35 | B. thunbergii, B. vulgaris | Roots | USA | E. coli, P. aeruginosa, S. aureus, S. mutans, and S. pyogenes | Ethanolic extracts more active against studied bacteria, strongest activity against S. pyogenes and S. aureus. | [107] | |
| 36 | B. vulgaris | Root bark | Algeria | Methanol and water | S. aureus, E. faecalis, E. coli, E. cloacae, K. pneumoniae, P. aeruginosa | The extracts of species root barks presented a strong activity against S. aureus (23.0 mm), a weak activity against E. faecalis (13.0 mm) and no activity toward other strains. | [108] |
5. Antioxidant Activities of Berberis Plants (In Vitro and In Vivo)
Free radicals ubiquitous in the environment affect human health by oxidative stress-induced damage. Finding exogenous sources with antioxidant activity is necessary in order to support the organism against the actions of free radicals. The fruits of most plants from Berberidaceae family have a sour taste which is due mainly to the presence of ascorbic acid or vitamin C. The vitamins and antioxidant compounds in barberry plant might be useful for treating diseases [109].
The antioxidant effect of B. vulgaris on oxidative systems, such as liver cells oxidation, red blood cells haemolysis, and haemoglobin non-enzymatic glycosylation was demonstrated, and the highest inhibitory effect was exerted on glycosylation. The extracts of B. vulgaris was the most promising as antioxidants, as well as anti-inflammatory and acetylcholinesterase (AChE) inhibitors. The capacity of B. vulgaris for scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), the inhibitions of lipoxygenase and AChE are mainly due to the phenol and flavonoid contents [110].
B. vulgaris root extract was evaluated for the alleviation of oxidative stress by using female Japanese quails. Moreover, B. vulgaris root extract exerted antioxidant effects through inhibiting NF-kB, which was activated and suppressed in the heat stress environment [111]. The antioxidant potential of 50% aqueous ethanolic root extract of B. aristata was examined on antioxidant enzymes of the liver in diabetic rats, along with its safety parameters. The root extract of B. aristata has strong potential to decrease oxidative stress [112].
Significant antioxidant effects, mainly on ABTS, hydroxyl radicals and DPPH, have been reported to berberine hydrochloride. The relationship among diabetes mellitus and the increase of formation of free radicals and a decrease in antioxidant potential is well known. Since berberine hydrochloride has significant radicals scavenging and protective effects against β-cell damage and antioxidant of the pancreas in diabetes mellitus, it seems reasonable that antioxidants can play an important role in the improvement of diabetes and in screening the novel treatment drug of diabetes mellitus [113].
The extracts from the inner stem bark of B. vulgaris exhibited high antioxidant activity, and most of the identified compounds were isoquinoline alkaloids. The values were higher than the standard antioxidant compounds (vitamin C and butylated hydroxytoluene (BHT)) [114].
It was widely investigated the composition of the major anthocyanins and the antioxidant activities of the fruit of B. heteropoda. The high anthocyanins content indicated that this fruit could be considered as an excellent source of natural colorants and a functional food that benefits human health [115]. Regarding the alkaloid extract of B. aetnensis roots, it is also possessed antioxidant properties, and all results are in agreement with other reports on the alkaloids from the roots of Berberis species [116].
The findings suggested that B. vulgaris fruits have an important potential for their antioxidant activities depends on the content of phenolic compounds and organic acids [117].
6. Anticancer Activities of Berberis Plants (In Vitro and In Vivo)
Cancer is the third leading cause of death worldwide, preceded by cardiovascular and infectious diseases. In medical science, there is need for effective and acceptable cancer therapeutics agents that are non-toxic, highly efficacious against multiple cancers, cost effective, and acceptable by human population [118]. The interest in natural products has increased because they are less toxic to normal cells, and they reduce side effects and drug resistance observed in synthetic drugs.
In India, medicinal plants have been used for treating disease since ancient times. Many of these belong to the genus Berberis. The fruit of B. vulgaris is rich in polyphenols, vitamins, proteins, ascorbic acid, and anthocyanin, which are important for human health. Moreover, they are rich in alkaloids which promote anticancer activity. In particular in hepatic cancer cell (Hepg2) ethanol extract of the fruit of B. vulgaris reduces cell vitality and promotes the selective increase of protein expression like alkaline phosphatase (ALP), a hepatic enzyme important in the diagnosis of disease [119]. Anticancer activity of fruit extract of B. vulgaris was also demonstrated on human breast cancer cells (MCF-7). The extract reduces cell proliferation in time and dose-dependent manner. It has also been evidenced the importance of the solvent for the extraction processes, because as it is reported in several studies, ethanol extract is more active than water extract, probably due to its capacity to extract more compounds responsible for anticancer activity like alkaloids [120]. B. vulgaris ethanol extract reduces viability in the breast, colon, hepatic and cervix cancer cell lines in a dose-dependent manner after incubation at 24, 48 and 72 h. The ethanol extract has a similar activity of berberine chloride [121]. This was also confirmed by El Khalki et al. [122], who studied cytotoxicity on human breast adenocarcinoma cell (MCF-7) of B. vulgaris and berberine.
The antioxidant activity might play a major role in increasing efficiency of such extracts to kill cancer cells and protect normal cells, besides the inhibition of cell growth. Choi et al. [123] demonstrated that treatment with berberine reduces p53 expression in the human prostate cancer cell. In fact, berberine promotes translocation of p53 in nuclei and arrest of the cell cycle in G0/G1. This was also confirmed in in vivo studies. In this sense, the intraperitoneal administration of berberine at 10 mg/kg caused a substantial decline in tumor volume and weight of prostate cancer. The effect is more evident in cancer expressing p53 (LNCaP) both in vivo and in vitro [123].
Berberine and curcumin have been tested on different types of cancer cell line models as A549 (lung cancer cell line), Hep-G2 (liver cancer cell line), MCF-7 (breast cancer cell line), Jurkat (leukemia cancer cell line) and K562 (kidney cancer cell line) by Balakrishna et al. [124]. This work can reveal the synergetic activity of these compounds. The anticancer effects in these cells are mediated by inducing apoptosis [124]. The synergistic effect of two different compounds was also evidenced by Ren et al. [125]. They showed as galangine and berberine together demonstrated an anticancer activity stronger than that showed when used singularly, on esophageal carcinoma cells. In fact, they induce apoptosis, promote cell cycle arrest in the G2/M phase and increase reactive oxygen species (ROS) in cancer cells [125]. The anticancer activity of B. aristata roots was also evaluated on human osteosarcoma cells [126].
B. libanotica root extract showed potential anti-inflammatory and anticancer activity, mostly due to alkaloids and other compounds. This effect was demonstrated on human colon cancer cells [127] in which berberine inhibits COX-2 transcriptional activity. B. libanotica extract reduced the viability of CD4 T-cells infected by the retrovirus HTLV1, a kind of cell characteristic of an aggressive form of leukaemia [128]. Moreover, root extract showed anticancer activity on different cell lines of prostate cancer; it reduces cell viability and promotes cell cycle arrest in G0/G1 [129]. Subsequent studies on human erythroleukemia cell lines investigated molecular pathways responsible for the anticancer activity. The extract induced apoptosis of cells through the modulation of Akt/NF-Kb/COX-2 signal transduction pathways [130]. B. libanotica extract showed a dominant effect on K562 cells by the activation of the late markers of apoptosis with caspase-3 activation, Poly (ADP-ribose) polymerase (PARP) cleavage and DNA fragmentation. The study demonstrated that treatment with the extract induces apoptosis in erythroleukemia cell line expressing COX-2 (HEL cells) or not (K562), especially at a dose of 300 μg/mL after 48h of treatment. In particular, B. libanotica extract induced activation of caspase-3 and -9, correlated with PARP cleavage and DNA fragmentation. In this process, the extract is more effective than berberine tested at a dose of 40 μg/mL. Moreover, the extract reduced significantly the expression of COX-2, which prevent apoptosis in cancer cells through the activation of Akt and NF-kB. A similar mechanism was shown for 4-chlorobenzoyl berbamine; and a synthetic compound derived from berbamine. It induces apoptosis in lymphoma cell lines and G2/M cell cycle arrest through PI3K/Akt and NF-kB signaling pathways [131]. Changes as esterification, etherification or sulfonylation on the structure of berbamine allowed to obtaining new molecules capable of solving the problem of resistant in many types of tumor [132]. Many synthetic derivatives of berbamine also demonstrated antineoplastic activity. Among these, BBMD3 was shown to be the most potent as an anticancer agent on human melanoma cells. It inhibits JAK/STA3 pathways reducing pro-apoptotic gene expression [133]. This was also confirmed in osteosarcoma and glioblastoma cell lines where BBMD3 induced inhibition of Jak2/STAT3 signaling pathway and activation of the stress response JNK pathway. Moreover, it increases the expression of miR-4284 involved in tumorigenesis and apoptosis [134,135].
Berbamine, which is contained in B. amurensis, has activity also tested on solid tumor. In fact, it arrests growth and migration in vitro and ex vivo of human lung cancer A549 cell line at low concentration trough down-regulation of anti-apoptotic protein Bcl-2 and up-regulation of the pro-apoptotic protein Bax [136]. B. amurensis extract arrests proliferation of Hepg2 and MCF-7 cells; extraction technique influences the presence of the active compound and consequently the extract activity [137].
B. orthobotrys is another species of this genus mainly grow in Iran. Roots bark leaves are used in traditional medicine in easy problem like menstrual pains, kidney stones, but the species have also demonstrated anticancer activity in HeLa cell line. As reported by Bavand et al. [138], ethanol root extract induces morphological change and apoptosis in HeLa cells then 72h of treatment. In particular treatment of cells with 1.25 mg/mL of extract, reduced the cell viability, inhibited the cell growth, changed cell adhesion to the substrate, pigmented the cells and formed apoptotic bodies [138]. Treatment with a low concentration of the extract also presents anticancer activity on other types of cancer cell line. Engel et al. [139] reported that the reduction of cell vitality of 60% caused by treatment with different doses ranged from 100 to 1 μg/mL of root extract of B. orthobotrys. They also studied the possible molecular mechanism responsible for cell death. Microscopic analysis of cells showed the accumulation of lysosome, which starts programmed cell death trough liberation of ROS and hydrolytic enzymes, granularization and formation of Golgi vesicles, as well as the diffuse distribution of neutral lipids. This is pronounced at 100 µg/mL, but also lower doses causes a slight formation of lysosome vesicles [139].
In addition to alkaloids there are other secondary metabolites with anticancer activity, for example, the triterpenoids the main active constituent of the trunk of B. koreana. They have been identified through mass spectrometry and nuclear magnetic resonance and tested in different cancer cell lines (A549, SK-OV-3, SK-MEL-2 and HCT-15) where they reduce cell proliferation [140,141].
7. Clinical Studies of Berberis Plants in Human
Currently available clinical trials regarding this group of plants point on their effects in various conditions related to cardiovascular diseases and associated risk factors, neurodegenerative diseases and inflammation.
One group of clinical trials conducting by Guiseppe Derosa et al. [142,143,144,145] had a specific interest in a fixed combination that included B. aristata and Silybum marianum (Berberol®). The reason for this combination lies in low bioavailability of B. aristata, while S. marianum is there to improve its intestinal absorption. A 52-week double-blind placebo-controlled study in 136 obese patients with type-2 diabetes mellitus (T2DM) and metabolic syndrome analyzed various parameters, including: Fasting blood glucose, insulin, total cholesterol, HDL, LDL, triglycerides, and body mass index (BMI). [146]. All of these parameters have been significantly improved in the treatment group compering with the baseline and in order to control group. Previously, comparing same fixed combination vs. B. aristata monotherapy in clinical trial conducting by Di Pierro et al. [147] with T2DM subjects, shown that combination is more effective in decreasing of HbA1c indicated that positive effects are partly due to S. marianum. In another study with the same combination of extracts in 102 dyslipidemia subjects after three months, it has been shown reducing of total cholesterol, triglycerides and LDL, with increasing of HDL from randomization and compared to the placebo group. The same result on lipid profile has been observed in a double-blind, randomized placebo-controlled trial that included 106 patients with metabolic syndrome treated with B. vulgaris [148]. Another two double-blind, randomized, placebo-controlled, 6-months clinical studies with Berberol® conducting by Derosa et al. [142,143], followed dyslipidaemic subjects intolerant to statins at high dosages. In both studies were included patients tolerant to a half dose of statins. The lipid profile of included patients did not significantly change in the active treatment group after reduction of statins dosage and the introduction of Barberol®. Meanwhile, in placebo group lipid profile was worsened compared to baseline and with active treatment.
The clinical trial with type 1 diabetes mellitus (T1DM) subjects treated with the same fixed combination (Berberol®) showed decreasing of insulin dose necessary to reach adequate glycemic control [144]. The clinical trial with subjects at low cardiovascular risk also confirmed hypocholesterolemia effects of a fixed combination of Berberol® [145].
Berberin has been shown to inhibit CYP3A4 in in vitro and animal models, as well as in humans, and that inhibition should increase blood levels of statins, cyclosporine, and calcium channel blockers, similar to the action of grapefruit [149]. The inhibition of enzyme CYP3A4 activity in humans has been observed in a two-phase randomized-crossover clinical study in healthy male subjects after two weeks of berberine administration (300 mg, p.o.) [150]. In a randomized double-blind placebo-controlled clinical trial, patients suffering from irritable bowel syndrome received berberine hydrochloride twice daily for two months [151]. The benefits from the treatment were observed as better IBS symptom and depression/anxiety scores.
One of the clinical trials examined the effect of aqueous extract of dried barberry taken orally as an anti-acne agent [152]. The results obtained from teenagers in this placebo-containing trial show the effectiveness of using barberry in the treatment of acne vulgaris, despite the treatment and control groups were small.
In all of these trials, no patients had serious adverse events. The limitations were the relatively small size of the sample, and relatively short follow-up period. However, the side effects of berberine have been reported in some in vitro and in vivo animal models, and observed effects were related to its neurotoxicity [153,154]. Despite that, in animal models of Alzheimer’s disease neuroprotective effects of berberine have been illustrated [155]. The published results in a review article from 2015 indicated that on web page www.clinicaltrials.gov there was 17 clinical trials on the efficacy of berberine [156], and currently there are 51 clinical trials which showing us increasing interest in beneficial effects of this compound [data obtained searching web page dated 24 May 2018].
8. Conclusions
Berberis is an important genus of wild plants with a multitude of uses in pharmacology and food industry. These species are the abundant source of important natural compounds, i.e., vitamins, minerals, alkaloids and antioxidants, which can be used in a wide array of pharmaceutical and nutraceutical products. Some of the species of the genus like B. vulgaris are also cultivated in Iran and other countries, but information regarding its cultivation, diseases and production technology is sparse. The present study has been carried out to report on the adaptation of different Berberis species, suitable agro-climatic conditions for the higher yield, its production technology, diseases and harvesting methods. However, further studies should be conducted to evaluate genetic diversity in the cultivated species for selection of high yielding genotypes, development of new varieties, yield enhancement through appropriate cultivation practices and integrated pest management (IPM) techniques.
Regarding food and pharmacological applications, Berberis is an important group of the plants having enormous potential in the food industry, and several reports of their antimicrobial activity have been found in the literature. Several phytochemicals found in fruits, leaves, stems and root have demonstrated biological activities. Phytochemicals present in EOs confer antimicrobial and antioxidant properties that make them useful as food preservatives. On the other hand, alkaloids present pharmacological properties, such as anticancer activities reported. However, not much information is available on the direct application of these plants in food products. On the other hand, the extracts of B. vulgaris were the most promising as antioxidants as well as inflammatory and neurological disorders protective. In addition, positive effects related to cancer targets have been reported to reduce cell proliferation without affecting a normal human cell. For this reason, Berberis spp. maybe considered an alternative for cancer treatment, but it is necessary to confirm their efficacy in vivo, especially investigating the toxicity during drug therapy.
Acknowledgments
This work was supported by CONICYT PIA/APOYO CCTE AFB170007.
Author Contributions
All authors contributed to the manuscript. Conceptualization, B.S. and J.S.-R.; Validation investigation, resources, data curation, writing–all authors; Review and editing, J.S.-R., M.d.l.L.C.-G., and W.C.C. All the authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Minaiyan M., Ghannadi A., Mahzouni P., Jaffari-Shirazi E. Comparative study of berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran. J. Pharm. Res. 2011;10:97–104. [PMC free article] [PubMed] [Google Scholar]
- 2.Mokhber-Dezfuli N., Saeidnia S., Gohari A., Kurepaz-Mahmoodabadi M. Phytochemistry and Pharmacology of Berberis Species. Pharmacogn. Rev. 2014;8:8. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahimi-Madiseh M., Lorigoini Z., Zamani-Gharaghoshi H., Rafieian-Kopaei M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017;20:569–587. doi: 10.22038/IJBMS.2017.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farhadi Chitgar M., Aalami M., Maghsoudlou Y., Milani E. Comparative Study on the Effect of Heat Treatment and Sonication on the Quality of Barberry (Berberis Vulgaris) Juice. J. Food Process. Preserv. 2017;41:e12956. doi: 10.1111/jfpp.12956. [DOI] [Google Scholar]
- 5.Aghbashlo M., Kianmehr M.H., Hassan-Beygi S.R. Specific heat and thermal conductivity of berberis fruit (Berberis vulgaris) Am. J. Agric. Biol. Sci. 2008;3:330–336. doi: 10.3844/ajabssp.2008.330.336. [DOI] [Google Scholar]
- 6.Birdsall T.C., Kelly G.S. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 1997;2:94–103. [Google Scholar]
- 7.Kang J., Kang Y., Ji X., Guo Q., Jacques G., Pietras M., Łuczaj N., Li D., Łuczaj Ł. Wild food plants and fungi used in the mycophilous Tibetan community of Zhagana (Tewo County, Gansu, China) J. Ethnobiol. Ethnomed. 2016;12:21. doi: 10.1186/s13002-016-0094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavakoli A., Sahari M.A., Barzegar M. Antioxidant activity of Berberis integerrima seed oil as a natural antioxidant on the oxidative stability of soybean oil. Int. J. Food Prop. 2018;20:S2914–S2925. doi: 10.1080/10942912.2017.1382509. [DOI] [Google Scholar]
- 9.Aliakbarlu J., Mohammadi S., Khalili S. A Study on Antioxidant Potency and Antibacterial Activity of Water Extracts of Some Spices Widely Consumed in Iranian Diet. J. Food Biochem. 2013;38:159–166. doi: 10.1111/jfbc.12034. [DOI] [Google Scholar]
- 10.Srivastava S., Srivastava M., Misra A., Pandey G., Rawat A. A review on biological and chemical diversity in Berberis (Berberidaceae) EXCLI J. 2015;14:247–267. doi: 10.17179/excli2014-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kafi M., Balandary A., Rashed-Mohasel M.H., Koochaki A., Molafilabi A. Berberis: Production and Processing. Zaban va adab Press; City, Iran: 2002. [Google Scholar]
- 12.Saied S., Begum S. Phytochemical studies of Berberis vulgaris. Chem. Nat. Compd. 2004;40:137–140. doi: 10.1023/B:CONC.0000033929.60336.bb. [DOI] [Google Scholar]
- 13.Yazdani A., Poorbaghi S.L., Habibi H., Nazifi S., Rahmani Far F., Sepehrimanesh M. Dietary Berberis vulgaris extract enhances intestinal mucosa morphology in the broiler chicken (Gallus gallus) Comp. Clin. Path. 2013;22:611–615. doi: 10.1007/s00580-012-1454-1. [DOI] [Google Scholar]
- 14.Bashir S., Gilani A.H., Siddiqui A.A., Pervez S., Khan S.R., Sarfaraz N.J., Shah A.J. Berberis vulgaris root bark extract prevents hyperoxaluria induced urolithiasis in rats. Phyther. Res. 2010;24:1250–1255. doi: 10.1002/ptr.3196. [DOI] [PubMed] [Google Scholar]
- 15.Hermenean A., Popescu C., Ardelean A., Stan M., Hadaruga N., Mihali C.V., Costache M., Dinischiotu A. Hepatoprotective effects of Berberis vulgaris L. extract/β cyclodextrin on carbon tetrachloride-induced acute toxicity in mice. Int. J. Mol. Sci. 2012;13:9014–9034. doi: 10.3390/ijms13079014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amjad M.S., Arshad M., Qureshi R. Ethnobotanical inventory and folk uses of indigenous plants from Pir Nasoora National Park, Azad Jammu and Kashmir. Asian Pac. J. Trop. Biomed. 2015;5:234–241. doi: 10.1016/S2221-1691(15)30011-3. [DOI] [Google Scholar]
- 17.Altundag E., Ozturk M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Procedia Soc. Behav. Sci. 2011;19:756–777. doi: 10.1016/j.sbspro.2011.05.195. [DOI] [Google Scholar]
- 18.Javadzadeh S., Fallah S. Therapeutic application of different parts of Berberis vulgaris. Int. J. Agric. Crop Sci. 2012;4:404–408. [Google Scholar]
- 19.Phillips R., Foy N. Herbs. Pan Books Ltd.; London, UK: 2002. [Google Scholar]
- 20.Kuo C.-L., Chi C.-W., Liu T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Sun D., Courtney H.S., Beachey E.H. Berberine sulfate blocks adherence of Streptococcus pyogenes to epithelial cells, fibronectin, and hexadecane. Antimicrob. Agents Chemother. 1988;32:1370–1374. doi: 10.1128/AAC.32.9.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajzadeh M.A.R., Rajaei Z., Shafiee S., Alavinejhad A., Samarghandian S., Ahmadi M. Effect of barberry fruit (Berberis Vulgaris) on serum glucose and lipids in streptozotocin-diabetic rats. Pharmacologyonline. 2011;1:809–817. [Google Scholar]
- 23.Meliani N., Dib M.E.A., Allali H., Tabti B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011;1:468–471. doi: 10.1016/S2221-1691(11)60102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X., Chan S.W., Tseng H.L., Deng Y., Hoi P.M., Choi P.S., Or P.M.Y., Yang J.M., Lam F.F.Y., Lee S.M.Y., et al. Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine. 2012;19:1263–1269. doi: 10.1016/j.phymed.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X., Bian H., Gao X. The Potential Mechanisms of Berberine in the Treatment of Nonalcoholic Fatty Liver Disease. Molecules. 2016;21:1336. doi: 10.3390/molecules21101336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J., Yin J., Gao H., Xu L., Wang Y., Xu L., Li M. Berberine Improves Insulin Sensitivity by Inhibiting Fat Store and Adjusting Adipokines Profile in Human Preadipocytes and Metabolic Syndrome Patients. Evid.-Based Complement. Altern. Med. 2012;2012:363845. doi: 10.1155/2012/363845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Ye J. Mitochondrial inhibitor as a new class of insulin sensitizer. Acta Pharm. Sin. B. 2012;2:341–349. doi: 10.1016/j.apsb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imenshahidi M., Hosseinzadeh H. Berberis Vulgaris and Berberine: An Update Review. Phyther. Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 29.West S., King V., Carey T.S., Lohr K.N., McKoy N., Sutton S.F., Lux L. Systems to rate the strength of scientific evidence. Evid. Rep. Technol. Assess. 2002;47:1–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Rounsaville T.J., Ranney T.G. Ploidy levels and genome sizes of berberis l. and mahonia nutt. species, hybrids, and cultivars. HortScience. 2010;45:1029–1033. doi: 10.21273/HORTSCI.45.7.1029. [DOI] [Google Scholar]
- 31.Mozaffarian V. A Dictionary of Iranian Plant Names. Farhang Mo’aser; Tehran, Iran: 2008. [Google Scholar]
- 32.Kafi M., Balandri A. Effects of gibberellic acid and ethephon on fruit characteristics and ease of harvest seed less barberry. Iran. Res. Organ. Sci. Technol. Cent. Khorasan. 1995;volume:page. [Google Scholar]
- 33.Peterson P., Leonard K., Miller J., Laudon R., Sutton T. Prevalence and distribution of common barberry, the alternate host of Puccinia graminis, in Minnesota. Plant Dis. 2005;89:159–163. doi: 10.1094/PD-89-0159. [DOI] [PubMed] [Google Scholar]
- 34.Łuczaj L. Archival data on wild food plants used in Poland in 1948. J. Ethnobiol. Ethnomed. 2008;4:4. doi: 10.1186/1746-4269-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Łuczaj Ł. Wild food plants used in Poland from the mid-19th century to the present. [Dziko rosnące rośliny jadalne użytkowane w Polsce od połowy XIX w. do czasów współczesnych] Etnobiologia Pol. 2011;1:57–125. [Google Scholar]
- 36.Bussmann R., Zambrana P., Narel Y., Sikharulidze S., Kikvidze Z., Kikodze D., Tchelidze D., Batsatsashvili K., Robbie E. Ethnobotany of Samtskhe-Javakheti, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017;12:7–24. [Google Scholar]
- 37.Kern F.D. Observations of the Dissemination of the Barberry. Ecology. 1921;2:211–214. doi: 10.2307/1929064. [DOI] [Google Scholar]
- 38.Dirr M. Manual of Woody Landscape Plants: Their Identification, Ornamental Characteristics, Culture, Propagation, and Uses. 5th ed. Stipes Publishing; Champaign, IL, USA: 1998. [Google Scholar]
- 39.Mack R.N., Erneberg M. The United States naturalized flora: Largely the product of deliberate introductions. Ann. Missouri Bot. Gard. 2002;89:176–189. doi: 10.2307/3298562. [DOI] [Google Scholar]
- 40.Fulling E.H. Plant life and the law of man IV barberry, currant and gooseberry, and cedar control. Bot. Rev. 1943;9:483–592. doi: 10.1007/BF02872487. [DOI] [Google Scholar]
- 41.Javadzadeh S. Effect of different methods of harvesting, drying and time on losses seedless barberry (Berberis vulgaris L) Int. J. Agron. Plant. 2013;4:254–260. [Google Scholar]
- 42.Sharma R. Medicinal plants of India: An Encyclopaedia. Indian Counc. Med. Res. New Delhi. 2003;1:33. [Google Scholar]
- 43.Li X., Zhang L., Li W., Yin X., Yuan S. New taxa of Berberis (Berberidaceae) with greenish flowers from a biodiversity hotspot in Sichuan Province, China. Plant Divers. 2017;39:94–103. doi: 10.1016/j.pld.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward J.S., Worthley T.E., Williams S.C. Controlling Japanese barberry (Berberis thunbergii DC) in southern New England, USA. For. Ecol. Manag. 2009;257:561–566. doi: 10.1016/j.foreco.2008.09.032. [DOI] [Google Scholar]
- 45.Adhikari B., Pendry C.A., Pennington R.T., Milne R.I. A revision of berberis S.S. (Berberidaceae) in Nepal. Edinburgh J. Bot. 2012;69:447–522. doi: 10.1017/S0960428612000261. [DOI] [Google Scholar]
- 46.Baytop T. Turkish Plant Names Dictionary. Atatürk Culture, Language and History High Foundation. Turkish Language Foundation; Ankara, Turkey: 1994. [Google Scholar]
- 47.Khan T., Khan I.A., Rehman A. A review on Berberis species reported from Gilgit- Baltistan and Central Karakoram National Park, Pakistan. J. Med. Plants Stud. 2014;2:16–20. [Google Scholar]
- 48.Ali M., Malik A.R., Sharma K.R. Vegetative propagation of Berberis aristata DC. An endangered Himalayan shrub. J. Med. Plants. 2008;2:374–377. [Google Scholar]
- 49.Ali M.N., Khan A.A. Pharmacognostic studies on Berberis lycium Royle, and its importance as a source of raw material for the manufacture of berberine in Pakistan [angiosperm trees] Pakistan J. For. 1978;28:25–27. [Google Scholar]
- 50.Kaur C., Miani S. Fruits and vegetables healthy foods for new millennium. Indian Hort. 2001;45:29–32. [Google Scholar]
- 51.Tewary D.K., Bhardwaj A., Shanker A. Pesticidal activities in five medicinal plants collected from mid hills of western Himalayas. Ind. Crops Prod. 2005;22:241–247. doi: 10.1016/j.indcrop.2005.01.004. [DOI] [Google Scholar]
- 52.Fallahi J., Moghaddam R.P., Nasiri-Mahallati M. Effect of harvest date on quantitative and qualitative indices of seedless barberry. Iran. J. F. Crop. Res. 2010;8:225–234. [Google Scholar]
- 53.Moghaddam P.R., Fallahi J., Shajari M.A., Mahallati M.N. Effects of harvest date, harvest time, and post-harvest management on quantitative and qualitative traits in seedless barberry (Berberis vulgaris L.) Ind. Crops Prod. 2013;42:30–36. doi: 10.1016/j.indcrop.2012.05.007. [DOI] [Google Scholar]
- 54.Arena M.E., Curvetto N. Berberis buxifolia fruiting: Kinetic growth behavior and evolution of chemical properties during the fruiting period and different growing seasons. Sci. Hortic. (Amsterdam) 2008;118:120–127. doi: 10.1016/j.scienta.2008.05.039. [DOI] [Google Scholar]
- 55.Chandra P., Todaria N.P. Maturation and ripening of three Berberis species from different altitudes. Sci. Hortic. (Amsterdam) 1983;19:91–95. doi: 10.1016/0304-4238(83)90048-1. [DOI] [Google Scholar]
- 56.Mahmoodi H.R., Zamani G.H., Balandary A. The study of qualitative characteristics of seedless barberry (Berberis vulgaris L.) as influenced by different fruit harvesting dates and two different climates; Proceedings of the 6th Congress of Iranian Horticultural Sciences; Isfahan, Iran. 2009; pp. 1486–1489. [Google Scholar]
- 57.Minore D., Rudolf P.O., Berberis L. In: The Woody Plant Seed Manual, Agriculture Handbook. Bonner F.T., Karrfalt R.P., editors. Volume 727. U.S. Department of Agriculture Forest Service; Washington, DC, USA: 2008. pp. 298–302. [Google Scholar]
- 58.Obesco J.R. Fruit removal and potential seed dispersal in a southern Spanish population of Berberis vulgaris subsp. australis (Berberidaceae) Acta Oecologica/Oecologia Pantarum. 1989;10:321–328. [Google Scholar]
- 59.Royer F., Dickinson R. Weeds of the Northern U.S. and Canada: A Guide for Identification. University of Alberta; Edmonton, AB, Canada: 1999. [Google Scholar]
- 60.Chapman W.K., Bessette A.E. Trees and Shrubs of the Adirondacks. North Country Books, Inc.; Utica, NY, USA: 1990. [Google Scholar]
- 61.Eriksson O., Ehrlén J. Phenological variation in fruit characteristics in vertebrate-dispersed plants. Oecologia. 1991;86:463–470. doi: 10.1007/BF00318311. [DOI] [PubMed] [Google Scholar]
- 62.Imanshahidi M., Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phyther. Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 63.Alemardan A., Asadi W., Rezaei M., Tabrizi L., Mohammadi S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crops Prod. 2013;50:276–287. doi: 10.1016/j.indcrop.2013.07.061. [DOI] [Google Scholar]
- 64.Potdar D., Hirwani R.R., Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83:817–830. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Dolezal M., Velisek J., Famfulikova P. Chemical composition of less-known wild fruits; Proceedings of the EUROFOODCHEM XI Meeting; Norwich, UK. 26–28 September 2001; pp. 241–244. [Google Scholar]
- 66.Hamedi A., Moheimani S.M., Sakhteman A., Etemadfard H., Moein M. An Overview on Indications and Chemical Composition of Aromatic Waters (Hydrosols) as Functional Beverages in Persian Nutrition Culture and Folk Medicine for Hyperlipidemia and Cardiovascular Conditions. J. Evid.-Based Complement. Altern. Med. 2017;22:544–561. doi: 10.1177/2156587216686460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonmezdag A.S., Kelebek H., Selli S. Volatile and key odourant compounds of Turkish Berberis crataegina fruit using GC-MS-Olfactometry. Nat. Prod. Res. 2018;32:777–781. doi: 10.1080/14786419.2017.1360882. [DOI] [PubMed] [Google Scholar]
- 68.Hashemi-Moghaddam H., Mohammadhosseini M., Azizi Z. Impact of amine- and phenyl-functionalized magnetic nanoparticles impacts on microwave-assisted extraction of essential oils from root of Berberis integerrima Bunge. J. Appl. Res. Med. Aromat. Plants. 2018;10:1–8. doi: 10.1016/j.jarmap.2018.03.007. [DOI] [Google Scholar]
- 69.Bonesi M., Loizzo M.R., Conforti F., Passalacqua N.G., Saab A., Menichini F., Tundis R. Berberis aetnensis and B. libanotica: A comparative study on the chemical composition, inhibitory effect on key enzymes linked to Alzheimer’s disease and antioxidant activity. J. Pharm. Pharmacol. 2013;65:1726–1735. doi: 10.1111/jphp.12172. [DOI] [PubMed] [Google Scholar]
- 70.Jay J. Modern Food Microbiology. Aspen Publishers Inc.; Gaithersburg, MD, USA: 1998. [Google Scholar]
- 71.Pszczola D.E. Emerging ingredients: Believe it or not! Food Technol. 1999;53:98–100. [Google Scholar]
- 72.Kaya M., Ravikumar P., Ilk S., Mujtaba M., Akyuz L., Labidi J., Salaberria A.M., Cakmak Y.S., Erkul S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018;45:287–297. doi: 10.1016/j.ifset.2017.11.013. [DOI] [Google Scholar]
- 73.Thusa R., Mulmi S. Analysis of Phytoconstituents and Biological Activities of Different Parts of Mahonia nepalensis and Berberis aristata. Nepal J. Biotechnol. 2017;5:5–13. doi: 10.3126/njb.v5i1.18864. [DOI] [Google Scholar]
- 74.Pokhrel N.R., Adhikari R.P., Baral M.P. In Vitro screening and evaluation of antimicrobial activities of some medicinal plants of Nepal. Nepal J. Sci. Technol. 2003;5:1. [Google Scholar]
- 75.Ebrahimi A., Chavoushpour M., Mahzoonieh M.R., Lotfalian S. Antibacterial activity and ciprofloxacin-potentiation property of Berberis vulgaris asperma stem extracts on pathogenic bacteria. J. HerbMed Pharmacol. 2016;5:112–115. [Google Scholar]
- 76.Singh S.K., Vishnoi R., Dhingra G.K., Kishor K. Antibacterial activity of leaf extracts of some selected traditional medicinal plants of Uttarakhand, North East India. J. Appl. Nat. Sci. 2012;4:47–50. doi: 10.31018/jans.v4i1.220. [DOI] [Google Scholar]
- 77.Saravanakumar T., Manonmani E., Venkatasubramanian P., Vasanthi N.S. Antimicrobial potential of Daruharidra (Berberis aristata DC) against the pathogens causing eye infection. Int. J. Green Pharm. 2014;8:153. doi: 10.4103/0973-8258.140170. [DOI] [Google Scholar]
- 78.Alamzeb M. Bioassay guided isolation and characterization of anti-microbial and anti-trypanosomal agents from Berberis glaucocarpa Stapf. African J. Pharm. Pharmacol. 2013;7:2564–2570. doi: 10.5897/AJPP2013.3444. [DOI] [Google Scholar]
- 79.Parvu M., Parvu A.E., Craciun C., Barbu-Tudoran L., Vlase L., Tamas M., Rosca-Casian O., PersecA O., Molnar A.M. Changes in Botrytis cinerea conidia caused by Berberis vulgaris extract. Not. Bot. Horti Agrobot. Cluj-Napoca. 2010;38:15–20. [Google Scholar]
- 80.Shah Z., Ilyas M., Khan M., Ahmad A., Khan M., Khan N. Antimicrobial activities of selected medicinal plants collected from Northern districts of Khyber Pakhtunkhwa, Pakistan. J. Pharm. Res. 2012;5:1729–1733. [Google Scholar]
- 81.Ghareeb D.A., El-Wahab A.E.A. Biological assessment of Berberis vulgaris and its active constituent, berberine: Antibacterial, antifungal and anti-hepatitis C virus (HCV) effect. J. Med. Plants Res. 2013;7:1529–1536. [Google Scholar]
- 82.Shahid T., Memon M., Malik R.A., Ikram N., Malik W., Ali A. A study of Antimicrobial Activity of Berberis vulgaris (Zirishk) Aqueous Plant Extract using Pathogenic Isolates from Patients of Islamabad and Rawalpindi. Imp. J. Interdiscip. Res. 2017;3:1365–1371. [Google Scholar]
- 83.Krisch J., Galgóczy L., Tölgyesì M., Papp T., Vágvölgyi C. Effect of fruit juices and pomace extracts on the growth of Gram-positive and Gram-negative bacteria. Acta Biol. Szeged. 2008;52:267–270. [Google Scholar]
- 84.Rasool S., Khan F.Z., Hassan S.U., Ahmed M., Ahmed M., Tareen R.B. Anticonvulsant, antimicrobial and cytotoxic activities of berberis calliobotrys aitch ex koehne (Berberidaceae) Trop. J. Pharm. Res. 2015;14:2031–2039. doi: 10.4314/tjpr.v14i11.12. [DOI] [Google Scholar]
- 85.Irshad A.H., Pervaiz A.H., Abrar Y.B., Fahelboum I., Awen B.Z.S. Antibacterial activity of Berberis lycium root extract. Trakia J. Sci. 2013;11:88–90. [Google Scholar]
- 86.Haouat A.C., Haggoud A., David S., Ibnsouda S., Iraqui M. In vitro evaluation of the antimycobacterial activity and fractionation of Berberis hispanica root bark. J. Pure Appl. Microbiol. 2014;8:917–925. [Google Scholar]
- 87.Mattana C.M., Satorres S.E., Juan V., Cifuente D., Tonn C., Laciar A.L. Antibacterial activity study of single and combined extracts of Berberis ruscifolia, Baccharis sagittalis, Euphorbia dentata and Euphorbia schikendanzii, native plants from Argentina. BLACPMA. 2012;11:428–434. [Google Scholar]
- 88.Joshi P.V., Shirkhedkar A.A., Prakash K., Maheshwari V.L. Antidiarrheal activity, chemical and toxicity profile of Berberis aristata. Pharm. Biol. 2011;49:94–100. doi: 10.3109/13880209.2010.500295. [DOI] [PubMed] [Google Scholar]
- 89.Singh M., Srivastava S., Rawat A.K.S. Antimicrobial activities of Indian Berberis species. Fitoterapia. 2007;78:574–576. doi: 10.1016/j.fitote.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Hussain M.A., Khan M.Q., Habib T., Hussain N. Antimicronbial activity of the crude root extract of berberis lycium royle. Adv. Environ. Biol. 2011;5:585–588. [Google Scholar]
- 91.Azimi G., Hakakian A., Ghanadian M., Joumaa A., Alamian S. Bioassay-directed isolation of quaternary benzylisoquinolines from Berberis integerrima with bactericidal activity against Brucella abortus. Res. Pharm. Sci. 2018;13:149–158. doi: 10.4103/1735-5362.223797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bukhari I., Hassan M., Abbasi F., Mujtaba G., Mahmood N., Fatima A., Afzal M., Rehman M., Perveen P., Khan T. A study on comparative pharmacological efficacy of Berberis lycium and penicillin G. African J. Microbiol. Res. 2011;5:725–727. [Google Scholar]
- 93.Sati S.C., Takuli P., Kumar P., Khulbe K. Antibacterial activity of three medicinal plants of Kumaun Himalaya against some pathogenic bacteria. Int. J. Pharma Sci. Res. 2015;6:1361–1368. [Google Scholar]
- 94.Malik Z., Jain K., Ravindran K., Sathiyaraj G. In vitro antimicrobial activity and preliminary phytochemical analysis of Berberis aristata. Int. J. Ethnobiol. Ethnomed. 2017;4:1–6. [Google Scholar]
- 95.Manosalva L., Mutis A., Urzúa A., Fajardo V., Quiroz A. Antibacterial activity of alkaloid fractions from berberis microphylla G. Forst and study of synergism with ampicillin and cephalothin. Molecules. 2016;21:76. doi: 10.3390/molecules21010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehmood A., Murtaza G., Bhatti T.M., Kausar R., Ahmed M.J. Biosynthesis, characterization and antimicrobial action of silver nanoparticles from root bark extract of Berberis lycium Royle. Pak. J. Pharm. Sci. 2016;29:131–137. [PubMed] [Google Scholar]
- 97.Anzabi Y. In vitro study of Berberis vulgaris, Actinidia deliciosa and Allium cepa L. antibacterial effects on Listeria monocytogenes. Crescent J. Med. Biol. Sci. 2015;2:111–115. [Google Scholar]
- 98.Thakur P., Chawla R., Narula A., Sharma R.K. Protective effect of Berberis aristata against peritonitis induced by carbapenem-resistant Escherichia coli in a mammalian model. J. Glob. Antimicrob. Resist. 2017;9:21–29. doi: 10.1016/j.jgar.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 99.Shahid M., Rahim T., Shahzad A., Latif T.A., Fatma T., Rashid M., Raza A., Mustafa S. Ethnobotanical studies on Berberis aristata DC. root extracts. African J. Biotechnol. 2009;8:556–563. [Google Scholar]
- 100.Rizwan M., Nasir H., Shah S.Z. Phytochemical and biological screening of Berberis aristata. Adv. Life Sci. 2017;57:1–7. [Google Scholar]
- 101.Amin A.H., Subbaiah T.V., Abbasi K.M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 1969;15:1067–1076. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 102.Freile M.L., Giannini F., Pucci G., Sturniolo A., Rodero L., Pucci O., Balzareti V., Enriz R.D. Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Fitoterapia. 2003;74:702–705. doi: 10.1016/S0367-326X(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 103.Hyun T.K., Kim H.C., Kim J.S. In vitro Screening for Antioxidant, Antimicrobial, and Antidiabetic Properties of Some Korean Native Plants on Mt. Halla, Jeju Island. Indian J. Pharm. Sci. 2015;77:668–674. doi: 10.4103/0250-474x.174984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kosalec I., Gregurek B., Kremer D., Zovko M., Sanković K., Karlović K. Croatian barberry (Berberis croatica Horvat): A new source of berberine—Analysis and antimicrobial activity. World J. Microbiol. Biotechnol. 2009;25:145–150. doi: 10.1007/s11274-008-9860-x. [DOI] [Google Scholar]
- 105.Malik T.A., Kamili A.N., Chishti M.Z., Ahad S., Tantry M.A., Hussain P.R., Johri R.K. Breaking the resistance of Escherichia coli: Antimicrobial activity of Berberis lycium Royle. Microb. Pathog. 2017;102:12–20. doi: 10.1016/j.micpath.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 106.Musumeci R., Speciale A., Costanzo R., Annino A., Ragusa S., Rapisarda A., Pappalardo M.S.S., Iauk L. Berberis aetnensis C. Presl. extracts: Antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents. 2003;22:48–53. doi: 10.1016/S0924-8579(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 107.Villinski J.R., Dumas E.R., Chai H.B., Pezzuto J.M., Angerhofer C.K., Gafner S. Antibacterial activity and alkaloid content of Berberis thunbergii, Berberis vulgaris and Hydrastis canadensis. Pharm. Biol. 2003;41:551–557. doi: 10.1080/13880200390500768. [DOI] [Google Scholar]
- 108.Bereksi M.S., Hassaïne H., Bekhechi C., Abdelouahid D.E. Evaluation of Antibacterial Activity of some Medicinal Plants Extracts Commonly Used in Algerian Traditional Medicine against some Pathogenic Bacteria. Pharmacogn. J. 2018;10:507–512. doi: 10.5530/pj.2018.3.83. [DOI] [Google Scholar]
- 109.Maznah I., Teoh S.L., Loh P. Determination of total antioxidant activity of selected local medicinal plants; Proceedings of the Herbs an International Conference and Exhibitions, Mines; Seri Kembangan, Malaysia. 9–11 November 1999; pp. 124–128. [Google Scholar]
- 110.Eddouks M., Maghrani M., Lemhadri A., Ouahidi M.-L., Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet) J. Ethnopharmacol. 2002;82:97–103. doi: 10.1016/S0378-8741(02)00164-2. [DOI] [PubMed] [Google Scholar]
- 111.Sahin K., Orhan C., Tuzcu M., Borawska M.H., Jabłonski J., Guler O., Sahin N., Hayirli A. Berberis vulgaris root extract alleviates the adverse effects of heat stress via modulating hepatic nuclear transcription factors in quails. Br. J. Nutr. 2013;110:609–616. doi: 10.1017/S0007114512005648. [DOI] [PubMed] [Google Scholar]
- 112.Komal S., Ranjan B., Neelam C., Birendra S., Kumar S.N. Berberis Aristata: A Review. Int. J. Res. Ayurveda Pharm. 2011;2:383–388. [Google Scholar]
- 113.Luo A., Fan Y. Antioxidant activities of berberine hydrochloride. J. Med. Plants Res. 2011;5:3702–3707. [Google Scholar]
- 114.Pyrkosz-Biardzka K., Kucharska A.Z., Sokół-Łȩtowska A., Strugała P., Gabrielska J. A comprehensive study on antioxidant properties of crude extracts from fruits of Berberis vulgaris L., Cornus mas L. and Mahonia aquifolium nutt. Polish J. Food Nutr. Sci. 2014;64:91–99. doi: 10.2478/v10222-012-0097-x. [DOI] [Google Scholar]
- 115.Sun L.L., Gao W., Zhang M.M., Li C., Wang A.G., Su Y.L., Ji T.F. Composition and antioxidant activit y of the anthocyanins of the fruit of berberis heteropoda schrenk. Molecules. 2014;19:19078–19096. doi: 10.3390/molecules191119078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Campisi A., Acquaviva R., Bonfanti R., Raciti G., Amodeo A., Mastrojeni S., Ragusa S., Iauk L. Antioxidant Properties of Berberis aetnensis C. Presl (Berberidaceae) Roots Extract and Protective Effects on Astroglial Cell Cultures. Sci. World J. 2014;2014:315473. doi: 10.1155/2014/315473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gundogdu M. Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. Fruits. Adv. Environ. Biol. 2013;7:344–348. [Google Scholar]
- 118.Zaorsky N.G., Churilla T.M., Egleston B.L., Fisher S.G., Ridge J.A., Horwitz E.M., Meyer J.E. Causes of death among cancer patients. Ann. Oncol. 2017;28:400–407. doi: 10.1093/annonc/mdw604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanachi P., Kua S.H., Asmah R., Motalleb G., Fauziah O. Cytotoxic Effect of Berberis vulgaris Fruit Extract on the Proliferation of Human Liver Cancer Cell line (HepG2) and Its Antioxidant Properties. Int. J. Cancer Res. 2006;2:1–9. [Google Scholar]
- 120.Hoshyar R., Mahboob Z., Zarban A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology. 2016;68:1207–1213. doi: 10.1007/s10616-015-9880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abd El-Wahab A.E., Ghareeb D.A., Sarhan E.E.M., Abu-Serie M.M., El Demellawy M.A. In vitro biological assessment of berberis vulgaris and its active constituent, berberine: Antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement. Altern. Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El Khalki L., Tilaoui M., Jaafari A., Ait Mouse H., Zyad A. Studies on the Dual Cytotoxicity and Antioxidant Properties of Berberis vulgaris Extracts and Its Main Constituent Berberine. Adv. Pharmacol. Sci. 2018;2018:3018498. doi: 10.1155/2018/3018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi M.S., Oh J.H., Kim S.M., Jung H.Y., Yoo H.S., Lee Y.M., Moon D.C., Han S.B., Hong J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009;34:1221–1230. [PubMed] [Google Scholar]
- 124.Balakrishna A., Kumar M.H. Evaluation of synergetic anticancer activity of berberine and curcumin on different models of A549, Hep-G2, MCF-7, Jurkat, and K562 cell lines. Biomed Res. Int. 2015;2015:354614. doi: 10.1155/2015/354614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ren K., Zhang W., Wu G., Ren J., Lu H., Li Z., Han X. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed. Pharmacother. 2016;84:1748–1759. doi: 10.1016/j.biopha.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 126.Sengupta P., Raman S., Chowdhury R., Lohitesh K., Saini H., Mukherjee S., Paul A. Evaluation of Apoptosis and Autophagy Inducing Potential of Berberis aristata, Azadirachta indica, and Their Synergistic Combinations in Parental and Resistant Human Osteosarcoma Cells. Front. Oncol. 2017;7:296. doi: 10.3389/fonc.2017.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fukuda K., Hibiya Y., Mutoh M., Koshiji M., Akao S., Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999;66:227–233. doi: 10.1016/S0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- 128.Safi S., Esseily F., El Ezzy M., Gali-Muhtasib H., Esseily J., Diab-Assaf M., Lampronti I., Saab A. The ethanol fraction from the stem of Berberis libanotica inhibits the viability of adult T cell leukemia. Minerva Biotecnol. 2012;24:129–133. [Google Scholar]
- 129.El-Merahbi R., Liu Y.N., Eid A., Daoud G., Hosry L., Monzer A., Mouhieddine T.H., Hamade A., Najjar F., Abou-Kheir W. Berberis libanotica ehrenb extract shows anti-neoplastic effects on prostate cancer stem/progenitor cells. PLoS ONE. 2014;9:e112453. doi: 10.1371/journal.pone.0112453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Diab S., Ftdanzi C., Léger D.Y., Ghezali L., Millot M., Martin F., Azar R., Esseily F., Saab A., Sol V., et al. Berberis libanotica extract targets NF-$κ$B/COX-2, PI3K/Akt and mitochondrial/caspase signalling to induce human erythroleukemia cell apoptosis. Int. J. Oncol. 2015;47:220–230. doi: 10.3892/ijo.2015.3012. [DOI] [PubMed] [Google Scholar]
- 131.Du H.P., Shen J.K., Yang M., Wang Y.G., Yuan X.G., Ma Q.L., Jin J. 4-Chlorobenzoyl berbamine induces apoptosis and G2/M cell cycle arrest through the PI3K/Akt and NF-κB signal pathway in lymphoma cells. Oncol. Rep. 2010;23:709–716. doi: 10.3892/or_00000688. [DOI] [PubMed] [Google Scholar]
- 132.Xie J., Ma T., Gu Y., Zhang X., Qiu X., Zhang L., Xu R., Yu Y. Berbamine derivatives: A novel class of compounds for anti-leukemia activity. Eur. J. Med. Chem. 2009;44:3293–3298. doi: 10.1016/j.ejmech.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 133.Nam S., Xie J., Perkins A., Ma Y., Yang F., Wu J., Wang Y., Zhen Xu R., Huang W., Horne D.A., et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol. Oncol. 2012;6:484–493. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang F., Nam S., Brown C.E., Zhao R., Starr R., Horne D.A., Malkas L.H., Jove R., Hickey R.J. A novel berbamine derivative inhibits cell viability and induces apoptosis in cancer stem-like cells of human glioblastoma, via up-regulation of miRNA-4284 and JNK/AP-1 signaling. PLoS ONE. 2014;9:e94443. doi: 10.1371/journal.pone.0094443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang F., Nam S., Zhao R., Tian Y., Liu L., Horne D.A., Jove R. A novel synthetic derivative of the natural product berbamine inhibits cell viability and induces apoptosis of human osteosarcoma cells, associated with activation of JNK/AP-1 signaling. Cancer Biol. Ther. 2013;14:1024–1031. doi: 10.4161/cbt.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duan H., Luan J., Liu Q., Yagasaki K., Zhang G. Suppression of human lung cancer cell growth and migration by berbamine. Cytotechnology. 2010;62:341–348. doi: 10.1007/s10616-009-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu J., Yu D., Sun H., Zhang Y., Zhang W., Meng F., Du X. Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crops Prod. 2015;69:68–75. doi: 10.1016/j.indcrop.2015.01.053. [DOI] [Google Scholar]
- 138.Bavand R., Nemati F. Cytotoxic effect of the root extract of Berberis orthobotrys on hela cell line. IIOAB J. 2016;7:204–208. [Google Scholar]
- 139.Engel N., Ali I., Adamus A., Frank M., Dad A., Ali S., Nebe B., Atif M., Ismail M., Langer P., et al. Antitumor evaluation of two selected Pakistani plant extracts on human bone and breast cancer cell lines. BMC Complement. Altern. Med. 2016;16:1. doi: 10.1186/s12906-016-1215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim K.H., Choi S.U., Lee K.R. Bioactivity-guided isolation of cytotoxic triterpenoids from the trunk of Berberis koreana. Bioorganic Med. Chem. Lett. 2010;20:1944–1947. doi: 10.1016/j.bmcl.2010.01.156. [DOI] [PubMed] [Google Scholar]
- 141.Kim K.H., Choi S.U., Lee K.R. Cytotoxic triterpenoids from Berberis koreana. Planta Med. 2012;78:86–89. doi: 10.1055/s-0031-1280127. [DOI] [PubMed] [Google Scholar]
- 142.Derosa G., Romano D., D’Angelo A., Maffioli P. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis. 2015;239:87–92. doi: 10.1016/j.atherosclerosis.2014.12.043. [DOI] [PubMed] [Google Scholar]
- 143.Derosa G., Romano D., D’Angelo A., Maffioli P. Berberis aristata/Silybum marianum fixed combination (Berberol®) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: A randomized, placebo-controlled, clinical trial. Phytomedicine. 2015;22:231–237. doi: 10.1016/j.phymed.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Derosa G., D’Angelo A., Maffioli P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin. Nutr. 2016;35:1091–1095. doi: 10.1016/j.clnu.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 145.Derosa G., D’Angelo A., Romano D., Maffioli P. Effects of a Combination of Berberis aristata, Silybum marianum and Monacolin on Lipid Profile in Subjects at Low Cardiovascular Risk; A Double-Blind, Randomized, Placebo-Controlled Trial. Int. J. Mol. Sci. 2017;18:343. doi: 10.3390/ijms18020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guarino G., Strollo F., Carbone L., Della Corte T., Letizia M., Marino G., Gentile S. Bioimpedance analysis, metabolic effects and safety of the association Berberis aristata/Bilybum marianum: A 52-week double-blind, placebo-controlled study in obese patients with type 2 diabetes. J. Biol. Regul. Homeost. Agents. 2017;31:495–502. [PubMed] [Google Scholar]
- 147.Di Pierro F., Putignano P., Villanova N., Montesi L., Moscatiello S., Marchesini G. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin. Pharmacol. Adv. Appl. 2013;5:167–174. doi: 10.2147/CPAA.S54308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zilaee M., Kermany T., Tavalaee S., Salehi M., Ghayour-Mobarhan M., Ferns G.A. Barberry treatment reduces serum anti-heat shock protein 27 and 60 antibody titres and high-sensitivity c-reactive protein in patients with metabolic syndrome: A double-blind, randomized placebo-controlled trial. Phytother. Res. 2014;28:1211–1215. doi: 10.1002/ptr.5117. [DOI] [PubMed] [Google Scholar]
- 149.Brenyo A., Aktas M.K. Review of complementary and alternative medical treatment of arrhythmias. Am. J. Cardiol. 2014;113:897–903. doi: 10.1016/j.amjcard.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 150.Guo Y., Chen Y., Tan Z.R., Klaassen C.D., Zhou H.H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur. J. Clin. Pharmacol. 2012;68:213–217. doi: 10.1007/s00228-011-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen C., Tao C., Liu Z., Lu M., Pan Q., Zheng L., Li Q., Song Z., Fichna J. A Randomized Clinical Trial of Berberine Hydrochloride in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Phyther. Res. 2015;29:1822–1827. doi: 10.1002/ptr.5475. [DOI] [PubMed] [Google Scholar]
- 152.Fouladi R.F. Aqueous extract of dried fruit of berberis vulgaris L. in acne vulgaris, a clinical trial. J. Diet. Suppl. 2012;9:253–261. doi: 10.3109/19390211.2012.726702. [DOI] [PubMed] [Google Scholar]
- 153.Shin K.S., Choi H.S., Zhao T.T., Suh K.H., Kwon I.H., Choi S.O., Lee M.K. Neurotoxic effects of berberine on long-term l-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch. Pharm. Res. 2013;36:759–767. doi: 10.1007/s12272-013-0051-4. [DOI] [PubMed] [Google Scholar]
- 154.Kwon I.H., Choi H.S., Shin K.S., Lee B.K., Lee C.K., Hwang B.Y., Lim S.C., Lee M.K. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci. Lett. 2010;486:29–33. doi: 10.1016/j.neulet.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 155.Qian C., Zhu F. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BioMed. 2006;3:1–9. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ahmed T., Gilani A.U.H., Abdollahi M., Daglia M., Nabavi S.F., Nabavi S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015;67:970–979. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]