Abstract
Background
Herpes zoster, commonly known as shingles, is a neurocutaneous disease caused by the reactivation of the virus that causes varicella (chickenpox). After resolution of the varicella episode, the virus can remain latent in the sensitive dorsal ganglia of the spine. Years later, with declining immunity, the varicella zoster virus (VZV) can reactivate and cause herpes zoster, an extremely painful condition that can last many weeks or months and significantly compromise the quality of life of the affected person. The natural process of aging is associated with a reduction in cellular immunity, and this predisposes older people to herpes zoster. Vaccination with an attenuated form of the VZV activates specific T‐cell production avoiding viral reactivation. The USA Food and Drug Administration has approved a herpes zoster vaccine with an attenuated active virus, live zoster vaccine (LZV), for clinical use amongst older adults, which has been tested in large populations. A new adjuvanted recombinant VZV subunit zoster vaccine, recombinant zoster vaccine (RZV), has also been approved. It consists of recombinant VZV glycoprotein E and a liposome‐based AS01B adjuvant system.
This is an update of a Cochrane Review last updated in 2016.
Objectives
To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Search methods
For this 2019 update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 1, January 2019), MEDLINE (1948 to January 2019), Embase (2010 to January 2019), CINAHL (1981 to January 2019), LILACS (1982 to January 2019), WHO ICTRP (on 31 January 2019) and ClinicalTrials.gov (on 31 January 2019).
Selection criteria
We included randomised controlled trials (RCTs) or quasi‐RCTs comparing zoster vaccine (any dose and potency) versus any other type of intervention (e.g. varicella vaccine, antiviral medication), placebo, or no intervention (no vaccine). Outcomes were incidence of herpes zoster, adverse events (death, serious adverse events, systemic reactions, or local reaction occurring at any time after vaccination), and dropouts.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 11 new studies involving 18,615 participants in this update. The review now includes a total of 24 studies involving 88,531 participants. Only three studies assessed the incidence of herpes zoster in groups that received vaccines versus placebo. Most studies were conducted in high‐income countries in Europe and North America and included healthy Caucasians (understood to be white participants) aged 60 years or over with no immunosuppressive comorbidities. Two studies were conducted in Japan. Fifteen studies used LZV. Nine studies tested an RZV.
The overall quality of the evidence was moderate. Most data for the primary outcome (incidence of herpes zoster) and secondary outcomes (adverse events and dropouts) came from studies that had a low risk of bias and included a large number of participants.
The incidence of herpes zoster at up to three years follow‐up was lower in participants who received the LZV (one dose subcutaneously) than in those who received placebo (risk ratio (RR) 0.49, 95% confidence interval (CI) 0.43 to 0.56; risk difference (RD) 2%; number needed to treat for an additional beneficial outcome (NNTB) 50; moderate‐quality evidence) in the largest study, which included 38,546 participants. There were no differences between the vaccinated and placebo groups for serious adverse events (RR 1.08, 95% CI 0.95 to 1.21) or deaths (RR 1.01, 95% CI 0.92 to 1.11; moderate‐quality evidence). The vaccinated group had a higher incidence of one or more adverse events (RR 1.71, 95% CI 1.38 to 2.11; RD 23%; number needed to treat for an additional harmful outcome (NNTH) 4.3) and injection site adverse events (RR 3.73, 95% CI 1.93 to 7.21; RD 28%; NNTH 3.6) of mild to moderate intensity (moderate‐quality evidence). These data came from four studies with 6980 participants aged 60 years or over.
Two studies (29,311 participants for safety evaluation and 22,022 participants for efficacy evaluation) compared RZV (two doses intramuscularly, two months apart) versus placebo. Participants who received the new vaccine had a lower incidence of herpes zoster at 3.2 years follow‐up (RR 0.08, 95% CI 0.03 to 0.23; RD 3%; NNTB 33; moderate‐quality evidence). There were no differences between the vaccinated and placebo groups in incidence of serious adverse events (RR 0.97, 95% CI 0.91 to 1.03) or deaths (RR 0.94, 95% CI 0.84 to 1.04; moderate‐quality evidence). The vaccinated group had a higher incidence of adverse events, any systemic symptom (RR 2.23, 95% CI 2.12 to 2.34; RD 33%; NNTH 3.0), and any local symptom (RR 6.89, 95% CI 6.37 to 7.45; RD 67%; NNTH 1.5). Although most participants reported that there symptoms were of mild to moderate intensity, the risk of dropouts (participants not returning for the second dose, two months after the first dose) was higher in the vaccine group than in the placebo group (RR 1.25, 95% CI 1.13 to 1.39; RD 1%; NNTH 100, moderate‐quality evidence).
Only one study reported funding from a non‐commercial source (a university research foundation). All of the other included studies received funding from pharmaceutical companies.
We did not conduct subgroup and sensitivity analyses
Authors' conclusions
LZV and RZV are effective in preventing herpes zoster disease for up to three years (the main studies did not follow participants for more than three years). To date, there are no data to recommend revaccination after receiving the basic schedule for each type of vaccine. Both vaccines produce systemic and injection site adverse events of mild to moderate intensity.
Plain language summary
Vaccines for preventing shingles in older adults
Review question
We evaluated the effectiveness and safety of vaccines to prevent shingles in healthy older people.
Background
Varicella zoster virus causes chickenpox and can remain inactive in nerve cells for many years. The virus can reactivate, travel through the nerve to the skin, and produce blisters along the nerve path. This condition is called shingles (herpes zoster), and mostly affects people with low immunity, such as older people. Before blisters appear, symptoms may include itching, numbness, tingling, or local pain. Shingles causes nerve inflammation and severe pain that can affect quality of life. The incidence rate of herpes zoster ranges from 2.08 cases to 6.20 cases per 1000 person‐years (i.e. the number of new cases per population at risk, in a given time period). This number is increasing, due in part to people living longer.
This is an update of a Cochrane Review last updated in 2016.
Search date
31 January 2019.
Study characteristics
We included 11 new studies involving 18,615 participants in this update; the review now includes evidence from 24 studies involving 88,531 participants. Most studies were conducted in high‐income countries in Europe and North America, whilst two studies were conducted in Japan. Study participants were healthy adults aged 60 years or over with no difficulty fighting infection, most of whom were Caucasian (understood to be white) women. Follow‐up ranged from 28 days to 7 years. All primary study reports were published in English.
Study funding sources
Most studies were funded by pharmaceutical companies; one study received funding from a university research foundation.
Key results
One large, high‐quality study including 38,546 participants aged 60 years or over compared LZV versus fake (placebo) vaccines (one dose administered as a subcutaneous (given under the skin) injection) and found that the active vaccine can prevent shingles for up to three years. The adverse effects of the vaccine were mostly mild to moderate, for systemic symptoms as well as for injection site reactions.
RZV is a new vaccine that contains a small part of the varicella zoster virus plus adjuvant. An adjuvant is a substance that enhances the response of the body against a stimulus (bacteria, viruses, and substances that appear foreign and harmful) to defend itself. This vaccine requires a total of two intramuscular doses, given two to six months apart. Two studies (29,311 participants for safety evaluation and 22,022 participants for efficacy evaluation) compared RZV versus placebo and reported that people who received the RZV had fewer episodes of herpes zoster but more systemic symptoms and injection site reactions. Most participants reported that these adverse effects were of mild to moderate intensity. It is important to note that the number of participants who did not receive the second dose was higher in the vaccine group than in the placebo group.
Quality of the evidence
We assessed the overall quality of evidence as moderate because the studies included many participants.
Summary of findings
Background
Description of the condition
Herpes zoster, or shingles, is a neurocutaneous disease that can be extremely painful. Symptoms often last for many weeks or months after complete healing of the lesions (Gilden 2000). Herpes zoster is caused by the reactivation of the varicella zoster virus (VZV) when immunity to VZV declines.
The geographical distribution of VZV indicates that it is a common human pathogen with worldwide occurrence (Cohen 2007). Although varicella occurs worldwide, the epidemiology of the disease is markedly different in tropical and temperate countries. In temperate countries, such as the UK and the USA, most people have seroconverted to VZV by adolescence (meaning they have had prior contact with the virus and developed antibodies). Serological studies of resident tropical populations and immigrants from tropical countries indicate that seroconversion generally occurs in late adolescence and adulthood (Lee 1998).
VZV is a highly contagious organism, and in the first contact with the virus, usually in childhood, the individual develops chickenpox (varicella). VZV can remain dormant for years in the dorsal sensory ganglia of the spinal cord. The latency of the virus is maintained by cellular immunity, which inhibits viral replication. Years later, during periods of decreased cell‐mediated immunity or simply because of aging, the virus can replicate in the dorsal sensory ganglia of the spinal cord and migrate along sensory nerves. Prodromal symptoms of viral reactivation include itching, numbness, tingling, or severe localised pain, which precede the appearance of skin lesions by one to five days. The typical cutaneous manifestations of an acute herpes zoster episode include clusters of vesicles that spread in a linear pattern along the path of nerves and do not cross the midline of the body (Cohen 2007; Moffat 2007). Within three to five days, these lesions progress to pustules, ulcerations, and crusting and go on to heal spontaneously within two to four weeks (Gnann 2002).
Herpes zoster causes substantial morbidity and has a significant impact on quality of life (Gnann 2002; Partridge 2009; Sampathkumar 2009). Schmader 2007 conducted a prospective observational study of 165 outpatients with acute herpes zoster who were enrolled within 14 days of onset of rash. Pain was moderate to severe, and discomfort was common during the acute rash phase. Acute herpetic neuralgia was associated with sleep disruption, impaired general activities, and enjoyment of life, especially after the onset of the rash, and had significant impact on quality of life. Although herpes zoster lesions and symptoms are transient in most individuals, some of those affected may develop postherpetic neuralgia that can last for months to years, which can cause substantial suffering and have a negative impact on quality of life (Coplan 2004; Dworkin 2003; Johnson 2014; Pickering 2011).
The incidence of herpes zoster varies greatly. In a systematic review based on data from prospective surveillance, medical record or administrative record with medical record review, the incidence rate of herpes zoster ranged between 3 and 5/1000 person‐years in North America, Europe and Asia‐Pacific (Kawai 2014). Another recent systematic review conducted in Spain reported an incidence rate of 2.08 to 5.46/1000 person‐years (Mareque 2019). In a cohort study conducted in Taiwan (66,453 participants), the incidence was 4.72 per 1000 person‐years (Chung 2016). In a study conducted in Germany involving 4751 participants mostly aged 60 to 69 years, the annual self‐reported incidence rate of herpes zoster was of 6.2 per 1000 person‐years (Caputo 2019).
Older adults (aged 60 years or older) are at increased risk of developing herpes zoster (Arvin 1996; Cho 2007; Heymann 2008; Jih 2009; Thomas 2004). A recent systematic review reported that the incidence of herpes zoster increased with age,from 5 to 8/1000 in people aged 50 years or over to 11/1000 in those aged 75 years and over (five studies) (Mareque 2019). A Canadian cohort study reported incidence of 8.2/1000 in adults aged 50 years or over (Marra 2016). Kawai 2014 reported herpes zoster incidence of 6 to 8/1000 person‐years at 60 years and 8 to 12/1000 person‐years at 80 years of age.
Several studies indicate that incidence is also increasing over time. Marra 2016 reported that in Canada, the incidence of herpes zoster increased from 2.9 per 1000 inhabitants in 1997 to 4.7 per 1000 inhabitants in 2012. In a cohort study in the USA (Kawai 2016), the incidence rate of herpes zoster adjusted by age and sex increased from 0.76 (95% confidence interval (CI) 0.63 to 0.89) per 1000 person‐years in 1945 to 1949 to 3.15 (95% CI 3.04 to 3.26) per 1000 person‐years in 2000 to 2007 which corresponds to a more than four‐fold increase over the 60‐year period. In the USA, the annual incidence of herpes zoster increased from 3.10 episodes per 1000 in older adults in 2000 to 5.22 in 2007 (Rimland 2010). Most people with herpes zoster are women (Caputo 2019; Mareque 2019; Marra 2016). Although family history of shingles suggests a possible genetic predisposition to the disease (Cho 2007; Haanpää 2002), results from available case‐control studies are conflicting (Gatti 2010; Hicks 2008).
Due to lengthening lifespans, there are increasing concerns about quality of life for older adults, who are a growing segment of the population.
Description of the intervention
Two different vaccines are currently available to prevent herpes zoster, as follows.
Live attenuated VZV zoster vaccine (LZV): this vaccine contains the same live attenuated virus used in the chickenpox vaccine, but it has over 14‐fold more plaque‐forming units of the attenuated virus per dose. The two vaccines are therefore not interchangeable (Oxman 2005). This vaccine was approved by the USA Food and Drug Administration (FDA) for older adults (aged 60 years and over) in May 2006 (FDA 2006), and was approved by the FDA for individuals aged 50 years or over in October 2018 (FDA 2018).
Adjuvanted recombinant subunit zoster vaccine (RZV) has also been tested (Leroux‐Roels 2012). It does not contain the live attenuated virus, but rather a small fraction of the virus that cannot replicate but can boost immunogenicity. This vaccine contains antigen gE (glycoprotein E), which is the most abundant glycoprotein on the surface of VZV and the most abundant antigen in VZV‐infected cells and the main target for VZV‐specific CD4 + T‐cell response (Arvin 1986; Arvin 1996). This vaccine also includes adjuvant AS01, which is a liposome‐based adjuvant system containing immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) plus saponin QS‐21 (Quillaja saponaria Molina, fraction 21) (Baldridge 2004; Kensil 1991). It was approved by the FDA for clinical use in October 2017 (FDA 2017).
How the intervention might work
Primary infection with VZV induces the production of specific memory T‐cells in sufficient numbers to keep the virus in its latent form. Host factors such as aging, or other conditions that affect cellular immunity, may reduce T‐cells to levels that can no longer inhibit viral replication, thereby increasing the likelihood of clinical manifestations of the disease.
Live attenuated VZV zoster vaccine (LZV) consists of live attenuated VZV that activates specific T‐cell production, thus increasing existing immunity and avoiding reactivation of viral replication (Arvin 2005). Several randomised controlled trials (RCTs) have evaluated the efficacy and safety of live attenuated virus vaccines in preventing herpes zoster (Beals 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Levin 2000; Levin 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013).
Adjuvanted recombinant VZV subunit zoster vaccine (RZV) is a new vaccine that contains the most abundant glycoprotein on the surface of VZV and the most abundant antigen in VZV‐infected cells. The adjuvant component is important because it helps to elicit an early, high, and long‐lasting immune response with less antigen (Rajesh 1995). This leads to additional stimulation of the immune system. The RZV improves immune stimulation against VZV, and its efficacy and safety have been tested in several RCTs (Chlibek 2013; Chlibek 2014; Cunningham 2016; Lal 2015; Lal 2018; Maréchal 2018; NCT02052596; Schwarz 2017; Vink 2017).
Why it is important to do this review
Herpes zoster is a disease that can have an important effect on the quality of life of affected individuals (Schmader 2007). The incidence of herpes zoster is increasing over time (Marra 2016), and is higher in the elderly population (Mareque 2019). The vaccination of healthy individuals is a way of preventing the disease. In this context, it is important to critically assess the best available evidence on the effectiveness of these vaccines, as well as their safety profile, since they are given to healthy individuals.
This review is also important to map the existing research gaps and to encourage scientists to pursue investigations in this area, including studies with longer follow‐ups of participants and to test new types of vaccines.
This is a second update of a Cochrane Review first published in 2012 and first updated in 2016 (Gagliardi 2012; Gagliardi 2016).
Objectives
To evaluate the effectiveness and safety of vaccination for preventing herpes zoster in older adults.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and quasi‐RCTs (studies in which participants are allocated to different arms of the trial using a method of allocation that is not truly random), regardless of publication date or language.
Types of participants
We included studies involving older adults (mean age 60 years and over). We excluded trials involving participants with immunosuppressive disorders.
Types of interventions
We included clinical trials that compared herpes zoster vaccine, of any dose and potency, with at least one of the following comparison groups.
Any other type of intervention (e.g. varicella vaccine, antiviral medication).
Placebo.
Nothing (no vaccine).
Types of outcome measures
Primary outcomes
Incidence of herpes zoster, diagnosed according to the criteria (clinical or laboratory, or both) established by the primary studies.
Secondary outcomes
-
Adverse events (occurring at any time after vaccination):
death (death was specified as a serious adverse event because of its importance in clinical studies and clinical practice);
serious adverse events (as defined by the FDA as: "Death, life‐threatening, hospitalisation (initial or prolonged), disability or permanent damage, congenital anomaly/birth defect, required intervention to prevent permanent impairment or damage (devices), other important medical events)" (FDA definition);
systemic reactions (e.g. fatigue, fever, gastrointestinal symptoms, headache, myalgia, shivering, or other); and
local reaction (e.g. pain, pruritus, swelling, or other).
Dropouts.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019 Issue 1, January), which includes the Cochrane Acute Respiratory Infections Group Specialised Register, in the Cochrane Library, MEDLINE (1948 to January 2019), Embase (2010 to January 2019), CINAHL (Cumulative Index to Nursing and Allied Health Literature) (1981 to January 2019), LILACS (Latin American and Caribbean Health Science Information database) (1982 to January 2019), WHO ICTRP (World Health Organization ‐ International Clinical Trials Registry Platform) (on 31 January 2019) and ClinicalTrials.gov (on 31 January 2019). We conducted all searches for this update on 31 January 2019.
We used the search strategy in Appendix 1 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (Appendix 2), LILACS (Appendix 3), and CINAHL (Appendix 4). We imposed no language or publication restrictions.
Searching other resources
We searched two trial registries, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and the USA National Institutes of Health Ongoing Trials Register (ClinialTrials.gov), for completed and ongoing studies (latest search 31 January 2019).
We checked the reference lists of relevant studies. We contacted trial authors for additional information and unpublished studies. We checked conference proceedings and thesis banks for unpublished studies. We also contacted vaccine manufacturers for unpublished data.
Data collection and analysis
The aim of intention‐to‐treat (ITT) analysis is to include all participants randomised into a trial irrespective of what subsequently occurred (Lewis 1993; Newell 1992). ITT analyses are generally preferred as they are unbiased, and also because they address a more pragmatic and clinically relevant question. We attempted to consider ITT when this was possible or available.
Selection of studies
Two review authors (AG, BNGA) independently assessed the titles and abstracts of all retrieved records against our inclusion criteria. We used the Kappa coefficient to test concordance amongst review authors (Latour 1997). Any discrepancies were resolved through consensus or by consulting a third review author (MRT) when necessary.
Data extraction and management
We created a data extraction form specifically for this review to collect relevant information such as study methods, participants, intervention group, control group, and outcomes.
Assessment of risk of bias in included studies
We evaluated the methodological quality of each included study in accordance with the criteria for judging risk of bias in the Cochrane ‘Risk of bias’ assessment tool (Higgins 2011). We evaluated the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We classified each of these domains as 'low risk of bias', 'unclear risk of bias', or 'high risk of bias'.
Measures of treatment effect
Dichotomous data
For binary data, we calculated the results for each study using the risk ratio (RR) with 95% confidence interval (CI) and number needed to treat for an additional beneficial outcome (NNTB) for efficacy, and number needed to treat for an additional harmful outcome (NNTH) for adverse events, where there were statistically significant differences.
Continuous data
For outcomes presented in other forms (e.g. reported as medians, quartiles, etc.) or without consistent statistical information (e.g. standard deviations (SDs), or number of participants), we inserted these data into an Additional table.
Unit of analysis issues
The participant was the unit of analysis, including participants undergoing more than one intervention in cross‐over trials. We used data from cross‐over studies (separated or grouped) when this information was available.
Dealing with missing data
For dichotomous data, we performed ITT analyses to include all participants randomised to the study groups. We contacted trial authors in order to obtain any missing data from the included studies. In studies for which reasons for withdrawal were not provided, we analysed data assuming the worst‐possible outcome, since imputation of data is a matter of personal judgement (Higgins 2011).
Assessment of heterogeneity
We assessed the consistency of results through visual inspection of the forest plots and by calculating the I² statistic (Higgins 2003), which estimates the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. We assumed substantial (significant) heterogeneity when the I² statistic was > 50%. We analysed data using a random‐effects model.
Assessment of reporting biases
It was not necessary to prepare a funnel plot since we included fewer than 10 studies in the meta‐analysis.
Data synthesis
We calculated the RR for dichotomous variables and the mean difference (MD) for continuous variables, when studies reported their results in the same units of measurement. When continuous data were reported in different units, we pooled the data through standardised mean differences (SMDs). We used 95% CIs for all statistical methods employed to pool data. We entered data into Cochrane Review Manager 5 software (Review Manager 2014), and conducted meta‐analyses using a random‐effects model.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: incidence of herpes zoster, adverse events (i.e. death, serious, systemic, potential immune‐mediated disease, and local symptoms), and dropouts. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias), Atkins 2004, to assess the quality of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2006a; Guyatt 2006b). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to downgrade or upgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Factors that can reduce the quality of the evidence (downgrade) include:
limitations in study design or execution (risk of bias): lower by one or two levels;
inconsistency of results: lower by one or two levels;
indirectness of evidence: lower by one or two levels;
imprecision: lower by one or two levels;
publication bias: lower by one or two levels.
Factors that can increase the quality of the evidence (upgrade) include:
large magnitude of effect: upgrade by one or two levels;
all plausible confounding that would reduce the demonstrated effect or increase the effect if no effect was observed: upgrade by one level;
dose‐response gradient: upgrade by one level.
Based on these factors, we classified the quality of evidence for each outcome as high, moderate, low, or very low (Schünemann 2011):
high‐quality evidence: RCTs or double‐upgraded observational studies;
moderate‐quality evidence: downgraded RCTs or upgraded observational studies;
low‐quality evidence: double‐downgraded RCTs or observational studies;
very low‐quality evidence: triple‐downgraded RCTs or downgraded observational studies; or case series/case reports.
Subgroup analysis and investigation of heterogeneity
We grouped results from studies according to methodological and clinical aspects such as vaccine dosage (plaque‐forming units per dose), vaccine conservation method (refrigerated or frozen), participant age, previous episode of herpes zoster, and simultaneous administration of other vaccines.
Sensitivity analysis
We performed sensitivity analyses where this was possible. We investigated the impact of quasi‐RCTs, studies with lower methodological quality, cross‐over studies, and unpublished data on the results of the review.
Results
Description of studies
This updated version of the review includes 24 RCTs (36 published reports) (Beals 2016; Berger 1998; Chlibek 2013; Chlibek 2014; Cunningham 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Lal 2015; Lal 2018; Levin 2000; Levin 2018; Maréchal 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Oxman 2005; Schwarz 2017; Tyring 2007; Vermeulen 2012; Vesikari 2013; Vink 2017).
We classified 11 studies as ongoing in the last version of this review (Gagliardi 2016). We included six of these studies in the current version of the review: Beals 2016 (formerly NCT01385566); Cunningham 2016 (formerly NCT01165177); Lal 2018 (formerly NCT01751165); NCT00886613; NCT01505647; and Vink 2017 (formerly NCT01777321). Of the remaining five studies, three did not meet our inclusion criteria and were excluded (Kovac 2018 (formerly NCT01165229); Strezova 2017 (formerly NCT02075515); Weinberg 2018 (formerly NCT02114333)), and two were retained as ongoing studies: NCT02180295 was withdrawn prior to enrolment, and NCT02526745 was completed, but results have not been posted on ClinicalTrials.gov or published elsewhere.
Lal 2015 presented efficacy data by age, and data for participants aged 60 years or over were included. However, in response to our enquiry, the study authors replied that safety data ordered by age were not available, therefore we used safety data provided for participants aged 50 years or over.
Results of the search
We included 11 new studies for this update. The process of study identification and selection for this update is shown in Figure 1.
1.
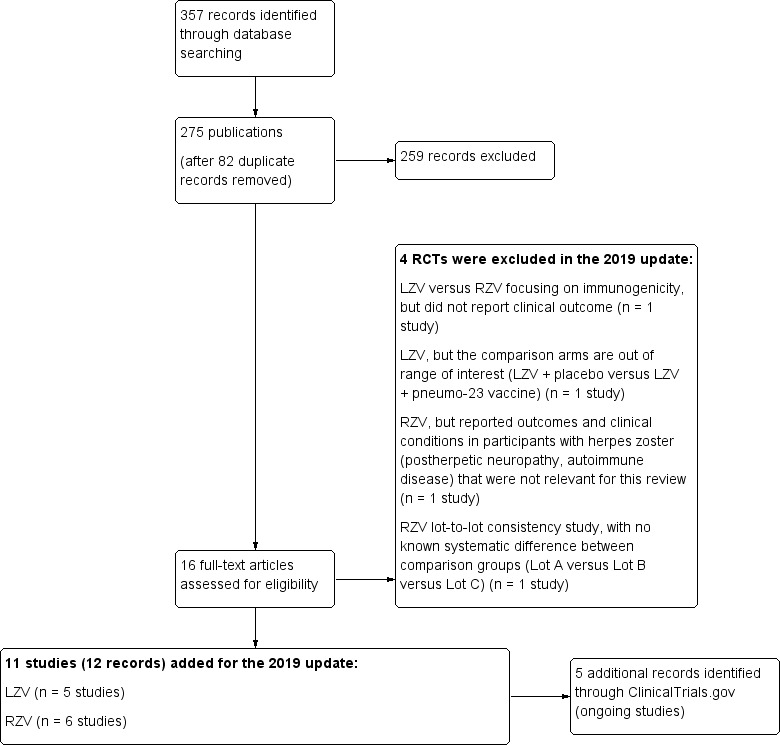
Study flow diagram 2019 update.
Included studies
The 24 included trials enrolled a total of 88,531 participants (Beals 2016; Berger 1998; Chlibek 2013; Chlibek 2014; Cunningham 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Lal 2015; Lal 2018; Levin 2000; Levin 2018; Maréchal 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Oxman 2005; Schwarz 2017; Tyring 2007; Vermeulen 2012; Vesikari 2013; Vink 2017).
Design
All included studies were RCTs. Of the 24 trials, 14 were double‐blinded (Berger 1998; Chlibek 2013; Cunningham 2016; Gilderman 2008; Hata 2016; Lal 2015; Levin 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; Oxman 2005; Tyring 2007; Vermeulen 2012); two were partly blinded (Beals 2016; Chlibek 2014); and eight were open‐label studies (Diez‐Domingo 2015; Lal 2018; Levin 2000; Maréchal 2018; NCT02052596; Schwarz 2017; Vesikari 2013; Vink 2017). Trial duration varied from 28 days to 7.0 years postvaccination.
Only Mills 2010 used a cross‐over design. We included this study because the cross‐over was design appropriate; it is clear that the order of receiving treatments was randomised ("subjects were enrolled and randomized in a 1:1 ratio to one of two vaccination groups"); it can be assumed that the trial was not biased from carry‐over effects; and unbiased data were available. This study reported outcome data (for adverse events and dropouts) separately for participants aged 50 to 59 years and 60 years or over. For this review, we only included data from these older participants who received zoster vaccines versus placebo.
Location
Six studies were conducted in the USA (Beals 2016; Gilderman 2008; Levin 2000; Levin 2018; Mills 2010; Oxman 2005); 15 studies were multicentre: France, Switzerland, and Ireland (Berger 1998); the Czech Republic, Spain, and the USA (Chlibek 2013); the Czech Republic, Germany, the Netherlands, and Sweden (Chlibek 2014); 18 countries in Europe, North America, Latin America, Asia, and Australia (Cunningham 2016); Germany and Spain (Diez‐Domingo 2015); 18 countries in Europe, North America, Latin America, Asia, and Australia (Lal 2015); the USA and Estonia (Lal 2018); the USA, Canada, and Estonia (Maréchal 2018); Canada, Germany, Spain, the UK, and the USA (Murray 2011); Canada, Germany, and the USA (Schwarz 2017); the USA, Canada, the UK, Germany, and Belgium (Tyring 2007); the USA and the Netherlands (Vermeulen 2012); Finland, Germany, Italy, Spain, and the Netherlands (Vesikari 2013). Two studies were conducted in Japan (Hata 2016; Vink 2017). Location information was not provided for three studies (NCT00886613; NCT01505647; NCT02052596).
Setting
All studies were conducted in outpatient settings.
Sample sizes
The mean sample size was 2175 participants and ranged from 54 to 38,546 participants. Four studies included more than 10,000 participants (Cunningham 2016; Lal 2015; Murray 2011; Oxman 2005).
Participants
Participants were healthy adults, with a mean age of 68 years. Most participants (> 88%) in the primary studies were Caucasian (understood to be white) and female (58%).
With one exception, all included studies enrolled healthy older adults with previous VZV contact, but without a history of herpes zoster (Mills 2010). Mills 2010 enrolled participants with a history of herpes zoster. Two studies included participants aged 70 or older (Cunningham 2016; Vesikari 2013). Hata 2016 included participants with diabetes and good glycaemic control.
Interventions
As there were several types of interventions, we grouped them as follows.
Vaccine versus placebo: LZV versus placebo (Mills 2010; Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012); RZV versus placebo (Cunningham 2016; Lal 2015).
Different routes of administration: LZV intramuscular (IM) route versus LZV subcutaneous (SC) route (Diez‐Domingo 2015); LZV intradermal route (ID) versus LZV SC route (Beals 2016); RZV IM route versus RZV SC route (Vink 2017).
Different storage modes: refrigerated versus frozen LZV (Gilderman 2008).
Different processing or composition: high‐potency LZV versus low‐potency LZV (Tyring 2007); LZV AMP (Alternative Manufacturing Process) versus LZV (NCT01505647); heat‐treated LZV versus LZV or placebo (NCT00886613).
Different administration intervals: two doses of a LZV versus a single dose and two doses given at different intervals (Vesikari 2013); adjuvanted recombinant VZV subunit zoster vaccine: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline (Chlibek 2013); adjuvanted recombinant VZV subunit zoster vaccine: three groups of VZV subunit gE in three different quantities versus unadjuvanted gE or saline (Chlibek 2014); RZV two doses given at three different intervals (Lal 2018).
Comparison with other vaccines or concomitant versus non‐concomitant administration: LZV versus 23‐valent pneumococcal polysaccharide vaccine (Berger 1998,Hata 2016); RZV versus 23‐valent pneumococcal polysaccharide vaccine (Maréchal 2018); LZV + inactivated quadrivalent influenza vaccines (IIV4) concomitant administration versus LZV + IIV4 sequential administration (Levin 2018); RZV + IIV4 co‐administration group versus non‐co‐administration group (Schwarz 2017); RZV + tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (TDaPV) co‐administration group versus RZV + TDaPV non‐co‐administration group (NCT02052596).
Subgroup and sensitivity analyses
We did not conduct subgroup analyses due to differences between study interventions.
We did not conduct sensitivity analyses because there were no quasi‐randomised studies or studies with lower methodological quality. We found only one small cross‐over study that had no impact on the results of the review.
Outcomes
Seven included studies reported incidence of herpes zoster (Cunningham 2016; Hata 2016; Lal 2015; Lal 2018; Maréchal 2018; Tyring 2007; Vink 2017).
All 24 included studies reported adverse events. These included or were defined as death, serious adverse events, unsolicited reports of adverse events, systemic adverse events, and injection site reactions (Beals 2016; Berger 1998; Chlibek 2013; Chlibek 2014; Cunningham 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Lal 2015; Lal 2018; Levin 2000; Levin 2018; Maréchal 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Oxman 2005; Schwarz 2017; Tyring 2007; Vermeulen 2012; Vesikari 2013; Vink 2017).
Excluded studies
We excluded a total of 11 RCTs (Hayward 1994; Hayward 1996; Irwin 2007; Kerzner 2007; Kovac 2018; Leroux‐Roels 2012; Macaladad 2007; MacIntyre 2010; Patterson‐Bartlett 2007; Strezova 2017; Weinberg 2018). Three of these studies evaluated LZV focusing on immunogenicity, but did not report clinical outcomes (Hayward 1994; Hayward 1996; Patterson‐Bartlett 2007). Weinberg 2018 compared LZV versus RZV, but focused only on immunogenicity. Irwin 2007 tested an intervention outside the scope of this review (Tai Chi). Kerzner 2007 evaluated LZV administered concomitantly with influenza vaccine. Kovac 2018 investigated RZV, but reported outcomes and clinical conditions in participants with herpes zoster (postherpetic neuropathy, autoimmune disease) that were not relevant for this review. Leroux‐Roels 2012 evaluated RZV, but included participants outside the age range of interest (55 to 57 years). Macaladad 2007 evaluated LZV, but included participants outside the age range of interest (adults aged up to 60 years). MacIntyre 2010 evaluated LZV, but the comparison arms did not match our inclusion criteria (antizoster + placebo versus antizoster + pneumo‐23 vaccine). Strezova 2017 conducted a multicentre, lot‐to‐lot consistency study (RZV), with no known systematic difference between comparison groups (Lot A versus Lot B versus Lot C).
Ongoing studies
We identified five ongoing studies (NCT02180295; NCT02526745; NCT03116594; NCT03120364; NCT03439657). We will check for completion of these studies for a future update. If complete, we will assess reports for inclusion in the review.
Risk of bias in included studies
Details of the 'Risk of bias' assessment for each trial are provided in Characteristics of included studies. The overall risk of bias is presented graphically in Figure 2 and is summarised in Figure 3.
2.
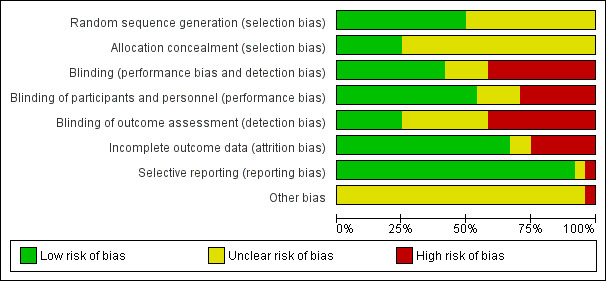
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
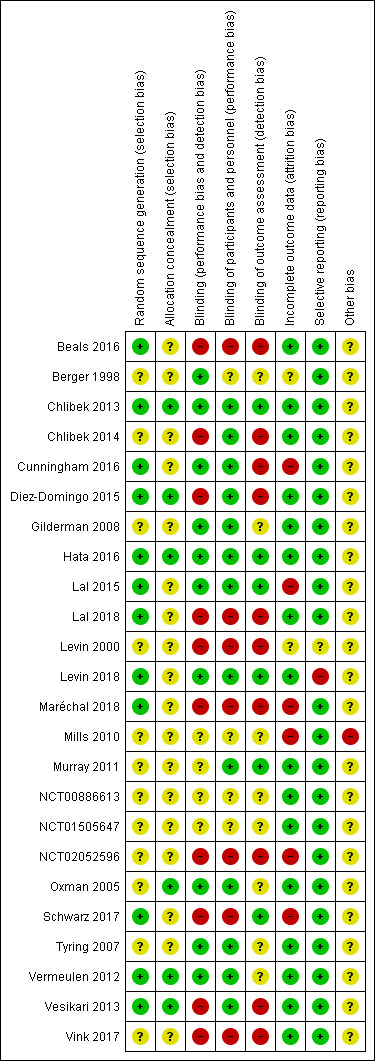
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assessed 12 studies as at low risk of bias for random sequence generation (selection bias) because they described how randomisation was performed (Beals 2016; Chlibek 2013; Cunningham 2016; Diez‐Domingo 2015; Hata 2016; Lal 2015; Lal 2018; Levin 2018; Maréchal 2018; Schwarz 2017; Vermeulen 2012; Vesikari 2013).
The other 12 included trials provided no details on the randomisation process and were thus classified as at unclear risk of bias for this domain (Berger 1998; Chlibek 2014; Gilderman 2008; Levin 2000; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Oxman 2005; Tyring 2007; Vink 2017).
Allocation concealment
We assessed seven trials as at low risk of bias because adequate allocation concealment was described in the study reports (Chlibek 2013; Diez‐Domingo 2015; Hata 2016; Lal 2015; Oxman 2005; Vermeulen 2012: Vesikari 2013).
Seventeen trials did not report details of allocation concealment and were thus classified as at unclear risk of bias for this domain (Beals 2016; Berger 1998; Chlibek 2014; Cunningham 2016; Gilderman 2008; Lal 2018; Levin 2000; Levin 2018; Maréchal 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Schwarz 2017; Tyring 2007; Vink 2017).
Blinding
We assessed 10 trials as at low risk of bias for this domain because it was clear that trial personnel were blinded to assignments (Berger 1998; Chlibek 2013; Cunningham 2016; Gilderman 2008; Hata 2016; Lal 2015; Levin 2018; Oxman 2005; Tyring 2007; Vermeulen 2012).
We judged four studies as at unclear risk of bias because the study reports did not describe the blinding process (Mills 2010; Murray 2011; NCT00886613; NCT01505647).
We assessed 10 studies as at high risk for this domain as they did not describe how blinding was performed or were open‐label studies (Beals 2016; Chlibek 2014; Diez‐Domingo 2015; Lal 2018; Levin 2000; Maréchal 2018; NCT02052596; Schwarz 2017; Vesikari 2013; Vink 2017).
Incomplete outcome data
We assessed 16 studies as at low risk of bias for this domain because the flow of participants was clear (Beals 2016; Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Lal 2018; Levin 2018; Murray 2011; NCT00886613; NCT01505647; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013; Vink 2017).
We classified Berger 1998 and Levin 2000 as at unclear risk of attrition bias due to insufficient information related to this domain.
We assessed the remaining six studies as at high risk for attrition bias: the participant flow was unclear (Cunningham 2016; Lal 2015; NCT02052596); the study report provided no data from the first arm of this cross‐over study (Mills 2010); and data for many outcomes were presented graphically only (Maréchal 2018; Schwarz 2017).
Selective reporting
We classified 22 studies as at low risk of reporting bias because the outcomes originally defined by the authors were presented for all groups (Beals 2016; Berger 1998; Chlibek 2013; Chlibek 2014; Cunningham 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Lal 2015; Lal 2018; Maréchal 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; NCT02052596; Oxman 2005; Schwarz 2017; Tyring 2007; Vermeulen 2012; Vesikari 2013; Vink 2017).
We assessed Levin 2000 as at unclear risk of bias due to insufficient information related to this domain. We judged Levin 2018 as at high risk of reporting bias because not all adverse events proposed in the methods section were presented in the results.
Other potential sources of bias
We only assessed Mills 2010 as having a high risk of bias for this domain because it had a cross‐over design. We classified all of the other included studies as having an unclear risk for other bias due to insufficient information for judgement.
Effects of interventions
Summary of findings for the main comparison. Live zoster vaccine versus placebo for preventing herpes zoster in older adults.
| Live zoster vaccine versus placebo for preventing herpes zoster in older adults | ||||||
| Patient or population: healthy older adults aged ≥ 60 years Settings: outpatients Intervention: live zoster vaccine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Live zoster vaccine versus placebo | |||||
| Incidence of herpes zoster, 3.1 years follow‐up Clinical or laboratory criteria Follow‐up: mean 3.1 years | 33 per 1000 | 16 per 1000 (14 to 19) | RR 0.49 (0.43 to 0.56) | 38,546 (1 study) | ⊕⊕⊕⊝ moderate1 | NNTB = 50 |
| Participants with adverse events Clinical or laboratory criteria Follow‐up: mean 3.1 years | 344 per 1000 | 584 per 1000 (553 to 615) | RR 1.71 (1.38 to 2.11) | 7119 (5 studies) | ⊕⊕⊕⊝ moderate1 | NNTH = 4.3 |
| Death Clinical criteria Follow‐up: mean 3.1 years | 32 per 1000 | 32 per 1000 (29 to 35) | RR 1.01 (0.92 to 1.11) | 50,820 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events: 1 or more serious adverse events regardless of type of storage of the vaccine Clinical or laboratory criteria Follow‐up: mean 3.1 years | 22 per 1000 | 23 per 1000 (21 to 26) | RR 1.08 (0.95 to 1.21) | 51,029 (6 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events ‐ systemic adverse events Clinical or laboratory criteria Follow‐up: mean 42 days | 227 per 1000 | 241 per 1000 (222 to 263) | RR 1.24 (0.82 to 1.87) | 7119 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events ‐ injection site adverse events Clinical criteria Follow‐up: mean 7 days | 161 per 1000 | 480 per 1000 (441 to 522) | RR 3.73 (1.93 to 7.21) | 7040 (4 studies) | ⊕⊕⊕⊝ moderate1 | NNTH = 3.6 |
| Dropouts Clinical or laboratory criteria Follow‐up: mean 3.1 years | 48 per 1000 | 47 per 1000 (43 to 51) | RR 0.99 (0.90 to 1.08) | 38,916 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| *The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Most data came from a large study, and the quality of the evidence was downgraded because the trial did not describe the method used for random sequence generation.
Summary of findings 2. Recombinant zoster vaccine versus placebo for preventing herpes zoster in older adults.
| Recombinant zoster vaccine versus placebo for preventing herpes zoster in older adults | ||||||
| Patient or population: healthy older adults aged ≥ 60 years Settings: outpatients Intervention: recombinant zoster vaccine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Recombinant zoster vaccine versus placebo | |||||
| Incidence of herpes zoster at least 3.2 years follow‐up Clinical or laboratory criteria Follow‐up: mean 3.2 years | 34 per 1000 | 3 per 1000 (2 to 4) | RR 0.08 (0.03 to 0.23) | 22,022 (2 studies) | ⊕⊕⊕⊝ moderate1 | NNTB = 33 |
| Participants with adverse events ‐ death Clinical criteria Follow‐up: mean 3.2 years | 43 per 1000 | 41 per 1000 (36 to 45) | RR 0.94 (0.84 to 1.04) | 29,311 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events ‐ serious adverse events Clinical or laboratory criteria Follow‐up: mean 3.2 years | 130 per 1000 | 126 per 1000 (118 to 133) | RR 0.97 (0.91 to 1.03) | 29,311 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events ‐ any systemic symptom Clinical criteria Follow‐up: mean 30 days | 291 per 1000 | 648 per 1000 (617 to 680) | RR 2.23 (2.12 to 2.34) | 9762 (2 studies) | ⊕⊕⊕⊝ moderate1 | NNTH = 3.0 |
| Participants with adverse events ‐ potential immune‐mediated disease Clinical or laboratory criteria Follow‐up: mean 3.2 years | 13 per 1000 | 12 per 1000 (9 to 14) | RR 0.88 (0.71 to 1.08) | 29,311 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Participants with adverse events ‐ any local symptom Clinical criteria Follow‐up: mean 7 days | 117 per 1000 | 807 per 1000 (746 to 873) | RR 6.89 (6.37 to 7.45) | 9769 (2 studies) | ⊕⊕⊕⊝ moderate1 | NNTH = 1.5 |
| Dropouts ‐ did not receive second dose Clinical or laboratory criteria Follow‐up: mean 3.2 years | 40 per 1000 | 50 per 1000 (50 to 50) | RR 1.25 (1.13 to 1.39) | 29,311 (2 studies) | ⊕⊕⊕⊝ moderate1 | NNTH = 100 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Both studies had limitations in study design or execution (allocation concealment, attrition or detection bias).
Primary outcome
1. Incidence of herpes zoster
Live attenuated varicella zoster virus (VZV) vaccine (LZV) versus placebo
Oxman 2005 (N = 38,546) evaluated the effectiveness of zoster vaccine versus placebo in reducing the incidence of herpes zoster with a median surveillance of 3.1 years and reported a significant reduction for this outcome in the vaccinated group (risk ratio (RR) 0.49, 95% confidence interval (CI) 0.43 to 0.56; Analysis 1.1.1). Although this was a significant difference in favour of the intervention, the magnitude of this effect was a risk difference (RD) of 2%, and the number needed to treat for an additional beneficial outcome (NNTB) was 50. The quality of evidence was moderate, downgraded due to risk of bias (no description of the randomisation process) (Table 1).
1.1. Analysis.
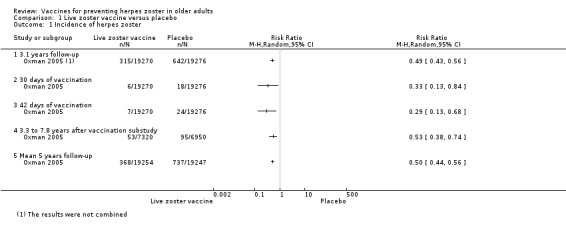
Comparison 1 Live zoster vaccine versus placebo, Outcome 1 Incidence of herpes zoster.
The vaccinated group had a reduced incidence of herpes zoster as early as 30 days postvaccination (RR 0.33, 95% CI 0.13 to 0.84; Analysis 1.1.2). These cases were excluded from the final intention‐to‐treat (ITT) analysis. At 42 days postvaccination, the benefits of vaccination are clear (RR 0.29, 95% CI 0.13 to 0.68; Analysis 1.1.3).
The continuation of the Oxman 2005 study was published in 2012 (Schmader 2012) (N = 14,270), and evaluated the effectiveness of the vaccine five years after participants had been vaccinated. However, the published data reported different dates for the collection of outcomes in the intervention and the placebo groups. The data from the zoster vaccine group were from December 2004 to March 2006 (16 months), whilst data from the placebo group were reported from December 2004 to September 2005 (10 months), since in October 2005 the zoster vaccine was also offered to participants in the placebo group, as stated by the authors: "Beginning in October 2005, open‐label zoster vaccine was offered without charge to Shingles Prevention Study placebo recipients". We contacted the study authors and asked for data corresponding to the period from December 2004 to September 2005 (10 months) for both groups (vaccine and placebo). The authors replied to our request but did not provide this information and suggested that we should instead assume a uniform rate of events and calculate the estimated number of cases from that. According to their suggestion, we calculated that the inferred rate of incidence of herpes zoster (from December 2004 to September 2005) would be 53 in the vaccine group at 10 months (total number of herpes zoster cases in the vaccine group 84 in 16 months, therefore 53 in 10 months), and the incidence of herpes zoster would be 95 cases in 10 months in the placebo group. The resulting RR was 0.53, 95% CI 0.38 to 0.74; RD −0.01, 95% CI −0.01 to −0.00; NNTB 100, in favour of the vaccinated group (Analysis 1.1.4). By the same reasoning, when considering the follow‐up period of five years, there was a significant decrease in the incidence of herpes zoster in the vaccine group compared to the placebo group (RR 0.50, 95% CI 0.44 to 0.56; RD −0.02, 95% CI −0.02 to −0.02; NNTB 50; Analysis 1.1.5). We did not include these data in the Table 1 as these data were inferred. Hata 2016 did not present any cases of herpes zoster (Analysis 1.1.6). See also Table 1.
Hata 2016 compared LZV versus placebo in people with controlled diabetes and did not report any confirmed cases of herpes zoster in the one year of follow‐up. However, this study was small (54 participants).
The overall quality of evidence for the primary effectiveness outcome (incidence of herpes zoster) up to three years of follow‐up was moderate for the comparison LZV versus placebo (Oxman 2005). We downgraded the quality of the evidence due to insufficient information about random sequence generation (Table 1).
Higher‐potency LZV versus lower‐potency LZV
Tyring 2007 compared higher‐potency LZV versus lower‐potency LZV and reported a higher incidence of herpes zoster (the polymerase chain reaction was positive for wild‐type VZV in two cases) in the first group, but this difference was not significant (RR 2.55, 95% CI 0.12 to 52.99).
Live versus inactivated zoster vaccine
Levin 2000 compared LZV versus an inactivated zoster vaccine and reported no difference in the incidence of herpes zoster (RR 0.96, 95% CI 0.06 to 15.17).
Adjuvanted recombinant zoster vaccine (RZV) versus placebo
Lal 2015 and Cunningham 2016 (N = 22,022) tested RZV efficacy. For a follow ‐up period of at least 3.2‐years, the pooled data showed a decrease in the incidence of herpes zoster in vaccinated participants compared to those who received placebo (RR 0.08, 95% CI 0.03 to 0.23; RD 3%; NNTB 33; Analysis 2.1). Heterogeneity (I² statistic) for this meta‐analysis was 82% (Analysis 2.1). The RR for herpes zoster from data provided by Cunningham 2016 for the follow‐up period of at least four years was 0.11, 95% CI 0.04 to 0.31; RD 6%; NNTB 16.7 (Analysis 2.2).
2.1. Analysis.
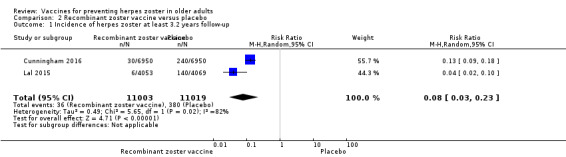
Comparison 2 Recombinant zoster vaccine versus placebo, Outcome 1 Incidence of herpes zoster at least 3.2 years follow‐up.
2.2. Analysis.
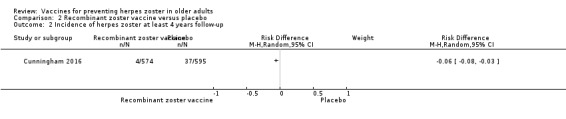
Comparison 2 Recombinant zoster vaccine versus placebo, Outcome 2 Incidence of herpes zoster at least 4 years follow‐up.
We assessed quality of evidence as moderate, downgrading due to insufficient information on allocation concealment and the flow of participants (Table 2).
Secondary outcomes
1. Adverse events
A summary of the adverse events associated with the use of the different types of herpes zoster vaccines compared to placebo is presented in Table 3.
1. Summary of adverse events for LZV versus placebo and RZV versus placebo.
| Comparison | Summary of adverse events |
| LZV versus placebo | The incidence of the following AEs did not differ significantly between the groups receiving LZV or placebo: 1 or more SAEs (including death), vaccine‐related SAEs, systemic AEs, AEs not related to vaccine, and haematoma at inoculation site. Participants of the vaccinated group had a higher incidence of vaccine‐related AEs and vaccine‐related systemic AEs beyond AEs at the injection site (erythema, pain, swelling, warmth, pruritus, rash, mass, and varicella‐like rash). The injection site AEs were erythema, pruritus, swelling, which lasted longer in the LZV group, and duration of rash lasted longer in the placebo group. |
| RZV versus placebo | The incidence of the following AEs did not differ significantly between the groups receiving RZV or placebo: SAEs (including death), SAEs (including death) related to vaccination, and potential immune‐mediated disease. Systemic AEs (myalgia, fatigue, headache, fever, shivering, and gastrointestinal symptom) as well as local AEs (redness, pain, and swelling) occurred more frequently in the RZV group than in the placebo group. |
AEs: adverse events LZV: live zoster vaccine RZV: recombinant zoster vaccine SAEs: serious adverse events
LZV versus placebo
Details of the adverse events for this comparison are provided in Table 4.
2. Adverse events live zoster vaccine (LZV).
| Comparison (studies) | Results |
|
LZV versus placebo (Hata 2016; Levin 2018; Mills 2010; Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012) |
The following adverse events did not differ significantly between groups receiving LZV or placebo: death (Hata 2016; Murray 2011; Oxman 2005), 1 or more SAE regardless of type of storage of the vaccine (Murray 2011; Oxman 2005), vaccine‐related serious adverse events (Murray 2011; Oxman 2005), hospitalisation (Oxman 2005), hospitalisation related to HZ (Oxman 2005), systemic adverse events (Hata 2016; Mills 2010; NCT00886613; Oxman 2005; Vermeulen 2012), systemic pruritus (Hata 2016; Vermeulen 2012), general malaise (Hata 2016), headache (NCT00886613), varicella‐like rash not at injection site (from day of vaccination to day 42) (NCT00886613; Oxman 2005; Vermeulen 2012), rash unrelated to HZ (from day of vaccination to day 42) (NCT00886613; Oxman 2005), haematoma at inoculation site (Oxman 2005), and adverse events not related to vaccine (Hata 2016). Participants of vaccinated group had a higher incidence of the following: 1 or more adverse events (RR 1.71, 95% CI 1.38 to 2.11; RD 0.23, 95% CI 0.14 to 0.32; NNTH 4.3, 95% CI 3.1 to 7.1) (Analysis 1.3.6) (Hata 2016; Mills 2010; NCT00886613; Oxman 2005; Vermeulen 2012); vaccine‐related adverse events (RR 2.64, 95% CI 1.21 to 5.75; RD 0.26, 95% CI 0.03 to 0.55; NNTH 3.8, 95% CI 1.8 to 33.3) (Analysis 1.3.7) (Hata 2016; NCT00886613; Vermeulen 2012); and vaccine‐related systemic adverse events (RR 1.30, 95% CI 1.07 to 1.58; RD 0.01, 95% CI 0.00 to 0.03; NNTH 100.0 95% CI 33.3 to 100.00) (Analysis 1.3.9) (Mills 2010; NCT00886613; Oxman 2005). The vaccinated group had a higher incidence of adverse events at the injection site (RR 3.73, 95% CI 1.93 to 7.21; RD 0.28, 95% CI 0.15 to 0.41; NNTH 3.6, 95% CI 2.4 to 6.7) (Analysis 1.3.15) (Hata 2016; Mills 2010; Oxman 2005; Vermeulen 2012). Specific injection site adverse events also occurred more frequently in the vaccinated group:
Varicella‐like rash at injection site (up to day 42) also occurred more frequently in the vaccinated group: RR 2.86, 95% CI 1.21 to 6.76, but without a significant RD due to the small number of events (Analysis 1.3.24) (Oxman 2005). The risk of herpes zoster‐like rash up to 42 days postvaccination was lower in the vaccinated group (RR 0.47, 95% CI 0.27 to 0.84) than in the placebo group, but without a significant RD (Analysis 1.3.26) (Oxman 2005). Duration of injection site adverse events Injection site adverse events generally lasted longer in the zoster vaccine group. There were significant differences with respect to the duration of the following local adverse events: erythema MD 2.40 days (95% CI 1.56 to 3.24) (Analysis 1.4.1); pruritus MD 2.40 days (95% CI 1.32 to 3.48) (Analysis 1.4.3); and swelling MD 1.90 days (95% CI 1.35 to 2.45) (Analysis 1.4.4). The duration of pain and haematoma did not differ significantly between the groups: MD 1.00 (95% CI −0.10 to 2.10) (Analysis 1.4.2) and MD −0.50 (95% CI −5.52 to 4.52) (Analysis 1.4.6), respectively. The duration of rash was longer in the placebo group than in the vaccine group: RR −16.60 (95% CI −33.68 to 0.48) (Analysis 1.4.5). |
| High‐potency versus low‐potency zoster vaccine (Tyring 2007) | The comparison of high‐ versus low‐potency zoster vaccine yielded no significant differences between groups for the following adverse events: vaccine‐related adverse events, systemic vaccine‐related adverse events, and vaccine‐related serious adverse events (death). |
|
Refrigerated versus frozen zoster vaccine (Gilderman 2008) |
There were no significant differences between the refrigerated versus the frozen zoster vaccine for the following adverse events: 1 or more adverse events, vaccine‐related adverse events, systemic adverse events, systemic vaccine‐related adverse events, serious adverse events, vaccine‐related serious adverse events or death. However, there were more injection site adverse events in the group receiving frozen vaccines (RR 0.77, 95% CI 0.60 to 0.98). |
|
2 doses versus a single dose of LZV and 2 doses given at different intervals (Vesikari 2013) |
Zoster vaccine 1‐month schedule versus zoster vaccine 3‐month schedule There was no statistical difference between participants who received the doses of zoster vaccine 2 months apart compared to those receiving them 3 months apart: SAE (RR 0.95, 0.14 to 6.70); withdrawal due to AE (RR 2.86, 95% CI 0.12 to 69.80); AE (RR 1.10, 95% CI 0.91 to 1.31); vaccine‐related AE (RR 1.00, 95% CI 0.81 to 1.24); systemic AE (RR 1.34, 95% CI 0.90 to 2.00); vaccine‐related systemic AE (RR 1.27, 95% CI 0.45 to 3.60); rash of interest non‐injection site rashes (RR 0.95, 95% CI 0.06 to 15.14); varicella/varicella‐like rash (RR 0.95, 95% CI 0.06 to 15.14); injection site reaction (RR 0.99, 95% CI 0.80 to 1.23); solicited injection site reaction (RR 1.00, 95% CI 0.81 to 1.25); unsolicited injection site reaction (RR 0.41, 95% CI 0.11 to 1.56); erythema injection site (RR 1.01, 95% CI 0.80 to 1.27); pain injection site (RR 0.84, 95% CI 0.57 to 1.25); swelling injection site (RR 1.05, 95% CI 0.75 to 1.47). No participants from either group reported the following AEs: vaccine‐related SAE; vaccine‐related withdrawal due to AE; non‐serious vaccine‐related withdrawal due to AE; and herpes zoster/zoster‐like rash. Zoster vaccine 1‐month schedule versus zoster vaccine single dose Only participants with systemic AE: there were significant differences in favour of the 2 doses 1 month apart, with a higher incidence in the single‐dose group: RR 0.74, 95% CI 0.56 to 0.97; RD −0.07, 95% CI −0.13 to −0.01; NNTH 14.3, 95% CI 7.6 to 100. There was no statistical difference for most adverse events: SAE (RR 0.72, 95% CI 0.16 to 3.30); withdrawal due to AE (RR 0.36, 95% CI 0.05 to 2.82); vaccine‐related withdrawal due to AE (RR 0.21, 95% CI 0.01 to 3.74); non‐serious vaccine‐related withdrawal due to AE (RR 0.21, 95% CI 0.01 to 3.74); AE (RR 0.92, 95% CI 0.80 to 1.05); vaccine‐related AE (RR 0.91, 95% CI 0.77 to 1.08); vaccine‐related systemic AE (RR 0.54, 95% CI 0.26 to 1.12); rash of interest non‐injection site rashes (RR 1.61, 95% CI 0.15 to 17.72); varicella/varicella‐like rash (RR 9.66, 95% CI 0.39 to 236.25); herpes zoster/zoster‐like rash (RR 0.64, 95% CI 0.03 to 13.36); injection site reaction (RR 0.93, 95% CI 0.78 to 1.10); solicited injection site reaction (RR 0.94, 95% CI 0.79 to 1.11); unsolicited injection site reaction (RR 0.35, 95% CI 0.11 to 1.13); injection site erythema (RR 0.98, 95% CI 0.81 to 1.17); injection site pain (RR 0.74, 95% CI 0.54 to 1.01); injection site swelling (RR 1.08, 95% CI 0.82 to 1.41). There were no participants with vaccine‐related SAE in either group. Zoster vaccine 3‐month schedule versus zoster vaccine single dose Participants in the single‐dose group had a higher incidence of the following AEs in comparison to the group that received 2 doses, 3 months apart: AE (RR 0.84, 95% CI 0.72 to 0.97; RD −0.09; 95% CI −0.17 to −0.02; NNTH 11.1, 95% CI 5.9 to 50); systemic AE (RR 0.55, 95% CI 0.39 to 0.76; RD −0.13, 95% CI −0.18 to −0.07; NNTH 7.6, 95% CI 5.6 to 14.3); vaccine‐related systemic AE (RR 0.42, 95% CI 0.18 to 0.98; RD −0.04, 95% CI −0.06 to −0.01; NNTH 25.0, 95% CI 16.6 to 100). There were no significant differences between groups for the following adverse events: SAE (RR 0.75, 95% CI 0.16 to 3.46); withdrawal due to AE (RR 0.18, 95% CI 0.01 to 3.04); vaccine‐related withdrawal due to AE (RR 0.23, 95% CI 0.01 to 3.93); non‐serious vaccine‐related withdrawal due to AE (RR 0.23, 95% CI 0.01 to 3.93); vaccine‐related AE (RR 0.91, 95% CI 0.77 to 1.08); rash of interest non‐injection site rashes (RR 1.69, 95% CI 0.15 to 18.60); varicella/varicella‐like rash (RR 10.14, 95% CI 0.41 to 247.92); herpes zoster/zoster‐like rash (RR 0.68, 95% CI 0.03 to 14.02); injection site reaction (RR 0.93, 95% CI 0.79 to 1.11); solicited injection site reaction (RR 0.93, 95% CI 0.78 to 1.11); unsolicited injection site reaction (RR 0.85, 95% CI 0.38 to 1.91); injection site erythema (RR 0.97, 95% CI 0.80 to 1.17); injection site pain (RR 0.87, 95% CI 0.65 to 1.17); injection site swelling (RR 1.03, 95% CI 0.77 to 1.36). There were no participants with vaccine‐related SAE in either group. |
| LZV AMP versus LZV (NCT01505647) | There were no significant differences between LZV AMP versus LZV for the following adverse events: participants with 1 or more adverse events; injection site adverse events; injection site erythema; injection site pain; injection site pruritus; and injection site swelling. It is important to note that there was a significant difference for participants with 1 or more serious adverse events (RR 0.25, 95% CI 0.08 to 0.82; RD −0.04, 95% CI −0.07 to −0.00; NNTH 25.0, and no RD favourable to LZV). There were no deaths in this study. |
| Heat‐treated LZV versus LZV or placebo (NCT00886613) |
Heat LZV versus LZV There was no SAE in this comparison. There were no significant differences between groups for the following adverse events: 1 or more AE, 1 or more vaccine‐related AE, 1 or more systemic AE, 1 or more vaccine‐related systemic AE, headache, injection site erythema, and injection site pruritus. On the other hand, for 1 or more injection site AE (RR 0.40, 95% CI 0.23 to 0.70; RD −0.40, 95% CI −0.60 to −0.20; NNTH 2.5, 95% CI 1.17 to 5.0); vaccine‐related 1 or more injection site AE (RR 0.48, 95% CI 0.27 to 0.85; RD −0.30, 95% CI −0.50 to −0.09; NNTH 3.3, 95% CI 2 to 11.1); injection site induration (RR 0.36, 95% CI 0.16 to 0.82; RD −0.26, 95% CI −0.45 to −0.08; NNTH 3.8, 95% CI 2.2 to 12.5); injection site pain (RR 0.18, 95% CI 0.07 to 0.48; RD −0.44, 95% CI −0.62 to −0.26; NNTH 2.3, 95% CI 1.6 to 3.8). All significant differences were favourable to heat LZV. Heat LZV versus placebo There was no SAE in this comparison. There was no significant difference between heat LZV and placebo for all adverse events reported. |
|
LZV IM route versus LZV SC route (Diez‐Domingo 2015) |
The participants who received SC vaccines had a significantly higher incidence of the following adverse events:
There were no significant differences between groups for the following adverse events: all systemic adverse events: RR 1.03, 95% CI 0.70 to 1.51; vaccine‐related systemic AE: RR 0.93, 95% CI 0.44 to 1.98; headache considered as vaccine‐related by the investigator: RR 0.75, 95% CI 0.17 to 3.32; unsolicited injection site reaction: RR 0.65, 95% CI 0.29 to 1.45; severe injection site erythema (> 10 cm): RR 0.67, 95% CI 0.11 to 3.96; severe injection site pain (inability to work or perform usual activity): RR 1.01, 95% CI 0.14 to 7.06; severe injection site swelling (> 10 cm): RR 0.25, 95% CI 0.03 to 2.23. |
| LZV intradermal route versus LZV SC route (Beals 2016) |
Full‐dose intradermal versus full‐dose subcutaneous There were significant differences in favour of LZV SC for 2 AEs: 1 or more injection site AEs (RR 1.53, 95% CI 1.12 to 2.09; RD 0.27, 95% CI 0.08 to 0.47; NNTH 3.7, 95% CI 2.1 to 12.5) and erythema (RR 2.49, 95% CI 1.59 to 3.89; RD 0.46, 95% CI 0.27 to 0.65; NNTH 2.2, 95% CI 1.5 to 3.7). There were no significant differences between groups for the following adverse events: pain, swelling, induration, pruritus, haematoma or anaesthesia or rash. 1/3 dose intradermal versus full‐dose subcutaneous There were significant differences in favour of full‐dose LZV SC for the following AEs: erythema (RR 1.95, 95% CI 1.20 to 3.18; RD 0.29, 95% CI 0.09 to 0.50; NNTH 3.4, 95% CI 2.0 to 11.1) and induration (RR 3.57, 95% CI 1.38 to 9.23; RD 0.25, 95% CI 0.07 to 0.42; NNTH 4.0, 95% CI 2.4 to 14.3). There was no significant difference between groups for the other adverse events. 1/10 dose intradermal versus full‐dose subcutaneous There was no significant difference between groups for any adverse events. 1/27 dose intradermal versus full‐dose subcutaneous Erythema (RR 1.72, 95% CI 1.03 to 2.88; RD 0.22, 95% CI 0.01 to 0.43; NNTH 4.5, 95% CI 2.30 to 100.0) and induration (RR 3.06, 95% CI 1.14 to 8.17; RD 0.20, 95% CI 0.03 to 0.37; NNTH 5.0, 95% CI 2.7.0 to 3.3). There was no significant difference between groups for the other adverse events. Full‐dose intradermal versus 1/3 dose subcutaneous There was a difference between the groups favourable to the subcutaneous 1/3 dose group, which had a significantly lower incidence of the following AEs:1 or more injection site adverse events (RR 3.86, 95% CI 1.95 to 7.63; RD 0.59, 95% CI 0.40 to 0.77; NNTH 1.7, 95% CI 1.3 to 2.5); erythema (RR 5.20, 95% CI 2.27 to 11.93; RD 0.62, 95% CI 0.43 to 0.80; NNTH 1.6, 95% CI 1.2 to 2.3); and induration (RR 6.00, 95% CI 1.45 to 24.81; RD 0.29, 95% CI 0.12 to 0.47; NNTH 3.4, 95% CI 2.1 to 8.3). There was no significant difference between groups for the other adverse events. 1/3 dose intradermal versus 1/3 dose subcutaneous There was no significant difference between groups for all adverse events reported. 1/10 dose intradermal versus 1/3 dose subcutaneous There were significant differences in favour of 1/3 dose SC for the following AEs: 1 or more injection site adverse events (RR 2.71, 95% CI 1.32 to 5.60; RD 0.35, 95% CI 0.14 to 0.57; NNTH 2.9, 95% CI 1.8 to 7.1); erythema (RR 3.20, 95% CI 1.32 to 7.75; RD 0.32, 95% CI 0.12 to 0.53; NNTH 3.1, 95% CI 1.9 to 8.3); and induration (RR 5.50, 95% CI 1.32 to 22.98; RD 0.26, 95% CI 0.09 to 0.44; NNTH 3.8, 95% CI 2.3 to 11.1). There was no significant difference between groups for the other adverse events. 1/27 dose intradermal versus 1/3 dose subcutaneous There were significant differences in favour of 1/3 dose SC for the following AEs: 1 or more injection site adverse events (RR 2.71, 95% CI 1.32 to 5.60; RD 0.35, 95% CI 0.14 to 0.57; NNTH 2.9, 95% CI 1.8 to 7.1); erythema (RR 3.60, 95% CI 1.51 to 8.59; RD 0.38, 95% CI 0.18 to 0.59; NNTH 2.6, 95% CI 1.7 to 5.6); and induration (RR 5.00, 95% CI 1.18 to 21.14; RD 0.24, 95% CI 0.06 to 0.41; NNTH 4.2, 95% CI 2.4 to 16.7). There was no significant difference between groups for the other adverse events. |
|
LZV versus pneumo‐23 vaccine (Berger 1998) |
1 study compared 3 different concentrations of plaque‐forming units (pfu) of live attenuated VZV and reported the following adverse events: 3200 pfu VZV/dose versus pneumo‐23 There was a lower incidence of 1 or more injection site reactions in the group vaccinated with the 3200 pfu/dose zoster vaccine (RR 0.61, 95% CI 0.41 to 0.91) as well as pain at the injection site (RR 0.49, 95% CI 0.30 to 0.81). There were no significant differences between the 3200 pfu/dose zoster vaccine and the pneumo‐23 vaccine for the following local adverse events: induration (≥ 2 cm diameter injection site), probably vaccine‐related injection site pain, redness (≥ 2 cm diameter injection site), pruritus or vesicles (no patients had vesicles in the 3200 pfu/dose zoster vaccine nor the pneumo‐23 groups). 8500 pfu VZV/dose versus pneumo‐23 There was a lower incidence of 1 or more injection site reaction in the group vaccinated with the 8500 pfu/dose zoster vaccine (RR 0.63, 95% CI 0.43 to 0.93). There were no significant differences for the following injection site adverse events between participants who received the 8500 pfu/dose VZV vaccine and those who received the pneumo‐23 vaccine: induration (≥ 2 cm diameter injection site), pain (injection site), probably vaccine‐related injection site pain, redness, pruritus and vesicles. 41,650 pfu VZV/dose VZV versus pneumo‐23 Participants receiving the 41,650 pfu/dose zoster vaccine had significantly lower rates of one or more injection site reaction (RR 0.41, 95% CI 0.24 to 0.68) and pain at injection site (RR 0.43, 95% CI 0.25 to 0.74) than those receiving the pneumo‐23 vaccine. There were no significant differences between the groups for the following injection site adverse events: induration (≥ 2 cm diameter injection site), probably vaccine‐related injection site pain, redness (≥ 2 cm diameter injection site), pruritus and vesicles (no patients had vesicles in the 41,650 pfu/dose zoster vaccine nor the pneumo‐23 vaccine groups). |
| LZV + IIV4 concomitant administration versus LZV + IIV4 sequential administration (Levin 2018) | There were no significant differences between groups for the following: death, serious adverse events, one or more adverse events, non injection‐site adverse events, non injection site vaccine‐related AE, injection‐site adverse events. There were no vaccine‐related adverse events. |
AE: adverse event or adverse experiences AMP: Alternative Manufacturing Process CI: confidence interval Elderly or older adults: aged ≥ 60 years old Frozen: −15 °C or colder gE: recombinant subunit VZV composed of glycoprotein E gE/saline: unadjuvanted gE Heat LZV: heat‐treated LZV HZ: herpes zoster ID: identification IIV4: inactivated quadrivalent influenza vaccines IM: intramuscular ISRs: injection site adverse reactions ITT: intention‐to‐treat LZV or ZV: live zoster vaccine (live attenuated Oka varicella zoster virus vaccine) MD: mean difference NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome pfu: plaque‐forming units pIMDs: potential immune‐mediated diseases pneumo‐23 vaccine: 23–valent pneumococcal polysaccharide vaccine RD: risk difference Refrigerated: 2 °C to 8 °C RR: risk ratio SAEs: serious adverse events SC: subcutaneously or subcutaneous VZV: varicella zoster virus
Seven studies (N = 51,952) compared herpes zoster vaccine versus placebo and presented safety data that could be pooled into a meta‐analysis (Hata 2016; Levin 2018; Mills 2010; Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012). Oxman 2005 presented a more detailed assessment of safety for a subgroup of participants (zoster vaccine N = 3345; placebo N = 3271). Murray 2011 assessed only serious adverse events.
There were no significant differences between groups receiving LZV or placebo for death (RR 1.01, 95% CI 0.92 to 1.11; Analysis 1.3.1) (Hata 2016; Mills 2010; Murray 2011; NCT00886613; Oxman 2005; N = 50,820); one or more serious adverse events (RR 1.08, 95% CI 0.95 to 1.21; Analysis 1.3.2) (Hata 2016; Mills 2010; Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012; N = 51,029); vaccine‐related serious adverse events (RR 0.99, 95% CI 0.24 to 4.15; Analysis 1.3.3) (Mills 2010; Murray 2011; NCT00886613; Oxman 2005; N = 50.766); hospitalised (Analysis 1.3.4) or hospitalisation related to herpes zoster (Analysis 1.3.5).
1.3. Analysis.
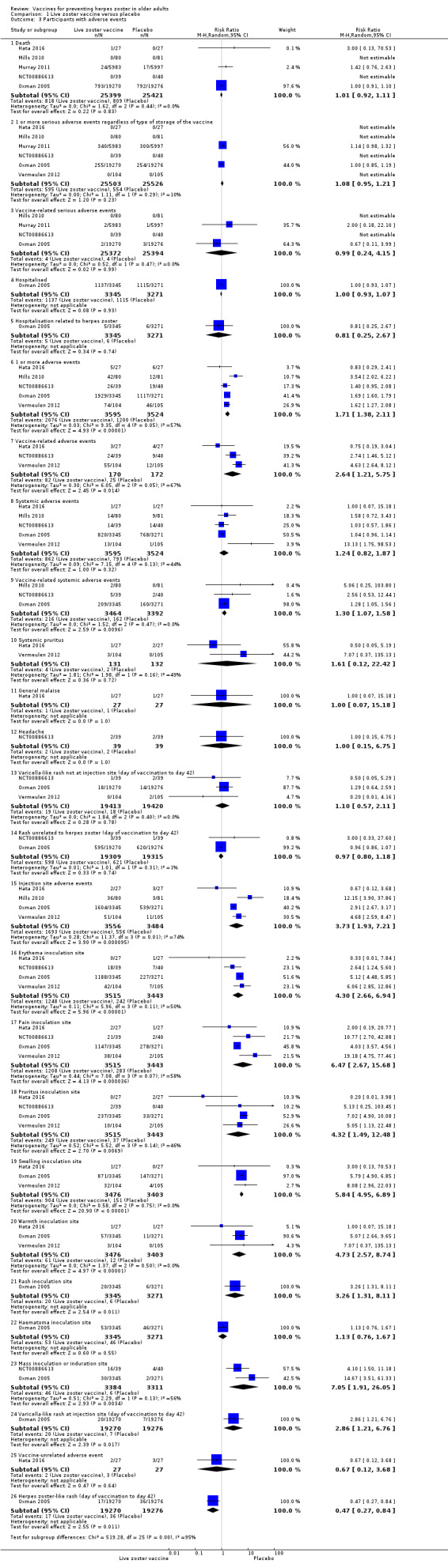
Comparison 1 Live zoster vaccine versus placebo, Outcome 3 Participants with adverse events.
Participants who received the active agent had a higher risk of adverse events than those in the placebo group. When we pooled data from studies reporting the number of participants with one or more adverse event (Hata 2016; Mills 2010; NCT00886613; Oxman 2005; Vermeulen 2012), we observed an increased risk in the vaccine group (RR 1.71, 95% CI 1.38 to 2.11; RD 0.23, 95% CI 0.14 to 0.32; number needed to treat for an additional harmful outcome (NNTH) 4.3, 95% CI 3.1 to 7.1; N = 7119; Analysis 1.3.6).
Vaccine‐related adverse events occurred more frequently in the vaccinated group than in the placebo group (RR 2.64, 95% CI 1.21 to 5.75; RD 0.26, 95% CI −0.03 to 0.55; NNTH 3.8, 95% CI 1.8 to 33.3; N = 342; Analysis 1.3.7) (Hata 2016; NCT00886613; Vermeulen 2012).
Systemic adverse events were more frequent in the vaccinated groups (N = 7119) RR 1.24, 95% CI 0.82 to 1.87 (Analysis 1.3.8) (Hata 2016; Mills 2010; NCT00886613; Oxman 2005; Vermeulen 2012). Regarding systemic adverse events (Analysis 1.3.8), there was a discrepancy between Vermeulen 2012 and the other studies. When we were reviewing data collection, we noted at the bottom of the table with this information, the authors clarified that the vaccine‐related systemic events were also included in "systemic events". This may have led to the assumption that the outcome systemic adverse events favoured the placebo group. However, pooled data showed no differences between groups for this adverse event.
Vaccine‐related systemic adverse events occurred more frequently in the vaccinated group than in the placebo group (pooled data RR 1.30, 95% CI 1.07 to 1.58; RD 0.01, 95% CI 0.00 to 0.03; NNTH 100.0, 95% CI 33.3 to 100.00; N = 6856; Analysis 1.3.9) (Mills 2010; NCT00886613; Oxman 2005).
The vaccinated group had a higher risk of injection site adverse events than the placebo group (N = 7040) (pooled RR 3.73, 95% CI 1.93 to 7.21; RD 0.28, 95% CI 0.15 to 0.41; NNTH 3.6, 95% CI 2.4 to 6.7; Analysis 1.3.15) (Hata 2016; Mills 2010; Oxman 2005; Vermeulen 2012).
Specific injection site adverse events occurred more frequently in the vaccinated group but were mild to moderate in intensity.
The most important adverse events (serious adverse events, hospitalisation, injection site adverse events, and death) are presented in Table 1. Although the vaccinated groups had a higher rate of injection site adverse events, this higher rate was not detected for serious adverse events, hospitalisation, or deaths.
For the safety studies with different formulations of LZV, Gilderman 2008; NCT00886613; NCT01505647; Tyring 2007, or LZV compared to pneumo‐23, Berger 1998, and LZV + IIV4 co‐administration concomitant versus sequential administration, Levin 2018, there were no significant differences between comparison groups. The administration of LZV using the SC route was associated with a higher incidence of adverse events compared to IM administration of the same vaccine (Diez‐Domingo 2015). There were fewer adverse events in participants who received the LZV using the SC route than the ID route (Beals 2016).
We judged the quality of evidence for safety outcomes up to three years of follow‐up (hospital admissions or participants with injection site adverse effects) as of moderate, downgrading by one level due to risk of bias related to insufficient information on random sequence generation (Table 1).
RZV versus placebo
Details of the adverse events for this comparison are provided in Table 5.
3. Adverse events: adjuvanted recombinant varicella zoster virus subunit zoster vaccine (RZV).
| Comparison (studies) | Results |
| RZV versus placebo (Cunningham 2016; Lal 2015) | The adverse events related to RZV versus placebo were:
|
|
RZV: lower or higher quantities of adjuvants plus gE subunit VZV versus unadjuvanted gE or saline (Chlibek 2013) |
The incidence of adverse events in participants randomised to 4 different groups was compared as follows:
We compared each of the groups with all of the other groups (total of 6 comparisons) as follows: 50 μg gE/AS01E versus 50 μg gE/AS01B There was a significantly higher incidence of adverse events in participants who received a higher quantity of adjuvant (AS01B):
There were no significant differences between groups for all other adverse events: any grade 3 symptom, any general symptom, any general grade 3 symptom, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, grade 3 headache, myalgia, grade 3 myalgia, any grade 3 local symptom, local grade 3 pain, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 50 μg gE/AS01E versus 50 μg gE/saline (unadjuvanted)
All these differences in incidence of adverse events favoured the unadjuvanted gE group. There were no significant differences between groups for the following adverse events: any grade 3 symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness and local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever or grade 3 headache in either group. 50 μg gE/AS01B versus 50 μg gE/saline (unadjuvanted)
All these differences in incidence of adverse events favoured unadjuvanted gE. There were no significant differences between groups for the following adverse events: any grade 3 symptom, any general grade 3 symptom, grade 3 fatigue, gastrointestinal symptoms, grade 3 headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness and local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever or grade 3 gastrointestinal symptoms in either group. 50 μg gE/AS01E versus saline
All these differences in incidence of adverse events favoured the saline group. There were no significant differences between groups for the following adverse events: any grade 3 symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, grade 3 headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local redness, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever or grade 3 headache in either group. 50 μg gE/AS01B versus saline
All these differences in incidence of adverse events favoured the saline group. There were no significant differences between groups for the following adverse events: any grade 3 symptom, any general grade 3 symptom, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, grade 3 headache, grade 3 myalgia, any local grade 3 symptom, local grade 3 pain, local grade 3 redness, local swelling and local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 50 μg gE/saline (unadjuvanted) versus saline
There were no significant differences between groups for the following adverse events: any grade 3 symptom, any general symptom, any general grade 3 symptom, fatigue, grade 3 fatigue, fever, gastrointestinal symptoms, grade 3 gastrointestinal symptoms, headache, myalgia, grade 3 myalgia, any local symptom, local pain, local redness and local swelling, or consent withdrawal. No participants in either group had grade 3 fever, grade 3 headache, any local grade 3 symptom, local grade 3 pain, local grade 3 redness, local grade 3 swelling, loss to follow‐up, and serious adverse events. |
|
RZV: 3 groups of VZV subunit gE in 3 different quantities versus unadjuvanted gE or saline (Chlibek 2014) |
The incidence of adverse events in participants randomised to 5 different groups was compared as follows:
We compared each of the groups to all other groups (total of 10 comparisons) as follow: 25 µg gE/AS01B versus 50 µg gE/AS01B There were no differences between groups in the incidence of the following adverse events: any fatigue, grade 3 fatigue, any fever, grade 3 fever, any headache, grade 3 headache, any myalgia, grade 3 myalgia, local pain, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. 25 µg gE/AS01B versus 100 µg gE/AS01B There were no differences between groups in the incidence of the following adverse events: any fatigue, grade 3 fatigue, any fever, any headache, grade 3 headache, any myalgia, grade 3 myalgia, local pain, grade 3 local pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. 50 µg gE/AS01B versus 100 µg gE/AS01B
There were no differences between groups in the incidence of other adverse events: any fatigue, grade 3 fatigue, any fever, grade 3 fever, any headache, grade 3 headache, grade 3 myalgia, local pain, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, and serious adverse events. 25 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences in incidence of adverse events favoured unadjuvanted gE. There were no differences in the incidence of the following adverse events: grade 3 fatigue, any fever, any headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 50 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences in incidence of adverse events favoured unadjuvanted gE. There were no differences in the incidence of the following adverse events: grade 3 fatigue, any fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All these differences in incidence of adverse events favoured unadjuvanted gE. There were no differences in the incidence of the following adverse events: grade 3 fatigue, any fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 25 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in incidence of adverse events favoured saline + 100 µg gE/AS01B. There were no differences in the incidence of the following adverse events: any fatigue, grade 3 fever, any headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. 50 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in incidence of adverse events favoured saline + 100 µg gE/AS01B. There were no differences in the incidence of the following adverse events: grade 3 fatigue, any fever, grade 3 fever, grade 3 headache, grade 3 myalgia, local grade 3 pain, local redness, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. 100 µg gE/AS01B versus saline + 100 µg gE/AS01B
All differences in incidence of adverse events favoured saline + 100 µg gE/AS01B. There were no differences in the incidence of the following adverse events: grade 3 fatigue, headache, grade 3 headache, grade 3 myalgia, local grade 3 pain, local grade 3 redness, local swelling, local grade 3 swelling, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever in either group. Saline + 100 µg gE/AS01B versus 100 µg gE/saline (unadjuvanted gE)
All differences in incidence of adverse events favoured 100 µg gE/saline. There were no differences in the incidence of the following adverse events: any fatigue, grade 3 fatigue, any fever, any headache, any myalgia, grade 3 myalgia, local grade 3 pain, local grade 3 redness, consent withdrawal, loss to follow‐up, and serious adverse events. No participants had grade 3 fever, grade 3 headache, or local grade 3 swelling in either group. |
| RZV: 2 doses given at 3 different intervals (Lal 2018) | There were no statistically significant differences between groups for any of the 3 comparisons (RZV 2 doses 2 months apart versus RZV 2 doses 6 months apart; RZV 2 doses 2 months apart versus RZV 2 doses 12 months apart; and RZV 2 doses 6 months apart versus RZV 2 doses 12 months apart) in incidence of the following adverse events: at least 1 unsolicited AE symptom, at least 1 unsolicited AE symptom related to vaccination. There were no significant differences between groups for the following general symptoms: fatigue, grade 3 fatigue, fever, grade 3 fever, headache, grade 3 headache, myalgia, grade 3 myalgia, gastrointestinal symptom, grade 3 gastrointestinal symptom. The average duration of solicited general symptoms was ≤ 2 days. There were no significant differences between groups for the following local symptoms: local pain, grade 3 local pain, local redness, grade 3 redness, local swelling, grade 3 local swelling. The average duration of local symptoms was ≤ 3 days. There were no significant differences between groups for the following: SAE, withdrawn due to an SAE, consent withdrawal, lost to follow‐up. There were no cases of suspected zoster or autoimmune disease throughout the study in any of the groups. |
| RZV IM route versus RZV SC route (Vink 2017) | There was a significant difference between groups favouring the IM route for the following adverse events: injection site redness (RR 1.73, 95% CI 1.18 to 2.55; RD 0.37, 95% CI 0.15 to 0.58; NNTH 2.7, 95% CI 1.7 to 6.7); injection site swelling (RR 2.00, 95% CI 1.25 to 3.21; RD 0.40, 95% CI 0.17 to 0.63; NNTH 2.5, 95% CI 1.6 to 5.9); grade 3 injection site swelling (RR 5.00, 95% CI 1.19 to 20.92; RD 0.27, 95% CI 0.08 to 0.46; NNTH 3.7, 95% CI 2.2 to 12.5); injection site pruritus (RR 2.10, 95% CI 1.20 to 3.67; RD 0.37, 95% CI 0.13 to 0.60; NNTH 2.7, 95% CI 1.7 to 7.7). There were no differences between groups for all other adverse events. There were no deaths or autoimmune diseases. |
| RZV versus pneumo‐23 (Maréchal 2018) |
Serious adverse events within 30 days after vaccination
There were no serious adverse events or pIMDs that were considered vaccine‐related. Serious adverse events from 30 days after last vaccination up to the end of study
There were no serious adverse events or pIMDs that were considered vaccine‐related. When comparing the group that received RZV + pneumo‐23 versus the group that received only pneumo‐23, the following systemic adverse events occurred within 7 days after vaccination:
Injection site AE
|
| RZV + TDaPV co‐administration group versus RZV + TDaPV not co‐administration group (NCT02052596) | There were no significant differences between groups for the following: death, serious adverse events, systemic adverse events, injection site adverse events, unsolicited vaccine‐related adverse events. There were no pIMDs. |
| RZV + IIV4 co‐administration group versus not co‐administration group (Schwarz 2017) | There were no deaths. There were no significant differences between groups except for the following AEs:
|
AE: adverse event or adverse experiences AS01: liposome‐based adjuvant system containing the immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and the saponin QS‐21 (Quillaja saponaria Molina, fraction 21) Adjuvanted gE/AS01B: 50 μg purified gE with adjuvant B (1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21) Adjuvanted gE/AS01E: 50 μg purified gE with adjuvant E (500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21) AS01B: adjuvant B composed of 1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21 AS01E: adjuvant E composed of 500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21 CI: confidence interval Elderly or older adults: aged ≥ 60 years old gE: recombinant subunit VZV composed of glycoprotein E gE/saline: unadjuvanted gE ID: identification IIV4: inactivated quadrivalent influenza vaccines IM: intramuscular MPL: immunoenhancer 3‐O‐desacyl‐4′‐monophosphoryl lipid A NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome pIMDs: potential immune‐mediated diseases pneumo‐23 vaccine: 23–valent pneumococcal polysaccharide vaccine QS‐21: immunoenhancer saponin Quillaja saponaria Molina, fraction 21 RD: risk difference RR: risk ratio RZV: adjuvanted recombinant zoster vaccine (contains 50 µg of recombinant VZV glycoprotein E, and the liposome‐based AS01B adjuvant system contains 50 µg of 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and 50 µg of Quillaja saponaria Molina, fraction 21 (QS21)) SAEs: serious adverse events SC: subcutaneously or subcutaneous TDaPV: tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine VZV: varicella zoster virus
We analysed adverse events among participants aged 50 years or over because data for adverse events by specific age groups were not available. We performed ITT analyses for adverse events that did not include all randomised participants. In other words, we considered the worst‐case scenario for the intervention group (we assumed that participants with missing information had adverse events) and the best‐case scenario for the placebo group (we assumed that participants with missing information did not experience adverse events). We detected no differences between groups in this analysis, therefore we decided to present the results for adverse events as they were published.
There were no significant differences between groups for death (N = 29,311) RR 0.94, 95% CI 0.84 to 1.04 (Analysis 2.3.1) (Cunningham 2016; Lal 2015); serious adverse events (N = 29,311) RR 0.97, 95% CI 0.91 to 1.03 (Analysis 2.3.3) (Cunningham 2016; Lal 2015).
2.3. Analysis.
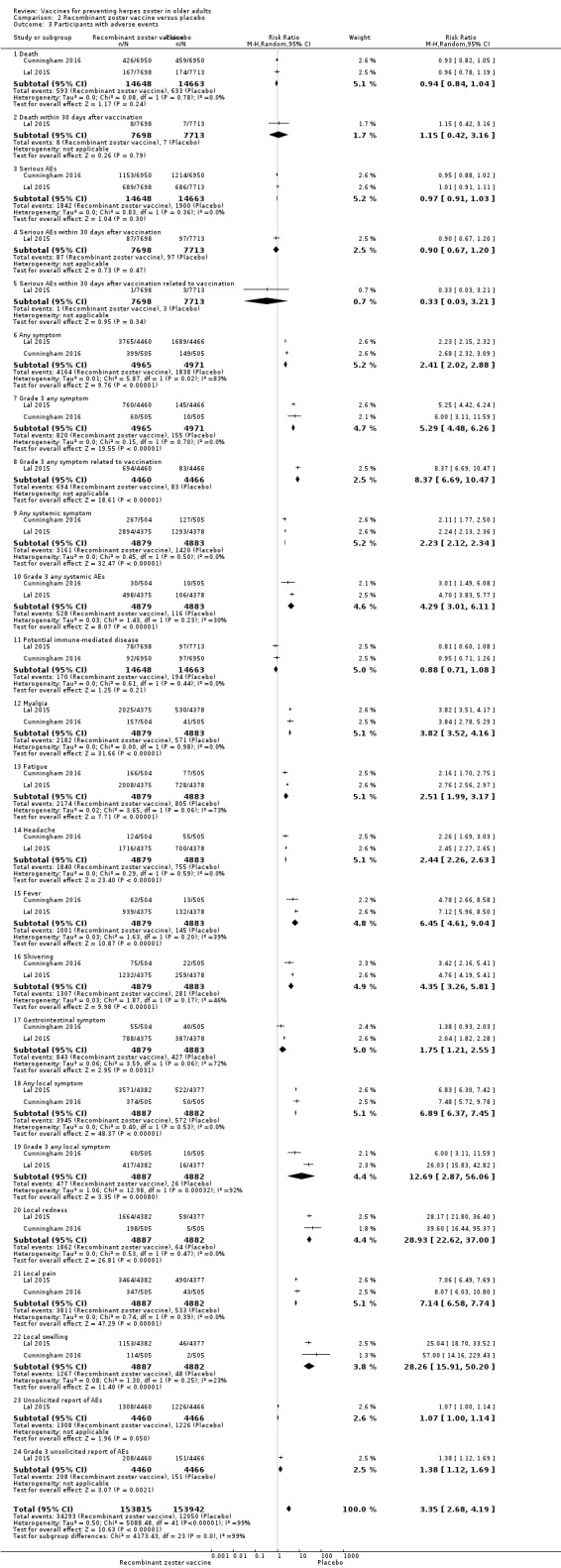
Comparison 2 Recombinant zoster vaccine versus placebo, Outcome 3 Participants with adverse events.
Two studies (N = 29,311) compared RZV versus placebo (Cunningham 2016; Lal 2015). The vaccinated group had a higher incidence of any symptom (RR 2.27, 95% CI 2.18 to 2.36; RD 0.47, 95% CI 0.45 to 0.49; NNTH 2.1, 95% CI 2.0 to 2.2; Analysis 2.3.6).
Any systemic symptoms occurred more frequently in participants who received the vaccine (RR 2.23, 95% CI 2.12 to 2.34; RD 0.33, 95% CI 0.24 to 0.41; NNTH 3.0, 95% CI 2.4 to 4.2; N = 9762; Analysis 2.3.9).
There were no significant differences between groups for potential immune‐mediated disease (N = 29,311) RR 0.88, 95% CI 0.71 to 1.08 (Analysis 2.3.11) (Cunningham 2016; Lal 2015) (Table 2).
The participants who received the vaccine had more gastrointestinal symptoms (N = 9762) RR 1.75, 95% CI 1.21 to 2.55; RD 0.06, 95% CI 0.00 to 0.12 (Analysis 2.3.17).
Vaccinated participants had a higher frequency of any local symptom (N = 9769) RR 6.89, 95% CI 6.37 to 7.45; RD 0.67, 95% CI 0.62 to 0.73; NNTH 1.5, 95% CI 1.4 to 1.6 (Analysis 2.3.18). The most important difference between adverse events was for injection site events. The participants in the vaccinated group had a much higher incidence of injection site adverse events than those in the placebo group (absolute risk of 80.7% in the vaccinated group versus 11.7% in the placebo group).
The most important adverse events (death, serious adverse events, any systemic symptom, potential immune‐mediated disease, and injection site adverse events) are presented in Table 2.
Heterogeneity was high in the following meta‐analyses: Analysis 2.3.6: any symptom (83%); Analysis 2.3.13: fatigue (73%); Analysis 2.3.17: gastrointestinal symptom (72%); and Analysis 2.3.19: any local symptom (92%).
The duration of adverse events was transient. Median duration was one to two days for systemic reactions and two to three days for injection site reactions.
We judged quality of evidence as of moderate due to insufficient information on allocation concealment and because the flow of participants was unclear (Table 2).
2. Dropouts
There were no significant differences between LZV and placebo for any reasons for dropouts (N = 38,856) RR 0.99, 95% CI 0.90 to 1.08 (Analysis 1.5.1) (Mills 2010; Oxman 2005; Vermeulen 2012), and also for participants with no follow‐up (N = 50,627) RR 1.05, 95% CI 0.74 to 1.48 (Analysis 1.6) (Mills 2010; Murray 2011; Oxman 2005).
1.5. Analysis.
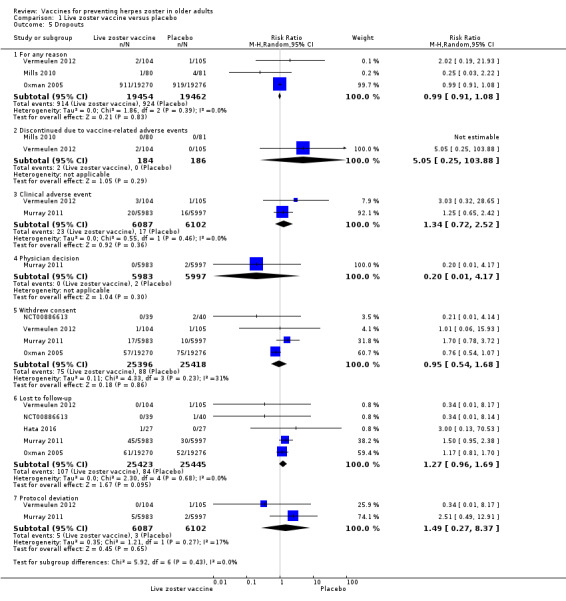
Comparison 1 Live zoster vaccine versus placebo, Outcome 5 Dropouts.
1.6. Analysis.
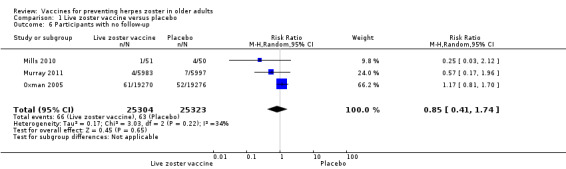
Comparison 1 Live zoster vaccine versus placebo, Outcome 6 Participants with no follow‐up.
Lal 2015 and Cunningham 2016 described four reasons for dropouts: "not receiving vaccine according to protocol" (no difference between groups) (Analysis 2.4.1); "receiving the wrong vaccine" (no difference between groups) (Analysis 2.4.2); "diagnosis of herpes zoster less than 30 days after the second dose" (RR 0.32, 95% CI 0.14 to 0.71 but no RD) (Analysis 2.4.3); and "did not receive second dose" (the vaccinated group had higher dropout rates than the placebo group for this reason: RR 1.25, 95% CI 1.13 to 1.39; RD 0.01; NNTH 100) (Analysis 2.4.4).
2.4. Analysis.
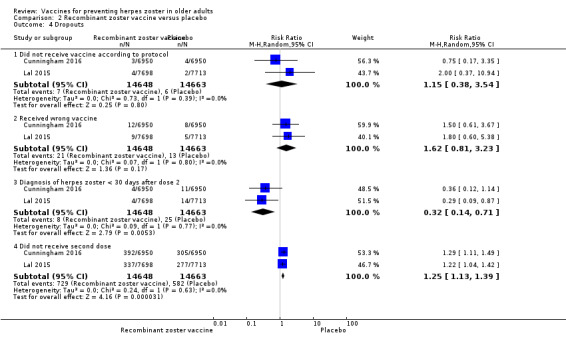
Comparison 2 Recombinant zoster vaccine versus placebo, Outcome 4 Dropouts.
Details of dropouts in the included studies for all comparisons are provided in Table 6.
4. Dropouts.
| Dropouts (all included studies) |
LZV versus placebo The pooled data from the studies that compared zoster vaccine and placebo showed no differences in reasons for dropout (Analysis 1.5): for any reason (RR 0.99, 95% CI 0.91 to 1.08) (Analysis 1.5.1) (Mills 2010; Oxman 2005; Vermeulen 2012); discontinued due to vaccine‐related adverse events (RR 5.05, 95% CI 0.25 to 103.88) (Analysis 1.5.2) (Mills 2010; Vermeulen 2012); for clinical AE (RR 1.34, 95% CI 0.72 to 2.52) (Analysis 1.5.3) (Murray 2011; Vermeulen 2012); for physician decision (RR 0.20, 95% CI 0.01 to 4.17) (Analysis 1.5.4) (Murray 2011); for withdrawal of consent (RR 0.95, 95% CI 0.54 to 1.68) (Analysis 1.5.5) (Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012); for loss to follow‐up (RR 1.27, 95% CI 0.96 to 1.69) (Analysis 1.5.6) (Hata 2016; Murray 2011; NCT00886613; Oxman 2005; Vermeulen 2012); and for protocol deviation (RR 1.49, 95% CI 0.27 to 8.37) (Analysis 1.5.7) (Murray 2011; Vermeulen 2012). In Mills 2010, Oxman 2005, and Vermeulen 2012, consent was withdrawn after the intervention. In Murray 2011, some participants apparently withdrew consent after randomisation, but the exact number that withdrew consent after the intervention is not stated. The pooled data from the studies that compared zoster vaccine versus placebo showed no differences in reasons for participants with no follow‐up (RR 1.05, 95% CI 0.74 to 1.48) (Analysis 1.6) (Mills 2010; Murray 2011; Oxman 2005). High‐potency versus low‐potency zoster vaccine: there were no differences between groups (Tyring 2007). Refrigerated versus frozen zoster vaccine: there were no differences between groups (Gilderman 2008). LZV IM route versus LZV SC route: there were no withdrawals due to adverse events in either group (Diez‐Domingo 2015). LZV intradermal route versus LZV SC route: there were no significant differences between full‐dose intradermal versus full‐dose SC; 1/3 dose intradermal versus full‐dose SC; 1/10 dose intradermal versus full‐dose SC; 1/27 dose intradermal versus full‐dose SC; 1/3 dose intradermal versus 1/3 dose SC. There were no dropouts for full‐dose intradermal versus 1/3 dose SC; 1/10 dose intradermal versus 1/3 dose SC; and 1/27 dose intradermal versus 1/3 dose SC (Beals 2016). 2 doses of a zoster vaccine versus a single dose and 2 doses given at different intervals: there were no differences between groups for participant withdrawals due to adverse events (Vesikari 2013). LZV AMP versus LZV: there were no differences between groups (NCT01505647). LZV + IIV4 concomitant administration versus LZV + IIV4 sequential administration: for this comparison there were no significant differences for dropouts between groups (Levin 2018) In all the comparisons of Chlibek 2013, there were no differences in dropouts between the groups. Similarly, in all the comparisons of Chlibek 2014, there were no differences in dropouts between the groups.. RZV versus placebo:Cunningham 2016 and Lal 2015 described 4 reasons for dropout: did not receive vaccine according to protocol (Analysis 2.4.1); received wrong vaccine (Analysis 2.4.2); had diagnosis of HZ less than 30 days after dose 2 (Analysis 2.4.3); and did not receive second dose (Analysis 2.4.4). There were no differences between groups for the first 2 outcomes. The third outcome had an RR of 0.32 (95% CI 0.14 to 0.71) but no RD. For the fourth outcome, the vaccine group had a higher dropout rate than the placebo group: RR 1.25, 95% CI 1.13 to 1.39; RD 0.01, 95% CI 0.01 to 0.01; NNTH 100, 95% 100.0 to 100.0. RZV IM route versus RZV SC route: there was no difference in participant withdrawal between groups (Vink 2017). RZV + TDaPV co‐administration group versus RZV + TDaPV not co‐administration group: there was no difference in dropouts between groups (NCT02052596). Co‐administration RZV + IIV4 versus not co‐administration group RZV + IIV4: there was no difference between groups for dropouts (Schwarz 2017). |
AE: adverse event or adverse experiences AMP: Alternative Manufacturing Process AS01: liposome‐based adjuvant system containing the immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and the saponin QS‐21 (Quillaja saponaria Molina, fraction 21) Adjuvanted gE/AS01B: 50 μg purified gE with adjuvant B (1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21) Adjuvanted gE/AS01E: 50 μg purified gE with adjuvant E (500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21) AS01B: adjuvant B composed of 1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21 AS01E: adjuvant E composed of 500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21 Elderly or older adults: aged ≥ 60 years old Frozen: ‐15 °C or colder gE: recombinant subunit VZV composed of glycoprotein E gE/saline: unadjuvanted gE HZ: herpes zoster ID: identification IIV4: inactivated quadrivalent influenza vaccines IM: intramuscular LZV or ZV: live zoster vaccine (live attenuated Oka varicella zoster virus vaccine) MPL: immunoenhancer 3‐O‐desacyl‐4′‐monophosphoryl lipid A NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome pneumo‐23 vaccine: 23–valent pneumococcal polysaccharide vaccine QS‐21: immunoenhancer saponin Quillaja saponaria Molina, fraction 21 Refrigerated: 2 °C to 8 °C RR: risk ratio RZV: adjuvanted recombinant zoster vaccine (contains 50 µg of recombinant VZV glycoprotein E, and the liposome‐based AS01B adjuvant system contains 50 µg of 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and 50 µg of Quillaja saponaria Molina, fraction 21 (QS21)) SAEs: serious adverse events SC: subcutaneously or subcutaneous TDaPV: tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine VZV: varicella zoster virus
The overall quality of evidence for dropouts up to three years of follow‐up was moderate for the comparison LZV versus placebo (Oxman 2005). The overall quality of evidence was also moderate for the comparison RZV versus placebo for up to 3.2 years of follow‐up (Cunningham 2016; Lal 2015). The reason for downgrading the evidence for the first comparison (LZV versus placebo) was insufficient information about random sequence generation (Table 1). We downgraded the evidence for the second comparison (RZV versus placebo) due to insufficient information on allocation concealment and flow of participants (Table 2).
Observation: the numbers of studies and participants for some analyses appear to be incorrect because some analyses include only safety subgroups, and not the total number of participants. Additionally, in some studies the flow of participants was not clear (attrition bias). We considered ITT analyses when these were possible or available.
Discussion
Summary of main results
Live attenuated zoster vaccine (LZV)
We included a total of 15 clinical trials that reported prespecified outcomes for LZV (incidence of herpes zoster, adverse events, and dropouts) (Beals 2016; Berger 1998; Cunningham 2016; Diez‐Domingo 2015; Gilderman 2008; Hata 2016; Levin 2000; Mills 2010; Murray 2011; NCT00886613; NCT01505647; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013).
Data from a major RCT, the Shingles Prevention Study (Oxman 2005), which included 38,546 participants, confirmed the effectiveness of the intervention when compared to placebo in older adults for at least 3.1 years (moderate‐quality evidence). The continuation of Oxman 2005 (Schmader 2012) had the longest duration of follow‐up, reporting an average five years of herpes zoster surveillance in older adults (aged 60 years and over). The available data suggest that the vaccine works for an average of five years to prevent herpes zoster in adults aged 60 years and over. However, these long‐term effect estimates for incidence of herpes zoster should be interpreted with caution since they were derived from inferred data.
Even with this unfavourable safety profile, most adverse events were of mild‐to‐moderate intensity. This is clearly reported in the adverse event sub study conducted by Oxman 2005. The interference of herpes zoster in activities of daily life (ADL) was measured by the zoster brief pain inventory (ZBPI ADL), in which scores greater than or equal to 300 indicate significant pain‐related interference in daily life and quality of life (Coplan 2004). There were no significant differences between the vaccinated and placebo groups for this outcome in the Oxman 2005 study (RR 0.63, 95% CI 0.34 to 1.16; Analysis 1.2).
1.2. Analysis.
Comparison 1 Live zoster vaccine versus placebo, Outcome 2 Interference of herpes zoster in activities of daily life.
Although the rate of adverse events (Table 4) was higher in the LZV group, dropout rates (Table 6) were similar in the vaccine and placebo groups, suggesting that these adverse events did not have important repercussions.
With one exception (Hata 2016), all included studies received funding from the pharmaceutical industry.
The FDA approved LZV for older adults (aged 60 years and over) in May 2006 (FDA 2006), and this was approved for individuals aged 50 years and over in October 2018 (FDA 2018).
Adjuvanted recombinant VZV subunit zoster vaccine (RZV)
We included nine trials that tested the effects of RZV on prespecified outcomes (incidence of herpes zoster, adverse events, and dropouts) (Chlibek 2013; Chlibek 2014; Cunningham 2016; Lal 2015; Lal 2018; Maréchal 2018; NCT02052596; Schwarz 2017; Vink 2017). We assessed two of these studies as having a low risk of bias (Chlibek 2013; Lal 2015). Lal 2015 and Cunningham 2016 evaluated the incidence of herpes zoster, adverse events, and dropouts using the final product of the vaccine composition, a vaccinated group versus a placebo group over an average of 3.2 years of follow‐up. There was a significant decrease in the incidence of herpes zoster in the vaccinated group (moderate‐quality evidence ).
The data on RZV suggest that the vaccine may be considered safe because there were no differences in serious adverse events between the vaccinated and placebo groups. Although systemic and injection sites adverse events (Table 5) occurred more frequently in the vaccinated group, these were transient.
Cunningham 2016 and Lal 2015 reported more dropouts for the second dose in the vaccine group than in the placebo group (Table 6).
There was high heterogeneity in the meta‐analyses that pooled the data of Cunningham 2016 and Lal 2015 for the following outcomes: incidence of herpes zoster (Analysis 2.1), any symptom (Analysis 2.3.6), fatigue (Analysis 2.3.13), gastrointestinal symptom (Analysis 2.3.17), and any local symptom (Analysis 2.3.19). Since the two studies had the same design and type of randomisation, differences in the characteristics of the participants should be considered. Lal 2015 included younger participants (60 years or older) than Cunningham 2016 (70 years or more). Moreover, the flow of patients in the Cunningham 2016 publication was unclear, with inconsistencies between the data presented in the publication and the supplementary appendix.
All nine studies received funding from the pharmaceutical industry.
The FDA approved RZV for clinical use in October 2017 (FDA 2017).
Overall completeness and applicability of evidence
We assessed the quality of evidence presented in this systematic review, based on studies that included a large number of healthy participants aged 60 years or older, as moderate. The two available vaccines (one SC dose of LZV and the recombinant vaccine, two IM doses two months apart) have been shown to produce a significant reduction in the incidence of herpes zoster over a period of at least three years.
The vaccines were safe, as there was no difference between groups in deaths or serious adverse events. The incidence of systemic adverse events was significantly higher amongst participants receiving RZV than in controls, which was not observed amongst participants receiving attenuated LZV. Both vaccines produced a higher incidence of injection site adverse events than controls.
There were no differences in withdrawals for any reason in the LZV group, but the number of participants not receiving the second dose of RZV was significantly higher in the vaccinated group than in the placebo group.
Readers should keep in mind that the majority of study participants were 60 years of age or older, Caucasian (understood to be white) (> 88%), and female (58%).
Quality of the evidence
As shown in Table 1, there is moderate‐quality evidence for the primary outcome, incidence of herpes zoster, for a follow‐up period of 3.1 years. The data for LZV come from a large study (38,546 participants), and the quality of the evidence was downgraded because the method used for random sequence generation was not described (Oxman 2005). Because it is a large study with a low risk of bias for five of the seven 'Risk of bias' domains, it provides consistent results showing that the LZV decreases the incidence of herpes zoster for at least 3.1 years postvaccination.
There is also moderate‐quality evidence that RZV reduces the incidence of herpes zoster over a 3.2‐year follow‐up period (Table 2). We downgraded the quality of evidence because allocation concealment was not described and the flow of patients was unclear. These data came from two studies that included 22,022 participants (Cunningham 2016; Lal 2015). One of these studies had a low risk of bias in four of the seven 'Risk of bias' domains (Cunningham 2016), and the other study had a low risk of bias in five of the seven domains, which strengthens the conclusion that this vaccine reduced the incidence of herpes zoster over a follow‐up period of 3.2 years (Lal 2015).
We also judged the quality of the evidence for adverse events for both LZV and RZV compared to placebo as moderate, for the same reasons as for the primary outcome. The quality of evidence for this outcome is strengthened by the definitions of adverse effects provided in the primary studies and the fact that systemic and injection site adverse events were collected prospectively. Participants who received LZV had a higher incidence of systemic adverse events (Hata 2016; Mills 2010; NCT00886613; Oxman 2005; Vermeulen 2012; totaling 7059 participants) and injection site reactions (Hata 2016; Mills 2010; Oxman 2005; Vermeulen 2012; totaling 6980 participants). We downgraded the quality of the evidence for this outcome to moderate due to insufficient information on random sequence generation. There were no differences between the vaccinated and placebo groups for death and serious adverse events.
There was moderate‐quality evidence (downgraded due to insufficient information on allocation concealment and attrition bias) based on two studies showing a higher incidence of adverse events in participants who received RZV versus placebo (Cunningham 2016; Lal 2015). There was a higher incidence of participants with any systemic symptoms (2 studies, 9762 participants) and any local symptom (2 studies, 9769 participants). There was no difference between groups for death, serious adverse events, and potential immune‐mediated disease (2 studies, 29,311 participants).
We found moderate‐quality evidence for dropouts for both vaccines (LZV and RZV). For LZV, there was no difference in the rate of dropouts between the vaccine and placebo groups (Mills 2010; Oxman 2005; Vermeulen 2012, totaling 38,856 participants). We downgraded this evidence due to insufficient information on random sequence generation. For RZV, there was a higher rate of dropouts for the second dose in the vaccinated groups. We downgraded the quality of the evidence to moderate due to insufficient information on allocation concealment and incomplete outcome data (Cunningham 2016; Lal 2015, totaling 29,311 participants).
Only the authors of Hata 2016 reported receiving grants not related to the conduct and results of the study. Berger 1998 and Levin 2000 did not describe any potential conflicts of interest. The following 12 studies described potential conflicts of interest: Beals 2016; Diez‐Domingo 2015; Gilderman 2008; Levin 2018; Mills 2010; Murray 2011; NCT00886613; NCT01505647; Oxman 2005; Tyring 2007; Vermeulen 2012; Vesikari 2013. The authors of these 12 studies were either employees (and employees may hold stock or stock options, or both, in the company) or former employees, or shared intellectual property rights on Zostavax (zoster vaccine live), or received speaker fees or consultancy payments or grants from Merck & Co Inc. The authors of nine studies were affiliated with GlaxoSmithKline Biologicals as employees (and employees may hold stock or stock options, or both, in the company), or former employees, or co‐inventor of a patent application related to the vaccine, or received lecture fees, or grant support, or owned GlaxoSmithKline stocks (Chlibek 2013; Chlibek 2014; Cunningham 2016; Lal 2015; Lal 2018; Maréchal 2018; NCT02052596; Schwarz 2017; Vink 2017).
Potential biases in the review process
We attempted to minimise the potential bias in the review process, within the control of the review authors. To do this, we searched all available databases using a highly sensitive search strategy without language restrictions. This led us to find a study published in Japanese (Ikematsu 2018). We also conducted duplicate data extraction to ensure that no data were lost and that all relevant information was accurate. We contacted the lead authors of included studies to obtain additional information and to clarify any pending doubts. Finally, we extracted data from unpublished studies in the "Study Results" section in ClinicalTrials.gov.
By including a cross‐over study in our meta‐analysis, risk of bias may have been introduced into the review process. However, this was a small study that assessed only adverse events and not effectiveness outcomes.
A limitation of our review is that we could not evaluate reporting bias (funnel plot) because none of our meta‐analyses included at least 10 studies per outcome. Since studies with positive results are more likely to be published, it is possible that studies with negative effects of zoster vaccines in older adults were conducted but not published (Kicinski 2013).
Agreements and disagreements with other studies or reviews
A cohort study followed 766,330 participants aged 65 years or over (a 5% random sample of Medicare patients) allocated according to whether or not they had received LZV between 1 January 2007 and 31 December 2009. Overall, the incidence rate of herpes zoster in vaccinated participants was 5.4 (95% CI 4.6 to 6.4) per 1000 person‐years compared to 10.0 (95% CI 9.8 to 10.2) per 1000 person‐years in those not vaccinated (Langan 2013).
A matched case‐control study that collected data from May 2006 to November 2014 was conducted by the Vaccine Adverse Event Reporting System (a national vaccine safety surveillance database maintained jointly by the US Centers for Disease Control and Prevention (CDC) and the FDA). The study aim was to clarify severe autoimmune adverse events after receiving LZV. The adverse events assessed were arthritis, vasculitis, systemic lupus erythematosus, thrombocytopenia, alopecia, Guillain‐Barre syndrome, optic neuritis, and multiple sclerosis. The study reported higher incidence of arthritis and alopecia after vaccination. Compared to people who were unexposed, participants who received zoster vaccination had 2.2 and 2.7 times the odds of developing arthritis (P < 0.001) and alopecia (P = 0.015) (Lay 2015).
Our main findings are similar to those reported by another review that also found a reduction in the incidence of herpes zoster and good tolerability of LZV (Sanford 2010).
Only one randomised study compared two vaccines (LZV versus RZV), but the only outcome reported was immunogenicity (Weinberg 2018).
Since no trial compared the effects of the two available vaccines on clinical outcomes (incidence of herpes zoster, adverse events, or dropouts), Tricco 2018 used the pair wise meta‐analysis methodology that is used when at least two studies examine the same intervention and comparator for a particular outcome and conducted a network meta‐analysis to compare the different shingles vaccines using the comparator placebo (Jansen 2013). In situations when both direct and indirect comparisons are available in a review, any use of multiple‐treatments meta‐analyses should be to supplement, rather than to replace, the direct comparisons. Direct evidence from good‐quality RCTs should be used wherever possible. Without this evidence, it may be necessary to look for indirect comparisons from RCTs (Glenny 2005). The conclusion of the systematic review and network meta‐analysis reached by Tricco 2018 that RZV is likely to be superior to LZV in reducing the incidence of herpes zoster should be interpreted with caution, since it comes from indirectly obtained data, and did not include all the aspects of the two vaccines. We should therefore take into consideration the balance between the benefits and harms of each vaccine.
The LZV is given in a single subcutaneous dose, whilst the administration schedule of RZV is two intramuscular doses two months apart. Although we found different NNTBs between the vaccines, other aspects should be considered, such as administration schedule, incidence of adverse events, and dropouts. The benefits of RZV may seem compelling, but it is also associated with a higher incidence of adverse events, and there was a higher incidence of participants who did not receive the second dose of RZV.
It is important to highlight that, although the CDC recommends two doses of RZV, separated by two to six months to prevent shingles (CDC), the efficacy and safety data found in this review were based on two randomised trials that administered two doses two months apart (Cunningham 2016; Lal 2015). The CDC has also stated that RZV is the preferred vaccine over LZV (CDC). However, as previously mentioned, whilst efficacy of RZV may be better, tolerance is lower.
Consequently, any statement about the superiority of LZV over RZV, or vice versa, should be avoided until there is a randomised trial directly comparing these two vaccines to prevent herpes zoster.
Authors' conclusions
Implications for practice.
There is a clear benefit for vaccination of older adults with both attenuated live zoster vaccine (LZV) and recombinant zoster vaccine (RZV) against herpes zoster with no major safety or tolerance concerns. Herpes zoster is more frequent amongst older adults, and its main clinical feature is pain, therefore preventing herpes zoster is desirable. Moderate‐quality evidence suggests that amongst people aged 60 years and over, zoster vaccines may reduce the incidence of herpes zoster for at least three years after vaccination.
Implications for research.
We suggest that future studies follow participants for more than three years to assess the effectiveness of vaccines in preventing herpes zoster over longer periods of time.
There is a need for more studies involving participants from different ethnicities in order to broaden the range of applicability of herpes vaccines to other populations. There is an ongoing study testing LZV versus placebo in participants of Asian ethnicity (NCT02526745).
Instead of relying on indirect comparisons, it is important to conduct randomised, double‐blind trials comparing the effects of the two available vaccines (LZV versus RZV) on clinical outcomes (herpes zoster incidence, adverse events) and dropouts. The findings of these trials will help to determine which intervention provides the most benefits and least harms for healthy adults aged 60 years or over.
The effectiveness of vaccines with lower concentrations of varicella zoster virus (< 18,700 plaque‐forming units/dose, the minimum dose used in Oxman 2005) could be tested in future studies.
Ongoing studies are testing the safety of different amounts of live varicella zoster virus, NCT02526745, or formulations of LZV, and a new vaccine LZV (NBP608, a single‐dose vaccine currently approved in Korea) versus LZV (NCT03116594; NCT03120364).
The effectiveness and safety of different adjuvants or formulations of the RZV could also be tested in future studies.
Feedback
Seeking efficacy and safety information for autoimmune cohort, 9 May 2018
Summary
Possibly the Institute, in consideration of recent developments in knowledge of immunology and adjuvants, may update, on behalf of millions of people diagnosed with autoimmune syndromes, the Institute's herpes zoster vaccine page, in consideration of more recent medical research into adjuvant‐induced autoimmunity, and the new herpes zoster vaccine, Shingrix, with the QS‐21 adjuvant, in view of current research, e.g., "The Autoimmune/inflammatory syndrome induced by adjuvants (ASIA), Descriptive Analysis of 300 Patients from the International Asia syndrome Registry," Watad, Quaresma M, Bragazzi NL, Cervera R, Tervaer, Amital, Shoenfeld, for a current review of Shingrix, which uses a markedly powerful immune stimulant called QS‐21 Quillaja saponaria ‐ GlaxoKlineSmith [sic] in their 2016 application to the FDA states they excluded "immunosuppressed" patients from their studies. Given the use of QS‐21 adjuvant in their Shingrix vaccine, it is unlikely GKS [sic] has funded no research of the autoimmune patient response to Shingrix.
Given the Shingrix use of this powerful immune stimulant, of interest is both GKS's [sic] use of the term, "immunosuppressed," rather than "immune‐compromised," and what does not appear are studies of the Shingrix use in autoimmune patients and varying potential in this population of millions of people, for QS‐21‐ induced autoimmunity... Some of these syndromes can be catastrophic. The lack of knowledge of, for example, non‐thrombotic antiphospholipid syndrome pathophysiology, prognosis, treatment, is very difficult for patients and doctors. Thank you for considering this suggestion.
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Suzanne Gorenfeld
Reply
Dear Suzanne
Thank you for the opportunity to provide brief review about the important issue of adverse events associated with this intervention. There is a consensus that vaccines and/or adjuvants may be related to autoimmune diseases/or ASIA. However, it is difficult to establish a causal relationship between autoimmune phenomena and prior vaccination (Agmon‐Levin 2009).
Vaccination has been implicated as the cause of many diseases, including ASIA and adjuvants can indeed improve the immunogenicity of the vaccine and have been associated with potential damage on rare occasions (Amanna 2007). However, so far, there is no high quality scientific evidence to support these hypotheses (Aps 2018). As with any other drug, the use of vaccines has been associated with adverse events. However, these adverse events are often less tolerated because they occur in healthy people (Balofsky 2010).
It is believed that autoimmunity develops when genetically predisposed individuals undergo modifications in response to environmental factors (Le Dantec 2015). Perricone 2013 highlights that genetic predisposition appears to be a prerequisite which predisposes certain individuals to develop vaccine‐related autoimmune syndromes and this may also explain their very low incidence. Watad 2018 reports that 89% of the patients had autoimmune/inflammatory syndrome along with another rheumatic/autoimmune disease. Hawkes 2015 emphasizes that the external stimuli that trigger ASIA need to be clearly defined. Which infections are related to the autoimmune condition? What doses of adjuvants?
The exact triggers that induce an individual to develop antiphospholipid antibodies are unknown. However, bacterial or viral infections are known factors that can lead certain individuals to develop the most severe form of the disease, known as catastrophic antiphospholipid syndrome. Indeed, 35% of severe cases were preceded by respiratory, skin or urinary infections (Asherson 2006).
Due to immunosenescence (Gruver 2007), with consequent atrophy of hematopoietic tissue and lymphoid organs, higher doses of antigens or the addition of adjuvants are required to increase the immunogenicity of the vaccine (Bruijn 2007). Since the immune response to the varicella‐zoster virus has a half‐life of approximately 50 years, elderly persons have a higher probability of reactivation of the virus and of having herpes zoster (Amanna 2007).
One of the vaccines in our review uses live attenuated varicella zoster virus. In a survey conducted on persons during the first 3 years after receiving a vaccine with smaller amounts of this same viral strain (Varivax), Wise 2000 reported only 400 possible self‐medication due to adverse reactions in 9.7 million doses sold. This confirms the safety of the vaccine in 99.96% of the immunisations. The safety is even higher if we take into account that only one third of the reactions (35%) were classified as serious.
Lay 2015 reported that compared to the non‐exposed population, exposed individuals had a higher incidence of arthritis and alopecia after vaccination. The relative risks of developing arthritis and alopecia were 2.2 and 2.7 (P < 0.001 and 0.015, respectively).
As for the recombinant vaccine adjuvanted with adjuvant AS01 (that is the adjuvant used for a vaccine of herpes zoster the reason for this adjuvant is to increase the cell‐mediated response, which is important in inducing protection in the case of shingles (Garçon 2007).
We agree that the pharmaceutical company GSK may no longer carry out surveys for the detection of late adverse events, but there are specialised entities for this type of surveillance. The Vaccine Adverse Event Reporting System (VAERS) is a national vaccine safety surveillance program co‐sponsored by the USA Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) that aims to detect possible signs of adverse events associated with vaccines. VAERS collects and analyses information from reports of adverse events (possible side effects) that occur after the administration of licensed vaccines in the USA. Reports are welcome from all people involved: patients, family members, health professionals, pharmacists and vaccine manufacturers (http://www.fda.gov/biologicsbloodvaccines/safetyavailability/reportaproblem/vaccineadverseevents/default.htm).
We cannot comment on the use of vaccines in immunosuppressed/immune‐compromised individuals because our review involved only healthy people.
Considering all these facts, there is no evidence at the moment to contraindicate the vaccination of older people against herpes zoster (using attenuated live virus or recombinant). Future studies may change our position.
Contributors
Anna MZ Gagliardi Juliana de Oliveira Gomes Eduardo Canteiro Cruz
What's new
| Date | Event | Description |
|---|---|---|
| 31 January 2019 | New search has been performed | In this 2019 update, we included 11 new trials (Beals 2016; Cunningham 2016; Hata 2016; Lal 2018; Levin 2018; Maréchal 2018; NCT00886613; NCT01505647; NCT02052596; Schwarz 2017; Vink 2017); excluded four new trials (Kovac 2018; MacIntyre 2010; Strezova 2017; Weinberg 2018); and identified five ongoing trials (NCT02180295; NCT02526745; NCT03116594; NCT03120364; NCT03439657). The 2019 update included a total of 24 trials that involved 88,531 participants. |
| 31 January 2019 | New citation required but conclusions have not changed | Our conclusions remain unchanged. Only one study evaluated the efficacy and safety of recombinant vaccine (Cunningham 2016). We pooled the results of this study with those of Lal 2015; there were no changes in the conclusions regarding the recombinant vaccine. The remaining 10 new included studies did not change our conclusions because most studies conducted isolated comparisons between different vaccine dosages, formulations, routes of administration, or interval schedules, Beals 2016; Lal 2018; NCT00886613; NCT01505647; Vink 2017, or conducted comparisons with other vaccines given in the other arm of the participant (Hata 2016; Levin 2018; Maréchal 2018; NCT02052596; Schwarz 2017). |
History
Protocol first published: Issue 12, 2010 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 31 October 2018 | Feedback has been incorporated | Feedback comment added. |
| 26 October 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 26 October 2015 | New search has been performed | In this 2015 update we included five new trials (Chlibek 2013; Chlibek 2014; Diez‐Domingo 2015; Lal 2015; Vesikari 2013), and excluded one new trial (Leroux‐Roels 2012). A new vaccine that contains a varicella zoster virus glycoproteic fraction plus adjuvant is under study. |
Acknowledgements
The authors thank the Cochrane Acute Respiratory Infections (ARI) Group for their support throughout the editorial process. We are especially grateful to Elizabeth Dooley for all of her support, plus commenting on our draft review update; Zohra Lassi for the feedback process; Ann Jones for commenting our draft review update; and Co‐ordinating Editor Mark Jones and Editor Mieke van Driel for commenting our final draft review update.
We would like to thank reviewers Erin Adams, Teresa Neeman, Jonathan Fuchs, and Anca Zalmanovici Trestioreanu who contributed to improve this review update for their valuable comments.
We thank Dr Myron J Levin for his prompt reply to our emails and for the valuable information that contributed to the quality of this review (Levin 2000; Mills 2010).
We thank Stéphane Thomas for her response to our emails (Vesikari 2013).
We thank Dr AL Cunningham, Cunningham 2016, Dr Atsuko Hata, Hata 2016, and Dr Himal Lal, Lal 2018, for their prompt responses to our requests and for providing additional information.
We thank Isabel dos Santos Figueiredo for conducting the Embase search and Pedro Luis Iwasaka Neder for helping us with the Japanese study.
Appendices
Appendix 1. CENTRAL (Wiley) and MEDLINE (Ovid) search strategy
1 exp Herpes Zoster/ 2 Herpesvirus 3, Human/ 3 shingles.tw. 4 zoster.tw. 5 (varicella adj3 virus*).tw. 6 Varicellovirus/ 7 varicellovir*.tw. 8 (hhv3 or hhv‐3).tw. 9 or/1‐8 10 exp Vaccines/ 11 exp Immunization/ 12 Vaccination/ 13 (vaccin* or immuni* or inocul*).tw. 14 or/10‐13 15 9 and 14 16 Herpes Zoster Vaccine/ 17 ((zoster or shingles) adj3 vaccin*).tw. 18 zostavax.tw,nm. 19 or/15‐18
Appendix 2. Embase (Elsevier) search strategy
#22. #18 AND #21 228 #21. #19 OR #20 856,507 #20. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 816,906 #19. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 241,010 #18. #14 OR #15 OR #16 OR #17 3,723 #17. zostavax:ab,ti AND [embase]/lim 22 #16. ((zoster OR shingles) NEAR/3 vaccin*):ab,ti AND [embase]/lim 425 #15. 'varicella zoster vaccine'/de AND [embase]/lim 1,065 #14. #8 AND #13 3,486 #13. #9 OR #10 OR #11 OR #12 375,972 #12. vaccin*:ab,ti OR immuni*:ab,ti OR inocul*:ab,ti AND [embase]/lim 315,836 #11. 'vaccination'/de AND [embase]/lim 60,243 #10. 'immunization'/exp AND [embase]/lim 127,614 #9. 'vaccine'/exp AND [embase]/lim 146,730 #8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 17,850 #7. hhv3:ab,ti OR 'hhv‐3':ab,ti AND [embase]/lim 6 #6. varicellovir*:ab,ti AND [embase]/lim 31 #5. 'varicellovirus'/de AND [embase]/lim 8 #4. (varicella NEAR/3 virus*):ab,ti AND [embase]/lim 5,290 #3. shingles:ab,ti OR zoster:ab,ti AND [embase]/lim 10,726 #2. 'varicella zoster virus'/de AND [embase]/lim 8,085 #1. 'herpes zoster'/exp AND [embase]/lim 10,650
Appendix 3. LILACS (BIREME VHL) search strategy
((MH:"herpes zoster" OR "herpes zoster" or shingles or zona or zoster OR Cobreiro OR Cobrelo OR MH:C02.256.466.423$ OR MH:"Herpesvirus 3, Human"OR "Herpesvirus Humano 3" OR "Varicella‐Zoster Virus" OR "Human herpesvirus 3" OR "Herpesvirus varicellae" OR "Virus de la Varicella‐Zoster" OR "Herpesvirus Humano Tipo 3" OR "Virus del Herpes Zoster" OR "Virus de la Varicela" OR "Vírus da Varicela" OR varicella OR varicela OR MH:varicellovirus OR hhv3 OR "hhv‐3") AND (MH:vaccines OR vacunas OR vacinas OR MH:D20.215.894$ OR MH:immunization OR Inmunización OR Imunização OR MH:E02.095.465.425.400$ OR MH:E05.478.550$ OR MH:N02.421.726.758.310$ OR MH:N06.850.780.200.425$ OR MH:N06.850.780.680.320$ OR MH:SP2.026.182.113$ OR MH:SP4.001.002.015.049$ OR MH:SP8.946.819.838$ OR MH:vaccination OR Vacunación OR Vacinação OR vaccin$ OR immuni$ OR inocul$)) OR (MH:"Herpes Zoster Vaccine" OR "Vacuna contra el Herpes Zoster" OR "Vacina contra Herpes Zoster" OR "shingles vaccine" OR "zoster vaccine" OR zostavax OR "Vacina contra Cobrelo") > clinical_trials
Appendix 4. CINAHL (EBSCO) search strategy
S26 S16 and S25 S25 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 S24 (MH "Quantitative Studies") S23 TI placebo* or AB placebo* S22 (MH "Placebos") S21 TI random* or AB random* S20 TI (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* blind* or doubl* blind* or tripl* blind* or trebl* blind* or singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) S19 TI clinic* trial* or AB clinic* trial* S18 PT clinical trial S17 (MH "Clinical Trials+") S16 S11 or S12 or S13 or S14 or S15 S15 TI zostavax or AB zostavax S14 TI zoster N3 vaccin* or AB zoster N3 vaccin* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost Search Screen ‐ Advanced Search Database ‐ CINAHL 123 Edit S14 S13 TI shingles N3 vaccin* or AB shingles N3 vaccin* Search modes ‐ Boolean/Phrase Interface ‐ EBSCOhost Search Screen ‐ Advanced Search Database ‐ CINAHL 52 Edit S13 S12 TI herpes zoster vaccin* or AB herpes zoster vaccin* S11 S6 and S10 S10 S7 or S8 or S9 S9 TI (vaccin* or immuni* or inocul*) or AB (vaccin* or immuni* or inocul*) S8 (MH "Immunization+") S7 (MH "Vaccines+") S6 S1 or S2 or S3 or S4 or S5 S5 TI (hhv3 or hhv‐3) or AB (hhv3 or hhv‐3) S4 TI varicella N3 virus* or AB varicella N3 virus* S3 TI zoster or AB zoster S2 TI shingles or AB shingles S1 (MH "Herpes Zoster+")
Data and analyses
Comparison 1. Live zoster vaccine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 3.1 years follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 days of vaccination | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 42 days of vaccination | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 3.3 to 7.8 years after vaccination substudy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Mean 5 years follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Interference of herpes zoster in activities of daily life | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Participants with adverse events | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Death | 5 | 50820 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.92, 1.11] |
| 3.2 1 or more serious adverse events regardless of type of storage of the vaccine | 6 | 51029 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.95, 1.21] |
| 3.3 Vaccine‐related serious adverse events | 4 | 50766 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.24, 4.15] |
| 3.4 Hospitalised | 1 | 6616 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.07] |
| 3.5 Hospitalisation related to herpes zoster | 1 | 6616 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.25, 2.67] |
| 3.6 1 or more adverse events | 5 | 7119 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.38, 2.11] |
| 3.7 Vaccine‐related adverse events | 3 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.21, 5.75] |
| 3.8 Systemic adverse events | 5 | 7119 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.82, 1.87] |
| 3.9 Vaccine‐related systemic adverse events | 3 | 6856 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.07, 1.58] |
| 3.10 Systemic pruritus | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.12, 22.42] |
| 3.11 General malaise | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 15.18] |
| 3.12 Headache | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.75] |
| 3.13 Varicella‐like rash not at injection site (day of vaccination to day 42) | 3 | 38833 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.57, 2.11] |
| 3.14 Rash unrelated to herpes zoster (day of vaccination to day 42) | 2 | 38624 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.18] |
| 3.15 Injection site adverse events | 4 | 7040 | Risk Ratio (M‐H, Random, 95% CI) | 3.73 [1.93, 7.21] |
| 3.16 Erythema inoculation site | 4 | 6958 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [2.66, 6.94] |
| 3.17 Pain inoculation site | 4 | 6958 | Risk Ratio (M‐H, Random, 95% CI) | 6.47 [2.67, 15.68] |
| 3.18 Pruritus inoculation site | 4 | 6958 | Risk Ratio (M‐H, Random, 95% CI) | 4.32 [1.49, 12.48] |
| 3.19 Swelling inoculation site | 3 | 6879 | Risk Ratio (M‐H, Random, 95% CI) | 5.84 [4.95, 6.89] |
| 3.20 Warmth inoculation site | 3 | 6879 | Risk Ratio (M‐H, Random, 95% CI) | 4.73 [2.57, 8.74] |
| 3.21 Rash inoculation site | 1 | 6616 | Risk Ratio (M‐H, Random, 95% CI) | 3.26 [1.31, 8.11] |
| 3.22 Haematoma inoculation site | 1 | 6616 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.76, 1.67] |
| 3.23 Mass inoculation or induration site | 2 | 6695 | Risk Ratio (M‐H, Random, 95% CI) | 7.05 [1.91, 26.05] |
| 3.24 Varicella‐like rash at injection site (day of vaccination to day 42) | 1 | 38546 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.21, 6.76] |
| 3.25 Vaccine‐unrelated adverse event | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.68] |
| 3.26 Herpes zoster‐like rash (day of vaccination to day 42) | 1 | 38546 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.84] |
| 4 Duration in days of adverse effects | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Erythema | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Pain | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Pruritus | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Swelling | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Rash | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Haematoma | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Dropouts | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 For any reason | 3 | 38916 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.91, 1.08] |
| 5.2 Discontinued due to vaccine‐related adverse events | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 5.05 [0.25, 103.88] |
| 5.3 Clinical adverse event | 2 | 12189 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.72, 2.52] |
| 5.4 Physician decision | 1 | 11980 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.17] |
| 5.5 Withdrew consent | 4 | 50814 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.54, 1.68] |
| 5.6 Lost to follow‐up | 5 | 50868 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.96, 1.69] |
| 5.7 Protocol deviation | 2 | 12189 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.27, 8.37] |
| 6 Participants with no follow‐up | 3 | 50627 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.41, 1.74] |
1.4. Analysis.
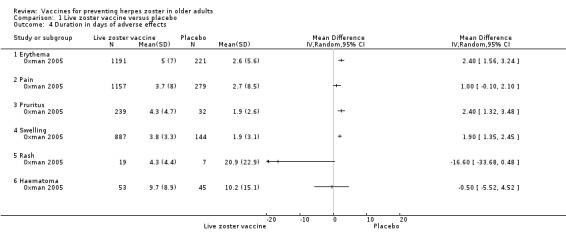
Comparison 1 Live zoster vaccine versus placebo, Outcome 4 Duration in days of adverse effects.
Comparison 2. Recombinant zoster vaccine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of herpes zoster at least 3.2 years follow‐up | 2 | 22022 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.03, 0.23] |
| 2 Incidence of herpes zoster at least 4 years follow‐up | 1 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Participants with adverse events | 2 | 307757 | Risk Ratio (M‐H, Random, 95% CI) | 3.35 [2.68, 4.19] |
| 3.1 Death | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.04] |
| 3.2 Death within 30 days after vaccination | 1 | 15411 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.42, 3.16] |
| 3.3 Serious AEs | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.03] |
| 3.4 Serious AEs within 30 days after vaccination | 1 | 15411 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.67, 1.20] |
| 3.5 Serious AEs within 30 days after vaccination related to vaccination | 1 | 15411 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 3.21] |
| 3.6 Any symptom | 2 | 9936 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [2.02, 2.88] |
| 3.7 Grade 3 any symptom | 2 | 9936 | Risk Ratio (M‐H, Random, 95% CI) | 5.29 [4.48, 6.26] |
| 3.8 Grade 3 any symptom related to vaccination | 1 | 8926 | Risk Ratio (M‐H, Random, 95% CI) | 8.37 [6.69, 10.47] |
| 3.9 Any systemic symptom | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [2.12, 2.34] |
| 3.10 Grade 3 any systemic AEs | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 4.29 [3.01, 6.11] |
| 3.11 Potential immune‐mediated disease | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.71, 1.08] |
| 3.12 Myalgia | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 3.82 [3.52, 4.16] |
| 3.13 Fatigue | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.99, 3.17] |
| 3.14 Headache | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [2.26, 2.63] |
| 3.15 Fever | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 6.45 [4.61, 9.04] |
| 3.16 Shivering | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 4.35 [3.26, 5.81] |
| 3.17 Gastrointestinal symptom | 2 | 9762 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.21, 2.55] |
| 3.18 Any local symptom | 2 | 9769 | Risk Ratio (M‐H, Random, 95% CI) | 6.89 [6.37, 7.45] |
| 3.19 Grade 3 any local symptom | 2 | 9769 | Risk Ratio (M‐H, Random, 95% CI) | 12.69 [2.87, 56.06] |
| 3.20 Local redness | 2 | 9769 | Risk Ratio (M‐H, Random, 95% CI) | 28.93 [22.62, 37.00] |
| 3.21 Local pain | 2 | 9769 | Risk Ratio (M‐H, Random, 95% CI) | 7.14 [6.58, 7.74] |
| 3.22 Local swelling | 2 | 9769 | Risk Ratio (M‐H, Random, 95% CI) | 28.26 [15.91, 50.20] |
| 3.23 Unsolicited report of AEs | 1 | 8926 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [1.00, 1.14] |
| 3.24 Grade 3 unsolicited report of AEs | 1 | 8926 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.12, 1.69] |
| 4 Dropouts | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Did not receive vaccine according to protocol | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.38, 3.54] |
| 4.2 Received wrong vaccine | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.81, 3.23] |
| 4.3 Diagnosis of herpes zoster < 30 days after dose 2 | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.71] |
| 4.4 Did not receive second dose | 2 | 29311 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.13, 1.39] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beals 2016.
| Methods |
Study design: randomised, partly blinded, parallel‐group study Duration: 42 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA, 3 centres, Aurora, CO (n = 1); Miami, FL (n = 2) Number: 223 participants; treatment (N = 171), control (N = 52) Participants' health status: history of varicella or who had resided in a country with endemic varicella zoster virus infection for ≥ 30 years; temperature < 38 °C on day of vaccination; good health Age: mean ˜ 61 years Sex: ˜ 56% female Other relevant information: aged ≥ 50 years 94% of participants had European ethnicity Exclusion criteria "Participants were excluded if they had either: a previous history of herpes zoster, received varicella vaccine, recent exposure to systemic immune suppressants, immune dysfunction, recent live virus vaccinations, antiviral drugs active against varicella‐zoster virus, or immune suppressed household members. Additional exclusion criteria included history of hypersensitivity reactions to any vaccine component, household exposure to pregnant women who had not had chickenpox and had not been vaccinated against varicella, household or workplace exposure to children 18 months and younger who have not been vaccinated against varicella, received immune globulin or blood products from 5 months before vaccination, receipt of inactivated vaccine from 7 days before study vaccine to 7 days postvaccination, except for inactivated influenza vaccine, not ambulatory, pregnant or breastfeeding, and active untreated tuberculosis". |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Systemic reactions for 42 days Local reactions from each injection site for 5 days (vaccine report cards recorded): ≥ 1 injection site adverse events, erythema, pain, swelling, induration, pruritus |
|
| Purpose of the study | "This exploratory study aimed to assess the immunogenicity and safety of intradermal zoster vaccination compared with the conventional subcutaneous route" | |
| Funding sources | Merck & Co Inc | |
| Conflicts of interest | "CRB, RAR, AKS, BKM, and RKE are employees of Merck & Co Inc; employees may hold stock and/or stock options in the company. KL, EAS, and MJL are investigators for the sponsor. MJL is a consultant to the sponsor and shares intellectual property rights on Zostavax. YL and EK are employees of NanoPass Technologies Ltd, the provider of the MicronJet600 device." | |
| Notes | "Zoster vaccine is a lyophilised preparation (ZOSTAVAX, Merck & Co Inc, Kenilworth, NJ, USA) of live, attenuated varicella‐zoster virus (Oka/Merck) stored frozen before reconstitution." Subcutaneous doses were given with a needle and syringe. "Intradermal injection used the NanoPass MicronJet600 device (NanoPass, Nes Ziona, Israel), which is equipped with three silicon microneedles, each 0.60 mm in length. Intradermal doses were reconstituted in the diluent used for subcutaneous administration except for the 1/27 dose, which was reconstituted with the sterile normal saline, because reconstituting in diluent would cause the dose to be too hypotonic." All doses were given over the deltoid muscle of the non‐dominant arm, and 39 participants across all groups received concomitant intradermal saline placebo in the dominant shoulder. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The two subcutaneous doses and the four intradermal doses were randomised (1·5:1:1:1:1:1) by a computer generated sequence." |
| Allocation concealment (selection bias) | Unclear risk | Despite the random sequence generation being appropriate, there were no details about allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study staff did not inform the participants of the dose of zoster vaccine or whether zoster vaccine or saline was injected into a given arm, but the method of the delivery was not concealed." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study staff did not inform the participants of the dose of zoster vaccine or whether zoster vaccine or saline was injected into a given arm, but the method of the delivery was not concealed." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "After 42 days, participants returned a completed vaccine report card, which records injection site reactions (for 5 days) and systemic safety." However, the participants were not totally blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The patient flow is clear. |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented for all groups. |
| Other bias | Unclear risk | Insufficient information |
Berger 1998.
| Methods |
Study design: RCT, double‐blind Duration: 42 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: France, Switzerland, and Ireland Number: 200 participants; treatment (N = 149), control (N = 49) Participants' health status: healthy adults with previous history of varicella confirmed by positive serology to VZV and a competent immune system (no signs of immunodeficiency). Age: mean ˜ 66 years Sex: ˜ 59% male Other relevant information: aged ≥ 55 years Exclusion criteria Fever at the time of selection, any previous zoster episode, seropositivity to HIV, any underlying immunodepressive condition, previous vaccination against varicella or zoster, any other recent vaccination, recent administration of any blood product, and sensitivity to neomycin. |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Local adverse reaction during 42 days (6 weeks): none, ≥ 1 reaction, induration (diameter ≥ 2 cm), pain (all), pain (probably vaccine‐related), redness (diameter ≥ 2 cm), pruritus and vesicles. | |
| Purpose of the study | "To evaluate the cell‐mediated and humoral immunogenicity and the safety of 1 of 3 doses of a live attenuated varicella‐zoster virus vaccine/OKA compared with a control vaccine." | |
| Funding sources | Pasteur Mérrieux Connaught, Lyon, France | |
| Conflicts of interest | Not described | |
| Notes | No participants had fever during the 72 hours following vaccination. 1 participant in the 8500 pfu VZV group presented with a mild vesicular rash after vaccination that lasted 7 days. Analysis of the vesicular fluid was negative for VZV (PCR analysis). No ITT analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Three groups of different concentrations of a live attenuated VZV/Oka vaccine under double‐blind conditions. 1 group of pneumococcal polysaccharide vaccine under single‐blind conditions and used as a control for a reactogenicity and immune response." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented for all groups. |
| Other bias | Unclear risk | Insufficient information |
Chlibek 2013.
| Methods |
Study design: RCT phase II, parallel group, placebo controlled, double‐blind Duration: 1 year after the last vaccination (14 months) |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 12 centres: USA (n = 7); Spain (n = 4), the Czech Republic (n = 1) Number: 410 participants; treatment (N = 372), control (N = 38) Participants' health status: healthy participants Age: mean age ˜ 65 years Sex: ˜ 57% female Other relevant information: aged ≥ 50 years ˜ 90% of participants were Caucasian (understood to be white) Exclusion criteria "Participants were excluded if they were using any investigational or non‐registered drug or vaccine within 30 days preceding the first dose of study vaccine or any non‐replicating vaccines within 2 weeks of enrolment, were receiving chronic (> 14 consecutive days) immunosuppressants or other immune‐modifying drugs within 3 months prior to enrolment (for corticosteroids, ≥0.5 mg/kg/day prednisone or equivalent), were previously vaccinated against herpes zoster or varicella, had a history of herpes zoster, allergic disease or reactions likely to be exacerbated by any component of the vaccine, had a confirmed or suspected immunosuppressive or immunodeficient condition, were administered immunoglobulins or any blood products within the 3 months preceding the first injection of study vaccine or planned to receive them during the study period, or had an acute disease at enrolment. In addition, women could not be pregnant or had to be using birth control or be of non‐childbearing potential" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | Immunogenicity and reactogenicity of recombinant gE in a representative older adult population | |
| Funding sources | GlaxoSmithKline Biologicals SA, Belgium | |
| Conflicts of interest | "R. C. has been the principal investigator in clinical studies supported by the GlaxoSmithKline group of companies and Novartis. He has also been a scientific consultant to Baxter, GSK, Novartis, Aventis Pasteur, and Pfizer and received sponsorship from GSK and Aventis Pasteur to attend scientific meetings. J. M. B. has been the principal investigator in clinical studies supported by GSK and Sanofi Pasteur MSD. He has also been a scientific consultant to GSK, Novartis, Sanofi Pasteur MSD, and Pfizer. H. C. has been principal investigator in clinical studies with GSK and other pharmaceutical companies M. L. R. D. has been the principal investigator in clinical studies supported by GSK and has received investigator fees from the Fundación Puerta de Hierro. E. L., J. F. M., and T. C. H. are employed by the GlaxoSmithKline group of companies. T. C. H. receives stock equity in GSK as part of his compensation. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed." | |
| Notes | "Of the 410 subjects, 395 completed the study. Of the 15 participants who discontinued the study early, 2 withdrew due to treatment related AEs (1 participants each in the gE/AS01E and gE/AS01B groups) and 2 withdrew for SAEs not considered treatment related (digestive tract haemorrhage in the gE/AS01E group and myocardial infarction in the gE/AS01B group), 2 vaccine‐related adverse events led to withdrawal from the study: 1 subject treated with gE/AS01B withdrew due to malaise beginning on the day of vaccination, and 1 participants treated with gE/AS01E withdrew due to injection site redness that lasted > 2 weeks. 2 lost to follow‐up (gE/AS01B), 8 consent withdrawal (4 in the gE/AS01B, 2 in the gE/AS01E, 1 in the gE/saline and 1 after second dose of vaccine in the group gE/AS01B). 1 protocol violation (gE/AS01E)" The only unsolicited symptom reported by > 3% of participants in any group was chills, which was reported by 5% (8/150) of participants treated with gE/AS01B and 2% (3/149) of those treated with gE/AS01E; this was not reported in participants treated with gE/saline or saline alone. No vaccine‐related serious adverse events or cases of herpes zoster were reported through month 14 of the study. We asked the study authors about adverse events by age or vaccination, but the response we received reiterated only the published data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was made using an algorithm that stratified by country, minimized for age, and included a block size of 11" |
| Allocation concealment (selection bias) | Low risk | "Treatments were allocated at each site using a central randomisation system on the Internet" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The person in charge of the vaccination accessed the randomisation system on Internet using the subject number and age" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both vaccine recipients and observers responsible for evaluations were blinded to which formulation was administered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both vaccine recipients and observers responsible for evaluations were blinded to which formulation was administered" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The patient flow is clear. |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented for all groups. |
| Other bias | Unclear risk | Insufficient information |
Chlibek 2014.
| Methods |
Study design: RCT phase II, single‐blind (participants) Duration: 36 months after first vaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 11 centres in the Czech Republic, Germany, the Netherlands, and Sweden Number: 714 participants; treatment (N = 495), control (N = 219) Participants' health status: healthy participants Age: mean ˜ 69.9 years Sex: ˜ 60% female Other relevant information: aged ≥ 60 years 99.3% Caucasian (understood to be white) Exclusion criteria "Participants were excluded if they had a history of herpes zoster; were previously vaccinated against herpes zoster or with any vaccine containing 3‐O‐desacyl‐ 4‐monophosphoryl lipid A(MPL) or Quillaja saponaria Molina, fraction 21 (QS21), were allergic to any of the vaccine components, had received a vaccine (except influenza) within 2 weeks, an investigational or non‐registered product, chronic immunosuppressants, corticosteroids within 30 days, or immunoglobulins or a blood product within 3 months before the first study vaccine dose, or had a history of drug or alcohol abuse." |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
Intensity of the solicited reactions was scored on a scale from 0 (absent) to 3 (severe). All solicited local reactions were considered vaccination‐related, and causality of the solicited general reactions, unsolicited adverse events, and serious adverse events was assessed by the investigators. |
|
| Purpose of the study | "The aim of the current study is to evaluate the safety and immunogenicity of different schedules and formulations of gE/AS01B in adults ≥ 60 years of age" | |
| Funding sources | GlaxoSmithKline Biologicals SA, Belgium | |
| Conflicts of interest | "Dr. Chlibek has been the principal investigator in clinical studies supported by GSK. He has received sponsorship to attend scientific meetings and reimbursement of other expenses from GSK and Pfizer, and receives payment for the development of educational presentations from Pears Health Cyber. Dr. Smetana has received consulting fees and honoraria/travel grants from GSK and Sanofi Pasteur in the past 3 years. Dr. Pauksens has been a principal investigator in clinical trials conducted by GSK, Pfizer, and Sanofi Pasteur. Dr. Rombo has received consulting fees from GSK. Dr. Van den Hoek has no conflict of interest to declare. Dr. Richardus has received grants from GSK for carrying out clinical trials and has received travel grants from GSK in the past 3 years. Dr. Plaßmann has received honoraria for conducting clinical trials from GSK. Dr. Schwarz received honoraria for consultancy, member ship of advisory boards and lecturing from GSK in the past 3 years. Mr. Ledent and Dr. Heineman are full time employees of the GSK group of companies. Dr. Heineman receives stock equity as part of his compensation." | |
| Notes | 715 participants were enrolled, but 714 were vaccinated. 701 completed the study through month 3. Most solicited reactions were transient (1.1 to 3.5 days on average) and were of mild‐to‐moderate intensity (grade 1 or 2), with ≤ 4.8% of participants in each group reporting grade 3 reactions. A total of 349 serious adverse events were reported in 205 participants during the study. 14 participants died due to an SAE, most due to cancer or heart failure. No serious adverse events were considered by the investigators to be related to the study vaccines. 47 participants (6.6%) were excluded from the according‐to‐protocol immunogenicity cohort. The most common reasons for exclusion were non‐compliance with the blood sampling schedule (N = 27) and the absence of essential serological data (N = 9). Of the 714 vaccinated participants, 685 (95.9%) were followed through month 12; 665 (93.1%) through month 24; and 646 (90.5%) through month 36. 8 participants were withdrawn from the 25 µg gE/AS01B group (3 not eligible, 2 lost to follow‐up, 2 consent withdrawal, and 1 death); 7 were withdrawn from the 50 µg gE/AS01B group (1 not eligible, 2 consent withdrawal, and 4 deaths); 6 were withdrawn from the 100 µg gE/AS01B group (2 not eligible, 2 consent withdrawal, and 2 deaths); 4 were withdrawn from the saline + 100 µg gE/AS01B group (1 lost to follow‐up, 1 consent withdrawal, and 2 deaths); and 4 were withdrawn from the 100 µg gE/saline group (2 lost to follow‐up and 2 deaths). "The proportion of subjects with solicited reactions was higher for groups receiving two doses of gE/AS01B but the proportion did not increase between the first and the second vaccination (data not shown)" We requested information about adverse events by age or vaccination from the study authors, but have only received the published data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were stratified by age (60–69 years and ≥70 years in a 1:4 ratio) and randomised"; the method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information was provided regarding this domain. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no mention of whether the outward appearance of the prepared injections was indistinguishable in all aspects. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind (only for participants), but the participants completed their diary cards themselves as follows: "solicited local reactions (pain, redness and swelling) and general reactions (fatigue, fever, headache and myalgia) were recorded by subjects on diary cards for seven days after each vaccination" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although the participants themselves completed their diary cards, the other adverse events were not blinded for the evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The patient flow is clear. |
| Selective reporting (reporting bias) | Low risk | The adverse events originally defined by the authors were presented. |
| Other bias | Unclear risk | Insufficient information |
Cunningham 2016.
| Methods |
Study design: randomised, double‐blind, placebo controlled Duration: mean follow‐up period of 3.7 years for efficacy and 4.0 years for safety |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 18 countries in Europe, North America, Latin America, Asia, and Australia Number: 13,900 participants; treatment (N = 6950), control (N = 6950) Participants' health status: healthy participants Age: mean 75.6 years Sex: ˜ 55% female Other relevant information: aged ≥ 70 years 76.9% Caucasian (understood to be white) Exclusion criteria History of herpes zoster, had been vaccinated previously against varicella or herpes zoster, or had an immunosuppressive condition |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Cases of herpes zoster Subgroup of participants recorded injection site reactions (pain, redness and swelling) and systemic reactions (fatigue, fever, gastrointestinal symptoms, headache, myalgia, and shivering) on diary cards for 7 days after each injection. "Unsolicited reports of adverse events were recorded for 30 days after each dose for all participants" "Serious adverse events were recorded for all participants for 12 months after the second dose" "Serious adverse events that were considered to be related to the study vaccine or to trial participation, events resulting in death, and potential immune‐mediated diseases were evaluated in all participants throughout the trial" |
|
| Purpose of the study | "The primary objective of ZOE‐70 was to evaluate the efficacy of HZ/su, as compared with placebo, in reducing the risk of herpes zoster among adults 70 years of age or older." "The secondary objectives included the evaluation of vaccine efficacy against postherpetic neuralgia and the evaluation of vaccine safety and reactogenicity." | |
| Funding sources | GlaxoSmithKline Biologicals | |
| Conflicts of interest | "Dr. Cunningham reports receiving consulting fees from Merck, BioCSL/Sequirus, and the GSK group of companies (GSK), all paid to his institution. Dr. Kovac, Dr. Campora, Ms. Vanden Abeele, Dr. Zahaf, and Dr. Oostvogels report being employees of GSK; Drs. Kovac, Zahaf, and Oostvogels also report holding stock in the company as part of their employee remuneration. Drs. Heineman, Lal, and Godeaux report being employees of and holding stock in GSK as part of their employee remuneration at the time of the study; Dr. Heineman is a current employee of Genocea Biosciences, Dr. Lal is a current employee of Pfizer, and Dr. Godeaux is a current employee of Crucell Holland. Dr. Chlibek reports receiving lecture fees from Pfizer and Gilead Sciences and grant support from Gilead Sciences; Dr. Díez‐Domingo, receiving fees for serving on advisory boards from GSK and Sanofi Pasteur MSD and grant support from Sanofi Pasteur MSD; Dr. Levin, receiving fees for serving on an advisory board from Merck, grant support from Merck and GSK, and royalties from a patent related to a zoster vaccine that he holds with Merck; Dr. McElhaney, receiving honoraria from GSK, Pfizer, Merck, and Sanofi Pasteur, paid to her institution, and travel support from Pfizer, Merck, and Sanofi Pasteur; Dr. Puig‐Barberà, receiving personal fees and grant support from GSK and Novartis; Dr. Vesikari, receiving fees for serving on an advisory board from Sanofi Pasteur MSD, lecture fees from GSK and Merck, and grant support from Merck; Dr. Watanabe, receiving consulting fees from Maruho and Japan Vaccines, lecture fees from Maruho and Mochida, and grant support from Maruho; Dr. de Looze, receiving grant support from GSK and Novartis; Dr. Gorfinkel, receiving lecture fees and grant support from GSK, Astellas, Ferring, Forest, Novo Nordisk, Janssen‐Ortho, Bayer, Wyeth, Combinator, Pfizer, Pharmanet, AstraZeneca, Lundbeck, Bristol‐Myers Squibb, Romark, McNeil, and Johnson & Johnson; Dr. McNeil, receiving consulting and lecture fees from Pfizer and Merck and grant support from Pfizer and GSK; Dr. Rombo, receiving lecture fees from GSK, Sanofi Pasteur, and Valneva; Dr. Smetana, receiving fees for serving on a board from Pfizer and lecture fees and travel support from GSK; and Dr. Weckx, receiving fees for serving on advisory boards from Novartis, GSK, AbbVie, and Wyeth." | |
| Notes | Recombinant zoster vaccine (herpes zoster subunit vaccine) contains 50 μg of recombinant VZV glycoprotein E and the liposome‐based AS01B adjuvant system (which contains 50 μg of 3‐O‐desacyl‐4′monophosphoryl lipid A (MPL) and 50 μg of Quillaja saponaria Molina, fraction 21 (QS21, licensed by GSK from Antigenics, a subsidiary of Agenus). A total of 14,816 participants were enrolled and randomised. 13 participants did not receive vaccine or placebo. 903 participants were excluded due to deviations from Good Clinical Practice standards. The remaining 13,900 participants made up the total of the vaccinated cohort, but not ITT analysis. Most participants received 2 doses of the study vaccines (94.4% of herpes zoster subunit vaccine recipients and 95.6% of placebo recipients). 1025 participants were randomly assigned to the reactogenicity subgroup (512 herpes zoster subunit vaccine recipients and 513 placebo recipients). "In this subgroup, solicited reports of reactions ('solicited reactions') that occurred within 7 days after each vaccination. a randomly selected subgroup of age stratified participants recorded injection‐site reactions (pain, redness and swelling) and systemic reactions (fatigue, fever, gastrointestinal symptoms, headache, myalgia, and shivering) on diary cards for 7 days after each injection. Redness and swelling at the injection site were scored as 0 if the affected area was less than 20 mm in diameter, 1 if the affected area was 20 to 50 mm, 2 if the affected area was more than 50 to 100 mm, and 3 if the affected area was more than 100 mm. Fever was scored as 0 for a body temperature lower than 37.5°C, 1 for 37.5°C to 38.0°C, 2 for 38.1°C to 39.0°C, and 3 for higher than 39.0°C (the preferred route for recording temperature was oral). All other symptoms were scored as 0 for absent, 1 for easily tolerated, 2 for interferes with normal activity, and 3 for prevents normal activity. Unsolicited reports of adverse events were recorded for 30 days after each dose for all participants. All serious adverse events were recorded for all participants for 12 months after the second dose. Serious adverse events that were considered to be related to the study vaccine or to trial participation, events resulting in death, and potential immune‐mediated diseases were evaluated in all participants throughout the trial." We asked the author, Dr Cunningham, for details of his study publication, and he kindly sent us the available information. Dr Cunningham responded promptly to our questions and provided us with what answers he could. There was a continuation of this study in Japan, which published a descriptive subgroup analysis in participants enrolled in this country throughout 4 years of follow‐up. Published as Ikamatsu 2018; the data were presented as Cunningham 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants "were randomly assigned in a 1:1 ratio to either the HZ/su group or the placebo group with the use of an online centralized randomization system". |
| Allocation concealment (selection bias) | Unclear risk | Whilst the sequence and random number generation were appropriate, no details were provided regarding allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The investigators were unaware of the study‐group assignments during the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk |
Cunningham 2016 followed the same methods used by Lal 2015: "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study "was monitored by an independent data and safety monitoring committee that met regularly during the course of the study to review all safety data in an unblinded manner". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No clear participant flow; the number of participants randomised to each group is not described for all outcomes |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results. |
| Other bias | Unclear risk | Insufficient information |
Diez‐Domingo 2015.
| Methods |
Study design: phase 3, open‐label, randomised Duration: participants were followed up for a maximum of 35 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 10 centres in Germany and Spain Number: 353 participants; treatment (N = 176), control (N = 177) Participants' health status: healthy participants with a history of varicella or resident for > 30 years in a country with endemic VZV infection Age: mean 62.6 years Sex: ˜ 55% female Other relevant information: aged ≥ 50 years Exclusion criteria "Previously been vaccinated with any VZV‐containing vaccine or had previously been diagnosed with HZ. In addition, were excluded: any subjects with a history of a febrile episode (≥38.3◦C) in the 72 h prior to study vaccination, those who had received any live vaccine within 28 days of study vaccination or inactivated vaccine within 14 days of study vaccination or immunoglobulins or other blood products within 5 months before vaccination and those who were taking systemic antiviral therapy or had an immune deficiency associated with disease (e.g. human immunodeficiency virus, cancer) or medical treatment (e.g. chemotherapy, transplant recipients)" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | "To evaluate the immunogenicity as measured by VZV antibody titres (gpELISA) at 4 weeks following ZOSTAVAX® administered by IM or SC route" "To evaluate the immune response as measured by a second assay, the VZV Interferon‐gamma (IFN‐Ȗ)‐ELISPOT at 4 weeks following ZOSTAVAX® administered by IM or SC route" "To describe the safety profile of ZOSTAVAX® administered by IM or SC route" |
|
| Funding sources | Sanofi Pasteur MSD | |
| Conflicts of interest | “JDD has been and is the principal investigator in trials sponsored by Sanofi Pasteur MSD, GSK, Merck, Baxter, Novartis and Pfizer. His institutions have received research grants from Sanofi Pasteur MSD, Pfizer and Baxter. He has received grants for attending meeting and has been a member of advisory boards for GSK, Pfizer and Sanofi Pasteur MSD. TW has received honoraria for lecturing and consulting activities from Novartis Vaccines and Sanofi Pasteur MSD. JGDL and CUM have no potential conflicts of interest to declare. IB, CE, ST and CS are employed by Sanofi Pasteur MSD, the company that commercialises the herpes zoster live‐attenuated vaccine (Zostavax®) in Europe.” | |
| Notes | This was basically an immunogenicity study; only the safety data were used in this review. Not ITT analyses More detailed unpublished data were kindly provided by Sanofi Pasteur MSD SNC. Data by age were not available. 1 participant in Group 1 (IM route) reported a zoster‐like rash (right thoracic dermatome) of mild intensity that occurred on day 12 after vaccine administration and lasted 6 days. No specimen was obtained for PCR testing. No participant was withdrawn due to an AE at any time after vaccine administration. No deaths were reported. 3 participants reported an SAE: 1 participant (hernia obstructive) in Group 1 (IM route) and 2 participants (humerus fracture and deep vein thrombosis) in Group 2 (SC route). None were assessed as vaccine‐related by the investigator. No participant was withdrawn due to an AE at any time after vaccine administration. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomised using an electronic case report form (e‐CRF)" |
| Allocation concealment (selection bias) | Low risk | "Allocation schedules were generated using a 1:1 ratio with permuted blocks of 4‐6" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Between visit 1 and 2, the participants were given a diary card to record their temperature if they were febrile (oral temperature ≥38.3 ◦C), occurrence of any solicited injection site (erythema, swelling and pain) adverse reactions (Days 0–4) and any unsolicited injection site adverse reactions, varicella, varicella‐like rashes, HZ and zoster‐like rashes and other systemic adverse events (AEs) (Days 0–28). They were also asked to report any serious AEs (SAEs) that occurred at any time during the study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The participants did not put any SAEs in their diary cards themselves, therefore this was not blinded for the staff. "They were also asked to report any serious AEs (SAEs) that occurred at any time during the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on adverse events that the authors proposed in their methodology were described in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
Gilderman 2008.
| Methods |
Study design: RCT, double‐blind Duration: 28 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA, multicentre Number: 367 participants; treatment (N = 182), control (N = 185) Participants' health status: healthy participants, immunocompetent individuals with a history of varicella or residence in a country where VZV infection is endemic Age: mean ˜ 63 years old Sex: ˜ 55% female Other relevant information: aged ≥ 50 years 68.1% white participants Exclusion criteria "Subjects were excluded if they had a clinical history of hypersensitivity or anaphylactic reactions to gelatin or neomycin, used any form of non topical antiviral therapy, had received a live vaccine within 4 weeks prior to the study dose or an inactivated vaccine within 1 week prior to the study dose, or another vaccination was planned before the subject was due to complete the study. Study exclusions also included a history of HZ, pregnancy, or breastfeeding; the plan to conceive within the duration of the study; known or suspected immune dysfunction; and alcohol or other substance abuse that might interfere with the evaluation required by the study." |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | "To support the development of a refrigerator‐stable formulation of Zostavax with a confirmatory clinical trial with varicella‐zoster virus antibody‐seropositive adults ≥50 years of age" | |
| Funding sources | Merck & Co Inc | |
| Conflicts of interest | “Other than those authors who are employees of Merck & Co., Inc. (as indicated in the affiliations), L.I.G., J.F.L., and T.M.N. have been investigators for the sponsor. Employees may hold stock and/or stock options in the company.” | |
| Notes | 1 participant withdrew consent prior to intervention. No ITT analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, with in‐house blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The formulations were visually indistinct, supplied in identical glass vials. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear participant flow |
| Selective reporting (reporting bias) | Low risk | Adverse events prespecified by the investigators were reported in the results section for both refrigerated and frozen zoster vaccines. |
| Other bias | Unclear risk | Insufficient information |
Hata 2016.
| Methods |
Study design: double‐blind, randomised, placebo controlled Duration: 3 months postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: Japan, single‐centre, Kitano Hospital, a general hospital in Osaka Number: 54 participants; treatment (N = 27), control (N = 27) Participants' health status: participants with diabetes mellitus had glycated haemoglobin levels within the range 6% to 9.5% (Japan Diabetes Society) or 6.4% to 9.9% (National Glycohemoglobion Standardization Program) and were without moderate or severe acute illness Age: mean 66.2 years Sex: 44.4% female Other relevant information: aged 60 to 70 years 100% Asians Exclusion criteria "Smokers, immunocompromised patients with any potential malignant disease, autoimmune disease, renal failure, users of steroids or other immunosuppressive drugs, those with heart disease treated by antiplatelet drugs, patients with dermatological disorders that might hinder judgement of a skin test reaction, and HZ patients were not included in the study." |
|
| Interventions |
Treatment group
Control group
Observation: 1 dose of PPSV23 was given subcutaneously on the other arm of each participant in both groups. |
|
| Outcomes |
|
|
| Purpose of the study | "To evaluate the immunogenicity and safety of a live Oka varicella zoster vaccine generally recommended for concurrent vaccination with PPSV23 in 60–70‐year‐old people with diabetes mellitus" | |
| Funding sources | "This work was supported by a grant from the Ministry of Health, Labour and Welfare of Japan, no. C2250068 during 2010–2015" | |
| Conflicts of interest | "YM has received a grant from the Research Foundation for Microbial Diseases of Osaka University (BIKEN). This grant is unrelated to the conduct and results of this study. TO has received a payment for lectures on the speaker’s bureau from Mitsubishi Tanabe Pharma Corp. related to cardiovascular diseases. All other authors affirm that no financial arrangement or other factor might present a potential conflict of interest related to this study.” | |
| Notes | "A live, attenuated Oka varicella vaccine (Lot No. VZ059, 068‐073,079) manufactured by the Research Foundation for Microbial Diseases of Osaka University (BIKEN) was used. The estimated potency was approximately 50 000 plaque forming units per dose." PPSV23 SC was administered in the other arm for all participants on day 0: "Each participant received one dose of the ZV or placebo and one dose of PPSV23 on day 0" We asked Dr Hata: "We would like to be clear whether the subcutaneous injection contained both vaccine (VZV and PPSV23) or those vaccine were administered in different arms. Also, was the same procedure done in the placebo group?" Dr Hata's answer: "We administered different vaccines in separate arms to confirm adverse reactions" "During follow up, one participant was unable to visit the hospital (we consider lost of follow up) and another died of acute cardiac insufficiency (heart failure) that was wholly unrelated to the vaccination". Both losses were in the intervention group (LZV group) "No zosteriform rash was reported during the observation period" "The secondary outcomes of safety were local and systemic adverse experiences on days 0–42 after vaccination, and severe adverse experiences and zoster events over a 1–year observational period" "VZ/Oka is a live, attenuated Oka varicella vaccine (Lot No. VZ059, 068‐073,079) manufactured by the Research Foundation for Microbial Diseases of Osaka University (BIKEN)" ITT analysis was used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After receiving the consent of participants, the study staff determined treatment allocation using a randomization system. An independent epidemiologist generated a series of randomization codes for varicella zoster vaccine solution or distilled water based on a random number table. Independent research staff at the hospital pharmacy were then informed of the codes" |
| Allocation concealment (selection bias) | Low risk | "A member of the medical staff was informed of the allocated participant code according to the order in the code table, but was blinded to the contents of the codes." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Pharmacists produced a vaccine solution or purified distilled water that were identical in appearance" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The medical staff member than administered the assigned vaccine solution or distilled water to the participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The contents of the code were concealed by the independent research staff until the study was completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events proposed by the authors in the methodology were described in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
Lal 2015.
| Methods |
Study design: randomised, double‐blind, placebo controlled Duration: mean follow‐up of 3.2 years |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 18 countries in Europe, North America, Latin America, Asia, and Australia Number: 15,411 participants; treatment (N = 7698), control (N = 7713) Participants' health status: healthy participants Age: mean age ˜ 62.4 years Sex: ˜ 61.2% female Other relevant information: aged ≥ 50 years ˜ 71.5% Caucasian (understood to be white) The majority from Europe: 51.2% Exclusion criteria A history of herpes zoster, previously vaccinated against varicella or herpes zoster, or had an immunosuppressive condition |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | "The primary objective of the study was to evaluate overall vaccine efficacy in reducing the risk of herpes zoster, as compared with placebo. Secondary objectives included determining the vaccine efficacy in reducing the incidence of herpes zoster in each age group (50 to 59 years, 60 to 69 years, and ≥70 years) and HZ/su safety and reactogenicity profiles." | |
| Funding sources | GlaxoSmithKline Biologicals | |
| Conflicts of interest | “The authors’ affiliations are as follows: GSK Vaccines, King of Prussia, PA (H.L., T.C.H.); Westmead Millennium Institute for Medical Research, Westmead, NSW, and the University of Sydney, Sydney — both in Australia (A.L.C.); GSK Vaccines, Wavre, Belgium (O.G., T.Z.); Faculty of Military Health Sciences, University of Defense, Hradec Kralove, Czech Republic (R.C.); Vaccine Research Unit, Fundación para el Fomento de la Investigación Sanitaria y Biomédica, Valencia, Spain (J.D.‐D., J.P.‐B.); Department of Family Medicine, Taipei Veterans General Hospital, and National Yang Ming University School of Medicine ‐ both in Taipei, Taiwan (S.‐J.H.); University of Colorado Anschutz Medical Campus, Aurora (M.J.L.); Advanced Medical Research Institute of Canada, Sudbury, ON (J.E.M.); Tartu University Hospital, Tartu, Estonia (A.P.); Vaccine Research Center, University of Tampere, Tampere, Finland (T.V.); Department of Dermatology, Aichi Medical University, Nagakute, Aichi, Japan (D.W.); and Centro de Referencia de Imunobiológicos Especiais, Universidade Federal de São Paulo, São Paulo (L.W.).” | |
| Notes |
We used the available data for efficacy by age ≥ 60 years (a total of 8,122 participants) and contacted the authors requesting adverse events by age, however the data were not provided. We therefore used the adverse events published for ≥ 50 years (a total of 15,411 participants). A total of 16,160 participants were enrolled, of which 749 were excluded from the efficacy analyses, mostly due to deviations from Good Clinical Practice standards at 2 study centres (involving 726 participants). The remaining 15,411 participants constituted the total vaccinated cohort for analysis, of which 14,759 (95.8%) were included in the modified vaccinated cohort, however we did not consider this last cohort since we used ITT analysis. Efficacy analysis only used data from participants aged 60 and over. Most participants received 2 doses of the study vaccines (95.6% of herpes zoster subunit vaccine recipients and 96.4% of placebo recipients). "A reactogenicity subgroup of participants. This subgroup included all participants who were 70 years of age or older and randomly selected participants in the two other age groups (50 to 59 years and 60 to 69 years). The participants rated the intensity of the solicited reactions on a scale from 0 (absent) to 3 (preventing normal everyday activities). Unsolicited adverse events were recorded for 30 days after each dose. Serious adverse events were recorded in all participants for up to 12 months after the second dose. Such events that were considered to be related to the study vaccine or study participation, any events resulting in death, and potentially immune‐mediated diseases were evaluated in all participants over the entire study period. (A full list of potentially immune‐mediated diseases is provided in the Supplementary Appendix.)" We contacted the authors of this study asking for details about why the participants did not receive dose 2. We received a response, but the authors could not provide this information because "the ZOE‐50 study, which was the subject of the NEJM report, is still ongoing and consequently blinded at the subject level. Therefore, information on the specific reasons for non‐receipt of the second vaccine or placebo dose is not presently available". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly assigned participants in a 1:1 ratio to receive either vaccine or placebo using an online centralized randomization system" |
| Allocation concealment (selection bias) | Unclear risk | Despite the sequence and random number generation being appropriate, there were no details about allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Because the appearance of the reconstituted HZ/su vaccine differed from the placebo solution, injections were prepared and administered by study staff who did not participate in any study assessment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigators, participants, and those who were responsible for the evaluation of any study end point were unaware of whether vaccine or placebo had been administered" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No clear participant flow; the number of participants randomised to each group is not described for all outcomes |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results. |
| Other bias | Unclear risk | Insufficient information |
Lal 2018.
| Methods |
Study design: phase 3, randomised, multicentre, open‐label Duration: 12 months post‐dose 2 |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA and Estonia Number: 354 participants; treatment (N = 235), control (N = 119) Participants' health status: healthy participants Age: mean ˜ 64.2 years Sex: 69.5% female Other relevant information: aged ≥ 50 years Majority of Caucasian/European (understood to be white) Exclusion criteria "Female participants had to be of non‐child bearing potential or have a negative pregnancy test on the day of vaccination and meet the contraceptive requirements as outlined in the protocol. Adults were excluded from participation in the study if they had taken any investigational or non‐registered product other than the study vaccine, were administered or planned to receive a live or non replicating vaccine for the protocol‐specified time period, had a history of HZ, received previous vaccination against varicella or HZ, or had a history of reaction or hypersensitivity likely to be exacerbated by any component of the vaccine. Chronic administration of immunosuppressants or other immune‐modifying drugs within 6 months prior to the first vaccine dose, or any confirmed or suspected immunosuppressive or immunodeficient condition resulting from disease or immunosuppressive/cytotoxic therapy also resulted in exclusion." |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | Immunogenicity, reactogenicity, and safety following administration of 2 HZ/su doses at intervals longer than 2 months (2 doses 6 months apart and 2 doses 12 months apart) | |
| Funding sources | GlaxoSmithKline Biologicals SA | |
| Conflicts of interest | “LC, BG, LO, CVA are employees, and TCH and HL former employees, of the GSK group of companies. BG, TCH, HL, LO hold shares in the GSK group of companies as part of their current or former employee remuneration. HL is employed by Pfizer Inc and receives stock as part of his remuneration. TCH is the co‐inventor of a patent application related to the vaccine used in this study and is currently a consultant for the GSK group of companies. AP declares that she has no conflict of interest.” | |
| Notes | For data analyses, only clinical safety outcomes were used. The intensity of all AEs was graded on a scale from 1 to 3. Grade 3 solicited symptoms were defined as ‘‘preventing normal every day activity” (pain, headache, fatigue, gastrointestinal symptoms, myalgia, shivering); surface diameter > 100 mm (redness/swelling); tympanic/oral/axillary temperature > 39.0 °C (fever). Grade 3 unsolicited AEs were also defined as ‘‘preventing normal, every day activities”. All solicited local reactions were considered causally related to vaccination. The causality of all other AEs was assessed by the investigator. We contacted Dr Lal requesting details about the reasons for the dropouts and if there was any consent withdraw. He promptly sent us a table with these data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1:1, to receive 2 doses of HZ/su according to a 0, 2‐month (group [Gr] 0–2), 0, 6‐month (Gr 0–6) or 0, 12‐month (Gr 0–12) schedule, using an online centralized randomisation system" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All of the adverse events listed in the methods section were described in the results. |
| Other bias | Unclear risk | Insufficient information |
Levin 2000.
| Methods |
Study design: RCT, non‐blinded Duration: 36 months postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA Number: 167 participants; treatment (N = 82), control (N = 85) Participants' health status: healthy participants with a history of varicella but not herpes zoster Age: mean ˜ 66 years Sex: ˜ 55% female Other relevant information: aged 55 to 89 years Exclusion criteria Immunosuppressive illness or medication |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Confirmed herpes zoster | |
| Purpose of the study | "To compare a live attenuated varicella vaccine versus heat‐inactivated varicella vaccine in relation the confirmed cases of HZ and immunogenicity in individuals aged 55‐89 years" | |
| Funding sources | Merck Research Laboratories, West Point, PA, USA | |
| Conflicts of interest | Not described | |
| Notes | Author answered our email and provided data for 1 clinical outcome. Most outcomes evaluated were immunologic. There is a misspelling of an author name on the paper: Dr Levin is referenced as Dr Levine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Unclear risk | Insufficient information |
Levin 2018.
| Methods |
Study design: phase 3, randomised, placebo‐controlled, blinded (participant, investigator, sponsor) Duration: 28 days following any vaccination and 4 months for serious AEs throughout the study (September 2015 to January 2016) |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA, 38 centres Number: 882 participants; treatment (N = 440), control (N = 442) Participants' health status: healthy participants with a history of varicella or residence in a VZV‐endemic country for 30 years Age: mean ˜ 61 years Sex: ˜ 59.9% female Other relevant information: aged ≥ 50 years ˜ 85.2% Caucasian (understood to be white) Exclusion criteria "Subjects were excluded if they had a history of: hypersensitivity to vaccine components; herpes zoster or prior receipt of any varicella or zoster vaccine; receipt of an influenza vaccine for the 2015–2016 influenza season; or other conditions that could influence the immunogenicity and safety assessments of either vaccines" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | Evaluate the immunogenicity, safety, and tolerability of 1 dose of LZV administered concomitantly with IIV4 | |
| Funding sources | Merck Sharp & Dohme Corp | |
| Conflicts of interest | Employees of Merck Sharp & Dohme Corp (UKB, JG, JM, JES, EB, ZP). Employees may hold stock or stock options, or both, in the company. All authors have been investigators for the sponsor. |
|
| Notes | IIV4 (open‐label) was administered in the left arm for all participants on day 1. IIV4 ‐ inactivated quadrivalent influenza vaccines. "IIV4 for the 2015–2016 influenza season was obtained from a commercial source and provided to the study sites as open‐label inventory (Fluzone Quadrivalent vaccine; Sanofi Pasteur, Swiftwater, PA, USA" All injection site adverse events were considered vaccine‐related. We used ITT analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central randomization procedure" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Blinded (subject, investigator, sponsor)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The lyophilized ZV and placebo were supplied in 0.65‐mL single‐dose vials and stored at 2‐to‐8 C. The ZV and matching placebo were reconstituted with sterile diluent immediately prior to administration, and were indistinguishable from each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinded (subject, investigator, sponsor)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | High risk | The results for adverse events proposed in the methodology were not all presented. |
| Other bias | Unclear risk | Insufficient information |
Maréchal 2018.
| Methods |
Study design: phase 3, open‐label, randomised and controlled Duration: 12 months after second dose |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 9 centres, USA (n = 3), Canada (n = 3), and Estonia (n = 3) Number: 865 participants; treatment (N = 432), control (N = 433) Participants' health status: healthy participants Age: mean 63.2 years Sex: 58.2% female Other relevant information: aged ≥ 50 years 93.8% Caucasian/European (understood to be white) Exclusion criteria "Adults were excluded from participation if they had previously received any pneumococcal, VZV or HZ vaccine, had a history of HZ, were administered or planning to use any investigational or non‐registered product or vaccine or non‐study vaccine from 30 days prior to inclusion in the study through 30 days after the second dose of RZV, had a documented pneumococcal infection within 5 years, had received immunosuppressants or other immune‐modifying drugs for more than 14 consecutive days within 6 months or had received long‐acting immune‐modifying drugs 6 months before first study vaccination. Adults with cerebrospinal fluid leaks, cochlear implants, chronic renal failure, nephrotic syndrome and functional or anatomic asplenia, were also excluded from participation in the study" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | Evaluate the immunogenicity and safety of RZV when the first dose was co‐administered with PPSV23 and compare it to a sequential administration Safety of the study vaccines was assessed as a secondary objective. |
|
| Funding sources | GlaxoSmithKline Biologicals SA | |
| Conflicts of interest | "TCH and HL were employed by the GSK group of companies at the time this study was designed and initiated. TCH and HL received salary and stock as part of their employee remuneration. TCH is co‐inventor of the patent application related to the vaccine used in this study and is currently a consultant for the GSK group of companies. HL is currently employed by Pfizer Inc. and receives salary and stock as part of his compensation. LO was employed by the GSK group of companies until end Feb2018 and is employee of CureVacAG as of March 1st 2018. LO owns GSK stock. CH, IE and DW are employed by the GSK group of companies. LO owns stock options as part of her employee remuneration. CM receives salary from Business & Decision LS for full time in sourcement at the GSK group of companies. MF is employed as Investigator at the Colchester Research Group, which is owned by his wife, Dr Linda Ferguson. PR receives Investigator stipends from Medicor Research Inc. AP and JT have nothing to disclose." | |
| Notes | Regarding the analysis of adverse events of this study, it was possible to compare injection site adverse events between participants who received the RZV at day 0 and those who received the PPSV23 vaccine at day 0.
In the analysis of systemic adverse events, participants who received RZV and PPSV23 at day 0 were compared to those who received PPSV23 vaccine at day 0. ITT analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomized (1:1) using a central randomization system on Internet (SBIR, GSK) to one of the two parallel study arms." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For many outcomes only graphs were presented. |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results. |
| Other bias | Unclear risk | Insufficient information |
Mills 2010.
| Methods |
Study design: RCT, double‐blind, cross‐over Duration: 28 days after each injection |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA, 9 centres Number: 101 participants; treatment (N = 51), control (N = 50) Participants' health status: healthy participants with physician‐documented history of herpes zoster ≥ 5 years prior to screening Age: mean ˜ 67.9 years Sex: ˜ 59% female Other relevant information: aged ≥ 50 years. Only data for participants aged ≥ 60 years were used in this review. ˜ 88.1% Caucasian (understood to be white) Exclusion criteria "Subjects were excluded if they had an episode of HZ <5 years before study entry; ≥ 2 prior episodes of HZ; previous vaccination with any VZV‐containing vaccine; immune deficiency associated with illness or medical treatments; received blood products within 5 months prior to the first study dose through 8 weeks after enrolment; had hypersensitivity or anaphylactic reactions to gelatin or neomycin; currently were using any form of non‐topical antiviral therapy; received any live vaccine 4 weeks prior to the first study dose or during the study period or received any inactivated vaccine 7 days prior to the first study dose or during the study period; or had a history of alcohol or drug abuse." |
|
| Interventions |
SC lyophilised (frozen) live zoster vaccine and 4 weeks later SC placebo SC placebo and SC lyophilised (frozen) live zoster vaccine 4 weeks later Treatment group
Control group
|
|
| Outcomes |
In participants aged ≥ 60 years
|
|
| Purpose of the study | "To determine the safety profile and immunogenicity of zoster vaccine in individuals who experienced a prior episode of herpes zoster" | |
| Funding sources | Merck & Co Inc | |
| Conflicts of interest | Janie Parrino, Xiaoming Li, Kathleen E Coll, Jon E Stek, Katia Schlienger, Ivan SF Chand, Jeffrey L Silber are employees of Merck Research Laboratories, PO Box 1000, North Wales, PA 19454, USA. Other authors have been investigators for the sponsor. Keith S Reisinger has also received speaker fees and consultancy payments from the sponsor. Employees may hold stock or stock options, or both, in the company. | |
| Notes | "The same subject may appear in different categories, but was counted only once in each category" Data were analysed with pooled data from cross‐over arms because separate data were not available. We contacted the author and received a reply. There was no separate analysis for the first arm prior to cross‐over. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, but it was not explained how this was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data from the first arm of this cross‐over study were reported. |
| Selective reporting (reporting bias) | Low risk | All of the adverse events listed in the methods section were described in the results. |
| Other bias | High risk | Cross‐over study |
Murray 2011.
| Methods |
Study design: randomised, double‐blind, placebo controlled Duration: 182 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 46 sites in Canada, Germany, Spain, the UK, and the USA Number: 11,980; treatment (N = 5983), control (N = 5997) Participants' health status: healthy participants Age: mean ˜ 69 years Sex: ˜ 58.7% female Other relevant information: aged ≥ 60 years ˜ 96.2% Caucasian (understood to be white) Exclusion criteria History of hypersensitivity reaction to gelatin, neomycin, or any other component of the vaccine; prior receipt of any varicella or zoster vaccine; live vaccinations from 4 weeks prior to vaccination or expected during the 42‐day postvaccination period; inactivated vaccinations within 7 d prior to vaccination or expected through the 42‐day postvaccination period with the exception of influenza vaccine; intercurrent illness that might interfere with the interpretation of the study or prevent the participant from completion of the study; immune dysfunction caused by a medical condition, use of immunosuppressive therapy; concomitant use of systemic antiviral therapy with activity against herpes viruses; and participation in an investigational drug or vaccine study within 30 d prior to vaccination or expected during the 42‐day postvaccination period |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | 1 or more serious side effect(s) occurring 26 weeks (182 days) after vaccination; vaccine‐related serious side effects, death, injection site adverse events, systemic adverse events; rashes and temperature were only reported if they were considered serious | |
| Purpose of the study | "To evaluate the general safety of zoster vaccine in adults ≥ 60 years old" | |
| Funding sources | Merck Sharp & Dohme Corp | |
| Conflicts of interest | Jon E Stek, Timothy A Sausser, Jin Xu, William W Wang, Ivan SF Chan, Paula W Annunziato, and Janie Parrino are employees of Merck & Co Inc, North Wales, PA, USA. All other authors have been investigators for the sponsor. Keith S Reisinger has also received speaker fees and consultancy payments from the sponsor. Employees may hold stock or stock options, or both, in the company. | |
| Notes | Non‐serious adverse events were not reported. The study reported 1 or more serious side effect(s) occurring 6 weeks (42 days) and 26 weeks (182 days) after vaccination. We included only the data reported for the second monitoring period, i.e. serious adverse event(s) detected at 182 days after vaccination, in our analyses. 36 participants discontinued because of adverse events; 27 participants withdrew consent; 75 participants were lost to follow‐up; 7 participants discontinued because of protocol deviation; and 2 participants were discontinued following physician's decision (both were in the placebo group). No ITT analysis "For all analyses, cross‐treated (i.e. randomised to ZV and received placebo, or randomised to placebo and received ZV) participants were considered according to the vaccine received and not the vaccine assigned" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The ZV and placebo were reconstituted with sterile diluent immediately prior to administration, and were indistinguishable from each other in appearance. Placebo was the vaccine stabiliser of ZV with no live virus." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "An independent data monitoring committee was established for continuous safety oversight during the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | The serious adverse events prespecified in the methods section were presented in the results. |
| Other bias | Unclear risk | Insufficient information |
NCT00886613.
| Methods |
Study design: phase 3, randomised, double‐blind Duration: 28 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: not provided Number: 120 participants; treatment (N = 80), control (N = 40) Participants' health status: healthy participants with prior history of varicella Age: between 60 and 88 years (mean not available) Sex: 61.7% female Other relevant information: aged ≥ 60 years Exclusion criteria Individuals with hypersensitivity reaction to any vaccine component, prior history of herpes zoster, have received any varicella or zoster vaccine including Zostavax, have a history of immunosuppression caused by disease, corticosteroids, cancer therapy, or organ transplant, have an active cancer, have received or will receive a live virus vaccine or an inactivated virus vaccine 4 weeks prior to participating in study (with the exception of influenza vaccine), and bedridden or homebound |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Adverse events 1 to 28 days post‐any vaccine dose | |
| Purpose of the study | A study to evaluate immunity to varicella zoster virus after immunisation with V212 vaccine or Zostavax (V212‐003) | |
| Funding sources | Merck Sharp & Dohme Corp | |
| Conflicts of interest | Not described | |
| Notes | HLZV (heat‐treated LZV) The data from this study where LZV and placebo were compared were evaluated in comparison 1 (LZV versus placebo). ITT analyses NCT00886613 Data were taken from clinicaltrials.gov/ct2/show/results/NCT00886613 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events proposed in the methodology were presented in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
NCT01505647.
| Methods |
Study design: phase 3, randomised, double‐blind Duration: 182 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: not provided Number: 498 participants; treatment (N = 331), control (N = 166) Participants' health status: healthy participants with history of varicella or residence in a VZV‐endemic area for ≥ 30 years. Females of reproductive potential must have a negative pregnancy test and must agree to use acceptable methods of birth control. Age: mean 62.8 years Sex: 59.2% female Other relevant information: aged ≥ 50 years Exclusion criteria History of hypersensitivity reaction to any vaccine component, prior receipt of any varicella or zoster vaccine, prior history of herpes zoster, have recently had another vaccination, pregnant or breastfeeding, use of immunosuppressive therapy, known or suspected immune dysfunction, concomitant antiviral therapy |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | Safety and immunogenicity of LZV made with an Alternative Manufacturing Process (AMP) compared to LZV. | |
| Funding sources | Merck Sharp & Dohme Corp | |
| Conflicts of interest | Not described | |
| Notes | Live attenuated zoster vaccine AMP ‐ live attenuated zoster vaccine manufactured with an alternative process Data were taken from clinicaltrials.gov/ct2/show/results/NCT01505647. ITT analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masking: Double (Participant, Investigator)", but the masking process is not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events proposed in the methodology were presented in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
NCT02052596.
| Methods |
Study design: phase 3, open‐label RCT Duration: February 2014 to April 2016 |
|
| Participants |
Inclusion criteria Setting: outpatient Country: USA Number: 830 participants; treatment (N = 412), control (N = 418) Participants' health status: healthy participants, females of non‐childbearing potential Age: mean 63.3 years Sex: 53.9% female Other relevant information: aged ≥ 50 years 86.9% Caucasian (understood to be white) Exclusion criteria Participants were excluded if "use of any investigational or non‐registered product (drug or vaccine) other than the study vaccines within 30 days preceding the first dose of study vaccine, or planned use during the study period; chronic administration (defined as more than 14 consecutive days) of immunosuppressants or other immune‐modifying drugs within six months prior to the first vaccine dose (for corticosteroids, this will mean prednisone ≥ 20 mg/day, or equivalent). A prednisone dose of < 20 mg/day is allowed. Inhaled, topical and intra‐articular corticosteroids are allowed; administration or planned administration of a vaccine not foreseen by the study protocol within the period starting 30 days before the first dose of study vaccine(s) and ending 30 days after the last dose of study vaccine. This includes any type of vaccine such as (but not limited to) live, inactivated and subunit vaccines (e.g. inactivated and subunit influenza vaccines); administration of long‐acting immune‐modifying drugs (e.g. infliximab) within six months prior to the first vaccine dose or expected administration at any time during the study period; concurrently participating in another clinical study, at any time during the study period, in which the person has been or will be exposed to an investigational or a non‐investigational vaccine/product (pharmaceutical product or device); previous vaccination against VZV or herpes zoster and/or planned administration during the study of an herpes zoster or VZV vaccine (including an investigational or non‐registered vaccine) other than the study vaccine; History of herpes zoster; vaccination against diphtheria, or tetanus in the last 5 years or planned vaccination against diphtheria or tetanus during the study period, other than the study vaccine(s); administration of a combined tetanus, diphtheria and pertussis (Tdap) vaccine at any time prior to study entry; any confirmed or suspected immunosuppressive or immunodeficient condition resulting from disease (e.g., malignancy, human immunodeficiency virus [HIV] infection) or immunosuppressive/cytotoxic therapy (e.g., medications used during cancer chemotherapy, organ transplantation or to treat autoimmune disorders); history of any reaction or hypersensitivity likely to be exacerbated by any component of the vaccines including prior severe allergic reaction following tetanus‐toxoid, diphtheria‐toxoid or pertussis‐containing vaccine; hypersensitivity to latex. Note: The investigational herpes zoster/su vaccine does not contain latex; acute disease and/or fever at the time of enrolment. Fever is defined as temperature ≥ 37.5°C /99.5°F by oral route. The preferred route for recording temperature in this study will be oral. People with a minor illness (such as mild diarrhoea, mild upper respiratory infection) without fever may, be enrolled at the discretion of the investigator; administration of immunoglobulins and/or any blood products within the 3 months preceding the first dose of study vaccine or planned administration during the study period; pregnant or lactating female; female planning to become pregnant or planning to discontinue contraceptive precautions before 2 months after the last dose of study vaccine; any condition which, in the opinion of the investigator, prevents the person from participating in the study. Any condition which, in the judgment of the investigator, would make intramuscular (IM) injection unsafe; encephalopathy (e.g. coma, decreased consciousness, prolonged seizures) not attributable to an identifiable cause within 7 days of administration of a previous pertussis antigen‐containing vaccine; progressive or unstable neurologic disorder; history of Arthus‐type hypersensitivity reaction following a prior dose of a tetanus‐toxoid containing vaccine within the last 10 years; history of Guillain‐Barré syndrome within 6 weeks of receipt of a prior vaccine containing tetanus toxoid." |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | "The purpose of this study is to assess immunogenicity, reactogenicity and safety of GSK Biologicals' HZ/su vaccine when its first dose is co‐administered with the Boostrix® vaccine in adults aged 50 years or older compared to administration of vaccines separately." | |
| Funding sources | GlaxoSmithKline | |
| Conflicts of interest | Not described | |
| Notes | ITT analyses Grade 3 pain was pain that prevented normal activity. Grade 3 redness/swelling was redness/swelling spreading beyond (>) 100 mm. Assessed solicited general symptoms were fatigue, gastrointestinal (symptoms included nausea, vomiting, diarrhoea, and/or abdominal pain), headache, myalgia, shivering, and fever (defined as oral, axillary, rectal, or tympanic temperature equal to or above 37.5 °C). Any = occurrence of the symptom regardless of intensity grade. Grade 3 symptoms = symptoms that prevented normal activity. Grade 3 fever = temperature above 39.0 °C. Related = general symptom assessed by the investigator as causally related to vaccination. The reason for withdrawal for 1 participant was incorrectly entered into the electronic case report form as “lost due to Crohn’s disease”. The person withdrew from the study due to a combination of irritable bowel syndrome and time constraints associated with employment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label RCT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label RCT |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participant flow not clear: "Not all subjects who were enrolled started the study due to elimination from statistical analyses or no vaccination received." |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were described in the results. |
| Other bias | Unclear risk | Insufficient information |
Oxman 2005.
| Methods |
Study design: randomised, placebo controlled, double‐blind Duration: at least 7 years of surveillance for herpes zoster |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 22 sites in the USA Number: 38,546 participants; treatment (N = 19,270), control (N = 19,276) Participants' health status: healthy participants with history of varicella or had resided in the continental USA for at least 30 years Age: mean 69 years Sex: ˜ 59% male Other relevant information: aged ≥ 60 years 95.4% Caucasian (understood to be white) Exclusion criteria "Immunocompromised persons and those unable to adhere to the study protocol" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes |
|
|
| Purpose of the study | "To determine whether vaccination with a live attenuated varicella‐zoster virus vaccine would decrease the incidence, severity, or both of HZ and postherpetic neuralgia in adults 60 years of age or older" | |
| Funding sources | "Supported by the Cooperative Studies Program, Department of Veterans Affairs, Office of Research and Development; by a grant from Merck (to the Cooperative Studies Program); and by a grant from the James R. and Jesse V. Scott Fund for Shingles Research (to Dr. Oxman). The vaccine and placebo used for the study were supplied by Merck; famciclovir was supplied by SmithKline Beecham and Novartis Pharmaceuticals" | |
| Conflicts of interest | "Supported by the Cooperative Studies Program, Department of Veterans Affairs, Office of Research and Development; by a grant from Merck (to the Cooperative Studies Program); and by a grant from the James R. and Jesse V. Scott Fund for Shingles Research (to Dr. Oxman)." "Drs. Crawford, Gershon, Griffin, Levin, Schmader, and Wright report having received consultation fees, lecture fees, or honoraria from Merck; Drs. Betts, Greenberg, Kauffman, Levin, Weinberg, and Wright report having received grant support from Merck; Drs. Annunziato, C.Y. Chan, I.S.F. Chan, Crawford, Harbecke, Keller, Silber, Simberkoff, and Wang and Ms. Williams report holding equity in Merck; Drs. Annunziato and Levin report having partial interests in relevant patents; and Drs. Annunziato, C.Y. Chan, I.S.F. Chan, Keller, Silber, and Wang are employees of Merck." | |
| Notes | "Zoster vaccine and placebo were lyophilised, held frozen at ‐15°C until reconstituted with sterile water, and administered within 30 minutes" 132 participants withdrew from the study and 113 were lost to follow‐up. 1588 participants died during the study, but it was not stated whether these were related to the protocol or not. Only a subgroup of participants had a safety assessment (zoster vaccine N = 3345; placebo N = 3271), being the adverse event sub study. This study performed 2 ITT analyses, with all individuals developing herpes zoster and with only those who developed herpes zoster after 30 days from the vaccine injection (modified ITT). For the meta‐analysis we considered the modified ITT. There was a break in surveillance for cases of herpes zoster of approximately 15 months between the completion of the Shingles Prevention Study surveillance in September 2003 and resumption of follow‐up in the Short‐Term Persistence Substudy in December 2004. Beginning in October 2005, open‐label zoster vaccine was offered without charge to Shingles Prevention Study placebo recipients. Placebo recipients enrolled in the Short‐Term Persistence Substudy completed the study upon receiving the zoster vaccine, since they could then no longer serve as unvaccinated controls. The Short‐Term Persistence Substudy participants who were zoster vaccine recipients in the Shingles Prevention Study continued to be followed until the initiation of the Long‐Term Persistence Substudy in March 2006. The 2012 publication evaluated the effectiveness of the vaccine for up to 7 years after the participants had been vaccinated. However, the data available in this publication report different dates for the collection of outcomes in the intervention and the placebo groups. The data from the zoster vaccine group are from December 2004 to March 2006 (16 months). The data from the placebo group are reported only from December 2004 to September 2005 (10 months), because in October 2005 the zoster vaccine was also offered to participants in the placebo group, as stated above. We contacted the authors of this study requesting data corresponding to the period from December 2004 to September 2005 (10 months) for both groups (vaccine and placebo). The authors replied to our email but did not provide this information, suggesting instead that we should "assume a uniform rate of events and calculate the estimated number of cases from that". We followed the advice received and calculated the incidence by inferring herpes zoster incidence data, but since these data lost their reliability, we decided not include them in Table 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Each study site received randomly ordered vials of zoster vaccine and placebo in separate boxes for each age stratum" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All other study personnel were blinded to study treatment assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Since the reconstituted zoster vaccine had a different appearance from the placebo, reconstitution and administration were performed by technicians who did not otherwise interact with participants, evaluate outcomes or adverse events, answer the telephone or enter study data." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events proposed in the methodology were presented in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
Schwarz 2017.
| Methods |
Study design: phase 3, randomised, open‐label Duration: 12 months after the second dose |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 20 centres in Canada (n = 2), Germany (n = 15), and the USA (n = 3) Number: 828 participants; treatment (N = 413), control (N = 415) Participants' health status: healthy participants Age: mean 63.4 years Sex: 51.8% female Other relevant information: aged ≥ 50 years 92% Caucasian (understood to be white) Exclusion criteria "They were excluded if they had taken (or planned to take) any investigational or non‐registered drug or vaccine, or any non study vaccine, from 30 days before study inclusion through 30 days after the second dose of HZ/su, had received influenza vaccine or had received long‐term treatment with immunosuppressant drugs or immune‐modifying drugs within 6 months before study inclusion, had received a previous VZV or HZ vaccination, or had a history of HZ" |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | "Primary outcomes: To evaluate the vaccine response rate (VRR) to HZ/su 1 month after the second dose of the vaccine in the coadministration group, to demonstrate the non‐inferiority of anti‐gE geometric mean concentrations (GMCs) after the second dose of HZ/su in coadministration versus control group, and to demonstrate the non‐inferiority of IIV4 immunogenicity in coadministration versus control groups for each vaccine strain by comparing the geometric mean titers (GMTs) of hemagglutination inhibition (HI) antibodies Secondary outcomes: to assess the non‐inferiority of HI antibody seroconversion rates (SCRs) in the coadministration group for each IIV4 strain versus those in control group, to assess IIV4 immunogenicity for each strain in terms of GMT and in terms of the age group–specific (age 50 to 64 or ≥ 65 years) Center for Biologics Evaluation and Research (CBER) criteria for seroprotection rates (SPRs) and SCRs [17], and to evaluate the safety and reactogenicity of both vaccines when co administered or sequentially administered Safety: Solicited local reactions were injection site pain, redness, and swelling; solicited general reactions were arthralgia, fatigue, fever, gastrointestinal symptoms (nausea, vomiting, diarrhoea, abdominal pain), head‐ ache, myalgia, and shivering. Reactogenicity of the herpes zoster subunit (HZ/su) and quadrivalent seasonal inactivated influenza (IIV4) immunizations. Solicited local and general reactions are presented for the total vaccinated cohort. The coadministration (Coadmin) group received the first dose of HZ/su and the IIV4 vaccine on day 0 and the second dose of HZ/su at month 2. The control group received the IIV4 vaccine on day 0, the first dose of HZ/su at month 2, and the second dose of HZ/su dose at month 4. A, Local reactions occurring in the coadministration group within 7 days after coadministration of the first dose of HZ/su (HZ) and IIV4 or in the control group within 7 days after each vaccine was administered separately are shown for each arm. Reactions for the coadministration group were recorded concurrently for 7 days after day 0; reactions for the control group were recorded for 7 days after day 0 for IIV4 and 7 days after the first dose of HZ/su was administered at month 2. B, General reactions occurring within 7 days after the first dose of HZ/su and IIV4 coadministration in the coadministration group or within 7 days after each vaccine was administered separately in the control group. General reactions for the coadministration group were recorded for 7 days after day 0 and were attributable to both vaccines given at the same time; reactions for the control group were recorded for 7 days after day 0 for IIV4 and 7 days after the first dose of HZ/su was administered at month 2, and so were attributable to each vaccine given separately. GI, gastrointestinal symptoms. C, General reactions occurring within 7 days after administration of the second dose of HZ/su in each group. Reactions were recorded in month 2 for the coadministration group and in month 4 for the control group. A local reaction for redness or swelling was recorded if the diameter was ≥20 mm; it was recorded as grade 3 intensity if the diameter was >100 mm. Fever was recorded if the oral temperature was ≥37.5°C; it was recorded as grade 3 intensity if it was >39.0°C. Other general reactions were recorded if they were mild or easily tolerated (no interference in normal daily activity), moderate (discomfort that interfered with normal daily activity), or severe (grade 3; significant discomfort that prevented normal daily activity). Error bars represent 95% confidence intervals" |
|
| Purpose of the study | To evaluate the immunogenicity and safety of an adjuvant herpes zoster subunit vaccine when co‐administered with a quadrivalent seasonal inactivated influenza vaccine (IIV4) in a phase 3, open‐label, randomised clinical trial in adults aged ≥ 50 years | |
| Funding sources | GSK Biologicals | |
| Conflicts of interest | "C. C., M. D., K. G., M. L. F., L. O., and P. V. d. S. are employees, and O. G., T. C. H., and H. L. are former employees of the GSK group of companies. C.C., O.G., T. C. H., L. O., and H. L. hold shares in the GSK group of companies as part of their actual or former employee remuneration. T. C. H. is the co inventor of a patent application related to the vaccine used in this study. T.C.H. is currently paid as a consultant for GSK and that H.L. is employed by Pfizer Inc and holds stock as part of his remuneration. T. F. S. reports receiving personal fees from GSK." | |
| Notes | No ITT analyses As there were many graphics in the journal article publication, we extracted data from clinicaltrials.gov/ct2/show/results/NCT01954251. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised 1:1 to 1 of the 2 parallel study arms using a central Internet‐based randomisation system (GSK Vaccines)" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Diary cards were provided to subjects at each vaccination to collect the solicited and unsolicited adverse events" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many outcomes were reported only as graphs. |
| Selective reporting (reporting bias) | Low risk | All data on effectiveness and adverse events proposed in the methodology were presented in the results for both groups. |
| Other bias | Unclear risk | Insufficient information |
Tyring 2007.
| Methods |
Study design: randomised clinical trial, blinded to participant, investigator, and sponsor Duration: 42 days postvaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 18 sites in the USA, Canada, the UK, Germany, and Belgium Number: 692 participants; treatment (N = 459), control (N = 233) Participants' health status: healthy participants with varicella history‐positive (or resident for more 30 years in a country with endemic VZV infection), herpes zoster history‐negative Age: mean 64.4 years Sex: ˜ 59.3% female Other relevant information: aged ≥ 50 years 92.6% Caucasian (understood to be white) Exclusion criteria History of hypersensitivity reaction to any component of the vaccine; prior receipt of any varicella or zoster vaccine; recent receipt of immune globulin or blood products, or both; live or inactivated vaccine during the study period; known immune dysfunction; concomitant use of antiviral therapy with activity against herpesviruses; and participation in an investigational drug or vaccine study within 30 days prior to vaccination |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Herpes zoster or herpes zoster‐like rash, varicella or varicella‐like rash, local and systemic clinical adverse events and tolerability of both interventions | |
| Purpose of the study | "To compare the safety and tolerability profile of a higher potency zoster vaccine (˜207,000 plaque forming units (PFU)/0.65‐mL dose) with that of a lower potency vaccine (˜58,000 PFU/0,65‐mL dose)" | |
| Funding sources | Merck Research Laboratories | |
| Conflicts of interest | Nickoya D Bundick, Jianjun Li, Ivan SF Chang, Jon E Stek, and Paula W Annunziato are representatives of Merck & Co Inc, West Point, PA, USA. | |
| Notes | Lower‐potency zoster vaccine in this study was similar to vaccine potencies studied in Oxman 2005. Randomised 2:1 ratio to receive 1 injection of each 3 participants were discontinued from the study: 2 participants were lost to follow‐up in the higher‐potency zoster vaccine group, and 1 participant in the lower‐potency zoster vaccine group withdrew consent prior to completion of the follow‐up period, but was included in the safety analyses. No ITT analyses (the participants who completed the study were considered) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded participants, investigator, and sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The 2 potency formulations were indistinguishable in appearance. All participants received a single 0.65 mL subcutaneous injection of either the higher‐potency zoster vaccine or the lower‐potency zoster vaccine. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | The adverse events prespecified in the methods section were reported in the results for both higher‐potency and lower‐potency zoster vaccines. |
| Other bias | Unclear risk | Insufficient information |
Vermeulen 2012.
| Methods |
Study design: randomised, double‐blind, placebo controlled Duration: 6 months after the second vaccination |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 6 sites: USA (n = 5) and the Netherlands (n = 1) Number: 209 participants; treatment (N = 104), control (N = 105) Participants' health status: healthy participants with a history of varicella and no prior herpes zoster Age: mean 69.7 years Sex: more than 60% female Other relevant information: aged ≥ 60 years 97.1% Caucasian (understood to be white) Exclusion criteria "Previous vaccination with any VZV‐containing vaccine, exposure to varicella or HZ within 4 weeks prior to study initiation, immune deficiency associated with illness or medical treatments (e.g. corticosteroids), neoplastic disease, receipt of blood products for 3 months prior to the first study dose, hypersensitivity or anaphylactic reactions to gelatin or neomycin (ingredients of the ZV), used any non‐topical antiviral therapy, or received any inactivated or live vaccine 6 weeks prior to the first study dose or during the study." |
|
| Interventions |
Treatment group
Control group
|
|
| Outcomes | Adverse events, both injection site and/or systemic. Swelling, redness, pain, or tenderness or rash at the injection site, or varicella(‐like) rash or herpes zoster(‐like) rash, any serious adverse events | |
| Purpose of the study | "To examine the safety, tolerability and immunogenicity after 1 and 2 doses of zoster vaccine in adults 60 years of age and older" | |
| Funding sources | Merck Sharp & Dohme Corp | |
| Conflicts of interest | Carrie Freeman, Ira Chalikonda, Jianjun Li, Jeffrey G Smith, Michael J Caulfield, Jon E Stek, Ivan SF Chan, Rupert Vessey, Florian P Schödel, Paula W Annunziato, Katia Schlienger, and Jeffrey L Silber are employees of Merck Sharp & Dohme Corp., and all other authors have been investigators for the sponsor. Dr Levin is a consultant for Merck and shares intellectual property in the zoster vaccine. Employees may hold stock or stock options, or both, in the company. | |
| Notes | The first and second doses were administered 42 days apart (post‐vaccination 1 and post‐vaccination 2). 1 participant in the vaccine group withdrew consent before vaccination. Discontinued after first vaccination (vaccine group): clinical AE = 3; withdrew consent = 1; no participants lost to follow‐up or due to protocol deviation; other = 2 Discontinued after first vaccination (placebo group): clinical AE = 1; withdrew consent = 1; no participants lost to follow‐up; protocol deviation = 1; other = 1 Discontinued after second vaccination (vaccine group): only 1 participant due to clinical AE Discontinued after second vaccination (placebo group): 1 lost to follow‐up and 2 for other reasons No ITT analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomised in a 1:1 ratio to receive 2 doses of either ZV or placebo, according to a computer‐generated, study‐centre specific allocation schedule" |
| Allocation concealment (selection bias) | Low risk | "Allocation numbers were assigned sequentially by the study site personnel to subjects who met the study eligibility criteria, beginning with the lowest number available at the study centre, after informed consent and medical history had been obtained. The allocation schedule was generated by a sponsor statistician not otherwise associated with the ZV program" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The subject, investigator, clinical study site personnel, and sponsor personnel directly involved in the study were blinded to whether the subject received zoster vaccine or placebo. They remained blinded until all subjects completed the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The clinical materials were prepared by an unblinded vaccine coordinator at each clinical site, because of differences in the turbidity of the study vaccine and placebo. Each vial of vaccine or placebo was labelled with a subject‐specific allocation number. The unblended vaccine coordinator reconstituted the study vaccine/placebo and wrapped the syringe in an opaque label containing subject allocation number and time of reconstitution. The unblinded vaccine coordinator did not have any contact with the subject and did not disclose the contents of the syringe to the person administering the study vaccine/placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All adverse events prespecified by the authors were described in the results for both vaccinations. |
| Other bias | Unclear risk | Insufficient information |
Vesikari 2013.
| Methods |
Study design: phase 3, randomised, open‐label Duration: 12 months after the last dose |
|
| Participants |
Inclusion criteria Setting: outpatient Country: 24 centres: Finland (n = 6), Germany (n = 13), Italy (n = 2), Spain (n = 2), and the Netherlands (n = 1) Number: 759 participants; treatment (N = 506), control (N = 253) Participants' health status: healthy participants with either a history of varicella or > 30 years residency in a country with endemic VZV infection Age: mean 76.1 years Sex: ˜ 56% female Other relevant information: aged ≥ 70 years Exclusion criteria "Individuals were excluded if they had: a history of herpes zoster, previous varicella or herpes zoster vaccination, exposure to varicella or herpes zoster during the preceding 4 weeks, fever (oral temperature 38.3°C) during the preceding 72 hours, live virus vaccination during the preceding 4 weeks and inactivated vaccination during the preceding 2 weeks." |
|
| Interventions |
Treatment groups
Control group
|
|
| Outcomes | Adverse events, immediate and not immediate, both at injection site and/or systemic:
|
|
| Purpose of the study | "The primary objective of the study was to demonstrate that a second dose of HZ vaccine, administered 1 mo or 3 mo after the first dose, elicits superior VZV antibody titres 4 weeks after vaccination compared with the first dose" "Secondary objectives of the study were to compare VZV antibody titres 12 mo after completion of each two‐dose schedule with those 12 mo after a single dose, and to describe the safety profile of all three HZ vaccination schedules" |
|
| Funding sources | Sanofi Pasteur MSD | |
| Conflicts of interest | "R.H. has received financial support from Sanofi Pasteur MSD for travel and accommodation costs related to meetings for the study; he has also participated in a Zostavax advisory board in Germany. The institutions of T.V. and H.C.R. received a grant from Sanofi Pasteur MSD for participating in the study; H.C.R.’s institution has also received payment for lectures organized by several pharmaceutical companies and academic institutions. G.I. has previously participated at speaker’s bureaus and advisory board meetings sponsored by GSK, Pfizer, Sanofi Pasteur and Sanofi Pasteur MSD and has received research funding as principal investigator from Crucell Berna, GSK, Pfizer, Sanofi Pasteur and Sanofi Pasteur MSD. J.M. has no conflicts of interest to declare. S.T. and C.S. are employees of Sanofi Pasteur MSD. A.F. was an employee of Sanofi Pasteur MSD when the study was performed but has since become an employee of Pfizer, a company which does not have any products relating to herpes zoster." | |
| Notes | This was an immunogenicity study. For safety analyses, 1 participant randomised to the 1 month between doses schedule was analysed as receiving the 3‐month schedule. More detailed unpublished data were kindly provided by Sanofi Pasteur MSD SNC. The data for the 3 groups were pooled for the period of the first vaccination. Randomised 1:1:1 ratio to receive: 1 injection only; 2 injections with 1 month between doses (day 28 to 35); and 2 injections with 3 months between doses (day 81 to 97) "Seventeen participants withdrew from study due to adverse events, of whom ten withdrew within 28 d after vaccination" The injection site reactions were generally mild to moderate in intensity and resolved in 3 to 7 days. 19 participants reported serious adverse events between screening and 12 months after the last vaccine dose. 1 participant reported 2 serious adverse events. None of the serious adverse events was considered to be vaccine‐related by the investigator. Serious adverse events occurred within 28 days of the first vaccine dose in 1.2% of participants (N = 9) and within 28 days of the second dose in 0.9% of participants (N = 4). In 7 participants serious adverse events occurred between 28 days and 12 months after the last dose. Before the study was stopped, 12 participants died, 7 within 12 months of the last vaccination and 5 more than 12 months after the last vaccination. No ITT analysis We asked the authors for the outcomes by age, but they kindly answered that there was no analysis of safety by age group. We only used the data for single doses since the authors state in their conclusion: "The results of this study demonstrate that there is no apparent advantage to administering a second dose of Zostavax on a one month or three month schedule among individuals aged ≥ 70 years". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used "blocks of randomisation" |
| Allocation concealment (selection bias) | Low risk | "The allocation schedule was generated using balanced permuted blocks of randomisation" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Solicited injection‐site reactions (erythema, swelling, and pain) occurring within 4 d of vaccination were recorded by participants in a diary card. Other injection‐site reactions and systemic AEs were recorded in the diary card for up to 28 d following each vaccination" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although participants completed their diary cards themselves, the other adverse events were not blinded for the evaluator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data proposed in the methodology were presented in the results. |
| Other bias | Unclear risk | Insufficient information |
Vink 2017.
| Methods |
Study design: randomised, open‐label Duration: 12 months after the last dose |
|
| Participants |
Inclusion criteria Setting: outpatient Country: Japan, single centre Number: 60 participants; treatment (N = 30), control (N = 30) Participants' health status: healthy participants Age: mean 61.9 years Sex: 50% female Other relevant information: aged ≥ 50 years 100% Asians Exclusion criteria "i) any investigational or non‐registered drug/vaccine within 30 days; ii) any immunosuppressants or immune‐modifying drugs within 6 months before study start; iii) allergic to any vaccine component, iv) history of herpes zoster, v) previously vaccinated against herpes zoster or varicella. vi) underlying illness, pregnancy, or planning to get pregnant" |
|
| Interventions |
Treatment group
Control group
Observation: 50 mg of recombinant VZV gE combined with the AS01B Adjuvant System per dose |
|
| Outcomes |
|
|
| Purpose of the study | "This study was conducted to evaluate the safety and immunogenicity of the HZ/su candidate vaccine in Japanese adults 50 years old or older when HZ/su was administered SC compared to IM" | |
| Funding sources | GlaxoSmithKline Biologicals SA, Rixensart, Belgium and Japan Vaccine Company, Tokyo, Japan | |
| Conflicts of interest | "Peter Vink and Martine Douha are employees of the GlaxoSmithKline group of companies and, as such, are compensated by GSK for work both related and unrelated to the submitted work. Peter Vink receives GSK stock equity as part of his compensation. Himal Lal and Thomas Heineman were employees of GSK and received salary and stock as compensation at the time of the study design, conduct, and interpretation of data and writing of manuscript. Himal Lal is currently an employee of Pfizer. Thomas Heineman is currently an employee of Genocea Biosciences. Masayuki Ogawa and Masahiro Eda are employees of the Japan Vaccine Company. Masanari Shiramoto declares having no potential conflicts of interest." | |
| Notes | ITT analyses In the publication of the results on ClinicalTrials.gov data "per participant" are provided. This is where we obtained the data for analyses. In the journal article the data were published as "per dose" and not "per participant". We extracted the published data from clinicaltrials.gov/ct2/show/results/NCT01777321. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label RCT |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label RCT |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear patient flow |
| Selective reporting (reporting bias) | Low risk | All data that the authors proposed in their methodology were presented in the results. |
| Other bias | Unclear risk | Insufficient information |
AE: adverse event or adverse experiences AMP: Alternative Manufacturing Process AS01: liposome‐based adjuvant system containing the immunoenhancers 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and the saponin QS‐21 (Quillaja saponaria Molina, fraction 21) Adjuvant gE/AS01B: 50 μg purified gE with adjuvant B (1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21) Adjuvant gE/AS01E: 50 μg purified gE with adjuvant E (500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21) AS01B: adjuvant B composed of 1 mg dioleoyl phosphatidylcholine, 250 μg cholesterol, 50 μg MPL, and 50 μg QS‐21 AS01E: adjuvant E composed of 500 μg dioleoyl phosphatidylcholine, 125 μg cholesterol, 25 μg MPL, and 25 μg QS‐21 Elderly or older adults: aged ≥ 60 years old Frozen: −15 °C or colder gE: recombinant subunit VZV composed of glycoprotein E gE/saline: unadjuvanted gE GSK: GlaxoSmithKline HLZV or heat LZV: heat‐treated LZV HZ: herpes zoster HZ/su: herpes zoster subunit vaccine ID: identification IIV4: inactivated quadrivalent influenza vaccines IM: intramuscular ISRs: injection site adverse reactions ITT: intention‐to‐treat Live zoster vaccine AMP: Alternative Manufacturing Process of live attenuated zoster vaccine LZV or ZV: live zoster vaccine (live attenuated Oka varicella zoster virus vaccine) mo: month MPL: immunoenhancer 3‐O‐desacyl‐4′‐monophosphoryl lipid A MSD: Merck Sharp & Dohme Corp NNTB: number needed to treat for an additional beneficial outcome NNTH: number needed to treat for an additional harmful outcome PCR: polymerase chain reaction pfu: plaque‐forming units pIMDs: potential immune‐mediated diseases PPSV23 or pneumo‐23 vaccine: 23–valent pneumococcal polysaccharide vaccine QS‐21: immunoenhancer saponin Quillaja saponaria Molina, fraction 21 RCT: randomised controlled trial Refrigerated: 2 °C to 8 °C RZV or HZ/su or GSK 1437173A: adjuvant recombinant zoster vaccine (contains 50 µg of recombinant VZV glycoprotein E, and the liposome‐based AS01B adjuvant system contains 50 µg of 3‐O‐desacyl‐4′‐monophosphoryl lipid A (MPL) and 50 µg Quillaja saponaria Molina, fraction 21 (QS21)) SAEs: serious adverse events SC: subcutaneously or subcutaneous TDaPV: tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine VZV: varicella zoster virus V212: heat‐treated VZV vaccine Zoster vaccine 1‐mo schedule: ZV 2 doses given 1 month apart Zoster vaccine 3‐mo schedule: ZV 2 doses given 3 months apart
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hayward 1994 | RCT evaluating LZV with no clinical outcome: focus on immunogenicity |
| Hayward 1996 | RCT evaluating LZV with no clinical outcome: focus on immunogenicity |
| Irwin 2007 | RCT; intervention tested was Tai Chi, not LZV |
| Kerzner 2007 | RCT evaluating LZV when administered concomitantly with influenza vaccine |
| Kovac 2018 | RCT, but outcomes and clinical condition not of interest to individuals with herpes zoster (postherpetic neuropathy, autoimmune disease) (RZV) |
| Leroux‐Roels 2012 | RCT evaluating RZV, but the mean of age was outside of our inclusion criteria (means ranged from 55 to 57 years) |
| Macaladad 2007 | RCT evaluating LZV, but the age was outside our range of interest: adults ≥ 30 years of age (adults less than 60 years of age) |
| MacIntyre 2010 | RCT, but the comparison arms were not within our range of interest (LZV + placebo versus LZV + pneumo‐23 vaccine) |
| Patterson‐Bartlett 2007 | RCT evaluating LZV with no clinical outcome: focus on immunogenicity |
| Strezova 2017 | RCT; multicentre, lot‐to‐lot consistency study, with no known systematic difference between comparison groups (Lot A versus Lot B versus Lot C) (RZV) |
| Weinberg 2018 | RCT: LZV versus RZV with no clinical outcome: focus on immunogenicity |
LZV: live zoster vaccine RCT: randomised controlled trial RZV: recombinant zoster vaccine
Characteristics of ongoing studies [ordered by study ID]
NCT02180295.
| Trial name or title | A lot‐to‐lot consistency study to evaluate safety, tolerability, and immunogenicity of inactivated varicella zoster virus (VZV) vaccine in healthy adults (V212‐014) |
| Methods | Allocation: randomised Endpoint classification: safety study Intervention model: parallel assignment Blinding: double‐blind (participant, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | 0 healthy volunteers, 50 years and over, both genders |
| Interventions |
|
| Outcomes | Geometric mean titre of VZV glycoprotein enzyme‐linked immunosorbent assay (gpELISA) antibody titres, number or percentage of participants with a serious adverse experience (time frame: up to 28 days post‐dose 4) |
| Starting date | July 2014 |
| Contact information | Medical Director, Merck Sharp & Dohme Corp |
| Notes | This study was withdrawn prior to enrolment. Sponsor: Merck Sharp & Dohme Corp |
NCT02526745.
| Trial name or title | Safety and immunogenicity study of live attenuated vaccine against herpes zoster in Chinese adults aged 50 years and older |
| Methods | Allocation: randomised Intervention model: parallel assignment Blinding: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 440 participants. Aged 50 to 80 years, both genders, accepts healthy volunteers |
| Interventions |
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | November 2015 |
| Contact information | Beijing Chaoyang District Centre for Disease Control and Prevention |
| Notes | Study evaluated the safety and immunogenicity of live attenuated vaccine in adults aged 50 years and over. Half of the participants received high doses of the vaccine, and the other half received low doses of the vaccine in phase I clinical trial. At the phase II clinical trial, participants were distributed equally into 4 groups (low, middle, high doses of the vaccine and placebo). Completed, but no results posted on ClinicalTrials.gov and no publications identified Sponsor: Beijing Chaoyang District Centre for Disease Control and Prevention |
NCT03116594.
| Trial name or title | Immunogenicity and safety of two lots of NBP608 compared to Zostavax in healthy adult aged 50 and over |
| Methods | Phase 2 and 3 Allocation: randomised Intervention model: parallel assignment Blinding: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 675 healthy participants aged 50 years and over |
| Interventions |
|
| Outcomes | Immunogenicity and safety of NBP608 LZV compared to Zostavax |
| Starting date | January 2014 |
| Contact information | Hee‐Jin Cheong PhD, Korea University Guro Hospital |
| Notes | Completed, but no results posted on ClinicalTrials.gov and no publications identified Sponsor: SK Chemicals Co Ltd |
NCT03120364.
| Trial name or title | Immunogenicity and safety of NBP608 compared to Zostavax in healthy adult aged 50 and over |
| Methods | Phase 3 Allocation: randomised Intervention model: parallel assignment Blinding: double‐blind (participant, investigator) Primary purpose: prevention |
| Participants | 824 healthy participants aged 50 years and over |
| Interventions |
|
| Outcomes | Immunogenicity and safety |
| Starting date | September 2015 |
| Contact information | Hee‐Jin Cheong PhD, Korea University Guro Hospital |
| Notes | Completed (April 2016), but no results posted on ClinicalTrials.gov and no publications identified Primary completion date: December 2015 "Launched for Herpes zoster (In the elderly, Prevention, In volunteers, In adults) in South Korea (SC) before May 2018" adisinsight.springer.com/drugs/800049347 Sponsor: SK Chemicals Co Ltd |
NCT03439657.
| Trial name or title | Immunogenicity and safety study of GSK Biologicals' herpes zoster vaccine GSK1437173A when co‐administered with Prevnar 13 in adults aged 50 years and older |
| Methods | Phase 3 Allocation: randomised Intervention model: parallel assignment Blinding: none (open‐label) Primary purpose: prevention |
| Participants | 912 participants, male or female, aged ≥ 50 years |
| Interventions |
|
| Outcomes |
|
| Starting date | April 2018 |
| Contact information | GSK Clinical Trials, GlaxoSmithKline |
| Notes | "The purpose of this study is to assess immunogenicity and safety of GSK Biologicals' HZ vaccine when its first dose is co‐administered with a pneumococcal polysaccharide conjugate vaccine (Prevnar 13) in adults aged ≥50 years old, as compared to the control group where the two HZ/su doses are administered subsequent to Prevnar 13." Sponsor: GlaxoSmithKline |
AE: adverse event HZ: herpes zoster pfu: plaque‐forming units pIMDs: potential immune‐mediated diseases SAE: serious adverse event VZV: varicella zoster virus
Differences between protocol and review
We eliminated the secondary outcome 'mean duration of vaccine protection'. We added 'dropouts' as a secondary outcome because this relates to the safety of the intervention.
We considered blinding of outcome assessment to be at low risk of bias when participants in double‐blind trials filled out cards received from the investigator themselves.
We considered the adverse event 'death' separately from serious adverse events as a secondary outcome for the review. We based this decision on the importance of death as a concept in both studies and clinical practice.
In Methods > Data collection and analysis > Measures of treatment effect > Continuous data, we added: "we could insert this data into an Additional table".
In Methods > Unit of analysis issues, we used data from cross‐over studies (separated or grouped) when available.
In Methods > Sensitivity analysis, we added cross‐over studies.
In Data collection and analysis > Data synthesis, we changed the text to: "we conducted meta‐analyses using a random‐effects model".
Brenda NG Andriolo was previously known as Brenda NG Silva.
Contributions of authors
Conceived the idea for the review: Anna Gagliardi (AG), Maria Regina Torloni (MT) and Brenda Nazaré Gomes Andriolo (BNGA) Co‐ordinating the review: AG Screening search results: AG, MT, BNGA, Regis Bruni Andriolo (RBA) Organising retrieval of papers: AG, Bernardo GO Soares (BS), Juliana de Oliveira Gomes (JOG), Eduardo Canteiro Cruz (ECC) Screening retrieved papers against inclusion criteria: AG, BS, MT, ECC Appraising quality of papers: AG, BNGA, MT Extracting data from papers: AG, BNGA Writing to authors of papers for additional information: AG, BNGA, MT Providing additional data about papers: AG, BS, JOG, ECC Obtaining and screening data on unpublished studies: AG, MT Data management for the review: AG, BNGA, MT, JOG, RBA, ECC Entering data into Review Manager 5: AG, BNGA, MT, JOG Review Manager 5 statistical data: AG, BNGA, RBA Other statistical analysis not using Review Manager 5: MT, RBA Interpretation of data: AG, BNGA, MT, BS, RBA, JOG Statistical inferences: AG, BNGA, MT Writing the review: AG, BNGA, MT, BS, JOG, RBA, ECC Guarantor for the review: AG Responsible for reading and checking the review before submission: AG, BNGA, BS, MT, JOG, RBA, ECC
Declarations of interest
Anna MZ Gagliardi: none known Brenda NG Andriolo: none known Maria R Torloni: none known Bernardo GO Soares: none known Juliana O Gomes: none known Regis B Andriolo: none known Eduardo C Cruz: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beals 2016 {published data only}
- Beals CR, Railkar RA, Schaeffer AK, Levin Y, Kochba E, Meyer BK, et al. Immune response and reactogenicity of intradermal administration versus subcutaneous administration of varicella‐zoster virus vaccine: an exploratory, randomised, partly blinded trial. Lancet Infectious Diseases 2016;16(8):915‐22. [DOI] [PubMed] [Google Scholar]
Berger 1998 {published data only}
- Berger R, Trannoy E, Holländer G, Bailleux F, Rudin C, Creusvaux H. A dose‐response study of a live attenuated varicella‐zoster virus (Oka strain) vaccine administered to adults 55 years of age and older. Journal of Infectious Diseases 1998;178(Suppl 1):99‐103. [0022‐1899/98/78S1‐0022$02.00] [DOI] [PubMed] [Google Scholar]
- Trannoy E, Berger R, Holländer G, Bailleux F, Heimendinger P, Vuillier D, et al. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose‐response trial. Vaccine 2000;18(16):1700‐6. [PII: S0264‐410X (99) 00510‐1] [DOI] [PubMed] [Google Scholar]
Chlibek 2013 {published data only (unpublished sought but not used)}
- Chlibek R, Bayas JM, Collins H, Pinta MLR, Ledent E, Johann F, et al. Safety and immunogenicity of an AS01 adjuvanted varicella‐zoster virus subunit candidate vaccine against herpes zoster in adults ≥ 50 years of age. Journal of Infectious Diseases 2013;208:1953–61. [DOI] [PubMed] [Google Scholar]
Chlibek 2014 {published data only (unpublished sought but not used)}
- Chlibek R, Smetana J, Pauksens K, Rombo L, Hoek JA, Richardus JH, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella‐zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine 2014;32(15):1745‐53. [DOI] [PubMed] [Google Scholar]
Cunningham 2016 {published data only}
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez‐Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. New England Journal of Medicine 2016;375(11):1019‐32. [DOI] [PubMed] [Google Scholar]
- Ikematsu H, Yamashita N, Ogawa M, Hirano M, Kovac M, Watanabe D. Efficacy, safety and immunogenicity of new adjuvanted herpes zoster subunit vaccine for Japanese over 50 years old and over 70 years old. Kansenshogaku Zasshi 2018;92(2):103‐14. [Google Scholar]
Diez‐Domingo 2015 {published and unpublished data}
- Diez‐Domingo J, Weinke T, Garcia de Lomas J, Meyer CU, Bertrand I, Eymin C, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live‐attenuated vaccine in adults aged ≥ 50 years: a randomised non‐inferiority clinical trial. Vaccine 2015;33(6):789‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Diez‐Domingo J, Weinke T, Kieninger‐Baum D, Eymin C, Thomas S, Sadorged C. A clinical study of a shingles (herpes zoster) vaccine (live) administered by intramuscular or subcutaneous routes in adults aged ≥ 50 years. European Geriatric Medicine 2013;4(Suppl):81–141. [Google Scholar]
Gilderman 2008 {published data only}
- Gilderman LI, Lawless JF, Nolen TM, Sterling T, Rutledge RZ, Fernsler DA, et al. A double‐blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator‐stable formulation of Zostavax. Clinical and Vaccine Immunology 2008;15(2):314‐9. [DOI: 10.1128/CVI.00310-07] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hata 2016 {published data only}
- Hata A, Inoue F, Hamamoto Y, Yamasaki M, Fujikawa J, Kawahara H, et al. Efficacy and safety of live varicella zoster vaccine in diabetes: a randomized, double‐blind, placebo‐controlled trial. Diabetes and Metabolism 2016;33(8):1094–101. [DOI: 10.1111/dme.13038] [DOI] [PubMed] [Google Scholar]
Lal 2015 {published data only}
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez‐Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. New England Journal of Medicine 2015;372(22):2087‐96. [DOI: 10.1056/NEJMoa1501184] [DOI] [PubMed] [Google Scholar]
Lal 2018 {published data only}
- Lal H, Poder A, Campora L, Geeraerts B, Oostvogels L, Abeele CV, et al. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: results of a phase III, randomized, open‐label, multicenter study. Vaccine 2018;36(1):148‐54. [DOI: 10.1016/j.vaccine.2017.11.019] [DOI] [PubMed] [Google Scholar]
Levin 2000 {published and unpublished data}
- Levin MJ, Ellison MC, Zerbe GO, Barber D, Chan C, Stinson D, et al. Comparison of a live attenuated and an inactivated varicella vaccine to boost the varicella‐specific immune response in seropositive people 55 years of age and older. Vaccine 2000;18(25):2915‐20. [PUBMED: 10812235] [DOI] [PubMed] [Google Scholar]
Levin 2018 {published data only}
- Levin MJ, Buchwald UK, Gardner J, Martin J, Stek JE, Brown E, et al. Immunogenicity and safety of zoster vaccine live administered with quadrivalent influenza virus vaccine. Vaccine 2018;36(1):179‐85. [DOI: 10.1016/j.vaccine.2017.08.029] [DOI] [PubMed] [Google Scholar]
Maréchal 2018 {published data only}
- Maréchal C, Lal H, Poder A, Ferguson M, Enweonye I, Heineman TC, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co‐administered with the 23‐valent pneumococcal polysaccharide vaccine in adults ≥ 50 years of age: randomized trial. Vaccine 2018;36(29):4278‐86. [DOI: 10.1016/j.vaccine.2018.05.110] [DOI] [PubMed] [Google Scholar]
Mills 2010 {published data only}
- Mills R, Tyring SK, Levin MJ, Parrino J, Li X, Coll KE, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine 2010;28(25):4204‐9. [DOI: 10.1016/j.vaccine.2010.04.003] [DOI] [PubMed] [Google Scholar]
Murray 2011 {published data only}
- Murray AV, Reisinger KS, Kerzner B, Stek JE, Sausser TA, Xu J, et al. Safety and tolerability of zoster vaccine in adults ≥ 60 years old. Human Vaccines 2011;7(11):1130‐6. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00886613 {published data only}
- NCT00886613. A study to evaluate immunity to varicella zoster virus after immunization with V212 vaccine or zostavax (V212‐003) [A double‐blind, randomized, placebo controlled, parallel group study to evaluate biomarkers of immunity to varicella zoster virus following immunization with V212/heat‐treated varicella‐zoster virus (VZV) vaccine or with ZOSTAVAX in healthy volunteers]. clinicaltrials.gov/ct2/show/NCT00886613 (first received 22 April 2009).
NCT01505647 {unpublished data only}
- NCT01505647. Safety and immunogenicity of zoster vaccine (ZOSTAVAX™) made with an alternative manufacturing process (AMP) (V211‐042 AM1) [A phase III double‐blinded, randomized, multicenter, controlled study to evaluate the safety, tolerability, and immunogenicity of ZOSTAVAX™ made with an alternative manufacturing process (AMP)]. clinicaltrials.gov/ct2/show/study/NCT01505647 (first received 4 January 2012).
NCT02052596 {published data only}
- NCT02052596. Immunogenicity and safety study of GSK Biologicals' herpes zoster vaccine GSK1437173A when co‐administered with Boostrix® in adults aged 50 years and older [Study to assess the immunogenicity and safety of GlaxoSmithKline (GSK) Biologicals' herpes zoster subunit (HZ/su) vaccine (GSK1437173A) when co‐administered with GSK Biologicals' diphtheria, tetanus and pertussis vaccine (Boostrix®) in adults aged 50 years and older]. clinicaltrials.gov/ct2/show/study/NCT02052596 date first submitted: 23 January 2014, date first posted: 3 February 2014.
Oxman 2005 {published data only}
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella‐zoster virus‐specific immune responses in elderly recipients of a herpes zoster vaccine. Journal of Infectious Diseases 2008;197:825‐35. [DOI: 10.1086/528696] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. New England Journal of Medicine 2005;352(22):2271‐84. [PUBMED: 15930418] [DOI] [PubMed] [Google Scholar]
- Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WWB, Zhang JH, et al. Effect of a zoster vaccine on herpes zoster‐related interference with functional status and health‐related quality‐of‐life measures in older adults. Journal of the American Geriatrics Society 2010;58(9):1634‐41. [DOI: 10.1111/j.1532-5415.2010.03021.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short‐term persistence substudy. Clinical Infectious Diseases 2012;55(10):1320–8. [DOI: 10.1093/cid/cis638] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff MS, Arbeit RD, Johnson GR, Oxman MN, Boardman KD, Williams HM, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Annals of Internal Medicine 2010;152(9):545‐54. [PUBMED: 20439572] [DOI] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caufield MJ, et al. Varicella‐zoster virus‐specific immune response to herpes zoster in elderly participants of a clinically effective zoster vaccine. Journal of Infectious Diseases 2009;200(7):1068‐77. [DOI: 10.1086/605611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwarz 2017 {published data only}
- Schwarz TF, Aggarwal N, Moeckesch B, Schenkenberger I, Claeys C, Douha M, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine co‐administered with seasonal influenza vaccine in adults aged 50 years or older. Journal of Infectious Diseases 2017;216(11):1352‐61. [DOI: 10.1093/infdis/jix481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyring 2007 {published data only}
- Tyring SK, Diaz‐Mitoma F, Padget LG, Nunez M, Poland G, Cassidy WM, et al. Safety and tolerability of a high‐potency zoster vaccine in adults ≥ 50 years of age. Vaccine 2007;25(10):1877‐83. [DOI: 10.1016/j.vaccine.2006.10.027] [DOI] [PubMed] [Google Scholar]
Vermeulen 2012 {published data only}
- Vermeulen JN, Lange JM, Tyring SK, Peters PH, Nunez M, Poland G, et al. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults ≥ 60 years of age. Vaccine 2012;30(5):904‐10. [DOI: 10.1016/j.vaccine.2011.11.096] [DOI] [PubMed] [Google Scholar]
Vesikari 2013 {published data only (unpublished sought but not used)}
- Vesikari T, Hardt R, Rümke HC, Icardi G, Montero J, Thomas S, et al. Immunogenicity and safety of a live attenuated shingles (herpes zoster) vaccine (Zostavax®) in individuals aged ≥ 70 years. A randomized study of a single dose vs. two different two‐dose schedules. Human Vaccines and Immunotherapeutics 2013;9(4):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vink 2017 {published data only}
- Vink P, Shiramoto M, Ogawa M, Eda M, Douha M, Heineman T. Safety and immunogenicity of a Herpes Zoster subunit vaccine in Japanese population aged ≥ 50 years when administered subcutaneously vs. intramuscularly. Human Vaccines and Immunotherapeutics 2017;13(3):574‐8. [DOI: 10.1080/21645515.2016.1232787] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Hayward 1994 {published data only}
- Hayward AR, Buda K, Levin MJ. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunology 1994;7(1):31‐6. [PUBMED: 7986334] [DOI] [PubMed] [Google Scholar]
Hayward 1996 {published data only}
- Hayward AR, Buda K, Jones M, White CJ, Levin MJ. Varicella zoster virus specific cytotoxicity following secondary immunization with live or killed vaccine. Viral Immunology 1996;9(4):241‐5. [PUBMED: 8978020] [DOI] [PubMed] [Google Scholar]
Irwin 2007 {published data only}
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of tai chi. Journal of the American Geriatrics Society 2007;55(4):511‐7. [DOI: 10.1111/j.532-5415.2007.01109.x] [DOI] [PubMed] [Google Scholar]
Kerzner 2007 {published data only}
- Kerzner B, Murray AV, Gheng E, Ifle R, Harvey PR, Tomlinson M, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. Journal of the American Geriatrics Society 2007;55(10):1499‐507. [DOI: 10.1111/j.1532-5415.2007.01397.x] [DOI] [PubMed] [Google Scholar]
Kovac 2018 {published data only}
- Kovac M, Lal H, Cunningham AL, Levin MJ, Johnson RW, Campora L, et al. ZOE‐50/70 Study Group. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine 2018;36(12):1537‐41. [DOI: 10.1016/j.vaccine.2018.02.029] [DOI] [PubMed] [Google Scholar]
Leroux‐Roels 2012 {published data only}
- Leroux‐Roels I, Leroux‐Roels G, Frédéric Clement F, Vandepapelière P, Vassilev V, Ledent E, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein E subunit vaccine candidate in young and older adults. Journal of Infectious Diseases 2012;206(8):1280–90. [DOI: 10.1093/infdis/jis497] [DOI] [PubMed] [Google Scholar]
Macaladad 2007 {published data only}
- Macaladad N, Marcano T, Guzman M, Moya J, Jurado F, Thompson M, et al. Safety and immunogenicity of a zoster vaccine in varicella‐zoster virus seronegative and low‐seropositive healthy adults. Vaccine 2007;25(11):2139‐44. [DOI: 10.1016/j.vaccine.2006.11.011] [DOI] [PubMed] [Google Scholar]
MacIntyre 2010 {published data only}
- MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, et al. Concomitant administration of zoster and pneumococcal vaccines in adults ≥ 60 years old. Human Vaccines 2010;6(11):894‐902. [PUBMED: 20980796] [DOI] [PubMed] [Google Scholar]
Patterson‐Bartlett 2007 {published data only}
- Patterson‐Bartlett J, Levin MJ, Lang N, Schödel FP, Vessey R, Weingerg A. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine 2007;25(41):7087‐93. [PUBMED: 17766015] [DOI] [PubMed] [Google Scholar]
Strezova 2017 {published data only}
- Strezova A, Godeaux O, Aggarwal N, Leroux‐Roels G, Lopez‐Fauqued M, Damme PV, et al. A randomized lot‐to‐lot immunogenicity consistency study of the candidate zoster vaccine HZ/su. Vaccine 2017;35(48 Part B):6700‐6. [DOI: 10.1016/j.vaccine.2017.10.017] [DOI] [PubMed] [Google Scholar]
Weinberg 2018 {published data only}
- Weinberg A, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, et al. Comparative immune responses to licensed herpes zoster vaccines. Journal of Infectious Diseases 2018;218(Suppl 2):81‐7. [DOI: 10.1093/infdis/jiy383] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02180295 {published data only}
- NCT02180295. A lot‐to‐lot consistency study to evaluate safety, tolerability, and immunogenicity of inactivated varicella zoster virus (VZV) vaccine in healthy adults (V212‐014). clinicaltrials.gov/ct2/show/NCT02180295 (first received 2 July 2014).
NCT02526745 {published data only}
- NCT02526745. Safety and immunogenicity study of live attenuated vaccine against herpes zoster in Chinese adults aged 50 years and older. clinicaltrials.gov/ct2/show/NCT02526745 (first received 18 August 2015).
NCT03116594 {published data only}
- NCT03116594. Immunogenicity and safety of two lots of NBP608 compared to Zostavax in healthy adult aged 50 and over. clinicaltrials.gov/ct2/show/NCT03116594 (first received 17 April 2017).
NCT03120364 {published data only}
- NCT03120364. Immunogenicity and safety of NBP608 compared to Zostavax in healthy adult aged 50 and over. clinicaltrials.gov/ct2/show/NCT03120364 (first received 19 April 2017).
NCT03439657 {published data only}
- NCT03439657. Immunogenicity and safety study of GSK Biologicals' herpes zoster vaccine GSK1437173A when co‐administered with Prevnar 13 in adults aged 50 years and older. clinicaltrials.gov/ct2/show/NCT03439657 (first received 20 February 2018).
Additional references
Agmon‐Levin 2009
- Agmon‐Levin N, Kivity S, Szyper‐Kravitz M, Shoenfeld Y. Transverse myelitis and vaccines: a multi‐analysis. Lupus 2009;18(13):1198–204. [DOI: 10.1177/0961203309345730] [DOI] [PubMed] [Google Scholar]
Amanna 2007
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. New England Jounal of Medicine 2007;357(19):1903–15. [DOI: 10.1056/NEJMoa066092] [DOI] [PubMed] [Google Scholar]
Aps 2018
- Aps LR, Piantola MA, Pereira SA, Castro JT, Santos FA, Ferreira LC. Adverse events of vaccines and the consequences of non‐vaccination: a critical review. Revista Saude Publica 2018;52(40):1‐13. [DOI: 10.11606/S1518-8787.2018052000384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arvin 2005
- Arvin A. Ageing, immunity, and the varicella‐zoster virus. New England Journal of Medicine 2005;352(22):2266‐7. [PUBMED: 15930416] [DOI] [PubMed] [Google Scholar]
Arvin 1986
- Arvin AM, Kinney‐Thomas E, Shriver K, Grose C, Koropchak CM, Scranton E, et al. Immunity to varicella‐zoster viral glycoproteins, gp I (gp 90/58) and gp III (gp 118), and to a nonglycosylated protein, p 170. Journal of Immunology 1986;137(4):1346‐51. [PUBMED: 3016094] [PubMed] [Google Scholar]
Arvin 1996
- Arvin AM. Varicella‐zoster virus. Clinical Microbiology Reviews 1996;9(3):361‐81. [PUBMED: 8809466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asherson 2006
- Asherson RA. The catastrophic antiphospholipid (Asherson's) syndrome. Autoimmunity Reviews 2006;6(2):64‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldridge 2004
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, el al. Taking a toll on human disease: toll‐like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opinion on Biological Therapy 2004;4(7):1129‐38. [PUBMED: 15268679] [DOI] [PubMed] [Google Scholar]
Balofsky 2010
- Balofsky A, Agmon‐Levin N, Shoenfeld Y. The new H1N1 and HPV vaccines and old fears. Current Opinion in Rheumatology 2010;22(4):431‐6. [DOI: 10.1097/BOR.0b013e32833a43c3] [DOI] [PubMed] [Google Scholar]
Bruijn 2007
- Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59‐adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 2007;26(1):119‐27. [DOI: 10.1016/j.vaccine.2007.10.051] [DOI] [PubMed] [Google Scholar]
Caputo 2019
- Caputo M, Horn J, Karch A, Akmatov MK, Becher H, Braun B, et al. Herpes zoster incidence in Germany ‐ an indirect validation study for self‐reported disease data from pretest studies of the population‐based German National Cohort. BMC Infectious Diseases 2019;19(1):99. [DOI: 10.1186/s12879-019-3691-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC
- Centers for Disease Control, shingles vaccination. www.cdc.gov/vaccines/vpd/shingles/public/shingrix/index.html accessed 24 October 2019.
Cho 2007
- Cho JW, Shin DH, Lee KS. Polymorphism of the IL‐10 gene is associated with susceptibility to herpes zoster in Korea. Journal of Dermatological Science 2007;45(3):213‐5. [PUBMED: 17204399] [DOI] [PubMed] [Google Scholar]
Chung 2016
- Chung WS, Lin HH, Cheng NC. The incidence and risk of herpes zoster in patients with sleep disorders: a population‐based cohort study. Medicine 2016;95(11):e2195. [DOI: 10.1097/MD.0000000000002195] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2007
- Cohen JI, Straus SE, Arvin AM. Varicella‐zoster virus replication, pathogenesis, and management. In: Knipe DM editor(s). Fields’ Virology. 5th Edition. Philadelphia: Lippincott Williams and Wilkins, 2007:2773‐818. [Google Scholar]
Coplan 2004
- Coplan PM, Schmader K, Nikas A, Chan ISF, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the Brief Pain Inventory. Journal of Pain 2004;5(6):344‐56. [DOI: 10.1016/j.jpain.2004.06.001] [DOI] [PubMed] [Google Scholar]
Dworkin 2003
- Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clinical Infectious Diseases 2003;36(7):877‐82. [DOI: 10.1086/368196] [DOI] [PubMed] [Google Scholar]
FDA 2006
- USA FDA approval letter ‐ Zostavax, 25 May 2006. https://wayback.archive‐it.org/7993/20170723093336/https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm132873.htm accessed 24 October 2019.
FDA 2017
- USA FDA approval letter ‐ zoster vaccine recombinant, adjuvanted. 20 October 2017. www.fda.gov/downloads/biologicsblood vaccines/vaccines/approvedproducts/ucm581750.pdf accessed 24 October 2019.
FDA 2018
- USA FDA approval letter ‐ Zostavax. www.fda.gov/vaccines‐blood‐biologics/vaccines/zostavax accessed 24 October 2019.
Garçon 2007
- Garçon N, Chomez P, Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Review of Vaccines 2007;6(5):723‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gatti 2010
- Gatti A, Pica F, Boccia MT, Antoni F, Sabato AF, Volpi A. No evidence of family history as a risk factor for herpes zoster in patients with post‐herpetic neuralgia. Journal of Medical Virology 2010;82(6):1007‐11. [PUBMED: 20419815] [DOI] [PubMed] [Google Scholar]
Gilden 2000
- Gilden DH, Kleinschmidt‐DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella zoster virus. New England Journal of Medicine 2000;342(9):635–45. [PUBMED: 10699164] [DOI] [PubMed] [Google Scholar]
Glenny 2005
- Glenny AM, Altman DG, Sakarovitch C, Deeks JJ, D'Amico R, Bradburn M, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9(26):1‐134. [PUBMED: 16014203] [DOI] [PubMed] [Google Scholar]
Gnann 2002
- Gnann JW Jr, Whitley RJ. Clinical practice: herpes zoster. New England Journal of Medicine 2002;347(5):340‐6. [PUBMED: 12151472] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 16 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gruver 2007
- Gruver AL, Hudson LL, Sempowsk GD. Immunosenescence of ageing. Journal of Pathology 2007;211(2):144–56. [DOI: 10.1002/path.2104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2006a
- Guyatt G, Gutterman D, Baumann MH, Addrizzo‐Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006;129(1):174‐81. [PUBMED: 16424429] [DOI] [PubMed] [Google Scholar]
Guyatt 2006b
- Guyatt G, Vist G, Falck‐Ytter Y, Kunz R, Magrini N, Schünemann H. An emerging consensus on grading recommendations?. ACP Journal Club 2006;144(1):A8‐9. [PUBMED: 16388549] [PubMed] [Google Scholar]
Haanpää 2002
- Haanpää M, Nurmikko T, Hurme M. Polymorphism of the IL‐10 gene is associated with susceptibility to herpes zoster. Scandinavian Journal of Infectious Diseases 2002;34(2):112‐4. [PUBMED: 11928840] [DOI] [PubMed] [Google Scholar]
Hawkes 2015
- Hawkes D, Benhamu J, Sidwell T, Miles R, Dunlop RA. Revisiting adverse reactions to vaccines: a critical appraisal of autoimmune syndrome induced by adjuvants (ASIA). Journal of Autoimmunity 2015;59:77‐84. [DOI: 10.1016/j.jaut.2015.02.005] [DOI] [PubMed] [Google Scholar]
Heymann 2008
- Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: results of a population‐based study in Israel. Infection 2008;36(3):226‐30. [PUBMED: 18454342] [DOI] [PubMed] [Google Scholar]
Hicks 2008
- Hicks LD, Cook‐Norris RH, Mendoza N, Madkan V, Arora A, Tyring SK. Family history as a risk factor for herpes zoster: a case‐control study. Archives of Dermatology 2008;144(5):603‐8. [PUBMED: 18490586] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ikematsu 2018
- Ikematsu H, Yamashita N, Ogawa M, Hirano M, Kovac M, Watanabe D. Efficacy, safety and immunogenicity of new adjuvanted herpes zoster subunit vaccine for Japanese over 50 years old and over 70 years old. Kansenshogaku Zasshi 2018;92(2):103‐14. [Google Scholar]
Jansen 2013
- Jansen JP, Naci H. Is network meta‐analysis as valid as standard pairwise meta‐analysis? It all depends on the distribution of effect modifiers. BioMed Central Medicine 2013;11:159. [DOI: 10.1186/1741-7015-11-159] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jih 2009
- Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Dermato‐Venereologica 2009;89(6):612‐6. [PUBMED: 19997693] [DOI] [PubMed] [Google Scholar]
Johnson 2014
- Johnson RW, Rice ASC. Postherpetic neuralgia. New England Journal of Medicine 2014;371(16):1526‐33. [DOI: 10.1056/NEJMcp1403062] [DOI] [PubMed] [Google Scholar]
Kawai 2014
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ 2014;4(6):e004833. [DOI: 10.1136/bmjopen-2014-004833] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawai 2016
- Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing incidence of herpes zoster over a 60‐year period from a population‐based study. Clinical Infectious Diseases 2016;63(2):221–6. [DOI: 10.1093/cid/ciw296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kensil 1991
- Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molinacortex. Journal of Immunology 1991;146(2):431‐7. [PUBMED: 1987271] [PubMed] [Google Scholar]
Kicinski 2013
- Kicinski M. Publication bias in recent meta‐analyses. PLOS ONE 2013;8(11):e81823. [10.1371/ journal.pone.0081823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langan 2013
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post‐herpetic neuralgia in an older US population: a cohort study. PLOS Medicine 2013;10(4):1‐11. [PUBMED: 23585738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Latour 1997
- Latour J, Abraira V, Cabello JB, López Sánchez J. Investigation methods in clinical cardiology (IV) clinical measurements in cardiology: validity and errors of measurement. Revista Española de Cardiología 1997;50(2):117‐28. [PUBMED: 9091999 ] [DOI] [PubMed] [Google Scholar]
Lay 2015
- Lai YC, Yew YW. Severe autoimmune adverse events post herpes zoster vaccine: a case‐control study of adverse events in a national database. Journal of Drugs in Dermatology 2015;14(7):681‐4. [PUBMED: 26151783] [PubMed] [Google Scholar]
Le Dantec 2015
- Dantec C, Brooks WH, Renaudineau Y. Epigenomic revolution in autoimmune diseases. World Journal of Immunology 2015;5(2):62‐7. [DOI: 10.5411/wji.v5.i2.62] [DOI] [Google Scholar]
Lee 1998
- Lee BW. Review of varicella zoster soroepidemiology in India and South‐East Asia. Tropical Medicine & International Health 1998;3(11):886‐90. [DOI: 10.1046/j.1365-3156.1998.00316.x; PUBMED: 9855401] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lewis 1993
- Lewis JA, Machin D. Intention to treat – who should use ITT?. British Journal of Cancer 1993;68(4):647‐50. [PUBMED: 8398686] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mareque 2019
- Mareque M, Oyagüez I, Morano R, Casado MA. Systematic review of the evidence on the epidemiology of herpes zoster: incidence in the general population and specific subpopulations in Spain. Public Health 2019;167(February):136‐46. [DOI: 10.1016/j.puhe.2018.10.015] [DOI] [PubMed] [Google Scholar]
Marra 2016
- Marra F, Chong M, Najafzadeh M. Increasing incidence associated with herpes zoster infection in British Columbia, Canada. BMC Infectious Diseases 2016;16(1):589. [DOI: 10.1186/s12879-016-1898-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moffat 2007
- Moffat J, Ku CC, Zerboni L, Sommer M, Arvin A. Pathogenesis and the disease consequences of primary infection. In: Arvin A, Campadelli‐Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, et al. editor(s). Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. [PUBMED: 21348076 ] 21348076 [Google Scholar]
NBP608
- SK Chemicals receives permission to market shingles vaccine. http://english.hani.co.kr/arti/english_edition/e_business/813943.html accessed 24 October 2019.
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI: 10.1093/ije/21.5.837] [DOI] [PubMed] [Google Scholar]
Partridge 2009
- Partridge DG, McKendrick MW. The treatment of varicella‐zoster virus infection and its complications. Expert Opinion on Pharmacotherapy 2009;10(5):797‐812. [PUBMED: 19351229] [DOI] [PubMed] [Google Scholar]
Perricone 2013
- Perricone C, Colafrancesco S, Mazor RD, Soriano A, Agmon‐Levin N, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) 2013: unveiling the pathogenic, clinical and diagnostic aspects. Journal of Autoimmunity 2013;47(December):1‐16. [DOI: 10.1016/j.jaut.2013.10.004] [DOI] [PubMed] [Google Scholar]
Pickering 2011
- Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Practice 2011;11(4):397–402. [DOI: 10.1111/j.1533-2500.2010.00432.x] [DOI] [PubMed] [Google Scholar]
Rajesh 1995
- Rajesh K, Gupta RK, Siber GR. Adjuvants for human vaccines ‐ current status, problems and future prospects. Vaccine 1995;13(14):1263–76. [DOI: 10.1016/0264-410X(95)00011-O] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rimland 2010
- Rimland D, Moanna A. Increasing incidence of herpes zoster among veterans. Clinical Infectious Diseases 2010;50(7):1000‐5. [PUBMED: 20178416] [DOI] [PubMed] [Google Scholar]
Sampathkumar 2009
- Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clinic Proceedings 2009;84(3):274‐80. [PUBMED: 19252116 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanford 2010
- Sanford M, Keating GM. Zoster vaccine (Zostavax®) a review of its use in preventing herpes zoster and postherpetic neuralgia in older adults. Drugs and Aging 2010;27(2):159‐76. [1170‐229X/10/0002‐0159/$49.95/0] [DOI] [PubMed] [Google Scholar]
Schmader 2007
- Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. Herpes zoster pain and discomfort on functional status and quality of life in older adults. Clinical Journal of Pain 2007;23(6):490‐6. [PUBMED: 17575488] [DOI] [PubMed] [Google Scholar]
Schmader 2012
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short‐term persistence substudy. Clinical Infectious Diseases 2012;55(10):1320–8. [DOI: 10.1093/cid/cis638] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thomas 2004
- Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster?. Lancet Infectious Diseases 2004;4(1):26‐33. [PUBMED: 14720565] [DOI] [PubMed] [Google Scholar]
Tricco 2018
- Tricco AC, Zarin W, Cardoso R, Veronik A, Khan PA, Nincic V, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta‐analysis. BMJ 2018;363(8173):k4029. [DOI: 10.1136/bmj.k4029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watad 2018
- Watad A, Quaresma M, Bragazzi NL, Cervera R, Tervaert JWC, Amital H, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)/Shoenfeld's syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clinical Rheumatology 2018;37(2):483‐93. [DOI: 10.1007/s10067-017-3748-9] [DOI] [PubMed] [Google Scholar]
Wise 2000
- Wise RP, Salive ME, Braun MM, Mootrey GT, Seward JF, Rider LG, et al. Postlicensure safety surveillance for varicella vaccine. JAMA 2000;284(10):1271‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gagliardi 2010
- Gagliardi AMZ, Gomes SBN, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008858] [DOI] [Google Scholar]
Gagliardi 2012
- Gagliardi AMZ, Silva BNG, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008858.pub2] [DOI] [PubMed] [Google Scholar]
Gagliardi 2016
- Gagliardi AMZ, Silva BNG, Torloni MR, Soares BGO. Vaccines for preventing herpes zoster in older adults. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD008858.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


