Abstract
Background
Age-related macular degeneration (AMD) induces irreversible loss of vision in older people. The exact physiopathology remains unclear, but numerous studies highlight the role of inflammation and multiple risk factors. Recent data show an altered periodontal condition subject to AMD. Periodontal diseases lead to the destruction of tooth-supporting tissues, mainly caused by the periodontal infection inducing a chronic inflammation. Periodontal diseases are known to be associated with several extraoral diseases such as diabetes, polyarthritis (rheumatoid arthritis), cardiovascular disease, and preeclampsia.
Objectives
To assess emerging evidence suggesting an association between periodontitis and AMD.
Methods
To support this review, we performed a literature search using PubMed, Cochrane, and Google Scholar databases, completed by manual searches in periodontology journals. We included only the original studies published before July 2017 reporting data on periodontal diseases and AMD. No restrictions were made on the language.
Results
Persons with AMD showed more periodontal diseases, fewer teeth, and more alveolar bone loss than those without AMD. Also, a significant association was observed between periodontal diseases and AMD, but only in the youngest individuals studied.
Conclusion
According to the studies included in this review, periodontal disease may be a plausible risk factor for AMD and may have a potential role in the earlier stages of this eye disease. Further studies should be encouraged for better understanding of this potential new relationship.
Keywords: age-related macular degeneration, periodontitis, periodontal disease, periodontal medicine, risk factors, systemic inflammation
INTRODUCTION
Periodontal diseases (PDs) are oral pathology that lead to the destruction of tooth-supporting tissues.1 They result from an inappropriate response by the host to a pathogenic bacterial challenge (bacterial biofilm) associated with genetic and environmental risk factors (genetic polymorphisms, tobacco, diabetes, stress, etc).2 Clinically, PDs are characterized by the presence of gingival bleeding (spontaneous or brushing), clinical gingival inflammation, gingival recessions, dental mobility, and loss of clinical attachment (presence of periodontal pockets and alveolar bone resorption).3 In the US, the prevalence of PDs (moderate to severe form) varies from 60% to 70% in adults older than age 65 years.4 In France, more than 50% of the adult population has mild, moderate, or severe periodontitis.5
In age-related macular degeneration (AMD), lesions of the macula at the center of the retina occur on a previously normal eye, inducing irreversible vision loss in older people.6 In industrialized countries, AMD is considered to be the leading cause of visual impairment in older adults.7 The disease is asymptomatic and evolves silently before the appearance of a scotoma (blind spot) in the center of the visual field during advanced AMD. This macular degeneration is subdivided into 2 types: 1) dry or atrophic AMD and 2) wet or exudative AMD.8 The dry type of AMD represents the majority (up to 90%) of cases.9
Presently, the etiology of AMD is not exactly known, and there is no curative treatment available for this disease. However, the management of AMD relies on palliative treatments (laser, dynamic phototherapy, surgery, antivascular endothelial growth factor therapy) and on identification of risk factors.10 In addition to the risk factors already known in its pathogenesis (older age, tobacco use, obesity, polymorphism of the complement factor, diet), recent studies have highlighted the role of systemic inflammatory biomarkers in the pathogenesis of AMD.9,11 Hong et al’s12 meta-analysis revealed that persons with C-reactive protein levels higher than 3 mg/L have a double risk of advanced AMD developing compared with persons having C-reactive protein levels lower than 1 mg/L. Similar observations have been reported by other studies.13,14 Moreover, pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor can promote pathologic changes in the retinal pigment epithelium.9
PDs induce a chronic inflammation and bacteremia in susceptible individuals and have also been associated with elevated systemic biomarkers such as tumor necrosis factor-α, C-reactive protein, IL-1β, and IL-6.15,16 Thus, PDs have been associated with several extraoral diseases such as polyarthritis (rheumatoid arthritis), diabetes, and cardiovascular disease, as well as preterm birth, low birth weight, and preeclampsia.17–20
This article aimed to assess, through a literature review, the emerging potential relationship between PD and AMD.
METHODS
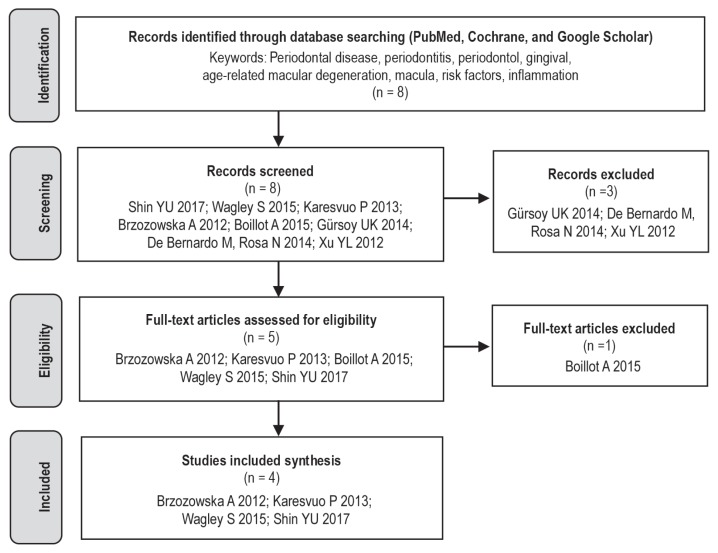
An electronic search was performed of PubMed, Cochrane, and Google Scholar databases by the combination of the following keywords: Periodontal disease, periodontitis, periodontal, gingival inflammation, eye disease, ocular disease, retinal disease, age-related macular degeneration, macular degeneration, and risk factors (Figure 1). A complementary search was made from the bibliographic references of the selected articles.
Figure 1.
Flowchart diagram.
No restrictions were made on the languages. The last search was conducted for all databases on July 2017. Initially, 8 articles were identified. After a thorough analysis of titles and abstracts, 2 articles were removed because they were letters to an editor, and another article was removed because it was out of our study context. Thus, 5 articles were selected for a full-text examination. Later, 1 more article was removed after a thorough reading because it was out of our study context.
RESULTS
From 8 articles identified, 4 articles were selected for the analysis.
The studies were conducted in Poland, Finland, the US, and Korea. Some characteristics of these studies are presented chronologically (Table 1).
Table 1.
Characteristics of studies included in review
| Source | Country | No. of participants/age/race | Methods | Main results |
|---|---|---|---|---|
| Brzozowska & Puchalska-Niedbał,21 2012 | Poland | 56 patients/45–90 y/white | Cross-sectional study Dental and periodontal checkups + radiography |
Patients with AMD had several inflammatory lesions in their oral cavity, which were mainly located in the periodontium (53.17%) |
| Karesvuo,22 2013 | Finland | 1751 patients/≥ 30 y/white | Cross-sectional study; national population-based Health 2000 survey Dental and periodontal checkups + radiography + laboratory analyses Self-report on AMD with questionnaire + interview |
Persons with AMD had fewer teeth (p < 0.001) and more alveolar bone loss (p = 0.004) compared with participants without AMD. Also, a significant independent association was found between alveolar bone loss and AMD in men ( p = 0.013) |
| Wagley,23 2015 | US | 5887 patients/> 40 y/white and nonwhite | Cross-sectional study; US NHANES III, 1988–1994 2 groups: ≤ 60 y, > 60 y Diagnosis of AMD: Ocular fundus photographs Diagnosis of PD: > 10% of sites with > 3 mm of CAL Multivariate regression analysis stratified with AMD confounders and risk factors |
PD is independently associated with AMD in those aged 40–60 y (odds ratio = 1.96, p = 0.006) but not for subjects > 60 y (p = 0.120) |
| Shin,24 2017 | Korea | 13,072 patients/> 40 y/Asian | Cross-sectional study; Korean NHANES, 2008–2010 and 2012 2 groups: ≤ 62 y; > 62 y Diagnosis of AMD: Ocular fundus photographs Diagnosis of PD: CP Index score 3 and 4 Mild PD: Score 3 (PPD = 4–5 mm) Severe PD: Score 4 (PPD ≥ 6 mm) Multivariate regression analysis stratified with AMD confounders and risk factors |
Persons with AMD in ≤ 62 y age group had more PD than participants without AMD (p = 0.031) Severe PD is independently associated with AMD in individuals 40–62 y No association between PD (both mild and severe) and AMD in participants > 62 y old |
AMD = age-related macular degeneration; CAL = clinical attachment level; CP = community periodontal; NHANES = National Health and Nutrition Examination Survey; PD = periodontal disease; PPD = periodontal pocket depth; + = plus.
In 2012, Brzozowska and Puchalska-Niedbał21 were the first to report about periodontal status and AMD. In their study, 56 patients with AMD between ages 45 and 90 years were recruited. Dental and periodontal checkups found many lesions in their oral cavities, which were mainly located in the periodontal tissues.21
In 2013, Karesvuo et al22 analyzed the relationship between AMD and oral status using 1751 individuals aged 30 years and older. The AMD diagnosis was obtained with a self-report using questionnaires before dental and periodontal examination. In this population, the prevalence of AMD was 3.1% (54 individuals). The authors reported that persons with AMD had fewer teeth (p < 0.001) and more alveolar bone loss (p = 0.004) compared with participants who did not have AMD.22 Also, they observed a significant association between alveolar bone loss and AMD in men (p = 0.013).22
In 2015, Wagley et al23 published their study results that focused on AMD and PD. They used data of 5887 participants aged 40 years or older from the US Third National Health and Nutrition Examination Survey (NHANES III) data (1988–1994). An AMD diagnosis was obtained using ocular fundus photographs according to international protocol.8 In this study, PD was defined according to loss of clinical attachment level (> 10% of sites with > 3 mm of loss of attachment).23 The participants were stratified into 2 groups: The “youngest group” (40–60 years) and the “oldest group” (> 60 years). After a logistic regression model controlled for AMD confounders and risk factors and was stratified by age, the authors observed that PD was independently associated with AMD only in the youngest group (odds ratio = 1.96, 95% confidence interval = 1.22–3.14, p = 0.006).23
In 2017, Shin et al24 published the results of their investigation concerning the same topic. From the Korean NHANES (2008–2010 and 2012), they enrolled 13,072 adults at least age 40 years. The AMD diagnosis and periodontal status were obtained. The AMD diagnosis was also determined using ocular fundus photographs, but PD was defined as the presence of periodontal pockets using the Community Periodontal Index score, in which mild PD was a score of 3 (periodontal pocket depth = 4–5mm) and severe PD was a score of 4 (periodontal pocket depth ≥ 6 mm). The prevalence of PD and AMD in the study was around 37.4% and 5.6%, respectively. All participants were divided into 2 groups: The youngest (≤ 62 years) and the oldest (> 62 years). The authors reported that individuals with AMD had more PD than participants without AMD in the youngest group (p = 0.031). Also, a multivariate logistic regression model after adjusting for all AMD confounding factors was performed. Shin and coworkers24 observed a significant link between severe PD and AMD only in the youngest group (odds ratio = 1.61, 95% confidence interval = 1.02–2.54). No significant association was observed between PD and AMD in the oldest age group and between mild PD and AMD in both age groups.
DISCUSSION
A potential association between PDs and AMD is a recent focus in periodontal medicine as assessed by 4 cross-sectional studies. According to these epidemiologic data, individuals with AMD showed more PDs than those without AMD (p = 0.031), and persons with AMD had fewer teeth (p < 0.001) and more alveolar bone loss (p = 0.004) than did individuals without AMD.22 This finding was observed in whites (p = 0.031) and in a heterogeneous population of whites and nonwhites.24 Also, a significant association was observed between PDs and AMD only in the youngest individuals.22 These observations can be explained by several reasons. Possibly, PDs could have a potential role in the earlier stages of AMD, or covariates increasing with age might dilute the effect of PDs on ages older than 60 years. These findings may be caused by a methodology bias because there were more subjects between ages 40 and 60 years than subjects older than age 60 years in both the US study (4432 vs 3876) and the Korean sample (8616 vs 4456). According to the literature, there could be a selection bias because AMD may start beyond age 60 years. Nevertheless, advanced age is the main risk factor for AMD.10 Multiple studies have shown that AMD is rare before age 55 years and is very common after age 75 years.7,25 Similarly, the prevalence and severity of PDs increase considerably with age.26
In addition to age, PD and AMD have other common risk factors. Scientific findings have suggested diabetes as a risk factor for advanced AMD.27 However, PD and diabetes have a bidirectional relationship.28,29 Indeed, the number of patients with diabetes increased in the presence of 2 or 3 risk factors of PD. In contrast, PD increases the susceptibility toward development of diabetes.30 Through diabetes, PD could have an indirect effect on AMD. Also, smokers have a higher risk of having PD.31 Several studies have demonstrated an association between smoking and both the wet and dry forms of AMD.30,32,33
In addition, PD and AMD occur in susceptible persons. Alteration of the complement pathway or its polymorphism is considered to play an important role in the pathogenesis of periodontitis and AMD.34,35 This dysfunction could be the common pathway for both diseases. In addition to genetic susceptibility, inflammatory deregulation/upregulation should be a potential key between a PD and AMD relationship. However, high levels of systemic inflammatory biomarkers have been associated with AMD and were significantly increased in patients with chronic PD.15,36
CONCLUSION
Recent epidemiologic data suggest that PDs are plausible additional risk factors for AMD. The link between the diseases remains unclear for the moment, but PD and AMD share similar risk factors such as inflammation disorders, advanced age, smoking habits, gene polymorphism, and diabetes. Other studies are necessary for better understanding of a potential PD and AMD relationship.
A New Way of Living
Going blind, especially later in life, presents one with a huge, potentially overwhelming challenge: to find a new way of living, of ordering one’s world, when the old way has been destroyed
— Oliver Sacks, CBE, FRCP, 1933–2015, British neurologist, naturalist, historian of science and author
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Author Contributions
Zocko Ange Désiré Pockpa, DDS, MSc, developed the idea and experimental design, performed research, and wrote the manuscript. Xavier Struillou, PhD, contributed substantially to the discussion. Assem Soueidan, PhD, contributed substantially to the discussion and proofread the manuscript. Zahi Badran, PhD, developed the idea and experimental design and contributed substantially to the discussion. Dramane Kone, DDS, performed critical proofreading and contributed to the discussion. Gnaba Samson Mobio, DDS, performed critical proofreading and contributed to the discussion. All authors have given final approval and take full responsibililty for the integrity and accuracy of this article.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005 Nov 19;366(9499):1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol 2000. 1997 Jun;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 3.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000. 2004 Jan;34(1):9–21. doi: 10.1046/j.0906-6713.2002.003421.x. [DOI] [PubMed] [Google Scholar]
- 4.Eke PI, Wei L, Borgnakke WS, et al. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol 2000. 2016 Oct;72(1):76–95. doi: 10.1111/prd.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeois D, Bouchard P, Mattout C. Epidemiology of periodontal status in dentate adults in France, 2002–2003. J Periodontal Res. 2007 Jun;42(3):219–27. doi: 10.1111/j.1600-0765.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 6.Coleman HR, Chan C-C, Ferris FL, III, Chew EY. Age-related macular degeneration. Lancet. 2008 Nov 22;372(9652):1835–45. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004 Mar;137(3):486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Bird AC, Bressler NM, Bressler SB, et al. International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995 Mar-Apr;39(5):367–74. doi: 10.1016/S0039-6257(05)80092-X. [DOI] [PubMed] [Google Scholar]
- 9.Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016 May;73:1765–86. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000 Dec;107(12):2224–32. doi: 10.1016/S0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmeggiani F, Romano MR, Costagliola C, et al. Mechanism of inflammation in age-related macular degeneration. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/546786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong T, Tan AG, Mitchell P, Wang JJ. A review and meta-analysis of the association between C-reactive protein and age-related macular degeneration. Surv Ophthalmol. 2011 May-Jun;56(3):184–94. doi: 10.1016/j.survophthal.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM. High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol. 2007 Mar;125(3):300–5. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitta VP, Christen WG, Glynn RJ, et al. C-reactive protein and the incidence of macular degeneration—Pooled analysis of 5 cohorts. JAMA Ophthalmol. 2013 Apr;131(4):507–13. doi: 10.1001/jamaophthalmol.2013.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal T, Pandey A, Deepa D, Asthana AK. C-reactive protein (CRP) and its association with periodontal disease: A brief review. J Clin Diagn Res JCDR. 2014 Jul;8(7):ZE21–4. doi: 10.7860/JCDR/2014/8355.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panezai J, Ghaffar A, Altamash M, Sundqvist K-G, Engström P-E, Larsson A. Correlation of serum cytokines, chemokines, growth factors and enzymes with periodontal disease parameters. PLoS One. 2017 Nov 30;12(11):e0188945. doi: 10.1371/journal.pone.0188945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang Q, Fu H, Qin B, et al. A possible link between rheumatoid arthritis and periodontitis: A systematic review and meta-analysis. Int J Periodontics Restorative Dent. 2017 Jan-Feb;37(1):79–86. doi: 10.11607/prd.2656. [DOI] [PubMed] [Google Scholar]
- 18.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012 Jan;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonetti MS, Van Dyke TE Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013 Apr;84(4 Suppl):S24–9. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 20.Sanz M, Kornman K Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013 Apr;40(Suppl 14):S164–9. doi: 10.1111/jcpe.12083. [DOI] [PubMed] [Google Scholar]
- 21.Brzozowska A, Puchalska-Niedbał L. Oral status as a potential source of infection in AMD patients—introduction [in Polish] Klin Oczna. 2012;114(1):29–32. [PubMed] [Google Scholar]
- 22.Karesvuo P, Gursoy UK, Pussinen PJ, et al. Alveolar bone loss associated with age-related macular degeneration in males. J Periodontol. 2012 Jan;84(1):58–67. doi: 10.1902/jop.2012.110643. [DOI] [PubMed] [Google Scholar]
- 23.Wagley S, Marra KV, Salhi RA, et al. Periodontal disease and age-related macular degeneration: Results From the National Health and Nutrition Examination Survey III. Retina. 2015 May;35(5):982–8. doi: 10.1097/IAE.0000000000000427. [DOI] [PubMed] [Google Scholar]
- 24.Shin YU, Lim HW, Hong EH, et al. The association between periodontal disease and age-related macular degeneration in the Korea National Health and Nutrition Examination Survey: A cross-sectional observational study. Medicine (Baltimore) 2017 Apr;96(14):e6418. doi: 10.1097/MD.0000000000006418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown GC, Brown MM, Sharma S, et al. The burden of age-related macular degeneration: A value-based medicine analysis. Trans Am Ophthalmol Soc. 2005 Dec;103:173–86. [PMC free article] [PubMed] [Google Scholar]
- 26.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: An overview. Periodontol 2000. 2002;29(1):7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Rong SS, Xu Q, et al. Diabetes mellitus and risk of age-related macular degeneration: A systematic review and meta-analysis. PLoS One. 2014 Sep 19;9(9):e108196. doi: 10.1371/journal.pone.0108196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Ann Periodontol. 1998 Jul;3(1):51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 29.Demmer RT, Holtfreter B, Desvarieux M, et al. The influence of type 1 and type 2 diabetes on periodontal disease progression: Prospective results from the Study of Health in Pomerania (SHIP) Diabetes Care. 2012 Oct;35(10):2036–42. doi: 10.2337/dc11-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakravarthy U, Augood C, Bentham GC, et al. Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology. 2007 Jun;114(6):1157–63. doi: 10.1016/j.ophtha.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Camelo-Castillo AJ, Mira A, Pico A, et al. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front Microbiol. 2015 Feb 24;6:119. doi: 10.3389/fmicb.2015.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton J, Edwards R, Mitchell P, Harrison RA, Buchan I, Kelly SP. Smoking and age-related macular degeneration: A review of association. Eye (Lond) 2005 Sep;19(9):935–44. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 33.Evans JR, Fletcher AE, Wormald RP. 28 000 Cases of age related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. Br J Ophthalmol. 2005 May;89(5):550–3. doi: 10.1136/bjo.2004.049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005 Apr 15;308(5720):419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 35.Hajishengallis G, Maekawa T, Abe T, Hajishengallis E, Lambris JD. Complement involvement in periodontitis: Molecular mechanisms and rational therapeutic approaches. Adv Exp Med Biol. 2015;865:57–74. doi: 10.1007/978-3-319-18603-0_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seddon JM, Gensler G, Rosner B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology. 2010 Aug;117(8):1560–6. doi: 10.1016/j.ophtha.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]