Abstract
Background
International travel is considered a risk factor for acquiring Chlamydia trachomatis; however, there are little empirical data to support this.
Aim
To examine the prevalence and risk factors for Chlamydia trachomatis infections among heterosexual international travellers (n = 28,786) attending the Melbourne Sexual Health Centre (MSHC), Australia, compared to Australian residents (n = 20,614).
Methods
We conducted a repeated cross-sectional study and analysed sexual behaviours and chlamydia positivity among heterosexual males and females aged ≤ 30 attending MSHC for the first time between January 2007 and February 2017. ‘Travellers’ were defined as individuals born outside of Australia who had resided in the country < 2 years. Associations between patient characteristics and chlamydia positivity were examined.
Results
Chlamydia positivity was higher among travellers (11.2%) compared with Australian residents (8.5%; p < 0.001). Male travellers had higher chlamydia positivity (12.1%) than Australian males (9.3%; p < 0.001), as did female travellers (10.4%) compared with Australian females (7.7%; p < 0.001). Travellers had a higher mean number of sexual partners than Australian residents among males (5.7 vs 4.7; p < 0.001) and females (3.6 vs 3.2; p < 0.001). Travellers from the United Kingdom, Europe, Ireland and New Zealand accounted for 29.6%, 21%, 8.5% and 5.8% of C. trachomatis infections, respectively. Chlamydia in males and females was associated with younger age (≤ 25), inconsistent condom use, a higher number of sexual partners (≥ 4 partners) and being a traveller (p < 0.001).
Conclusions
We found that international travel is an independent risk factor for chlamydia among young heterosexual travellers in Australia, who should therefore be a target group for chlamydia prevention.
Keywords: chlamydia, risk factors for chlamydia, travellers, sexually transmitted infections, STIs, sexually transmitted diseases
Introduction
Chlamydia trachomatis infection is one of the most common bacterial sexually transmitted infections (STIs) worldwide, with an estimated 131 million global cases in 2012 [1]. While most genital chlamydial infections are asymptomatic in both males and females, they are the main cause of non-gonococcal urethritis in males [2]. In females, chlamydia is a known cause of pelvic inflammatory disease and complications such as infertility [3]. Newman et al. estimated the pooled global prevalence of chlamydia among those 15–49 years to be 4.2% among females and 2.7% among males, with regional variations [4]. Travellers may be at particular risk of acquiring STIs such as chlamydia, as well as transmitting STIs to local populations [5,6].
A number of studies have found travel to be associated with increased high-risk sexual behaviours such as condomless sex, multiple sex partners, casual sex partners and sex with partners from countries with higher STI prevalence than one’s home country [6-11]. To date, however, there are few studies that have compared the prevalence of STIs between travellers and local residents, or that have assessed travel as an independent risk factor for STI acquisition [12,13]. A previous study showed that chlamydia prevalence was 1.4 times higher among backpackers attending a sexual health clinic in Sydney compared to local residents [7]. However, other studies comparing chlamydia prevalence in backpackers and local residents in Australia and the United Kingdom (UK) had differing results, with some studies showing no significant differences in prevalence [13-15]. This could be partly due to limited sample sizes in these studies. Therefore, larger studies are required to assess the association between travel and STI acquisition.
The aim of this study was to identify risk factors for C. trachomatis infections and compare chlamydia positivity between young heterosexual international travellers and young heterosexual Australian residents attending the Melbourne Sexual Health Centre (MSHC, the only major public STI service in Victoria, Australia) using a large, retrospective dataset. Travellers to Australia do not typically have access to Medicare (Australia’s government-funded universal healthcare), unless they are from a country that has a reciprocal healthcare agreement with Australia, and therefore need to pay for medical services; however, patients attending MSHC are not required to have Medicare and are treated free of charge. MSHC operates as a walk-in clinic, whereby patients are assessed and prioritised based on the presence of symptoms suggestive of an STI, and risk factors for acquiring an STI, and are offered testing and treatment of STIs. Therefore, it is imperative to understand the risk factors associated with travel to provide appropriate and inclusive service to the travellers. It is also important to know if travellers are a high-risk population that does require testing and prioritising to justify the high expenditure associated with STI testing and treatment. Our hypotheses are that C. trachomatis infections are higher in travellers and that travel is an independent risk factor for chlamydia.
Methods
Study design and population
This was a repeated cross-sectional study of heterosexual men and women aged ≤ 30 years who attended MSHC between 1 January 2007 and 28 February 2017. During the study period, patients who presented to the MSHC with a higher sexual risk or reported genital symptoms suggestive of an STI, e.g. urethral discharge or dysuria in males and vaginal discharge in females, or who requested asymptomatic STI screening were offered STI screening. Patients were assessed first (brief history and/or limited examination) by nurses to allocate them in order of priority for consultation with a clinician. Patients reporting sexual contact with a partner diagnosed with chlamydia were recorded as ‘contact with chlamydia’ in the MSHC’s database.
Prior to STI testing, patients completed a series of questions regarding their recent sexual behaviours using computer-assisted self-interview (CASI), including how many sexual partners they had in the last 3 months and 12 months, if condoms were used during vaginal sex and if they had sex with a partner from outside Australia in the last 12 months. Patients were informed that data were routinely collected and de-identified data might be used in research. At the time of the study, the web-based partner notification service Let Them Know (https://letthemknow.org.au/) was operational and Health Department contact tracers were rarely involved in partner notification for chlamydia; however, we did not capture how contacts were informed. Only a patient’s first visit and data related to this consultation were included in this study.
Case definition for travellers and Australian residents
As most travellers attending MSHC held working holiday visas, allowing them to stay in Australia for up to 2 years, in this study ‘travellers’ were defined as individuals born outside of Australia who had resided in Australia for less than 2 years. As it can take up to 5 years to obtain Australian residency, ‘Australian residents’ were defined as individuals who were born in Australia or, if born overseas, who had resided in Australia for more than 5 years.
Microbiological sample collection
Specimens obtained for chlamydia testing in women included a cervical or vaginal swab or first pass urine, while men provided first pass urine or urethral swab. Women reporting pelvic pain received bimanual examination; diagnosis of pelvic inflammatory disease was made on clinical grounds and was recorded in the clinic’s database. C. trachomatis was diagnosed by nucleic acid amplification test (NAAT) using Aptima Combo-2 (Hologic, Marlborough, Massachusetts, United States (US)) from March 2015 and, prior to that, BD ProbeTec ET (Becton Dickinson, Maryland, US) amplified DNA Assays using strand displacement amplification. C. trachomatis infections were defined as those testing positive by either method.
Data on demographic characteristics and sexual behaviour variables were extracted, together with data on chlamydia testing and results data, for all new patients aged ≤ 30 years who attended the MSHC during the study period. This study only included individuals aged ≤ 30 years because Australian guidelines recommend opportunistic screening of sexually active males and females in this age group, as it has the highest prevalence of C. trachomatis [14]. Patients were excluded from the analysis if they reported sex with same-sex individuals, transgender individuals or sex workers.
Chlamydia positivity, expressed as a percentage, was calculated as the number of positive cases divided by the number of chlamydia tests. It was compared between Australian residents and travellers.
Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM, Chicago, US). Chlamydia positivity included positive test results from asymptomatic patients that were screened for chlamydia, as well as those who reported genital symptoms before being tested for chlamydia. Wilson’s confidence interval (CI) for proportion was used to calculate CI. A chi-squared test was used to compare chlamydia positivity between different subgroups, categorised according to chlamydia risk factors. These subgroups included: (i) contacts with C. trachomatis infection, (ii) age, (iii) number of sex partners in the last 12 months, (iv) condom use, (v) presentation with genital symptoms, (vi) sex with overseas partners in the last 12 months and (vii) traveller status. Univariable and multivariable logistic regression were performed to examine factors associated with chlamydia positivity. Factors were also compared between travellers and residents. Factors with p < 0.05 in the univariable analysis were included in the multivariable analysis. A chi-squared trend test was used to examine the annual trend of chlamydia positivity.
Ethical approval
Ethical approval for the study was obtained from the Alfred Hospital Research Ethics Committee, Melbourne, Australia (number 541/17).
Results
Demographic characteristics
During the study period, between 1 January 2007 and 28 February 2017, there were 55,840 clinical consultations for heterosexual patients aged ≤ 30 years; of these, 6,440 (11.5%) were excluded, as no chlamydia testing was performed. Of the 49,400 patients included in the analysis, 28,786 were travellers (12,657 men and 16,129 women) and 20,614 were Australian residents (10,913 men and 9,701 women). The median age was 24 years (interquartile range (IQR): 22–27 years) in both travellers and Australian residents. Travellers had a higher mean number of sexual partners in the last 12 months than Australian residents, among both males (5.7 vs 4.7; p < 0.001) and females (3.6 vs 3.2; p < 0.001).
Chlamydia positivity
Chlamydia positivity among travellers and residents is shown in Table 1. The chlamydia positivity was higher among travellers 11.2% (3,218/28,786; 95% CI: 10.8–11.6), compared with Australian residents 8.5% (1,762/20,614; 95% CI: 8.2–8.9; p < 0.001). Among males, travellers had higher chlamydia positivity than Australian residents (12.1% vs 9.3%; p < 0.001). This applied to both men with genital symptoms (16.1% vs 12.6%; p < 0.001) and asymptomatic men (10.2% vs 7.5%; p < 0.001). Similarly, chlamydia positivity among females was higher in travellers compared with Australian residents (10.4% vs 7.7; p < 0.001). This also applied to females with genital symptoms (10.0% vs 6.8%; p < 0.001) and asymptomatic females (11.1% vs 8.6%; p < 0.001).
Table 1. Comparison of chlamydia positivity between international travellers and Australian residents, Melbourne, Australia, 2007–2017 (n = 49,400).
| Characteristics | Travellers (n = 28,786) |
Residents (n = 20,614) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Tested positive for chlamydia | Tested for chlamydia | Positivity % (95% CI) |
Tested positive for chlamydia | Tested for chlamydia | Positivity % (95% CI) |
||
| Males | |||||||
| All males | 1,537 | 12,657 | 12.1 (11.6–12.7) | 1,020 | 10,913 | 9.3 (8.8–9.9) | < 0.001 |
| Genital symptoms | 667 | 4,154 | 16.1 (15.0–17.2) | 499 | 3,957 | 12.6 (11.6–13.7) | < 0.001 |
| No genital symptoms | 870 | 8,503 | 10.2 (9.6–10.9%) | 521 | 6,956 | 7.5 (6.9–8.1) | < 0.001 |
| Reported contact with chlamydia | 414 | 1,537 | 26.9 (24.8–29.2) | 271 | 1,020 | 26.6 (24.0–29.4) | 0.90 |
| Females | |||||||
| All females | 1,681 | 16,129 | 10.4 (10.0–10.9) | 742 | 9,701 | 7.7 (7.1–8.2) | < 0.001 |
| Genital symptoms | 704 | 7,062 | 10.0 (9.3–10.7) | 295 | 4,348 | 6.8 (6.1–7.6) | < 0.001 |
| No genital symptoms | 1,028 | 9,234 | 11.1 (10.5–11.8) | 463 | 5,416 | 8.6 (7.8–9.3) | < 0.001 |
| Reported contact with chlamydia | 317 | 805 | 39.4 (36.1–42.8) | 162 | 342 | 47.4 (42.1–52.7) | 0.01 |
CI: confidence interval.
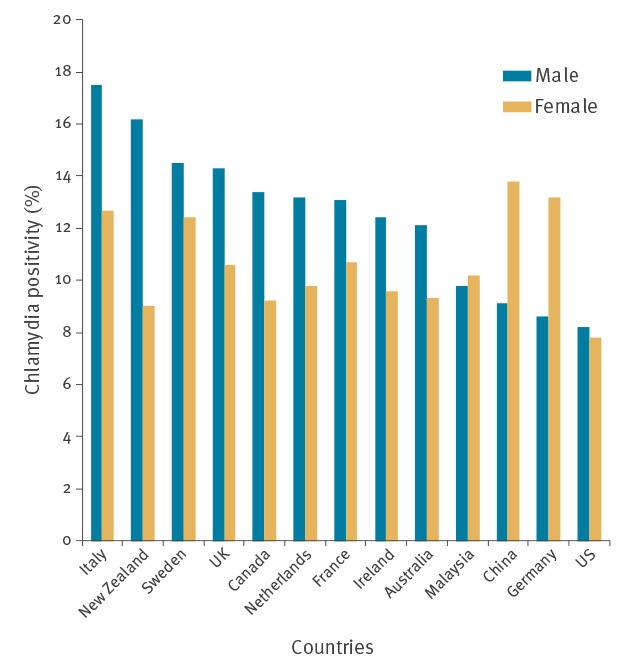
Chlamydia positivity among travellers, by sex and country of origin, are shown in Table 2 and Figure 1. The largest group of travellers was from the UK, which accounted for 26.3% of all travellers in the study. Chlamydia positivity among travellers from the UK was 14.3% (95% CI: 13.2–15.5) for males and 10.6% (95% CI: 9.7–11.6) for females. The travellers who made up the next largest group were from European Union (EU) countries other than the UK, which accounted for 23% of travellers. Chlamydia positivity among EU travellers, excluding UK travellers, was 13.5% (95% CI: 12.3–14.7) in males and 10.8% (95% CI: 10.0–11.8) in females.
Table 2. Chlamydia positivity and proportion of total chlamydia cases among travellers, by country of origin, Melbourne, Australia, 2007–2017 (n = 49,400).
| Country | Malesa (n = 23,570) |
Femalesb (n = 25,830) |
||||
|---|---|---|---|---|---|---|
| Tested positive for chlamydia | Tested for chlamydia | Chlamydia positivity % (95% CI) |
Tested positive for chlamydia | Tested for chlamydia | Chlamydia positivity % (95% CI) |
|
| European Union countries | ||||||
| France | 77 | 586 | 13.1 (10.6–16.1) | 83 | 779 | 10.7 (8.7–13.0) |
| Germany | 30 | 349 | 8.6 (6.1–12.1) | 67 | 508 | 13.2 (10.5–16.4) |
| Ireland | 158 | 1,270 | 12.4 (10.7–14.4) | 109 | 1,196 | 9.6 (7.6–10.9) |
| Italy | 47 | 268 | 17.5 (13.5–22.5) | 28 | 220 | 12.7 (9.0–17.8) |
| Netherlands | 23 | 174 | 13.2 (9–19.1) | 31 | 316 | 9.8 (7.0–13.6) |
| Sweden | 25 | 172 | 14.5 (10.0–20.6) |
95 | 765 | 12.4 (10.3–15.0) |
| UK | 518 | 3,615 | 14.3 (13.2–15.5) | 435 | 4,117 | 10.6 (9.7–11.6) |
| North America | ||||||
| Canada | 36 | 269 | 13.4 (9.8–18.0) | 77 | 838 | 9.2 (7.4–11.3) |
| US | 33 | 403 | 8.2 (5.9–11.3) | 67 | 864 | 7.8 (6.2–9.7) |
| Asia Pacific | ||||||
| China | 51 | 558 | 9.1 (7.1–11.8) | 98 | 711 | 13.8 (11.4–16.5) |
| Malaysia | 17 | 174 | 9.8 (6.2–15.1) | 25 | 245 | 10.2 (7.0–14.6) |
| New Zealand | 114 | 704 | 16.2 (13.7–19.1) | 72 | 798 | 9.0 (7.2–11.0) |
| Other countriesd | 609 | 5,995 | 10.2 (9.4–11.0) | 777 | 7,689 | 10.1 (9.5–10.1) |
UK: United Kingdom; US: United States.
a Total chlamydia cases among male travellers: 1,537.
b Total chlamydia cases among female travellers: 1,681.
d The 12 countries accounting for the greatest number of chlamydia cases are listed. The remaining cases of chlamydia among travellers are grouped under ‘Other countries’, which includes 179 countries. In total, 191 countries are included.
Figure 1.
Comparison of chlamydia positivity between males and females, by country of birth, Melbourne, Australia, 2007–2017
UK: United Kingdom; US: United States.
The 12 countries accounting for the greatest number of chlamydia cases are listed. Chlamydia positivity of Australian residents was included for comparison.
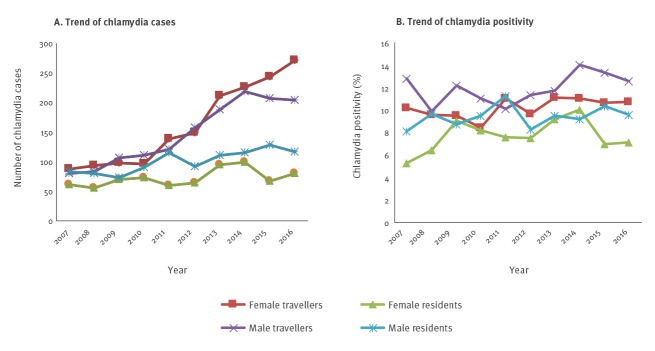
The number of C. trachomatis infections diagnosed in travellers and residents during the study period, by year, is shown in Figure 2A. Over the course of the study period, there was a 2.5-fold increase in the number of infections diagnosed among male travellers at the clinic and a 3-fold increase among female travellers, while the number remained relatively steady among male and female residents. Trends in chlamydia positivity over time in travellers and residents, by sex, are shown in Figure 2B. Overall, there was no significant increase in chlamydia positivity among travellers or residents during the study period, with the exception of chlamydia positivity among male travellers, which showed a significant upward trend (p = 0.01). Among chlamydia-positive women, pelvic inflammatory disease was diagnosed in 4.8% of the travellers (80/1,681; 95% CI: 3.8–5.9; p = 0.3) and 5.9% of the residents (44/742; 95% CI: 4.5–7.9; p = 0.3).
Figure 2.
Number of chlamydia infections and chlamydia positivity among international travellers and Australian residents, by sex, Melbourne, Australia, 2007–2016
Chlamydia positivity was determined by dividing the number of chlamydia-positive cases by the total number of chlamydia tests. It is expressed as a percentage.
Risk factors for chlamydia
Potential risk factors were compared between travellers and residents, with males and females combined in each group. Travellers were more likely to have ≥ 4 partners in the last 12 months compared with Australian residents (49.0% (14,099/28,786) vs 42.4% (8,736/20,614); p < 0.001), as well as sexual partners from overseas in the last 12 months (62.8% (7,570/12,050) vs 34.2% (3,547/10,368); p < 0.001). There were no significant differences between travellers and residents, respectively (data not shown), for the following potential risk factors: age groups (for example, 62.9% (18,118/28,786) vs 63.7% (13,141/20,614); p = 0.07, for those aged ≤ 25), consistent condom use in the last 12 months (15.5% (4,207/27,174) vs 15.2% (2,915/19,176); p = 0.4) or contact with chlamydia (6.3% (1,805/28,786) vs 6.3% (1,289/20,6114); p = 0.9).
Multivariate logistic regression analysis
Associations between potential risk factors and chlamydia positivity among all males and all females (travellers and residents combined) were assessed by multivariate analysis, as shown in Table 3.
Table 3. Associations between potential risk factors and chlamydia positivity among all heterosexual males and females aged ≤ 30 years, Melbourne, Australia, 2007–2017(n = 49,400).
| Characteristics | Males (n = 23,570)a |
Females (n = 25,830) a |
||||
|---|---|---|---|---|---|---|
| No. of individuals | OR (95% CI, p value) |
aOR (95% CI, p value) |
No. of individuals | OR (95% CI, p value) |
aOR (95% CI, p value) |
|
| Chlamydia contact | ||||||
| Yes | 1,785 | 6.6 (6.0–7.4, p < 0.001) | 9.4 (8.3–10.6, p < 0.001) | 1,309 | 6.7 (5.9–7.6, p < 0.001) | 7.0 (6.2–8.0, p < 0.001) |
| No | 21,785 | 1 (ref) | 1 (ref) | 24,521 | 1 (ref) | 1 (ref) |
| Age (years) | ||||||
| ≤ 20 | 2,028 | 1.2 (1.0–1.4, p = 0.04) | 1.3 (1.1–1.5, p = 0.008) | 3,603 | 1.9 (1.7–2.2, p < 0.001) | 2.0 (1.7–2.3, p < 0.001) |
| 21–25 | 11,765 | 1.4 (1.3–1.6, p < 0.001) | 1.4 (1.3–1.5., p < 0.001) | 13,863 | 1.5 (1.4–1.7, p < 0.001) | 1.5 (1.3–1.6, p < 0.001) |
| 26–30 | 9,777 | 1 (ref) | 1 (ref) | 8,364 | 1 (ref) | 1 (ref) |
| Number of partners in last 12 months | ||||||
| ≤ 3 | 11,063 | 1 (ref) | 1 (ref) | 15,502 | 1 (ref) | 1 (ref) |
| ≥ 4 | 12,507 | 1.7 (1.6–1.9, p < 0.001) | 1.6 (1.4–1.7, p < 0.001) | 10,328 | 1.6 (1.5–1.7, p < 0.001) | 1.4 (1.3–1.5, p < 0.001) |
| Condom use in last 12 months | ||||||
| Inconsistent | 18,506 | 3.2 (2.7–3.8, p < 0.001) | 2.7 (2.3–3.2, p < 0.001) | 20,722 | 2.1 (1.8–2.5, p < 0.001) | 2.0 (1.7–2.3, p < 0.001) |
| Always or non-penetrative sex | 3,721 | 1 (ref) | 1 (ref) | 3,401 | 1 (ref) | 1 (ref) |
| Genital symptoms | ||||||
| Yes | 8,111 | 1.7 (1.6–1.8, p < 0.001) | 2.8 (2.5–3.1, p < 0.001) | 11,410 | 0.9 (0.8–1.0, p = 0.002) | 1.1 (1.0–1.3, p = 0.004) |
| No | 15,459 | 1 (ref) | 1 (ref) | 14,420 | 1 (ref) | 1 (ref) |
| Sex overseas in last 12 months | ||||||
| Yes | 11,117 | 1.2 (1.1–1.3, p < 0.001) | 1.1 (1.0–1.2, p = 0.3) | 11,624 | 1.3 (1.2–1.4, p < 0.001) | 1.1 (1.0–1.2, p = 0.02) |
| No | 11,301 | 1 (ref) | 1 (ref) | 13,021 | 1 (ref) | 1 (ref) |
| Traveller | ||||||
| Yes | 12,657 | 1.3 (1.2–1.5, p < 0.001) | 1.4(1.2–1.5, p < 0.001) | 16,129 | 1.4 (1.3–1.5, p < 0.001) | 1.4 (1.3–1.6, p < 0.001) |
| No | 10,913 | 1 (ref) | 1 (ref) | 9,701 | 1 (ref) | 1 (ref) |
aOR: adjusted odds ratio; CI: confidence interval; OR: odds ratio; ref: reference.
a There are missing data for characteristics such as condom use in last 12 months (1,707 female and 1,343 males) and sex overseas in last 12 months (1,185 females and 1,152 males). The total may not add up due to incomplete computer-assisted self-interviews.
aOR is adjusted for (i) being a sexual contact of person with chlamydia infection, (ii) age, (iii) number of partners in the last 12 months, (iv) condom use in the last 12 months, (v) presence of genital symptoms suggestive of sexually transmitted infection, (vi) having had sexual partners from overseas in the last 12 months and (vii) being a traveller.
Among males, chlamydia positivity was independently associated with: (i) reporting sexual contact with a partner with chlamydia (adjusted odds ratio (aOR): 9.4; 95% CI: 8.3–10.6), (ii) younger age (aOR: 1.3; 95% CI: 1.1–1.5 for those aged ≤ 20 ), (iii) ≥ 4 female partners in the last 12 months (aOR: 1.6; 95% CI: 1.4–1.7), (iv) inconsistent condom use in the last 12 months (aOR: 2.7; 95% CI: 2.3–3.2), (v) genital symptoms (aOR: 2.8; 95% CI: 2.5–3.1) and (vi) being a traveller (aOR: 1.4; 95% CI: 1.2–1.5).
Among females, chlamydia positivity was independently associated with: (i) reporting sexual contact with a partner with chlamydia (aOR: 7.0; 95% CI: 6.2–8.0), (ii) younger age (aOR: 2.0; 95% CI: 1.7–2.3 for those aged ≤ 20), (iii) ≥ 4 male partners in the last 12 months (aOR: 1.4; 95% CI: 1.3–1.5), (iv) inconsistent condom use in the last 12 months (aOR: 2.0; 95% CI: 1.7–2.4), (v) genital symptoms (aOR: 1.1; 95% CI: 1.0–1.3) and (vi) being a traveller (aOR: 1.4; 95% CI: 1.3–1.6).
Sex with a partner from overseas in the last 12 months was significantly associated with chlamydia infection in females (aOR: 1.1; 95% CI: 1.0–1.2; p = 0.02), but not in males (aOR: 1.1; 95% CI: 1.0–1.2; p = 0.10).
Potential risk factors for C. trachomatis infection and their association with chlamydia positivity among travellers and residents are shown in Table 4.
Table 4. Association of risk factors for chlamydia infection among travellers and Australian residents, Melbourne, Australia, 2007–2017 (n = 49,400).
| Characteristics | Travellersa (n = 28,786) |
Australian residentsa (n = 20,614) |
||||
|---|---|---|---|---|---|---|
| No. of individuals | OR (95% CI, p value) |
aOR (95% CI, p value) | No. of individuals | OR (95% CI, p value) |
aOR (95%CI) |
|
| Chlamydia contact | ||||||
| Yes | 1,805 | 6.7 (6.1–7.4, p < 0.001) | 7.8 (6.9–8.9, p < 0.001) | 1,289 | 6.9 (6.0–7.8, p < 0.001) | 9.3 (7.7–11.1, p < 0.001) |
| No | 26,981 | 1 (ref) | 1 (ref) | 19,325 | 1 (ref) | 1 (ref) |
| Age (years) | ||||||
| ≤ 20 | 2,368 | 1.5 (1.3–1.8, p < 0.001) | 1.6 (1.4–2.0, p < 0.001) | 3,263 | 1.7 (1.4–1.9, p < 0.001) | 1.5 (1.3–1.9, p < 0.001) |
| 21–25 | 15,750 | 1.5 (1.4–1.6, p < 0.001) | 1.4(1.4–1.7, p < 0.001) | 9,878 | 1.4 (1.2 -1.6, p < 0.001) | 1.4 (1.2–1.6, p < 0.001) |
| 26–30 | 10,668 | 1 (ref) | 1 (ref) | 7,474 | 1 (ref) | 1 (ref) |
| Number of partners in last 12 months | ||||||
| ≤ 3 | 14,687 | 1 (ref) | 1 (ref) | 11,878 | 1 (ref) | 1 (ref) |
| ≥ 4 | 14,099 | 1.6 (1.5–1.7, p < 0.001) | 1.5 (1.3–1.6, < 0.001) | 8,736 | 1.7 (1.6–1.9, p < 0.001) | 1.6 (1.4–1.8, p < 0.001) |
| Condom use in last 12 months | ||||||
| Inconsistent | 22,967 | 2.6 (2.2–3.0, p < 0.001) | 2.3 (2.0–2.8, < 0.001) | 16,261 | 2.7 (2.2–3.3, p < 0.001) | 2.4 (1.9–3.1, p < 0.001) |
| Always or non-penetrative sex | 4,207 | 1 (ref) | 1 (ref) | 2,915 | 1 (ref) | 1 (ref) |
| Genital symptoms | ||||||
| Yes | 11,216 | 1.2 (1.1–1.3, p < 0.001) | 1.9 (1.7–2.1, < 0.001) | 8,305 | 1.2 (1.1–1.4, p < 0.001) | 2.6 (2.3–3.0, p < 0.001) |
| No | 17,570 | 1 (ref) | 1 (ref) | 12,309 | 1 (ref) | 1 (ref) |
| Sex overseas in last 12 months | ||||||
| Yes | 7,570 | 1.2 (1.1–1.3, p = 0.0.004) | 1.0 (0.9–1.2, p = 0.6) | 3,547 | 1.0 (0.9–1.2, p = 0.6) | 1.1 (0.9–1.2, p = 0.4) |
| No | 4,480 | 1 (ref) | 1 (ref) | 6,821 | 1 (ref) | 1 (ref) |
| Sex | ||||||
| Male | 12,657 | 1.2 (1.1–1.3, p < 0.001) | 1.2 (1.0–1.3, p = 0.005) | 10,913 | 1.2 (1.2–1.4, p < 0.001) | 1.2 (1.0–1.5, p = 0.06) |
| Female | 16,129 | 1 (ref) | 1 (ref) | 9,701 | 1 (ref) | 1 (ref) |
aOR: adjusted odds ratio; CI: confidence interval; OR: crude odds ratio; ref: reference.
a There are missing data for characteristics such as condom use in last 12 months (1,612 travellers and 1,418 residents) and sex overseas in last 12 months (16,736 travellers and 10,246 residents). The total may not add up due to incomplete computer-assisted self-interview.
aOR is adjusted for (i) being a sexual contact of person with chlamydia infection, (ii) age, (iii) number of partners in the last 12 months, (iv) condom use in the last 12 months, (v) presence of genital symptoms suggestive of sexually transmitted infection, (vi) having had sexual partners from overseas in the last 12 months and (vii) sex.
Discussion
To our knowledge, this is the largest study of chlamydia among international travellers, with over 3,000 travellers diagnosed with chlamydia over a 10-year period. Chlamydia positivity was significantly higher among heterosexual male and female travellers attending the MSHC between 2007 and 2017 compared with Australian residents; travel was an independent risk factor for C. trachomatis infection among both males and females, justifying maintaining ongoing free access to healthcare for travellers attending our service.
We found that younger age (≤25 years), inconsistent condom use and a higher number of sexual partners during the last 12 months were risk factors for chlamydia, findings that are consistent with other studies [16-18]. Over the study period, between 2007 and 2017, the number of chlamydia infections diagnosed among travellers attending MSHC increased, overtaking the number of diagnoses among Australian residents in the same age group. These results suggest that young international travellers visiting Australia should be a target group for chlamydia prevention and screening strategies. It is notable that 4.8% of female travellers with chlamydia were also diagnosed with pelvic inflammatory disease, a serious complication of C. trachomatis infection in females. Therefore, screening and prompt treatment of C. trachomatis infection should be encouraged to prevent morbidity arising from the infection among female travellers.
Consistent with previous studies, we also found that young, heterosexual international travellers were more likely to engage in higher risk sexual behaviours, such as having a high number of partners [8-10,12,15,19,20]. These studies have suggested that more casual sexual relationships during travel may be a result of physical separation from regular partners in an individual’s country of origin and removal of social factors that might inhibit such sexual behaviours [6,8,13,19-21]. Higher alcohol consumption, increased sexual activity with multiple partners, and partners from countries with higher prevalence of STIs have also been identified as risk behaviours for chlamydia among travellers [7,10,15,18,21]. It has been estimated that the risk of developing an STI increases up to 3-fold in people who engage in casual sex [8], with previous studies reporting the prevalence of travel-associated casual sex ranging between 20% and 35%, with the prevalence of condomless sex between 17% and 49% [8,19].
In our study, being a traveller was associated with chlamydia infection, independent of sexual risk factors such as having a higher number of partners or inconsistent condom use; this suggests that travellers are exposed to additional risk factors for chlamydia acquisition. One hypothesis is that travellers in our study had sex with other travellers (i.e. sexual network factors) and, therefore, were more likely to have sex with individuals who also have a higher likelihood of being infected with chlamydia [22]. One of the factors most strongly associated with chlamydia in both travellers and residents was sex with a partner who reported having chlamydia. MSHC did not routinely record whether sexual partners were travellers or not; therefore, we were not able to examine this.
Limitations
There are several limitations to this study. As it was conducted at a single clinic (MSHC), the findings may not be generalisable to international travellers around the world. We were only able to investigate prevalence of chlamydia, as screening for other STIs, e.g. gonorrhoea, is not routinely recommended among heterosexuals in Australia according to national guidelines [23]. Patients with chlamydia infection in the study included asymptomatic individuals and those presenting with chlamydia-associated symptoms. Patients that were high risk for chlamydia infection or those presenting with genital symptoms were more likely to attend MSHC than patients requesting anonymous screening; this may have resulted in a higher number of chlamydia infections reported in this study. Furthermore, we did not examine changes in sexual behaviour over time, which could influence changes in positivity rates for chlamydia.
The number of international visitors to Australia increased 1.6-fold over the study period, from 5 million in 2007 to 8 million in 2017; approximately 50% of these visitors were in Australia for holidays [24]. It is not known to what extent the travellers in this study acquired chlamydia infections in their home countries or during travel to other countries, as opposed to in Australia. Therefore, this could have resulted in a higher number of C. trachomatis infections among travellers.
Conclusions
Our study suggests that young, heterosexual travellers are at risk of chlamydiainfection and would benefit from pre-travel advice aimed at reducing the risk of STIs, including safe sex education and promotion of condom use. This could be administered in primary care settings, travel clinics or sexual health clinics, with follow-up STI screening at post-travel consultations. Sexually active travellers should be encouraged to attend STI screening and should seek STI testing if they develop genital symptoms in their destination country. When planning sexual health service delivery, policymakers should consider young, heterosexual international travellers as a high-risk population and should implement strategies to increase international travellers’ access to sexual health services abroad. Future research incorporating epidemiological typing of C. trachomatis with data on travel could help define the extent of transmission of chlamydial strains between countries and subsequent dissemination within destination countries.
Acknowledgements
We would like to acknowledge and thank Afrizal Afrizal for data extraction from the electronic database. EPFC was supported by the National Health and Medical Research Council Early Career Fellowship (GNT1091226). DAW was supported by the National Health and Medical Research Council Early Career Fellowship (GNT1123854).
Conflict of interest: None declared.
Authors’ contributions: ETA and MYC conceived the study and design. ETA, EPFC and MYC performed data analyses. ETA conducted chart review and wrote the first draft of the manuscript. All authors helped with interpretation of data, manuscript editing and approval of the final version.
References
- 1.World Health Organization (WHO). WHO guidelines for the treatment of chlamydia trachomatis. Geneva: WHO; 2012. Available from: http://www.who.int/reproductivehealth/publications/rtis/chlamydia-treatment-guidelines/en/ [PubMed]
- 2.Bradshaw CS, Tabrizi SN, Read TRH, Garland SM, Hopkins CA, Moss LM, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis. 2006;193(3):336-45. 10.1086/499434 [DOI] [PubMed] [Google Scholar]
- 3.Reekie J, Donovan B, Guy R, Hocking JS, Jorm L, Kaldor JM, et al. Hospitalisations for pelvic inflammatory disease temporally related to a diagnosis of Chlamydia or gonorrhoea: a retrospective cohort study. PLoS One. 2014;9(4):e94361. 10.1371/journal.pone.0094361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS One. 2015;10(12):e0143304. 10.1371/journal.pone.0143304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabada MM, Echevarria JI, Seas C, Gotuzzo E. High prevalence of sexually transmitted infections among young Peruvians who have sexual intercourse with foreign travelers in Cuzco. J Travel Med. 2009;16(5):299-303. 10.1111/j.1708-8305.2009.00324.x [DOI] [PubMed] [Google Scholar]
- 6.Sundbeck M, Agardh A, Östergren P-OO. Travel abroad increases sexual health risk-taking among Swedish youth: a population-based study using a case-crossover strategy. Glob Health Action. 2017;10(1):1330511. 10.1080/16549716.2017.1330511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNulty AM, Egan C, Wand H, Donovan B. The behaviour and sexual health of young international travellers (backpackers) in Australia. Sex Transm Infect. 2010;86(3):247-50. 10.1136/sti.2009.038737 [DOI] [PubMed] [Google Scholar]
- 8.Vivancos R, Abubakar I, Hunter PR. Foreign travel associated with increased sexual risk-taking, alcohol and drug use among UK university students: a cohort study. Int J STD AIDS. 2010;21(1):46-51. 10.1258/ijsa.2009.008501 [DOI] [PubMed] [Google Scholar]
- 9.Lewis CT, de Wildt G. Sexual behaviour of backpackers who visit Koh Tao and Koh Phangan, Thailand: a cross-sectional study. Sex Transm Infect. 2016;92(6):410-4. 10.1136/sextrans-2015-052301 [DOI] [PubMed] [Google Scholar]
- 10.Hughes K, Downing J, Bellis MA, Dillon P, Copeland J. The sexual behaviour of British backpackers in Australia. Sex Transm Infect. 2009;85(6):477-82. 10.1136/sti.2009.036921 [DOI] [PubMed] [Google Scholar]
- 11.Misson J, Chow EPF, Chen MY, Read TRH, Bradshaw CS, Fairley CK. Trends in gonorrhoea infection and overseas sexual contacts among females attending a sexual health centre in Melbourne, Australia, 2008-2015. Commun Dis Intell (2018). 2018;42: S2209-6051(18)00024-6. [PubMed] [Google Scholar]
- 12.Matteelli A, Schlagenhauf P, Carvalho ACC, Weld L, Davis XM, Wilder-Smith A, et al. GeoSentinel Surveillance Network Travel-associated sexually transmitted infections: an observational cross-sectional study of the GeoSentinel surveillance database. Lancet Infect Dis. 2013;13(3):205-13. 10.1016/S1473-3099(12)70291-8 [DOI] [PubMed] [Google Scholar]
- 13.Hawkes S, Hart GJ, Bletsoe E, Shergold C, Johnson AM. Risk behaviour and STD acquisition in genitourinary clinic attenders who have travelled. Genitourin Med. 1995;71(6):351-4. 10.1136/sti.71.6.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies SC, Karagiannis T, Headon V, Wiig R, Duffy J. Prevalence of genital chlamydial infection among a community sample of young international backpackers in Sydney, Australia. Int J STD AIDS. 2011;22(3):160-4. 10.1258/ijsa.2010.010354 [DOI] [PubMed] [Google Scholar]
- 15.Fischer JA, Debattista J, Rostami S, Peet AR, Dean JA, Allen KE, et al. Sexual risk taking in a community sample of international backpackers visiting Brisbane, Australia. Asia Pac J Public Health. 2015;27(2):NP2400-9. 10.1177/1010539513483822 [DOI] [PubMed] [Google Scholar]
- 16.Veličko I, Ploner A, Sparén P, Marions L, Herrmann B, Kühlmann-Berenzon S. Sexual and testing behaviour associated with Chlamydia trachomatis infection: a cohort study in an STI clinic in Sweden. BMJ Open. 2016;6(8):e011312-011312. 10.1136/bmjopen-2016-011312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro C, Jolly A, Nair R, Chen Y. Risk factors for genital chlamydial infection. Can J Infect Dis. 2002;13(3):195-207. 10.1155/2002/954837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wand H, Guy R, Donovan B, McNulty A. Population attributable risk for chlamydia infection in a cohort of young international travellers (backpackers) and residents in Australia. BMJ Open. 2011;1(1):e000004. 10.1136/bmjopen-2010-000004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svensson P, Sundbeck M, Persson KI, Stafström M, Östergren PO, Mannheimer L, et al. A meta-analysis and systematic literature review of factors associated with sexual risk-taking during international travel. Travel Med Infect Dis. 2018;24(March):65-88. 10.1016/j.tmaid.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 20.Ward BJ, Plourde P. Travel and sexually transmitted infections. J Travel Med. 2006;13(5):300-17. 10.1111/j.1708-8305.2006.00061.x [DOI] [PubMed] [Google Scholar]
- 21.Ericsson CD, Steffen R, Matteelli A, Carosi G. Sexually transmitted diseases in travelers. Clin Infect Dis. 2001;32(7):1063-7. 10.1086/319607 [DOI] [PubMed] [Google Scholar]
- 22.Chow EPF, Read TRH, Law MG, Chen MY, Bradshaw CS, Fairley CK. Assortative sexual mixing patterns in male-female and male-male partnerships in Melbourne, Australia: implications for HIV and sexually transmissible infection transmission. Sex Health. 2016;13(5):451-6. 10.1071/SH16055 [DOI] [PubMed] [Google Scholar]
- 23.Australasian Society for HIV (ASHM). Viral Hepatitis and Sexual Health Medicine. HIV, viral hepatitis and STIs: Australian STI management guidelines for use in primary care. Sydney: ASHM; 2018. [Accessed: 1 Dec 2018]. Available from: http://www.sti.guidelines.org.au
- 24.The Australian Trade and Investment Commission (Austrade). Tourism Research Australia. Results of international visitor survey, year ending September, 2017 data. Canberra: Austrade. [Accessed: 1 Dec 2018]. Available from: https://www.tra.gov.au/data-and-research