Abstract
Background
Excessive respiratory muscle effort during mechanical ventilation may cause patient self-inflicted lung injury and load-induced diaphragm myotrauma, but there are no non-invasive methods to reliably detect elevated transpulmonary driving pressure and elevated respiratory muscle effort during assisted ventilation. We hypothesized that the swing in airway pressure generated by respiratory muscle effort under assisted ventilation when the airway is briefly occluded (ΔPocc) could be used as a highly feasible non-invasive technique to screen for these conditions.
Methods
Respiratory muscle pressure (Pmus), dynamic transpulmonary driving pressure (ΔPL,dyn, the difference between peak and end-expiratory transpulmonary pressure), and ΔPocc were measured daily in mechanically ventilated patients in two ICUs in Toronto, Canada. A conversion factor to predict ΔPL,dyn and Pmus from ΔPocc was derived and validated using cross-validation. External validity was assessed in an independent cohort (Nanjing, China).
Results
Fifty-two daily recordings were collected in 16 patients. In this sample, Pmus and ΔPL were frequently excessively high: Pmus exceeded 10 cm H2O on 84% of study days and ΔPL,dyn exceeded 15 cm H2O on 53% of study days. ΔPocc measurements accurately detected Pmus > 10 cm H2O (AUROC 0.92, 95% CI 0.83–0.97) and ΔPL,dyn > 15 cm H2O (AUROC 0.93, 95% CI 0.86–0.99). In the external validation cohort (n = 12), estimating Pmus and ΔPL,dyn from ΔPocc measurements detected excessively high Pmus and ΔPL,dyn with similar accuracy (AUROC ≥ 0.94).
Conclusions
Measuring ΔPocc enables accurate non-invasive detection of elevated respiratory muscle pressure and transpulmonary driving pressure. Excessive respiratory effort and transpulmonary driving pressure may be frequent in spontaneously breathing ventilated patients.
Keywords: Mechanical ventilation, Artificial respiration, Acute lung injury, Myotrauma, Respiratory monitoring
Introduction
Patient inspiratory effort during mechanical ventilation may have both beneficial and deleterious effects. Inspiratory effort increases tidal volume and global dynamic lung stress (quantified by transpulmonary driving pressure, ΔPL) in pressure-targeted modes of ventilation, potentially leading to lung injury. Vigorous inspiratory efforts can generate pendelluft and amplify regional lung stress and strain, causing regional lung injury even in volume-cycled modes of ventilation [1, 2]. The amplitude of this regional stress is reflected by the dynamic transpulmonary driving pressure, ΔPL,dyn [3]. Excess diaphragmatic loading may impair systemic oxygen delivery and cause diaphragm muscle injury [4, 5]. The level of inspiratory effort during the first 3 days of ventilation was recently shown to predict the duration of ventilation and ICU admission [6]. Respiratory drive and effort are frequently elevated in patients with respiratory failure because of pain, anxiety, delirium, inadequate ventilatory assistance, and dyspnea [7, 8]. Therefore, patient inspiratory effort merits close attention during mechanical ventilation.
Inspiratory effort (quantified by respiratory muscle pressure, Pmus) is not routinely monitored during mechanical ventilation. Although several monitoring techniques are available (e.g., esophageal manometry [9], diaphragm electrical activity (Edi) [10], diaphragm ultrasound [11]), they require appropriate equipment, proficiency, and time, making it difficult for busy clinicians to assess inspiratory effort as part of routine respiratory monitoring. P0.1 is a simple and widely available method for estimating respiratory drive during mechanical ventilation [12], but it provides little information about the magnitude of dynamic lung stress generated by the combined effects of the ventilator and patient respiratory effort. Plateau pressure and driving pressure are used to detect excess lung stress during controlled mechanical ventilation [13], but these measurements may not be reliable in the presence of inspiratory effort as they can underestimate the true magnitude of stress and strain applied to the lung both globally and regionally [14]. Moreover, these measurements represent the total elastic pressure of the respiratory system (combining the lung and the chest wall); elevated values therefore do not necessarily entail excess lung stress when chest wall elastance is increased. A rapid and non-invasive technique for detecting excess respiratory effort and dynamic lung stress would substantially increase the feasibility of detecting injurious spontaneous breathing during mechanical ventilation.
During a randomly applied end-expiratory airway occlusion on the ventilator, the airway pressure deflection generated by the patient’s respiratory effort against the occluded airway (ΔPocc) is correlated with the pressure generated by the respiratory muscles to expand the lungs and chest wall during mechanically assisted breaths because a single end-expiratory occlusion does not alter respiratory drive [15]. Hence, ΔPocc may provide a non-invasive means of detecting excessive inspiratory effort and dynamic lung stress during assisted mechanical ventilation.
We hypothesized that excessive patient inspiratory effort (Pmus) and excessive dynamic lung stress (ΔPL,dyn) could be detected rapidly and non-invasively by measuring ΔPocc.
Methods
This study was conducted in two medical-surgical intensive care units at the University Health Network, Toronto, Canada. The findings presented in this paper represent an ancillary analysis on an ongoing clinical study (MYOTRAUMA, ClinicalTrials.gov NCT03108118) characterizing diaphragm activity and function longitudinally during mechanical ventilation. Informed consent was obtained from substitute decision makers prior to enrolment. If no substitute decision maker was available, eligible patients were enrolled by deferred consent and consent for the use of study data was obtained from study participants once they regained capacity. The Research Ethics Board at University Health Network approved the study protocols, and the study was performed in accordance with the ethical standards laid down in the 2008 Declaration of Helsinki. The study findings were validated in a dataset collected from a previously published cohort of patients in China [16].
Study subjects
Patients were enrolled in the MYOTRAUMA study if they were intubated for fewer than 36 h and if the reason for intubation was one of acute brain injury (i.e., stroke or traumatic brain injury), acute respiratory distress syndrome (ARDS), septic shock, or pneumonia. Patients were excluded if they were deemed unlikely to remain on the ventilator for at least 7 days, if there was a contraindication to esophageal catheterization (recent upper GI surgery, bleeding varices), or if they had a concomitant acute exacerbation of obstructive airways disease. Recordings obtained in MYOTRAUMA study subjects were included from days when the subjects were breathing spontaneously (triggering the ventilator).
Study protocol
Study methods are detailed in an online supplement (see Additional file 1). Flow, airway pressure (Paw), esophageal pressure (Pes), and diaphragm electrical activity (Edi) were recorded for 10 min on a daily basis. During each recording, 15–20 expiratory airway occlusions were applied on the Servo-I ventilator (Getinge, Solna, Sweden) at random intervals. Each occlusion was maintained for the duration of a single breath (confirmed by the return of Paw and Edi to baseline, see Fig. 1). The maximal deflection in Paw from PEEP during each occlusion was recorded as a measurement of occlusion pressure (ΔPocc) (note: not to be confused with the airway occlusion pressure at 100 milliseconds after the onset of inspiration, P0.1).
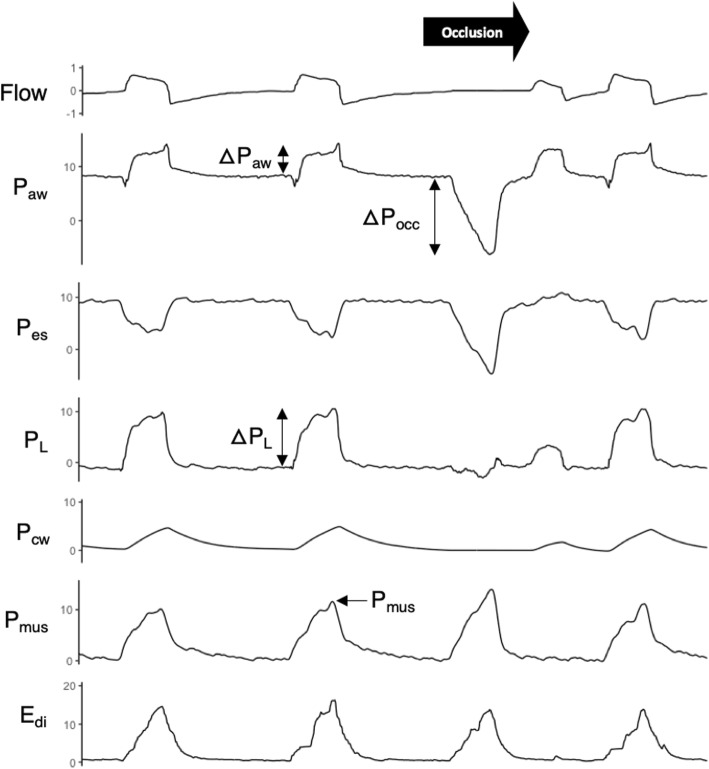
Fig. 1.
Representative tracings obtained during the airway occlusion maneuver. Flow, airway pressure (Paw), esophageal pressure (Pes), and diaphragm electrical activity (Edi) were recorded while a one-way end-expiratory occlusion permitting expiratory flow but not inspiratory flow (black arrow) was applied at a random interval. Transpulmonary pressure (PL), obtained by digital subtraction of Pes from Paw, signifies the dynamic stress applied to the lung. Chest wall elastic recoil pressure (ΔPcw) was estimated by multiplying tidal volume by predicted chest wall elastance. Inspiratory effort was quantified by the peak inspiratory muscle pressure, Pmus, estimated as the difference between ΔPcw and ΔPes (baseline Pmus is 0 cm H2O by definition). Note that peak Edi did not differ between occluded and non-occluded breaths
Signal analysis
Transpulmonary pressure (PL) was measured by real-time digital subtraction of Pes from Paw. The airway driving pressure (ΔPaw,dyn) was quantified as the difference between peak Paw and PEEP. The dynamic transpulmonary driving pressure (ΔPL,dyn) was quantified for each breath as the increase in PL from onset to peak during inspiration. Chest wall elastic recoil pressure at end-inspiration (Pcw) was estimated for each breath from the product of tidal volume and the empirically estimated chest wall elastance (see Additional file 1, Additional file 2, Additional file 3, Additional file 4, and Additional file 5). The pressure generated by the respiratory muscles during inspiration (Pmus) (i.e., the pressure that expands the lung and chest wall during inspiration) was quantified for each breath as the peak difference between Pcw and Pes during inspiration. Pressure-time product of Pmus per breath (PTPmus)—the reference standard for quantifying inspiratory effort [17]—was computed from Pcw and the integral of Pes during inspiration (see Additional file 1, Additional file 2, Additional file 3, Additional file 4, and Additional file 5).
To avoid measurement error due to inaccurate Pes measurements, recordings where the ratio of ΔPocc/ΔPes was greater than 1.3 or less than 0.7 were excluded from analysis.
Defining excessive Pmus and ΔPL
Thresholds defining excessive Pmus and ΔPL,dyn were selected a priori based on available physiological and clinical observations (see Additional file 1 for detailed rationale). Pmus normally ranges between 4 and 10 cm H2O, and ΔPL,dyn normally ranges between 4 and 8 cm H2O [18–20]. Given some uncertainty in the optimal definitions for excessive Pmus and ΔPL,dyn, discriminative accuracy was assessed for two different possible definitions of “excessive” values: for Pmus, 10 cm H2O and 15 cm H2O, and for ΔPL,dyn, 15 cm H2O and 20 cm H2O.
Statistical analysis
The goal of the analysis was to determine whether ΔPocc measured during airway occlusions could be used to predict the average values of ΔPL,dyn and Pmus for non-occluded (assisted) breaths during each daily 10-min recording and to detect when the average values of ΔPL,dyn and Pmus for non-occluded (assisted breaths) exceeded the cut-off values defined above.
For internal validation, we employed a cross-validation procedure (100 repetitions). During each cross-validation, patients were randomly divided into derivation (n = 10, 50%) and internal validation (n = 10, 50%) cohorts. In the derivation cohort (step 1), the ratios of mean Pmus (during all non-occluded breaths) to the mean ΔPocc (k1 = Pmus/ΔPocc) and mean ΔPes (during all non-occluded breaths) to the mean ΔPocc (k2 = ΔPes/ΔPocc) were computed in each daily recording using linear mixed-effects models to account for repeated recordings within subjects.
In the internal validation cohort (step 2), the derived values of k1 and k2 were used to predict Pmus and ΔPes (and hence ΔPL,dyn as ΔPaw − ΔPes) from three randomly selected measurements of ΔPocc in each recording (to mimic the use of just three occlusion maneuvers for prediction in clinical practice) according to Eqs. 1 and 2.
| 1 |
| 2 |
Predicted and observed values of Pmus and ΔPL,dyn were compared using Bland-Altman limits of agreement. To account for repeated measures within patients, linear mixed-effects models were employed to estimate within-patient limits of agreement as a proportion of the estimated value (LA%,within) [21]. Values were log-transformed because of non-normality in the distribution of differences between predicted and estimated values [22]. The mean and between-patient standard deviation of the bias between measured and predicted Pmus and ΔPL (SDbias,btw) were also computed in linear mixed-effects models. Total limits of agreement for Pmus and ΔPL across the range of estimated values were estimated as 1.96 × SDbias,btw + LA%,within × estimated value. The ability of predicted Pmus and ΔPL,dyn to detect excessive Pmus and ΔPL,dyn (defined by above threshold values) was evaluated in the internal validation cohort by receiver operating characteristic curve analysis and by computing sensitivity and specificity.
The cross-validation procedure (steps 1 and 2) was repeated 100 times to evaluate the stability of validity estimates during repeated random sampling [23]. All statistical analyses were conducted using R version 3.4.3 (www.r-project.org).
External validation
The discriminative validity and sensitivity and specificity of predicted Pmus and ΔPL to detect excessive Pmus and ΔPL,dyn were independently quantified in a separate previously published cohort of patients studied in a different center (Nanjing, China) receiving partially assisted ventilation in whom random expiratory airway occlusions were applied at varying levels of ventilator support (n = 13) [16].
Results
Prevalence of excessive respiratory effort and dynamic lung stress
After excluding 30 recordings because the ratio of ΔPocc/ΔPes was greater than 1.3 or less than 0.7, a total of 52 daily recordings were available in 16 subjects (median 3, IQR 2–5 daily recordings per patient); representative tracings are shown in Fig. 1. Twelve patients were available in the external validation cohort. Patient characteristics in both cohorts are summarized in Table 1.
Table 1.
Patient characteristics
| Patient characteristic | Primary cohort (n = 16) | External validation cohort (n = 12) |
|---|---|---|
| N measurements in cohort | 52 | 46 |
| N measurements per patienta | 3 (2–5) | 3 (1–7) |
| Age (years) (mean, SD) | 63 (10) | 60 (57–73) |
| Sex (n, % female) | 7 (44%) | 10 (83%) |
| Cause of respiratory failure (n, %) | ||
| Pneumonia | 10 (62%) | 10 (83%) |
| Non-pulmonary sepsis | 2 (13%) | 0 (0%) |
| Cardiogenic shock | 0 (0%) | 2 (17%) |
| Intracranial hemorrhage | 3 (19%) | 0 (0%) |
| Ischemic stroke | 1 (6%) | 0 (0%) |
| Sedation-Agitation Scale scoreb | 2 (2–3) | Not reported |
| Baseline nadir PaO2/FiO2 (mm Hg) | 148 (105–173) | Not reported |
| Mode of ventilation (n days, %) | ||
| Volume assist-control | 1 (2%) | – |
| Pressure assist-control | 9 (17%) | – |
| Pressure support | 39 (75%) | – |
| Not recorded | 3 (6%) | – |
| Neurally adjusted ventilatory assist | 0 (0%) | 12 (100%) |
| ΔPaw (cm H2O)b | 5 (3–7) | 10 (9–17) |
| Pmus (cm H2O)b | 16 (12–22) | 7 (5–9) |
| ΔPL (cm H2O)b | 18 (14–23) | 18 (14–22) |
Results are presented as median and interquartile range unless otherwise reported
aIn the primary cohort, one measurement was obtained per day; in the external validation cohort, multiple measurements were obtained on the same day at varying NAVA support levels
bValues reported include repeated measurements within subjects over different study days
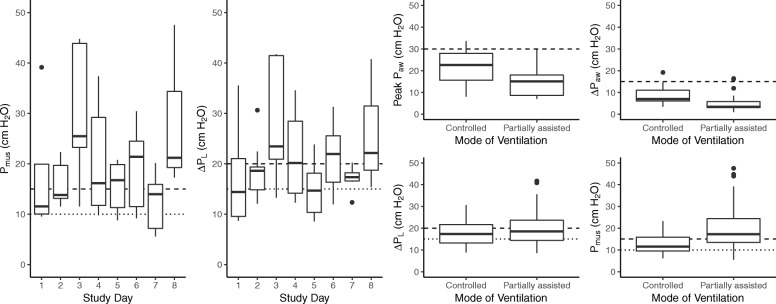
Pmus and ΔPL,dyn during assisted ventilation ranged widely in the cohort (Fig. 2). Pmus exceeded 10 cm H2O on 84% of patient-days in the study and exceeded 15 cm H2O on 53% of patient-days. In 14 patients (88%), Pmus exceeded 10 cm H2O on at least one study day. There was no evidence of a correlation between Pmus and pH (p = 0.21) or PaO2 (p = 0.57). The correlations between Pmus and SAS score (p = 0.07, R2 = 0.06) and SOFA score (p = 0.08, R2 = 0.09) did not reach significance. Pmus was inversely correlated with PaCO2 (p = 0.03, R2 = 0.11). Pmus was higher under partially assisted modes (mean difference 8 cm H2O, p = 0.02) and higher in patients admitted for pneumonia compared to patients with non-pulmonary admission diagnoses (mean difference 11 cm H2O, p = 0.05).
Fig. 2.
Distribution of ΔPL (dynamic transpulmonary driving pressure) and Pmus (respiratory muscle pressure) during mechanical ventilation. Pressures frequently exceeded “probably excessive” and “definitely excessive” thresholds (dotted and dashed lines, respectively) irrespective of the duration of the study or the mode of ventilation. While peak and driving airway pressures were lower under partially assisted modes of ventilation (p < 0.001 for both comparisons), transpulmonary pressure swings were not significantly different (p = 0.16)
ΔPL,dyn exceeded 15 cm H2O on 69% of patient-days and exceeded 20 cm H2O on 40% of patient-days. In 13 patients (81%), ΔPL,dyn exceeded 15 cm H2O on at least one study day. ΔPL,dyn was generally substantially higher than ΔPaw because pleural pressure (represented by Pes) decreases during inspiration even while Paw increases (median difference 12 cm H2O, IQR 8–18 cm H2O, p < 0.001). Although peak airway pressure and airway driving pressure were lower on days when patients were ventilated in pressure support ventilation mode compared to volume or pressure-control ventilation (p < 0.005 for both comparisons), ΔPL,dyn was not significantly different (p = 0.16) (Fig. 2). ΔPL,dyn was higher in patients admitted for pneumonia compared to patients with a non-pulmonary diagnosis (mean difference 9 cm H2O, p = 0.05).
Pmus and ΔPL,dyn were both within ideal limits (Pmus ≤ 10 cm H2O and ΔPL,dyn < 15 cm H2O) on only 8% of patient-days.
Validity of ΔPocc as a non-invasive marker of respiratory effort
There was no systematic difference in peak Edi between occluded and non-occluded breaths (mean difference 0 μV, limits of agreement ± 4 μV) confirming that respiratory drive was unaffected by the randomly applied intermittent airway occlusion. ΔPocc was highly correlated with PTPmus (Additional file 2: Figure S1, between-subjects R2 = 0.71, within-subjects R2 = 0.85).
Detecting excessive Pmus and ΔPL from ΔPocc
In the derivation cohorts, k1 (ratio of Pmus/ΔPocc) was − 0.74 (95% CI − 0.69, − 0.78) and k2 (ratio of ΔPes/ΔPocc) was 0.66 (0.61–0.70).
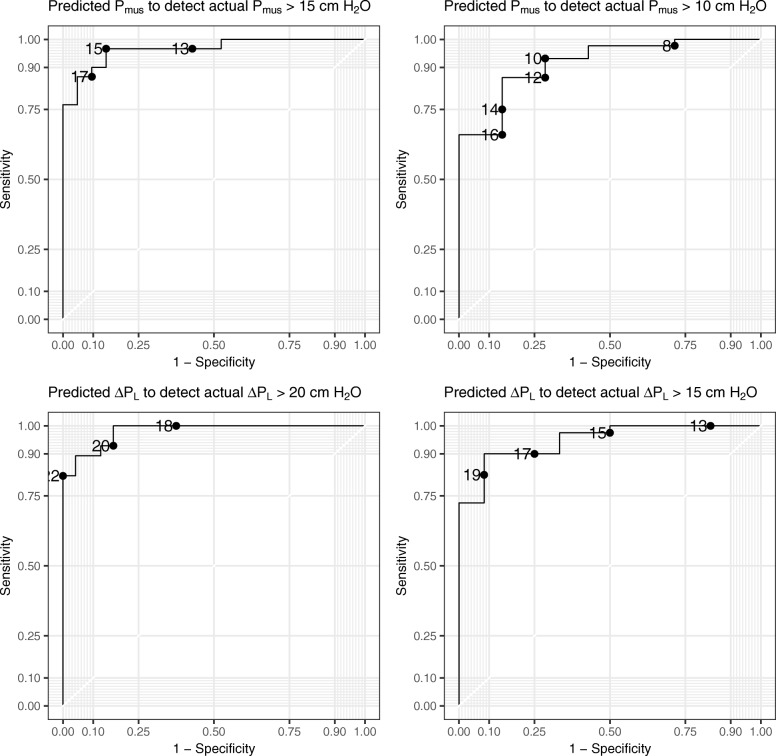
Agreement between predicted and measured values of Pmus and ΔPL,dyn in the internal validation cohorts was marginally acceptable: bias (the magnitude of difference between predicted and measured values) varied between subjects and the within-subject limits of agreement were relatively wide (Additional file 3: Figure S2, Additional file 4: Table S1). Nevertheless, predicted Pmus and ΔPL,dyn accurately detected excessive Pmus and ΔPL,dyn with areas under the receiver operating characteristic curves (AUROC) suggesting strong discriminative performance (AUROC > 0.9 in all cases, Fig. 3, Additional file 5: Table S2). Sensitivity and specificity of different cut-off values of predicted Pmus and ΔPL,dyn for excessive measured Pmus and ΔPL,dyn are shown in Additional file 5: Table S2.
Fig. 3.
Discriminative accuracy assessed by receiver operating characteristic curves. Threshold values are shown as points on the ROC curves. Pmus, respiratory muscle pressure; ΔPL, dynamic transpulmonary driving pressure
Based on the findings in the primary cohort, the utility of ΔPocc was tested in the external validation cohort using values of k1 = -3/4 and k2 = 2/3. Discriminative performance, sensitivity, and specificity for excessive Pmus and ΔPL,dyn were similarly strong (AUROC ≥ 0.94 for both excessive Pmus and ΔPL,dyn, Additional file 5: Table S2).
Discussion
We demonstrate for the first time that measurement of ΔPocc from three randomly applied end-expiratory occlusion maneuvers can detect excessive Pmus and ΔPL,dyn with high sensitivity and specificity, even though agreement between predicted and measured values are not sufficiently reliable to provide direct estimates of Pmus and ΔPL,dyn. Second, we report for the first time that in spontaneously breathing patients under mechanical ventilation, inspiratory effort and dynamic lung stress frequently exceed putative safe thresholds, irrespective of the depth of sedation or mode of ventilation. Patients only infrequently exhibited the “ideal” combination of lung and diaphragm-protective ventilation parameters (Pmus ≤ 10 cm H2O and ΔPL,dyn ≤ 15 cm H2O). The magnitude of dynamic lung stress during spontaneous breathing was often seriously underestimated by airway pressures available on the ventilator, confirming that airway pressures on the ventilator are an unreliable marker of dynamic lung stress when patients are spontaneously breathing.
Our method relies on predicting the swing in pleural pressure (quantified by Pes) under dynamic conditions (airway open) from the swing in airway pressure under quasi-static conditions (airway occluded). Under quasi-static conditions, the swing in pleural pressure matches the swing in airway pressure exactly. The swing in pleural pressure is smaller during inspiration than under quasi-static conditions because of the force-velocity relation of muscle and because of differences in chest wall mechanics and thoracoabdominal motion [24, 25]. Despite these sources of heterogeneity, we found that the conversion factors k1 and k2 for converting quasi-static conditions to dynamic conditions were fairly stable between patients and over time. These conversion factors provide the physiological basis for predicting Pmus and ΔPL,dyn from ΔPocc. Of note, a substantial proportion of recordings had to be excluded because ΔPocc differed from ΔPes during the occlusion maneuver—this highlights the importance of carefully considering esophageal balloon catheter placement when using Pes for monitoring.
Inspiratory effort and dynamic lung stress during assisted mechanical ventilation
The transition to partially assisted modes of ventilation is often regarded as a sign of recovery and progress towards liberation from the ventilator. However, important new insights about the potential for lung injury due to excessive inspiratory effort and the associated increase in global and regional lung stress (a phenomenon referred to as patient self-inflicted lung injury, P-SILI [26]) motivate efforts to avoid excessive effort and lung stress.
Our data suggest that greater attention should be paid to the potential risks of excessive inspiratory effort and dynamic lung stress during assisted mechanical ventilation. Observed Pmus and ΔPL,dyn frequently exceeded putative safe levels of inspiratory effort and lung stress. Reliable and feasible clinical monitoring systems are essential to ensure safe and effective ventilation. Although clinicians ordinarily rely on plateau, peak, and driving airway pressures, these parameters can seriously underestimate the true magnitude of lung stress during spontaneous breathing due to the negative pleural pressure generated by the respiratory muscles, which is usually not measured. Clinicians should therefore avoid relying on airway pressure measurements alone to assess the safety of mechanical ventilation in spontaneously breathing patients.
Measurement of ΔPocc as described for the first time in this study offers a highly feasible and sensitive means of detecting excessive inspiratory effort and dynamic lung stress. It is important to note that ΔPL,dyn can only be predicted if ΔPocc < 0 cm H2O; when inspiratory effort is absent, the inspiratory swing in ΔPes will be positive, and hence, ΔPL,dyn will not be correlated to ΔPocc.
Limitations
This study has several limitations. First, Pmus and ΔPL,dyn were estimated from measurements of airway and esophageal pressure. Due to the presence of active inspiratory efforts, we did not perform end-inspiratory holds to obtain quasi-static pressure measurements although some observations suggest that such measurements are feasible in the presence of inspiratory effort provided expiratory muscle activity is minimal [27, 28]. Consequently, ΔPL,dyn as measured in this study represents a “dynamic” measure that may overestimate the actual mechanical stress applied to the lung during tidal ventilation. On the other hand, while ΔPL at the inspiratory plateau corresponds to the time of maximal alveolar distension in the non-dependent lung, peak ΔPL,dyn is reached at the time point when dependent lung—the region most at risk during spontaneous breathing—is maximally distended by vigorous spontaneous efforts [3]. It may therefore be the more clinically relevant marker of dynamic lung stress in this context. Future studies should determine whether dynamic or quasi-static measurements of ΔPL best reflect regional distending pressures.
Second, Pmus measurements require measurement of elastic chest wall recoil pressure; owing to the absence of recordings of passive ventilation in most subjects, we relied on empirical estimates of chest wall elastance derived from predicted lung volumes. The reliability of this empiric approach is uncertain, but reassuringly, we found that predicted values of chest wall elastic recoil pressure approximated measured values in patients where direct measurements of chest wall elastance were available (as reported in the Additional file 1).
Third, the number of patients in the primary dataset (n = 16) is relatively small, possibly limiting the generalizability of the validation findings. The study population is representative of a broad range of ventilated patients with acute hypoxemic respiratory failure. To avoid overfitting the predicted values of k1 and k2 to our dataset and to estimate the precision of our estimates of the limits of agreement, we employed a cross-validation technique. Importantly, the approach to detecting excessive Pmus and ΔPL,dyn from ΔPocc performed extremely well in the independent external validation cohort from a different country (China). The generalizability of these findings is also supported by the fact that the value of k1 estimated in this study (median 0.74) corresponds closely to the value estimated by Bellani et al. when they derived the Pmus − Edi index (0.66) in an Italian study [29].
Clinical implications
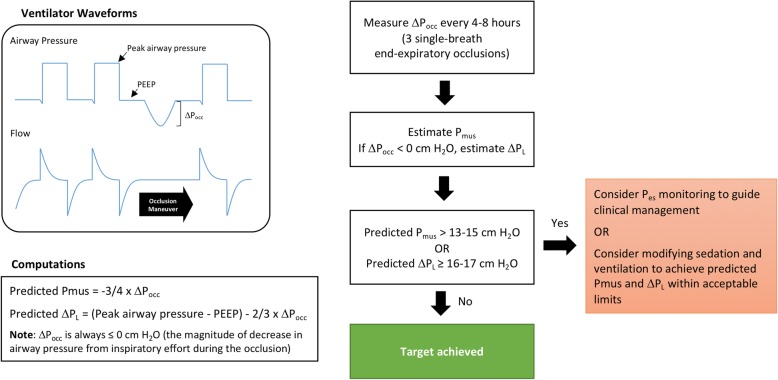
Regular measurements of ΔPocc to estimate Pmus and ΔPL,dyn during mechanical ventilation provide a highly feasible means of detecting excessive respiratory effort and excessive dynamic lung stress directly from ventilator waveforms. Most modern ventilators have capacity to apply an end-expiratory occlusion during ventilation in controlled or partially assisted modes. Pmus and ΔPL,dyn values predicted from ΔPocc are not sufficiently accurate to replace direct clinical monitoring (i.e., esophageal pressure) if desired by clinicians. Rather, these estimates could be used as a highly feasible, rapid, non-invasive “screening test” for excessive Pmus and ΔPL,dyn. These data could be employed as an indication to deploy more direct monitoring techniques (i.e., esophageal manometry) or to guide adjustments to ventilator assist level, sedation, and opioids (Fig. 4). The maneuver was well-tolerated in our study.
Fig. 4.
Proposed clinical algorithm for monitoring respiratory muscle pressure (Pmus) and dynamic transpulmonary pressure swings (ΔPL) based on the negative deflection in airway pressure during an end-expiratory airway occlusion maneuver (ΔPocc). Pes, esophageal pressure
Conclusions
Inspiratory effort and dynamic lung stress often exceed safe limits in patients breathing spontaneously under mechanical ventilation. The airway pressure deflection resulting from patient inspiratory effort during a transient end-expiratory occlusion maneuver (ΔPocc) can be used to detect excessive (potentially injurious) inspiratory effort and dynamic lung stress.
Supplementary information
Additional file 1. Supplemental description of methods.
Additional file 2: Figure S1. ΔPocc is correlated with inspiratory effort quantified by the pressure-time product of Pmus.
Additional file 3: Figure S2. Accuracy of predicting Pmus and ΔPL from ΔPocc assessed by Bland-Altman plots.
Additional file 4: Table S1. Agreement between measured and predicted Pmus and ΔPL.
Additional file 5: Table S2. Discriminative performance of predicted Pmus and ΔPL values to detect excessive Pmus and ΔPL.
Abbreviations
- ΔPaw,dyn
Inspiratory swing in pressure (difference between peak Paw and PEEP)
- ΔPL,dyn
Dynamic transpulmonary driving pressure; increase in PL from onset to peak during inspiration
- ΔPL,dyn
Dynamic transpulmonary driving pressure swing from end-expiration to peak transpulmonary pressure
- ΔPocc
Airway pressure deflection generated by the patient’s respiratory effort against the occluded airway; airway pressure from PEEP. The time of occlusion is as long as the duration of a single breath
- Edi
Diaphragm electrical activity
- k1
The ratio of mean Pmus (during all non-occluded breaths) to the mean ΔPocc (k1 = Pmus/ΔPocc)
- k2
The ratio of mean ΔPes (during all non-occluded breaths) to the mean ΔPocc (k2 = ΔPes/ΔPocc)
- P0.1
The airway occlusion pressure at 100 milliseconds after the onset of inspiration
- Paw
Airway pressure
- Pcw
Chest wall elastic recoil pressure at end-inspiration
- Pes
Esophageal pressure
- PL
Transpulmonary pressure
- Pmus
Respiratory muscle pressure (inspiratory effort); peak difference between Pcw and Pes during inspiration
- PTPmus
Pressure-time product of Pmus per breath
- SAS score
Sedation-Agitation Scale score
- SOFA score
Sequential Organ Failure Assessment score
Authors’ contributions
The study was conceived by MB and EG. Measurements were collected by EG. Signal analysis was conducted by MB and EG. Statistical analysis was conducted by EG. MB and EG drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This research was sponsored by PSI Foundation. Dr. Goligher is supported by an Early Career Investigator Award from the Canadian Institutes of Health Research. Dr. Fan is supported by a New Investigator Award from the Canadian Institutes of Health Research. The funders played no role in the design, analysis, or reporting of the results. The authors have no financial relationship with the organizations that funded the research.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to ongoing analysis in the primary study but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Approval for the study procedures and data collected for this report was obtained from the research ethics board at the University Health Network (12-5582).
Consent for publication
Not applicable.
Competing interests
Dr. Goligher’s laboratory receives support in the form of equipment from Getinge, and Dr. Goligher has received speaking honoraria from Getinge. The other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-019-2617-0.
References
- 1.Yoshida T, Nakahashi S, Nakamura M, Koyama Y, Roldan R, Torsani V, et al. Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. Am J Resp Crit Care. 2017;196:590–601. doi: 10.1164/rccm.201610-1972OC. [DOI] [PubMed] [Google Scholar]
- 2.Papazian L, Forel J-M, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. New Engl J Medicine. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida T, Amato MB, Kavanagh BP. Understanding spontaneous vs ventilator breaths: impact and monitoring. Intens Care Med. 2018;164:43. doi: 10.1007/s00134-018-5145-5. [DOI] [PubMed] [Google Scholar]
- 4.Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas J, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Resp Crit Care. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- 5.Hussain S, Roussos C. Distribution of respiratory muscle and organ blood flow during endotoxic shock in dogs. J Appl Physiol. 1985;59:1802–1808. doi: 10.1152/jappl.1985.59.6.1802. [DOI] [PubMed] [Google Scholar]
- 6.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Resp Crit Care. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 7.Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients: pathophysiology and clinical implications. Am J Respir Crit Care Med 2019;0. [DOI] [PubMed]
- 8.Gentzler Eliza R., Derry Heather, Ouyang Daniel J., Lief Lindsay, Berlin David A., Xu Cici Jiehui, Maciejewski Paul K., Prigerson Holly G. Underdetection and Undertreatment of Dyspnea in Critically Ill Patients. American Journal of Respiratory and Critical Care Medicine. 2019;199(11):1377–1384. doi: 10.1164/rccm.201805-0996OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intens Care Med. 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 10.Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, ROSSINI M, et al. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Resp Crit Care. 2001;164:419–424. doi: 10.1164/ajrccm.164.3.2009018. [DOI] [PubMed] [Google Scholar]
- 11.Goligher EC, Laghi F, Detsky ME, Farias P, Murray A, Brace D, et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intens Care Med. 2015;41:734. doi: 10.1007/s00134-015-3724-2. [DOI] [PubMed] [Google Scholar]
- 12.Telias I, Damiani F, Brochard L. The airway occlusion pressure (P0.1) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intens Care Med. 2018;195:438–434. doi: 10.1007/s00134-018-5045-8. [DOI] [PubMed] [Google Scholar]
- 13.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. New Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 14.Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20:142. doi: 10.1186/s13054-016-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xirouhaki N, Kondili E, Mitrouska I, Siafakas N, Georgopoulos D. Response of respiratory motor output to varying pressure in mechanically ventilated patients. Eur Respiratory J. 1999;14:508–516. doi: 10.1034/j.1399-3003.1999.14c06.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Liu S, Xie J, Yang Y, Slutsky AS, Beck J, et al. Assessment of patient-ventilator breath contribution during neurally adjusted ventilatory assist in patients with acute respiratory failure. Crit Care. 2015;19:43. doi: 10.1186/s13054-015-0775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jubran A, Tobin M. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Resp Crit Care. 1997;155:906–915. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- 18.Carteaux G, Mancebo J, Mercat A, Dellamonica J, Richard J-CM, Aguirre-Bermeo H, et al. Bedside adjustment of proportional assist ventilation to target a predefined range of respiratory effort. Crit Care Med. 2013;41:2125–2132. doi: 10.1097/CCM.0b013e31828a42e5. [DOI] [PubMed] [Google Scholar]
- 19.Mancebo J, Isabey D, Lorino H, Lofaso F, Lemaire F, Brochard L. Comparative effects of pressure support ventilation and intermittent positive pressure breathing (IPPB) in non-intubated healthy subjects. Eur Respir J. 1995;8:1901–1909. doi: 10.1183/09031936.95.08111901. [DOI] [PubMed] [Google Scholar]
- 20.Jubran Amal, Grant Brydon J. B., Laghi Franco, Parthasarathy Sairam, Tobin Martin J. Weaning Prediction. American Journal of Respiratory and Critical Care Medicine. 2005;171(11):1252–1259. doi: 10.1164/rccm.200503-356OC. [DOI] [PubMed] [Google Scholar]
- 21.Myles P, CUI J. Using the Bland-Altman method to measure agreement with repeated measures. Bja Br J Anaesth. 2007;99:309–311. doi: 10.1093/bja/aem214. [DOI] [PubMed] [Google Scholar]
- 22.Euser AM, Dekker FW, le Cessie S. A practical approach to Bland-Altman plots and variation coefficients for log transformed variables. J Clin Epidemiol. 2008;61:978–982. doi: 10.1016/j.jclinepi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(SICI)1097-0258(20000229)19:4<453::AID-SIM350>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Grassino A, Goldman, Mead J, Sears T. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol. 1978;44:829–839. doi: 10.1152/jappl.1978.44.6.829. [DOI] [PubMed] [Google Scholar]
- 25.Goldman GA, Mead J, Sears T. Mechanics of the human diaphragm during voluntary contraction: dynamics. J Appl Physiol. 1978;44:840–848. doi: 10.1152/jappl.1978.44.6.840. [DOI] [PubMed] [Google Scholar]
- 26.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Resp Crit Care. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 27.Bellani G, Grassi A, Sosio S, Gatti S, Kavanagh BP, Pesenti A, et al. Driving pressure is associated with outcome during assisted ventilation in acute respiratory distress syndrome. Anesthesiology. 2019;131:594–604. doi: 10.1097/ALN.0000000000002846. [DOI] [PubMed] [Google Scholar]
- 28.Bellani G, Grassi A, Sosio S, Foti G. Plateau and driving pressure in the presence of spontaneous breathing. Intens Care Med. 2019;45:97–98. doi: 10.1007/s00134-018-5311-9. [DOI] [PubMed] [Google Scholar]
- 29.Bellani G, Mauri T, Coppadoro A, Grasselli G, Patroniti N, Spadaro S, et al. Estimation of patient’s inspiratory effort from the electrical activity of the diaphragm. Crit Care Med. 2013;41:1483–1491. doi: 10.1097/CCM.0b013e31827caba0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental description of methods.
Additional file 2: Figure S1. ΔPocc is correlated with inspiratory effort quantified by the pressure-time product of Pmus.
Additional file 3: Figure S2. Accuracy of predicting Pmus and ΔPL from ΔPocc assessed by Bland-Altman plots.
Additional file 4: Table S1. Agreement between measured and predicted Pmus and ΔPL.
Additional file 5: Table S2. Discriminative performance of predicted Pmus and ΔPL values to detect excessive Pmus and ΔPL.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to ongoing analysis in the primary study but are available from the corresponding author on reasonable request.