Abstract
Purpose of the Review
In the present paper, we overview emerging research examining the autonomic nervous system (ANS), especially the parasympathetic nervous system as indexed by heart rate variability (HRV), and the impact of psychosocial factors on hypertension-related disease in African Americans.
Recent Findings
A growing corpus of studies has shown that (1) usual patterns of compensatory sympathetic-parasympathetic regulation differ between African Americans and European Americans; (2) despite their enhanced cardiovascular disease risk profile, African Americans tend to exhibit higher HRV relative to European Americans; and (3) racial discrimination and other forms of psychosocial stress are associated with diminished HRV among African Americans.
Summary
Significant disparities in hypertension-related disease exist such that African Americans have greater risk. The underlying factors associated with this increased risk are, to date, not fully understood. The present review provides evidence for a unique pattern of ANS regulation in African Americans and shows that psychosocial factors such as racial discrimination may contribute to this paradoxical situation.
Keywords: Hypertension, Autonomic imbalance, Heart rate variability, Discrimination, African Americans
Introduction
Hypertension is one of the leading risk factors for cardiovascular disease morbidity and mortality [1]. Importantly, significant ethnic differences exist such that African Americans have the highest prevalence of hypertension globally [2] as well as increased hypertension-related disease burden for both cardiovascular and non-cardiovascular diseases [3, 4]. African Americans also develop hypertension at an earlier age, have a differential developmental trajectory of high blood pressure, have higher average blood pressure, and worse prognosis compared to their European American counterparts [5, 6].
Despite decades of research, ethnic differences in hypertension persist without much insight into the causes for this disparity. These disparities appear to persist even after adjustment for many socioeconomic, behavioral, and biomedical risk factors. The autonomic nervous system (ANS) has been implicated in hypertension with elevated sympathetic nervous system (SNS) activity and decreased parasympathetic nervous system (PNS) activity associated with the etiology, course, progression, and consequences of hypertension [7]. The present paper reviews the role of the ANS in hypertension and related diseases with special emphasis on ethnic differences and the potential role of psychosocial factors.
Taking A Closer Look at Autonomic (Im) Balance
Autonomic nervous system dysfunction has long been considered an important pathophysiological mechanism underlying cardiovascular disease (CVD) risk, especially hypertension. Indeed, the etiology of hypertension has been characterized in terms of autonomic imbalance, wherein central and peripheral activity of the sympathetic branch of the ANS is heightened while activity of the parasympathetic branch is diminished [7, 8]. The relative sympathetic dominance observed in hypertension has been further described as a pattern of “hyperkinetic” circulation [9], in which, initial elevations in blood pressure (BP) are driven by predominately central mechanisms (i.e., increased heart rate and cardiac output). Over time, this heightened central drive shifts to become more peripheral in nature, with sustained elevations in BP maintained through elevated systemic vascular resistance (SVR). Mean arterial pressure (MAP) is a function of cardiac output (CO), which is stroke volume (SV) times heart rate (HR), and SVR. The ANS and baroreflex mediate this relationship such that greater PNS activity including lower HR is associated with lower MAP and SVR. To maintain MAP at a constant level, increases in SVR must be offset by decreases in CO and HR. When increases in SVR are not compensated by decreased CO and HR, then MAP increases.
SNS-mediated vascular dysfunction has been proposed as the predominant mechanism underpinning the higher burden of hypertension among African Americans [10–12]. Indeed, several lines of evidence provide converging support for this view including observations that African Americans tend to exhibit elevated SVR in early-stage hypertension, as well as greater levels of SVR at similar levels of BP, relative to European Americans [13–15]. SVR also has been shown to play a stronger role in diurnal BP regulation among African Americans relative to European Americans [16]. Moreover, recent evidence has further shown that the well-known non-dipping phenomenon in nighttime BP, which is also more common among African Americans and is associated with elevated CVD morbidity and mortality risk, also is characterized by a corresponding blunted decline in nighttime SVR [17]. Importantly, research examining the role of psychosocial stress in CVD risk has consistently revealed that African Americans tend to exhibit exaggerated or enhanced vasoconstrictive, and/or impaired vasodilatory responses to a range of behavioral and psychological stressors [14, 15]. Psychosocial stress including hostility and past experiences of racial discrimination have further been linked to enhanced alpha- and blunted beta-adrenergic receptor sensitivity, respectively, among African Americans providing further support for the view that SNS-mediated mechanisms play an important role in hypertension and CVD etiology and progression among this group [18, 19].
Given such consistent evidence regarding the role of the SNS, it is not entirely surprising that parasympathetic functioning among African Americans has been relatively overlooked.
Heart rate variability, commonly defined as variations in time in the interval between consecutive heart beats (i.e., inter-beat of RR intervals), is regarded as an important marker of autonomic nervous system functioning, and particularly, of parasympathetic (i.e., vagal) influence on the heart. Importantly, lower HRV, reflecting minimal or limited fluctuations in heart rate from one beat to the next, is an established risk factor for cardiovascular and all-cause mortality, and has been independently linked to established CVD risk factors including age, obesity, smoking, total and low density cholesterol, positive family history of CVD, and diabetes [20]. Recently, in a large sample of European men and women, HRV was inversely associated with four cardiovascular disease risk scores including the Hard Coronary Heart Disease Framingham score [21]. Low HRV also has been shown to predict hypertension onset [22] and is inversely associated with target organ damage including left ventricular hypertrophy [23–26] which also occurs more commonly among African Americans relative to European Americans [27–30].
Indeed, based on their well-documented greater CVD risk profile, as well as established associations of lower HRV with heightened CVD morbidity and mortality risk, it has been suggested low HRV may represent an additional significant pathway underlying the excessive CVD burden faced by African Americans [31]. In contrast to this notion, findings have emerged which demonstrate that African Americans may simultaneously exhibit both elevated SVR and higher HRV [32] relative to European Americans, a pattern which has been termed the “Cardiovascular Conundrum” [33•, 34]. Whereas the elevated SVR is consistent with the known increased risk in African Americans, the elevated HRV should be cardioprotective. In the remainder of this article, we will overview research examining relative ethnic differences in HRV, primarily between African Americans and European Americans, as well as research examining the impact of unique and common forms of psychosocial stress on HRV among African Americans. We will close with a discussion of candidate mechanisms which may offer further insights into this potential alternate pattern of autonomic-hemodynamic regulation as well as important recommendations for future research.
Ethnic Differences in Heart Rate Variability
While consistent research examining ethnic differences in HRV has only emerged within the last decade, there has been some previous evidence indicating higher HRV among African Americans relative to European Americans. For example, a 1995 investigation conducted in a randomly selected subsample of middle-aged (age range 45–64) adults (N > 1900), from the Atherosclerotic Risk in Communities (ARIC) study, observed that African Americans exhibited higher HRV relative to European Americans, in models adjusted for both age and gender [35]. A later study reported findings in a subsample of adolescent males drawn from the Bogalusa Heart Study [36]. Notably, these researchers examined ethnic differences in autonomic responses to several laboratory stressors (i.e., Valsalva maneuver, cold pressor). Although they exhibited higher resting and ambulatory blood pressure, African American adolescent males also displayed higher HRV relative to European American adolescent males in this study [36]. Another study also reported higher HRV among African Americans relative to subsamples of European, Hispanic, and Chinese Americans in the Multi-Ethnic Study of Atherosclerosis (MESA) sample [37].
Other research has indicated that African Americans exhibit lower HRV relative to European Americans and other groups. For example, a study comprised of an all-male sample found that young, healthy, African American men exhibited significantly lower HRV compared to an age-matched group composed of European, Hispanic, and Asian American men [38]. Another investigation conducted in a middle-aged (mean age = 48 ± 17 years) outpatients undergoing 24-h heart rate monitoring observed lower HRV among African Americans compared to age-matched European Americans [31]. In addition, a later analysis of a subsample from the ARIC study found African Americans to exhibit lower HRV, a pattern consistent with levels observed among older European American participants [39]. Still, other research has observed no differences in HRV between African and European Americans [40–42].
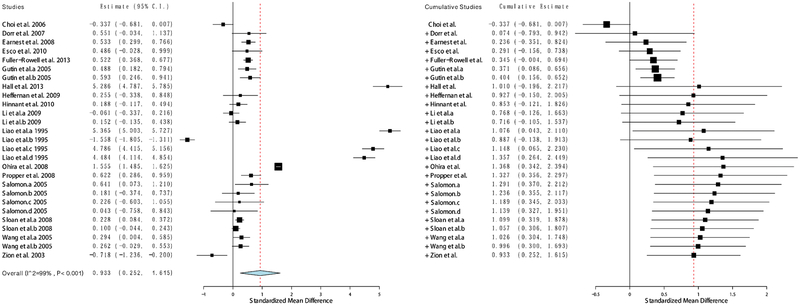
To begin to address these mixed findings, we conducted a meta-analysis of the available studies examining ethnic differences in HRV [33•]. In a sample spanning 17 published studies and over 11,000 participants, we observed a consistent effect indicating higher HRV among African Americans compared to European Americans. Importantly, the magnitude of this difference equated to nearly one standard deviation, Hedges’ g = 0.93, 95% CI (0.25–1.62), and remained robust even accounting for the potential influence of age, sex, and health status (see Fig. 1). Other research has further demonstrated that this phenomenon also may be present among other cultural groups. Notably, researchers recently observed a similar pattern of ethnic differences in HRV in a large sample (N = 11,989) of Brazilian civil service employees [43•]. Consistent with national census approaches in Brazil, participants in this study self-identified as either Black, Brown, or White. Intriguingly, participants who self-identified as Black had higher HRV than both Brown and White participants, while Brown participants also exhibited higher HRV relative to White participants. In addition to being consistent with the overall pattern observed in the earlier meta-analysis, this pattern of findings is notable because Black and Brown individuals in Brazil face a similar cardiovascular disease and mortality burden, as well as experience similar adverse social conditions as African Americans in the USA. In fact, these differences in HRV were attenuated when exposure to discrimination was added to the models. Importantly and consistent with the elevated vascular resistance, the Blacks in this sample had higher levels of hypertension relative to Browns and Whites, and Browns had higher levels of hypertension relative to Whites. As we discuss below, psychosocial stress, and particularly, race-related stress such as experiences of racial discrimination may be uniquely associated with HRV among African Americans.
Fig. 1.
Forest plot of effect size estimates (g) obtained from studies examining Black-White ethnic differences in resting-state vagally mediated heart rate variability (reprinted with permission from Hill et al. Psychosomatic Medicine, 2015;77(1):16–25. Copyright 2015 by Wolters Kluwer) [33•]
Psychosocial Stressors and Heart Rate Variability
Socioeconomic Factors
Autonomic dysfunction has been characterized as one potential mechanism linking lower socioeconomic status (SES) to racial disparities in health [44–47]. There is growing evidence that low SES is associated with lower basal HRV [46], as well as impaired recovery in HRV following stressful tasks [45, 48]. For example, Fuller-Rowell and colleagues examined race- and age-related differences in basal HRV and the change in HRV following a laboratory stressor (i.e., mental arithmetic and Stroop task) task in African and European American participants from the Midlife in the United States (MIDUS) study. These researchers found that HRV reactivity, the change in HRV during the stressor, decreased more rapidly with increasing age and that this pattern was more pronounced among African Americans. Importantly, a similar trend was observed for SES such that individuals with lower SES also exhibited less reactivity with increasing age, and SES was shown to partially account for the race difference in HRV reactivity. These findings provide support for a link between SES and HRV but also suggest that this relationship may vary as a function of age, race, and assessment condition (i.e., stressful versus non-stressful). Whereas low SES has previously been linked to impaired HRV recovery following a stressful task among Europeans in the Whitehall II cohort [49], a recent study observed a negative association between adulthood SES and HRV following as stressor in 246 African American and European American men followed since 1988 as part of the Pittsburgh Youth Study [44]. This latter finding raises the possibility that the association between SES and both resting and stress-related HRV may be more nuanced and warrants further study.
Racial Discrimination
The ANS has been described as the “first line of defense” in the stress response [50], and researchers have long suggested that the ANS, primarily the sympathetic branch, is an important link between the experience of race-related stress and poorer cardiovascular and overall health among African Americans [51]. Nearly 20 years ago, this idea was formally conceptualized into a broader biopsychosocial model proposing that the experience of racial discrimination represents a unique form of chronic psychosocial stress that contributes to dysregulation across multiple allostatic (i.e., cardiovascular, immune, neuroendocrine) systems [52]. At the same time, researchers were also beginning to consider that racism and discrimination may have a deleterious impact on health through diminished parasympathetic nervous system activity [53].
Hypertension and related complications have been implicated as the largest contributors to CVD-related deaths among African Americans [54]. Emerging data has also linked both structural and institutional forms of racial discrimination to greater CVD and all-cause mortality among African Americans [55, 56]. Contemporary research from population-based investigations including the Cardiovascular Risk Development in Young Adults (CARDIA) and Jackson Heart Study has shown that racial discrimination is consistently associated with hypertension prevalence, but not necessarily with BP, among African Americans [57, 58]. This raises the possibility that racial discrimination may be more strongly related with underlying autonomic-hemodynamic mechanisms implicated in CVD risk. In addition to structural and biochemical measures of vascular function such as carotid intima-media thickness [59], and Endothelin-1 [60], as well as vascular and systemic inflammatory markers (i.e., C-reactive protein) [61, 62], growing evidence also indicates that racial discrimination is inversely associated with HRV among African Americans. Notably, findings from laboratory-based studies have shown that African Americans exhibit blunted HRV recovery following exposure to race-related stressors [32] [63]. Other evidence suggests that the inverse relationship between past experiences of racial discrimination and HRV may impact other groups as well. Notably, Wagner and colleagues reported that lifetime discrimination was negatively associated with HRV recorded during a stressful speech task in a combined sample of European and African American women [64].
Research also has considered the impact of inter- versus intragroup discrimination on HRV. For example, a study by Hoggard and colleagues [64] showed that African American women exposed to discriminatory statements involving an African American confederate, paradoxically, exhibited an increase in HRV following the interaction. In contrast, African American women exposed to the same stimuli, but from a European American confederate exhibited no change in HRV following the task; however, these women displayed lower HRV and higher HR upon returning to the laboratory environment for follow-up on the very next day [65]. As these findings suggest, exposure to environments where previous experiences of racial discrimination have occurred may be an additional source of stress associated with alterations in cardiac autonomic functioning.
Other work has shown that discrimination related to threats or the experience of actual harm predicted lower HRV among African Americans [34], while exposure to explicitly discriminatory information about one’s own racial/ethnic group (i.e., stereotype threat) has been associated with impaired HRV recovery following a cognitively demanding task [66]. Importantly, cognitive processes associated with increased risk for both poorer mental and physical health also may influence the association between discrimination and HRV. For example, a recent investigation found that rumination, or a repetitive focus on the origins, indicators, and consequences of one’s negative emotions and experiences, significantly moderated the association between racial discrimination and heart rate variability in a sample of African Americans [67]. Increased rumination has previously been identified as one potential maladaptive response to chronic discrimination stress [52] and several studies have shown that rumination mediates the relationship between racial discrimination and depressive symptoms among African Americans and other stigmatized groups [68–70]. Importantly, rumination is one component of a larger pattern of repetitive, perseverative thinking that has been consistently linked to lower HRV, but also to elevated BP and SVR in numerous studies conducted in both US and European populations [71].
Characterizing the Conundrum
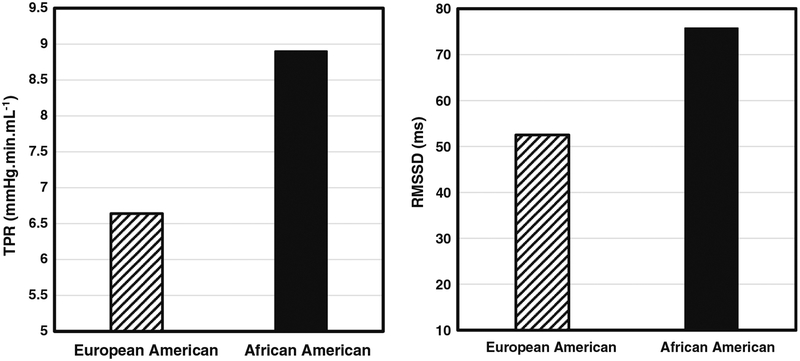
Classical perspectives on CVD etiology conceptualize autonomic imbalance as a state characterized by SNS hyperactivity and attenuated parasympathetic cardiac influence (i.e., lower HRV) [7]. African Americans face the greatest burden of hypertension in the world, and the contribution of SNS-mediated vascular dysfunction is one of the primary mechanisms thought to underlie this disparity [14, 19]. Yet, there is growing evidence of jointly elevated SNS and PNS activity in African Americans, a pattern we have termed the “Cardiovascular Conundrum.” Notably, in several studies in which African Americans have been shown to exhibit higher HRV, they also have evinced higher BP, relative to European Americans [32, 35, 36, 72]. This pattern also appears to extend to underlying hemodynamic mechanisms (for example, see Fig. 2a and b), as a study by Dorr and colleagues [32] observed both greater basal vascular resistance and HRV among African Americans.
Fig. 2.
Mean resting total peripheral resistance and vagally mediated heart rate variability in European (N = 26) and African American (N = 24) men (adapted with permission from Dorr et al. International Journal of Psychophysiology, 2007;66(2):125–34. Copyright 2007, with permission from Elsevier) [32]
Other studies have shown that typically inverse associations between HRV with psychosocial and pathophysiological CVD risk markers also appear to be reversed among African Americans. For instance, depression is a well-established predictor of lower HRV and increased CVD risk [20, 73]; yet, a recent investigation found that depressive symptoms were positively associated with HRV in a sample of middle-aged African Americans [74]. Moreover, increased left ventricular mass (LVM) is an early manifestation of hypertension-related target organ damage that is more common among African Americans and is further associated with increased CVD morbidity and mortality risk [27, 28]. However, in a recent study, researchers found that resting HRV was positively associated with LVM among African American, but not European American adults with normal and untreated high blood pressure [75]. While intriguing, these findings also underscore the relatively limited nature of the available studies characterizing potentially unique associations between HRV and both mental and physical health outcomes in African Americans. Additional research will be essential in ultimately elucidating whether the higher HRV observed among African Americans is clinically meaningful either as evidence of an alternative state of autonomic imbalance, or as a relative marker of stress resilience. With respect to the latter, based on converging evidence from neuroimaging, and laboratory-based investigations linking HRV to self-regulatory processes [76–78], researchers have speculated that higher HRV among African Americans may represent a form of psychophysiological compensation, or a greater coping demand subsequent to greater stress (i.e., racial discrimination) burden [33•, 34, 66].
Another possibility is that higher HRV among African Americans may be early evidence of diminished or failed compensation by the arterial baroreceptors. The baroreceptors play a central role in short- and long-term BP regulation, particularly in modulating the dynamic autonomic-hemodynamic mechanisms necessary to respond to shifting metabolic demands as well as maintain a stable BP. Baroreflex sensitivity is inversely associated with ambulatory BP, and prolonged stimulation of the baroreceptors has been associated with decreased long-term BP [79–81]. Moreover, measures of baroreflex function correlate strongly with indices of HRV that are most clearly vagal (i.e., parasympathetic) in nature [82, 83]. Research has shown that lower HRV is inversely associated with elevated BP and greater hypertension incidence up to 3 years later [84, 85]; however, it is unclear whether this pattern is consistent in African Americans as previous studies were conducted in predominately European, or European American samples, or did not report separate results by race/ethnicity. Thus, it remains to be determined whether higher HRV among African Americans may reflect a divergence in the typically compensatory relationship between vagal modulation and BP, as well as to discern the potential implications of this dissociation. While explanations for the conundrum of dually-elevated SNS and PNS activity among African Americans remain speculative, the emerging evidence, nonetheless, provides clear indication that current assumptions and interpretations regarding the relative meaning of high HRV may not be universal, or, at the very least, that HRV may hold different informative value as an indicator of disease risk across diverse populations.
Moving Forward: Implications for Future Research
While there has been a tremendous increase in research examining numerous aspects of HRV in association with both optimal and diminished health, few investigations have considered its potential role in accounting for ethnic disparities in cardiovascular and other health outcomes. Collectively, the studies reviewed here provide consistent support for the notion that African Americans exhibit higher HRV relative to European Americans. However, it is clear that additional research is needed to examine whether HRV is universally informative as an indicator of health, and to determine whether social and cultural stressors uniquely interact with HRV in exacerbating or attenuating CVD risk. There is some indication that higher HRV among African Americans may begin to emerge within the first 3–6 months of life [86]; nonetheless, this pattern has been observed across developmental strata including among middle-aged and older populations [33•]. One broad question that is raised is whether there is any potential benefit of higher HRV among African Americans. Notably, if higher HRV among African Americans reflects a type of psychophysiological adaptation, is this pattern stable? Additionally, what factors are associated with the decline of this potentially cardioprotective mechanism, over time? The available evidence indicates that socioeconomic factors may play a role in ethnic differences in stress-related changes in HRV. Thus, future research incorporating HRV measurements during stressful conditions in African Americans may be invaluable in further deciphering the impact of socioeconomic disadvantage. Moreover, the association between discrimination and HRV also appears to depend on several factors including whether past or anticipated experiences of discrimination involve threats or actual harm, and research also is needed to examine whether the association between discrimination and HRV is potentially influenced by individual differences in coping.
Conclusions
Hypertension is a leading risk factor for CVD. Significant ethnic differences exist such that African Americans are at greater risk than European Americans. The ANS plays a major role in the regulation of blood pressure and a growing body of research suggests ethnic differences in this ANS regulation. We have reviewed this research and found evidence for both elevated peripheral vascular resistance and elevated HRV in African Americans. This so-called cardiovascular conundrum will require further research to understand the role that psychosocial factors may play in this unexpected pattern of blood pressure regulation and its implications for disparities in CVD.
Funding Information
LaBarron K. Hill is supported by funding from the National Heart Lung and Blood Institute (#121708).
Footnotes
Conflict of Interest LaBarron K. Hill and Julian F. Thayer declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article is based on published papers and does not contain any examination with human or animal subjects performed by any of the authors.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy eople 2020 goals. Circulation. 2014;130(19):1692–9. 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3): e28–e292. 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keenan NL, Shaw KM. Centers for disease C, prevention. Coronary heart disease and stroke deaths - United States, 2006. MMWR Suppl. 2011;60(1):62–6. [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 5.Yano Y, Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S, et al. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol. 2017;2(4):381–9. 10.1001/jamacardio.2016.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–41. 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Julius S. Autonomic imbalance, hypertension, and cardiovascular risk. Am J Hypertens. 2000;13(6 Pt 2):112S–22S. [DOI] [PubMed] [Google Scholar]
- 8.Amerena J, Julius S. The role of the autonomic nervous system in hypertension. Hypertens Res. 1995;18(2):99–110. [DOI] [PubMed] [Google Scholar]
- 9.Julius S, Krause L, Schork NJ, Mejia AD, Jones KA, van de Ven C, et al. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens. 1991;9(1):77–84. [DOI] [PubMed] [Google Scholar]
- 10.Patel PD, Velazquez JL, Arora RR. Endothelial dysfunction in African-Americans . Int J Cardiol. 2009;132(2):157–72. 10.1016/j.ijcard.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez C, Sander GE, Giles TD. Prehypertension: defining the transitional phenotype. Curr Hypertens Rep. 2016;18(1):2 10.1007/s11906-015-0611-8. [DOI] [PubMed] [Google Scholar]
- 12.Oparil S, Zaman MA, Calhoun DA. Pathogenesis of hypertension. Ann Intern Med. 2003;139(9):761–76. [DOI] [PubMed] [Google Scholar]
- 13.Hill LK, Sherwood A, Blumenthal JA, Hinderliter AL. Hemodynamics and vascular hypertrophy in African Americans and Caucasians with high blood pressure. Am J Hypertens. 2016;29(12):1380–5. 10.1093/ajh/hpw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taherzadeh Z, Brewster LM, van Montfrans GA, VanBavel E. Function and structure of resistance vessels in black and white people. J Clin Hypertens (Greenwich). 2010;12(6):431–8. 10.1111/j.1751-7176.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alpert BS, Barnard M. Prevention of essential hypertension in minority populations. Prog Pediatr Cardiol. 2001;12(2):189–93. [DOI] [PubMed] [Google Scholar]
- 16.Sherwood A, Hughes JW, McFetridge J. Ethnic differences in the hemodynamic mechanisms of ambulatory blood pressure regulation. Am J Hypertens. 2003;16(4):270–3. 10.1016/S0895-7061(02)03269-7. [DOI] [PubMed] [Google Scholar]
- 17.Sherwood A, Hill LK, Blumenthal JA, Hinderliter AL. Circadian hemodynamics in men and women with high blood pressure: dipper vs. nondipper and racial differences. J Hypertens. 2018;36(2):250–8. 10.1097/Hjh.0000000000001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas KS, Nelesen RA, Malcarne VL, Ziegler MG, Dimsdale JE. Ethnicity, perceived discrimination, and vascular reactivity to phenylephrine. Psychosom Med. 2006;68(5):692–7. 10.1097/01.psy.0000238214.80871.e6. [DOI] [PubMed] [Google Scholar]
- 19.Hill LK, Sherwood A, McNeilly M, Anderson NB, Blumenthal JA, Hinderliter AL. Impact of racial discrimination and hostility on adrenergic receptor responsiveness in African American adults. Psychosom Med. 2018;80(2):208–15. 10.1097/PSY.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–31. 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 21.Schuster AK, Fischer JE, Thayer JF, Mauss D, Jarczok MN. Decreased heart rate variability correlates to increased cardiovascular risk. Int J Cardiol. 2016;203:728–30. 10.1016/j.ijcard.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder EB, Liao D, Chambless LE, Prineas RJ, Evans GW, Heiss G. Hypertension, blood pressure, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Hypertension. 2003;42(6):1106–11. 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 23.Alter P, Grimm W, Vollrath A, Czerny F, Maisch B. Heart rate variability in patients with cardiac hypertrophy–relation to left ventricular mass and etiology. Am Heart J. 2006;151(4):829–36. 10.1016/j.ahj.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Kilit C, Pasali Kilit T, Onrat E. Autonomic modulation in hypertension without hypertrophy. Acta Cardiol. 2015;70(6):721–7. 10.2143/AC.70.6.3120186. [DOI] [PubMed] [Google Scholar]
- 25.Kohara K, Hara-Nakamura N, Hiwada K. Left ventricular mass index negatively correlates with heart rate variability in essential hypertension. Am J Hypertens. 1995;8(2):183–8. 10.1016/0895-7061(94)00190-M. [DOI] [PubMed] [Google Scholar]
- 26.Melillo P, Izzo R, De Luca N, Pecchia L. Heart rate variability and target organ damage in hypertensive patients. BMC Cardiovasc Disord. 2012;12:105 10.1186/1471-2261-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, et al. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2005;112(6):819–27. 10.1161/CIRCULATIONAHA.104.518498. [DOI] [PubMed] [Google Scholar]
- 28.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46(1):124–9. 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 29.Hanevold C, Waller J, Daniels S, Portman R, Sorof J, International Pediatric Hypertension A. The effects of obesity, gender, and ethnic group on left ventricular hypertrophy and geometry in hypertensive children: a collaborative study of the International Pediatric Hypertension Association. Pediatrics. 2004;113(2):328–33. [DOI] [PubMed] [Google Scholar]
- 30.Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, et al. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64(15):1580–7. 10.1016/j.jacc.2014.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150(1):153–60. 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Dorr N, Brosschot JF, Sollers JJ, Thayer JF. Damned if you do, damned if you don’t: the differential effect of expression and inhibition of anger on cardiovascular recovery in Black and White males. Int J Psychophysiol. 2007;66(2):125–34. 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 33.•.Hill LK, Hu DD, Koenig J, Sollers JJ 3rd, Kapuku G, Wang X, et al. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom Med. 2015;77(1):16–25. 10.1097/PSY.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]; This meta-analysis reviews and quantifies the existing literature on ethnic(i.e., Black-White) differences in resting vagally mediated heart rate variability (HRV). Results of the meta-analysis indicate that African Americans tend to exhibit higher HRV relative to European Americans..
- 34.Hill LK, Hoggard LS, Richmond AS, Gray DL, Williams DP, Thayer JF. Examining the association between perceived discrimination and heart rate variability in African Americans. Cultur Divers Ethnic Minor Psychol. 2017;23(1):5–14. 10.1037/cdp0000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao DP, Barnes RW, Chambless LE, Simpson RJ, Sorlie P, Heiss G. Age, race, and sex-differences in autonomic cardiac-function measured by spectral-analysis of heart-rate-variability - the Aric study. Am J Cardiol. 1995;76(12):906–12. 10.1016/S0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- 36.Urbina EM, Bao WH, Pickoff AS, Berenson GS. Ethnic (black-white) contrasts in heart rate variability during cardiovascular reactivity testing in male adolescents with high and low blood pressure - the Bogalusa Heart Study. Am J Hypertens. 1998;11(2):196–202. 10.1016/S0895-7061(97)00314-2. [DOI] [PubMed] [Google Scholar]
- 37.Ohira T, Roux AVD, Prineas RJ, Kizilbash MA, Carnethon MR, Folsom AR. Associations of psychosocial factors with heart rate and its short-term variability: multi-ethnic study of atherosclerosis. Psychosom Med. 2008;70(2):141–6. 10.1097/PSY.0b013e318160686a. [DOI] [PubMed] [Google Scholar]
- 38.Zion AS, Bond V, Adams RG, Williams D, Fullilove RE, Sloan RP, et al. Low arterial compliance in young African-American males. Am J Physiol Heart Circ Physiol. 2003;285(2):H457–62. 10.1152/ajpheart.00497.2002. [DOI] [PubMed] [Google Scholar]
- 39.Choi JB, Hong S, Nelesen R, Bardwell WA, Natarajan L, Schubert C, et al. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med. 2006;68(3):421–6. 10.1097/01.psy.0000221378.09239.6a. [DOI] [PubMed] [Google Scholar]
- 40.Arthur CM, Katkin ES, Mezzacappa ES. Cardiovascular reactivity to mental arithmetic and cold pressor in African Americans, Caribbean Americans, and white Americans. Ann Behav Med. 2004;27(1):31–7. 10.1207/s15324796abm2701_5. [DOI] [PubMed] [Google Scholar]
- 41.Franke WD, Lee K, Buchanan DB, Hernandez JP. Blacks and whites differ in responses, but not tolerance, to orthostatic stress. Clin Auton Res. 2004;14(1):19–25. 10.1007/s10286-004-0155-5. [DOI] [PubMed] [Google Scholar]
- 42.Stein PK, Freedland KE, Skala JA, Carney RM, DavilaRoman V, Rich MW, et al. Heart rate variability is independent of age, gender, and race in congestive heart failure with a recent acute exacerbation. Am J Cardiol. 1997;79(4):511–&. 10.1016/S0002-9149(96)00798-9. [DOI] [PubMed] [Google Scholar]
- 43.•.Kemp AH, Koenig J, Thayer JF, Bittencourt MS, Pereira AC, Santos IS, et al. Race and resting-state heart rate variability in Brazilian civil servants and the mediating effects of discrimination: an ELSA-Brasil Cohort Study. Psychosom Med. 2016;78(8):950–8. 10.1097/PSY.0000000000000359 [DOI] [PubMed] [Google Scholar]; This study examines differences in resting vagally mediated heart rate variability in a large Brazilian cohort. Significant differences are revealed such that Brazilians self-identifying as either Black or Brown exhibit higher resting HRV relative to individuals identifying as White. In addition, racial discrimination was shown to indirectly contribute to group differences in HRV.
- 44.Boylan JM, Jennings JR, Matthews KA. Childhood socioeconomic status and cardiovascular reactivity and recovery among Black and White men: mitigating effects of psychological resources. Health Psychol. 2016;35(9):957–66. 10.1037/hea0000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuller-Rowell TE, Williams DR, Love GD, McKinley PS, Sloan RP, Ryff CD. Race differences in age-trends of autonomic nervous system functioning. J Aging Health. 2013;25(5):839–62. 10.1177/0898264313491427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloan RP, Huang MH, Sidney S, Liu K, Williams OD, Seeman T. Socioeconomic status and health: is parasympathetic nervous system activity an intervening mechanism? Int J Epidemiol. 2005;34(2):309–15. 10.1093/ije/dyh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill LK, Hoggard LS. Cultural influences on parasympathetic activity. In: The Handbook of Culture and Biology; 2017. 10.1002/9781119181361.ch14. [DOI] [Google Scholar]
- 48.Steptoe A, Feldman RJ, Kunz S, Owen N, Willemsen G, Marmot M. Stress responsivity and socioeconomic status - a mechanism for increased cardiovascular disease risk? Eur Heart J. 2002;23(22): 1757–63. 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- 49.Steptoe A, Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. Eur Heart J. 2002;23(1):13–25. 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- 50.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11(3):199–205. 10.1007/s11906-009-0035-4. [DOI] [PubMed] [Google Scholar]
- 51.Anderson NB, McNeilly M, Myers H. Toward understanding race difference in autonomic reactivity Individual differences in cardiovascular response to stress. Berlin: Springer; 1992. p. 125–45. [Google Scholar]
- 52.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans - a biopsychosocial model. Am Psychol. 1999;54(10):805–16. 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- 53.Brosschot JF, Thayer JF. Anger inhibition, cardiovascular recovery, and vagal function: a model of the link between hostility and cardiovascular disease. Ann Behav Med. 1998;20(4):326–32. 10.1007/BF02886382. [DOI] [PubMed] [Google Scholar]
- 54.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–92. 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 55.Chae DH, Clouston S, Hatzenbuehler ML, Kramer MR, Cooper HL, Wilson SM, et al. Association between an internet-based measure of area racism and black mortality. PLoS One. 2015;10(4): e0122963 10.1371/journal.pone.0122963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greer S, Kramer MR, Cook-Smith JN, Casper ML. Metropolitan racial residential segregation and cardiovascular mortality: exploring pathways. J Urban Health. 2014;91(3):499–509. 10.1007/s11524-013-9834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Din-Dzietham R, Nembhard WN, Collins R, Davis SK. Perceived stress following race-based discrimination at work is associated with hypertension in African-Americans. The metro Atlanta heart disease study, 1999–2001. Soc Sci Med. 2004;58(3):449–61. [DOI] [PubMed] [Google Scholar]
- 58.Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, et al. Perceived discrimination and hypertension among African Americans in the Jackson Heart study. Am J Public Health. 2012;102(Suppl 2):S258–65. 10.2105/AJPH.2011.300523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22(3):300–9. [DOI] [PubMed] [Google Scholar]
- 60.Cooper DC, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE. The effects of ethnic discrimination and socioeconomic status on endothelin-1 among blacks and whites. Am J Hypertens. 2009;22(7):698–704. 10.1038/ajh.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beatty DL, Matthews KA, Bromberger JT, Brown C. Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: study of women’s health across the nation. J Soc Issues. 2014;70(2):298–314. 10.1111/josi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24(3):438–43. 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neblett EW, Roberts SO. Racial identity and autonomic responses to racial discrimination. Psychophysiology. 2013;50(10):943–53. 10.1111/psyp.12087. [DOI] [PubMed] [Google Scholar]
- 64.Wagner J, Lampert R, Tennen H, Feinn R. Exposure to discrimination and heart rate variability reactivity to acute stress among women with diabetes. Stress Health. 2015;31(3):255–62. 10.1002/smi.2542. [DOI] [PubMed] [Google Scholar]
- 65.Hoggard LS, Hill LK, Gray DL, Sellers RM. Capturing the cardiac effects of racial discrimination: do the effects “keep going”? Int J Psychophysiol. 2015;97(2):163–70. 10.1016/j.ijpsycho.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams DP, Joseph N, Hill LK, Sollers JJ 3rd, Vasey MW, Way BM, et al. Stereotype threat, trait perseveration, and vagal activity: evidence for mechanisms underpinning health disparities in Black Americans. Ethn Health. 2017:1–18. 10.1080/13557858.2017.1378803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams DP, Pandya KD, Hill LK, Kemp AH, Way BM, Thayer JF, et al. Rumination moderates the association between resting high-frequency heart rate variability and perceived ethnic discrimination. J Psychophysiol. 2017:No Pagination Specified-No Pagination Specified. 10.1027/0269-8803/a000201. [DOI] [Google Scholar]
- 68.Borders A, Liang CTH. Rumination partially mediates the associations between perceived ethnic discrimination, emotional distress, and aggression. Cult Divers Ethn Min. 2011;17(2):125–33. [DOI] [PubMed] [Google Scholar]
- 69.Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How does stigma “get under the skin”?: the mediating role of emotion regulation. Psychol Sci. 2009;20(10):1282–9. 10.1111/j.1467-9280.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miranda R, Polanco-Roman L, Tsypes A, Valderrama J. Perceived discrimination, ruminative subtypes, and risk for depressive symptoms in emerging adulthood. Cultur Divers Ethnic Minor Psychol. 2013;19(4):395–403. 10.1037/a0033504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, et al. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol Bull. 2016;142(3):231–59. 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Thayer JF, Treiber F, Snieder H. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96(8):1166–72. 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 73.Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. 2013;89(3):288–96. 10.1016/j.ijpsycho.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Keen L 2nd,Turner AD, Mwendwa D, Callender C, Campbell A Jr. Depressive symptomatology and respiratory sinus arrhythmia in a non-clinical sample of middle-aged African Americans. Biol Psychol. 2015;108:56–61. 10.1016/j.biopsycho.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill LK, Watkins LL, Hinderliter AL, Blumenthal JA, Sherwood A. Racial differences in the association between heart rate variability and left ventricular mass. Exp Physiol. 2017;102(7):764–72. 10.1113/EP086228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoo HJ, Thayer JF, Greening S, Lee TH, Ponzio A, Min J, et al. Brain structural concomitants of resting state heart rate variability in the young and old: evidence from two independent samples. Brain Struct Funct. 2018;223(2):727–37. 10.1007/s00429-017-1519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holzman JB, Bridgett DJ. Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: a meta-analytic review. Neurosci Biobehav Rev. 2017;74(Pt A):233–55. 10.1016/j.neubiorev.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 78.Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille LK, et al. Heart rate variability and self-control–a meta-analysis. Biol Psychol. 2016;115:9–26. 10.1016/j.biopsycho.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 79.Lohmeier TE, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43(2):306–11. 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 80.Lohmeier TE. The sympathetic nervous system and long-term blood pressure regulation. Am J Hypertens. 2001;14(6 Pt 2): 147S–54S. [DOI] [PubMed] [Google Scholar]
- 81.Hesse C, Charkoudian N, Liu Z, Joyner MJ, Eisenach JH. Baroreflex sensitivity inversely correlates with ambulatory blood pressure in healthy normotensive humans. Hypertension. 2007;50: 41–6. 10.1161/hypertensionaha.107.090308. [DOI] [PubMed] [Google Scholar]
- 82.Benarroch EE. The arterial baroreflex functional organization and involvement in neurologic disease. Neurology. 2008;71(21):1733–8. 10.1212/01.wnl.0000335246.93495.92. [DOI] [PubMed] [Google Scholar]
- 83.Duschek S, del Paso GAR. Quantification of cardiac baroreflex function at rest and during autonomic. J Physiol Sci. 2007;57(5): 259–68. 10.2170/physiolsci.RP008807. [DOI] [PubMed] [Google Scholar]
- 84.Liao D, Cai J, Barnes RW, Tyroler HA, Rautaharju P, Holme I, et al. Association of cardiac autonomic function and the development of hypertension: the ARIC study. Am J Hypertens. 1996;9(12 Pt 1): 1147–56. [DOI] [PubMed] [Google Scholar]
- 85.Palatini P, Longo D, Zaetta V, Perkovic D, Garbelotto R, Pessina AC. Evolution of blood pressure and cholesterol in stage 1 hypertension: role of autonomic nervous system activity. J Hypertens. 2006;24(7):1375–81. 10.1097/01.hjh.0000234118.25401.1c. [DOI] [PubMed] [Google Scholar]
- 86.Propper C, Moore GA, Mills-Koonce WR, Halpern CT, Hill-Soderlund AL, Calkins SD, et al. Gene-environment contributions to the development of infant vagal reactivity: the interaction of dopamine and maternal sensitivity. Child Dev. 2008;79(5):1377–94. 10.1111/j.1467-8624.2008.01194.x. [DOI] [PubMed] [Google Scholar]