Abstract
Background
Zika virus (ZIKV) is believed to be endemic in Southeast Asia. However, there have been few Zika cases reported to date in Malaysia, which could be due to high pre-existing levels of population immunity.
Methods
To determine Zika virus (ZIKV) seroprevalence in Kuala Lumpur, Malaysia, 1085 serum samples from 2012, 2014–2015 and 2017 were screened for anti-ZIKV antibodies using a ZIKV NS1 blockade-of-binding assay. Reactive samples were confirmed using neutralization assays against ZIKV and the four dengue virus (DENV) serotypes. A sample was possible ZIKV seropositive with a ZIKV 50% neutralization (NT50) titre ≥20. A sample was probable ZIKV seropositive if, in addition, all DENV NT50 titres were <20 or the ZIKV NT50 titre was >4-fold greater than the highest DENV NT50 titre.
Results
We found low rates of possible ZIKV seropositivity (3.3% [95% confidence interval {CI} 2.4 to 4.6]) and probable ZIKV seropositivity (0.6% [95% CI 0.3 to 1.4]). Possible ZIKV seropositivity was independently associated with increasing age (odds ratio [OR] 1.04 [95% CI 1.02 to 1.06], p<0.0001) and male gender (OR 3.5 [95% CI 1.5 to 8.6], p=0.005).
Conclusions
The low ZIKV seroprevalence rate, a proxy for population immunity, does not explain the low incidence of Zika in dengue-hyperendemic Kuala Lumpur. Other factors, such as the possible protective effects of pre-existing flavivirus antibodies or reduced transmission by local mosquito vectors, should be explored. Kuala Lumpur is at high risk of a large-scale Zika epidemic.
Keywords: dengue virus, Malaysia, neutralization test, NS1 blockade-of-binding ELISA, seroprevalence, Zika virus
Introduction
Zika virus (ZIKV) is a flavivirus transmitted by Aedes aegypti and Aedes albopictus mosquitoes that has re-emerged in the last decade to cause extensive epidemics. While ZIKV causes relatively mild illness in most people, the latest emergence has also been associated with severe neurological disease and congenital malformations. ZIKV was first isolated from a monkey in Uganda in 1947 and was detected soon thereafter in Southeast Asia, where it was isolated from A. aegypti in Malaysia in 1966.1 In the following decades, there were sporadic reports of cases in Southeast Asia and in travellers who had been to Southeast Asia, as well as surveys showing high rates of seropositivity (reviewed by Lim et al.2). All these studies indicated that ZIKV is endemic in Southeast Asia. The more recent availability of genetic sequences of Asian strains has provided supportive phylogenetic evidence that ZIKV circulated and evolved in Southeast Asia before being introduced to Yap Island in 2007, other Pacific islands in 2013 and to the Americas in 2014–2015,3,4 resulting in explosive epidemics affecting hundreds of thousands of people.
A limited outbreak affecting 455 people occurred in Singapore in August–November 2016,5 the only outbreak described in Southeast Asia to date. A notable question is why there have not been more Zika cases and outbreaks described in Southeast Asia, despite its likely endemicity, the abundance of mosquito vectors and the presence of hyperendemic transmission of dengue virus (DENV). A frequent suggestion is that endemic circulation has led to levels of population immunity that limit the likelihood of huge epidemics as seen in susceptible populations in the Americas, where ZIKV was not known to exist previously. Few recent seroprevalence data exist in Southeast Asia to address this theory, partly because the well-documented serologic cross-reactivity between flaviviruses makes it difficult to carry out and interpret such studies.
Kuala Lumpur has one of the highest dengue incidences in Malaysia,6 at 444 per 100 000 in 2017.7 Historical serosurveys have shown age-related ZIKV seroprevalence rates of up to 70% in older adults in Malaysia,8,9 and ZIKV infections have been diagnosed in travellers from Malaysia.10 Yet, as of September 2018, only eight cases of Zika have ever been diagnosed in Malaysia, all in 2016, with at least three of these epidemiologically linked to the Singapore outbreak and no detections in a further 2360 dengue-negative contemporary serum samples tested by the Ministry of Health.7 Three of the eight confirmed Zika cases occurred within the densely populated Klang Valley conurbation centred around Kuala Lumpur. Here we used a recently described sensitive and specific ZIKV NS1 blockade-of-binding (BOB) enzyme-linked immunosorbent assay (ELISA) that has been extensively evaluated using well-characterized specimen panels and correlates well with the gold-standard neutralization assay that detects anti-E protein responses (11–13and data not shown). We screened serum samples from before, during and after the recent Zika emergences for anti-ZIKV antibodies using the ZIKV NS1 BOB assay and confirmed reactive samples with both ZIKV and DENV neutralization assays. We asked whether the relative lack of Zika cases in Kuala Lumpur could be explained by high pre-existing levels of population immunity.
Materials and methods
Patient samples
Residual serum samples from hospital inpatients (suspected of various infectious diseases) and healthy blood donors in 2012, 2014–2015 and 2017 were obtained from the diagnostic microbiology laboratory of the University of Malaya Medical Centre, a teaching hospital in Kuala Lumpur. Ethical approval was obtained from the hospital’s Medical Research Ethics Committee (2017116-5794).
Cells and viruses
Vero cells (ECACC 88020401) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Waltham, MA, USA) in the presence of 10% heat-inactivated foetal bovine serum (FBS; Life Technologies), 2 mM L-glutamine (Life Technologies), 1 mM sodium pyruvate (HyClone Laboratories, Logan, UT, USA), 100 U/ml penicillin (Life Technologies) and 100 μg/ml streptomycin (Life Technologies) and incubated at 37°C in 5% carbon dioxide.
The ZIKV strain used in this study was MRS_OPY_Martinique_PaRi_2015 (provided by the European Virus Archive, Marseille, France). The DENV strains used were DENV-1 Western Pacific, DENV-2 New Guinea C and clinical strains DENV-3 S78 and DENV-4 VP (provided by Chandramathi Samudi Raju of the University of Malaya), which were propagated in Vero cells with 2% FBS DMEM. Virus supernatants were harvested after 7–9 d, pre-cleared by centrifugation, aliquoted and stored at −80°C. All the viruses were titrated by focus immunoassay and stained with anti-flavivirus monoclonal antibody D1-4G2-4-15 (Absolute Antibody, Oxford, UK).14
ZIKV NS1 BOB ELISA (ZIKA35-HRP version)
The ZIKV NS1 BOB ELISA was performed as previously described,11 with the modification that the ZKA35 monoclonal antibody was directly conjugated to horseradish peroxidase (HRP) rather than to biotin. Briefly, polystyrene plates were coated overnight with 1 μg/mL ZIKV NS1 (Native Antigen, Kidlington, UK) and then blocked for 1 h with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (Rocky Mountain Biologicals, Missoula, MT, USA). Serum samples (1:10 dilution) were added to NS1-coated ELISA plates. After 1 h, an equal volume of anti-NS1 ZKA35 conjugated to HRP (a gift from Davide Corti, Humabs Biomed, Zurich, Switzerland) was added and the mixture was incubated at room temperature for 15 min. Plates were washed and the substrate 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO, USA) was added for 5–6 min, then the reaction was stopped with 2N sulphuric acid. Optical density (OD) was read at 450 nm in an ELx808 absorbance microplate reader (BioTek Instruments, Winooski, VT, USA). The percentage of inhibition was calculated as follows: [1−([OD sample−OD negative control]/[OD positive control−OD negative control])]×100. A cut-off value of 50% inhibition was used to denote a reactive ZIKV NS1 BOB ELISA result.
Virus neutralization assays
Samples that were reactive for anti-ZIKV antibodies by the ZIKV NS1 BOB assay were then tested by a plaque reduction neutralization test (PRNT) to screen for anti-ZIKV neutralizing antibodies, as previously described.15 Heat-inactivated serum samples were serially diluted 2-fold in 1X Dulbecco’s PBS from 1:20 to 1:1280 and mixed with 75–90 plaque-forming units of ZIKV pre-diluted in 2% FBS DMEM in equal volumes to a final volume of 200 μl. The virus–antibody mixture was incubated for 1 h at 37°C before inoculation into 1.5×105 Vero cells per well in a 24-well plate. The plate was further incubated for 1.5 h at 37°C before the plaque medium was replaced with the overlay (3 parts of 3.5% FBS DMEM mixed with 2 parts of 3% carboxymethylcellulose [Sigma-Aldrich]). After 3 d of incubation, the cells were fixed with 3.7% formaldehyde and stained with 0.5% crystal violet. The neutralizing titres of the samples were expressed as the PRNT50, which is the serum dilution that reduced plaque formation by 50%. ZKA185, a neutralizing human monoclonal antibody (Absolute Antibody, Redcar, UK), served as the positive control for the ZIKV neutralization assay.
Samples with a PRNT50 titre ≥20 were titrated for anti-ZIKV and anti-DENV neutralizing antibodies using a focus reduction neutralization test (FRNT). A rapid screen was first carried out for anti-DENV antibodies by mixing serum at a 1:20 dilution with 65–80 focus-forming units of DENV using a similar procedure to that described above, except that methylcellulose (Sigma-Aldrich) was used in the plaque medium. After 4 d (DENV-1, -3, -4) or 5 d (DENV-2) of incubation, plaque medium was removed and cells were rinsed with 1X PBS prior to fixation with a chilled 1:1 mixture of methanol:acetone. The cells were blocked with 1% bovine serum albumin–PBS and stained with 250 ng of anti-flavivirus antibody D1-4G2-4-15 per well for 1 h, followed by goat anti-mouse immunoglobulin G (IgG) HRP (Merck Millipore, Burlington, MA, USA) at 1:500 dilution for 1 h. KPL TrueBlue Peroxidase substrate (SeraCare, Milford, MA, USA) was used to visualize the foci. Serum samples that reduced the number of plaques by >50% were retested at dilutions of 1:40 to 1:2560 against both ZIKV and DENV. Neutralizing titres were expressed as FRNT50, which is the serum dilution that reduced focus formation by 50% relative to virus control in the absence of serum.
Definitions
A sample was defined as possible ZIKV seropositive if it was reactive using the ZIKV NS1 BOB assay and had ZIKV PRNT50 or FRNT50 titres ≥20. A sample was considered probable ZIKV seropositive if it fulfilled the criteria of possible ZIKV seropositive and additionally all DENV FRNT50 titres were <20 or the ZIKV PRNT50 or FRNT50 titre was ≥4-fold higher than the highest DENV FRNT50 titre.
Statistical analysis
We analysed factors associated with possible ZIKV seropositivity as the outcome using logistic regression with the likelihood ratio test. The factors tested were age, gender, source of the sample (inpatient or blood donor) and year of the sample (2012, 2014–2015 or 2017). Factors found to be significantly associated with the outcome in univariate analysis were used for multivariate analysis. The resulting model was tested for collinearity and interactions. To assess the final model, the Hosmer and Lemeshow goodness-of-fit test was performed and the area under the curve of the receiver operating characteristics curve was calculated. SPSS Statistics version 25 (IBM, Armonk, NY, USA) was used and p<0.05 was considered significant. The odds ratio (OR) and 95% confidence interval (CI) were reported for each factor.
Results
A total of 1085 samples were analysed, comprising 726 (66.9%) residual diagnostic samples collected from inpatients at the University of Malaya Medical Centre and 359 (33.1%) samples from healthy blood donors from the hospital’s blood bank (Supplementary Table 1). Samples were collected in 2012 (before the Pacific outbreaks of Zika), August 2014–March 2015 (the early stages of the Americas epidemics) and 2017 (after the outbreak in neighbouring Singapore). The number of samples from 2012, 2014–2015 and 2017 were 239 (all inpatients), 426 (58.2% inpatients and 41.8% blood donors) and 420 (56.9% inpatients and 43.1% blood donors), respectively. At least 30 samples were collected for each 10-y age group from <10 to >70 y of age for each sampling period, resulting in a total of 91–154 samples for each 10-y age group (Supplementary Table 1). There were 458 (42.2%) female and 627 (57.8%) male cases.
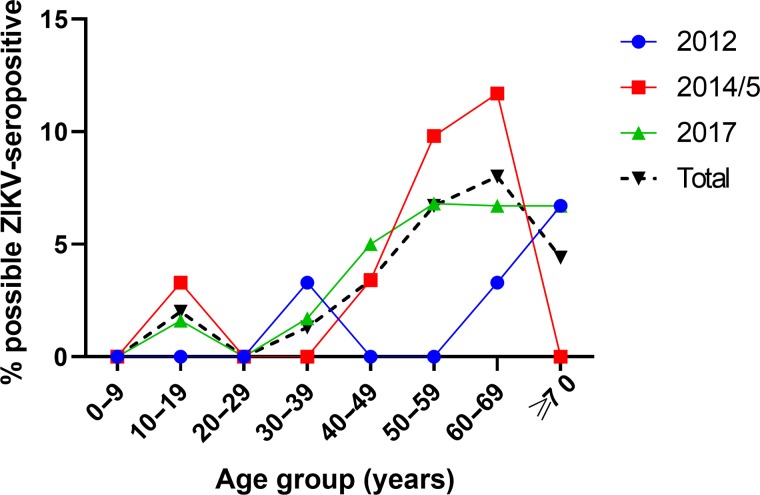
Eighty-two of the 1085 samples (7.6% [95% CI 6.1 to 9.3]) were reactive by the screening ZIKV NS1 BOB assay. Of these 82 samples, 36 (43.9%) had detectable anti-ZIKV neutralizing antibodies and were considered as possible ZIKV seropositive, comprising 3.3% (95% CI 2.4 to 4.6) of the total samples tested (Figure 1). A total of 74/82 (90.2%) screen-reactive samples also had detectable antibodies to at least one DENV serotype. Only 7 samples (0.6% [95% CI 0.3 to 1.4]) were considered probable ZIKV seropositive.
Figure 1.
Possible ZIKV-seropositive rates in different age groups in 2012, 2014–2015 and 2017 in Kuala Lumpur, Malaysia. Possible ZIKV-seropositive samples were reactive using the ZIKV NS1 BOB ELISA and had confirmatory ZIKV PRNT50 or FRNT50 titres ≥20.
Multivariate analysis was performed to identify factors associated with possible ZIKV seropositivity (Table 1). The year of sample collection (2012, 2014–2015, 2017) and the source of the sample (inpatient or blood donor) were not associated factors. Possible ZIKV seropositivity was independently associated with increasing age per year (OR 1.04 [95% CI 1.02 to 1.06], p<0.0001) and male gender (OR 3.5 [95% CI 1.5 to 8.6], p=0.005). This model had satisfactory fit (χ2=5.1, p=0.75) and discrimination (area under the curve=0.76 [95% CI 0.69 to 0.82], p <0.0001).
Table 1.
Univariate and multivariate analysis of factors associated with possible ZIKV seropositivity
| Factor | Cases, n | Possible ZIKV seropositive, n (%) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Age (years) | 1085 | 82 (7.6) | 1.04 (1.02 to 1.06) | <0.0001 | 1.04 (1.02 to 1.06) | <0.0001 |
| Gender | ||||||
| Male | 627 | 30 (4.8) | 3.8 (1.6 to 9.2) | 0.003 | 3.5 (1.5 to 8.6) | 0.005 |
| Female | 458 | 6 (1.3) | Reference | |||
| Year of sample | ||||||
| 2012 | 239 | 4 (1.7) | Reference | – | ||
| 2014–2015 | 426 | 17 (4.0) | 2.4 (0.8 to 7.3) | 0.11 | – | NS |
| 2017 | 420 | 15 (3.6) | 2.2 (0.7 to 6.6) | 0.17 | – | NS |
| Source of sample | ||||||
| Inpatient | 726 | 19 (2.6) | Reference | – | ||
| Blood donor | 359 | 17 (4.7) | 1.9 (0.95 to 3.6) | 0.07 | – | NS |
NS: not significant.
Discussion
Determination of ZIKV seroprevalence in dengue-endemic areas is difficult because of cross-reactivity among flaviviruses. Cross-neutralization of ZIKV is detectable early after DENV infection, but over time (≥6 months), neutralizing responses become more specific and most DENV-infected patients show either no or markedly lower ZIKV cross-neutralization.16,17 DENV antibody depletion experiments16 and antigenic maps17 suggest that ZIKV and DENV belong to different serocomplexes.
Therefore, in a region like Southeast Asia where DENV and potentially ZIKV are endemic, the presence of neutralizing titres to both viruses may indicate previous exposure to both. As the cumulative seroprevalence of DENV in Malaysia exceeds 90% by 70 y of age,18 it would be difficult to find serum showing ZIKV seropositivity alone. We therefore employed a strategy utilizing a highly specific screening ELISA (89–93%) to capture potential ZIKV infections,11–13 followed by neutralization tests to ZIKV and all four DENV serotypes. We report low possible and probable ZIKV seropositivity rates of 3.3% and 0.6%, respectively, which refutes the theory that high levels of population immunity explain the low incidence of Zika, at least in Kuala Lumpur. Increasing age and male gender were associated with seropositivity, as seen with DENV.6 This may reflect increased exposure to the shared Aedes vectors due to longer duration of exposure (older age) and gender-associated behaviours.
Several theories may explain the low ZIKV incidence in Southeast Asia.3,19–21 Diagnosis of Zika was likely missed in the past due to its similar clinical presentation with other tropical fevers, such as dengue, and a lack of diagnostic tools and surveillance programs. However, despite recent heightened surveillance, including reverse transcription polymerase chain reaction testing of thousands of dengue-negative cases, a number of Southeast Asian countries have reported low rates of Zika of 0.2–1.3%, including Cambodia,22 Laos,23 the Philippines,24 Vietnam25 and, as mentioned earlier, Malaysia.7 However, Thailand has reported the highest rates of ZIKV identified by surveillance, with 21% of suspect cases confirmed, suggesting persistent endemic transmission.26
Another possibility is that ZIKV is less transmissible by mosquitoes in Asia. There is evidence that A. aegypti and A. albopictus have lower competence for the Asian genotype of ZIKV compared with the African genotype.27,28 Within the Asian genotype, a virus from the recent Americas outbreaks was more infectious in A. aegypti than a virus from earlier ancestry circulating in Southeast Asia. This may be due to an NS1-A188V mutation (not seen in earlier Asian viruses) that increases NS1 antigenaemia in the mammalian host, increasing ZIKV infectivity in A. aegypti.29 Notably, ZIKV isolates from the Singapore outbreak in 2016 contained NS1-188V. During investigations of ZIKV cases in Malaysia, ZIKV was not detected in 122 Aedes mosquitoes (species not stated),30 in 255 A. albopictus captured around patients’ residences or in 37 contacts of patients in Sabah, East Malaysia.31 Monkeys are believed to be a sylvatic host of ZIKV in Africa, but there is also little recent evidence of this in long-tailed macaques, the most common macaque species in Malaysia.15,31 This may suggest a low level of transmission in Malaysia, but it is not clear whether this is due to the lower infectivity of the causative strains, as the identities of the NS1-188 residues are not available, or whether A. albopictus (at least in Sabah, where no A. aegypti was caught) has reduced competence for ZIKV. The most relevant study in the latter regard showed that A. albopictus from Singapore is highly competent for African ZIKV.32
Recent ZIKV seroprevalence studies from Southeast Asia are shown in Table 2. Although the number of studies and sample sizes are small and methodologies differ, two patterns are apparent. Countries such as Malaysia (this study), Laos33 and Vietnam34 may have lower levels of immunity of <10% and are at risk of large outbreaks. Other countries such as the Philippines,34 Cambodia,35 Thailand34,36 and Indonesia34,37 may have high population immunity, which may restrict transmission to small clusters.
Table 2.
Recent ZIKV seroprevalence studies in Southeast Asia
| Location | ZIKV assays used (criteria for positive result) | Other flaviviruses tested | Population | Sampling period | Seroprevalence |
|---|---|---|---|---|---|
| Kuala Lumpur, Malaysia (this study) | NS1 BOB ELISA, PRNT, FRNT (possible ZIKV-seropositive, PRNT50 or FRNT50 titres ≥20) | FRNT DENV1–4 | 1085 urban adults and children | 2012, 2014–2015, 2017 | 3.3% |
| Vientiane, Laos33 | Commercial IgG ELISA and virus neutralization test (titre ≥40) | None | 359 urban adults | 2003–2004 | 4.5% |
| 687 urban adults | 2015 | 9.9% | |||
| Indonesia (30 sites)37 | PRNT (ZIKV PRNT90 titres ≥10 and ≥4-fold DENV PRNT90 or negative DENV PRNT90) | PRNT DENV1–4 | 662 urban children, 1–4 y old | 2014 | 9.1% (0–18.2% across different sites) |
| Kampong Cham province, Cambodia35 | Western blot | None | 200 adults and children | 2016 | 63% |
| Bangkok, Thailand36 | PRNT (PRNT50 ≥10) | PRNT JEV, DENV1–4 | 135 urban adults | 2017 | 70.4% (PRNT50 ≥10), 55.6% (PRNT50 ≥20) or 22.2% (PRNT90 ≥20) |
| Migrant workers in Taiwan34 from | Commercial IgG ELISA; only a subset tested with PRNT | DENV IgG ELISA and a subset tested with PRNT | Adults >20 y of age; 150 from each country | 2017 | |
| Indonesia | 43.3% | ||||
| Philippines | 55.3% | ||||
| Thailand | 50.7% | ||||
| Vietnam | 6.0% |
JEV: Japanese encephalitis virus.
A recent model estimated up to 785 million ZIKV infections in Asia should an epidemic occur in immunologically naïve populations; this included 8.5 (range 7.1–9.6) million cases in Malaysia out of a current population of 31 million.38 Since immunity may vary substantially even within countries due to differing populations, the intensity of flavivirus transmission, the order of DENV serotype infections and many other factors, local seroprevalence studies are important to identify at-risk groups or areas for planning preparedness and responses.
The limitations of our study include the testing of samples from only Kuala Lumpur, which was chosen because it is an area with one of the highest DENV transmission rates in Malaysia. Extending this study nationwide would be important, particularly in rural areas where A. albopictus is in greater abundance,39 in view of the possible involvement of A. albopictus in ZIKV transmission in Sabah, East Malaysia.31 The duration of detectable neutralizing antibodies after ZIKV infection is unknown, but antibodies are present at least 1 y later11,13 and are believed to persist for life.
The strengths of our study include the large sample size, comprehensive age coverage and testing for potentially cross-reacting DENV antibodies. We reaffirm that a good approach for seroprevalence studies is to screen with ELISA and confirm with more labour-intensive, specific neutralization assays.40 We used a well-characterised ZIKV NS1 BOB assay to screen a dengue-hyperendemic population expected to have high rates of DENV seropositivity, yet only 7.6% were reactive, confirming the high specificity of this assay.11,12 In contrast, the commercial ZIKV IgG ELISA used to screen samples in similarly dengue-endemic Laos resulted in 28.8% reactive/equivocal samples needing neutralization testing.33 We also showed no differences in ZIKV seropositivity in healthy blood donors or ill inpatients. This is a useful finding, as it allays concerns that using residual serum from inpatients (which is more readily available) for ZIKV seroprevalence studies may be affected by undiagnosed acute flaviviral infections.
In conclusion, the low rate of ZIKV neutralizing antibodies in a Kuala Lumpur population indicates high vulnerability to a future epidemic. There is a surprisingly low incidence of ZIKV infections in Southeast Asia, the source of ZIKV strains causing recent large epidemics in the Pacific and the Americas. The reasons for this remain unclear and warrant further investigation for a fuller understanding of the epidemiology of this emerging virus.
Supplementary Material
Authors’ contributions: I-CS, EH and YFC conceived the study. I-CS, EH, MM and YFC designed the study protocol. MM, CLC and AP implemented the study. All authors analysed and interpreted the data. I-CS and EH drafted the manuscript. All authors read, revised and approved the final manuscript. I-CS and EH are guarantors of the paper.
Acknowledgments: We thank Davide Corti at Humabs Biomed, a subsidiary of Vir Biotechnology, for his generous donation of HRP-conjugated ZKA35 monoclonal antibody. We also thank the European Virus Archive for providing the ZIKV isolate.
Funding: This study was funded in part by a National Institute of Allergy and Infectious Diseases/National Institutes of Health grant (P01 AI106695 to EH) and a grant from the Malaysia One Health University Network and the US Agency for International Development (to I-CS).
Competing interests: None declared.
Ethical approval: This study was approved by the Medical Research Ethics Committee of the University of Malaya Medical Centre (2017116-5794).
References
- 1. Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411–5. [DOI] [PubMed] [Google Scholar]
- 2. Lim SK, Lim JK, Yoon IK. An update on Zika virus in Asia. Infect Chemother. 2017;49(2):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pettersson JH, Bohlin J, Dupont-Rouzeyrol M, et al. Re-visiting the evolution, dispersal and epidemiology of Zika virus in Asia. Emerg Microbes Infect. 2018;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faria NR, Quick J, Claro IM, et al. Establishment and cryptic transmission of Zika virus in Brazil and the Americas. Nature. 2017;5(7658):406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singapore Zika Study Group Outbreak of Zika virus infection in Singapore: an epidemiological, entomological, virological, and clinical analysis. Lancet Infect Dis. 2017;17(8):813–21. [DOI] [PubMed] [Google Scholar]
- 6. Mohd-Zaki AH, Brett J, Ismail E, et al. Epidemiology of dengue disease in Malaysia (2000–2012): a systematic literature review. PLoS Negl Trop Dis. 2014;8(11):e3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ministry of Health Malaysia Press statement of the Director-General of Health Malaysia: current situation for dengue, Zika and chikungunya in Malaysia for 2017. Putrajaya, Malaysia: Ministry of Health; 2017. http://www.moh.gov.my/index.php/database_stores/store_view_page/21/965 [accessed 20 June 2019]. [Google Scholar]
- 8. Pond WL. Arthropod-borne virus antibodies in sera from residents of Southeast Asia. Trans R Soc Trop Med Hyg. 1963;57(5):364–71. [DOI] [PubMed] [Google Scholar]
- 9. Wolfe ND, Kilbourn AM, Karesh WB, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64(5–6):310–6. [DOI] [PubMed] [Google Scholar]
- 10. Tappe D, Nachtigall S, Kapaun A, et al. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis. 2015;21(5):911–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balmaseda A, Stettler K, Medialdea-Carrera R, et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci USA. 2017;114(31):8384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balmaseda A, Zambrana JV, Collado D, et al. Comparison of four serological methods and two reverse transcription-PCR assays for diagnosis and surveillance of Zika virus infection. J Clin Microbiol. 2018;56(3):e01785-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zambrana JV, Bustos Carrillo F, Burger-Calderon R, et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc Natl Acad Sci USA. 2018;115(37):9294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henchal EA, Gentry MK, McCown JM, et al. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg. 1982;31(4):830–6. [DOI] [PubMed] [Google Scholar]
- 15. Chua CL, Chan YF, Rovie-Ryan JJ, et al. Little evidence of Zika virus infection in wild long-tailed macaques, Peninsular Malaysia. Emerg Infect Dis. 2019;25(2):374–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins MH, McGowan E, Jadi R, et al. Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis. 2017;23(5):773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montoya M, Collins M, Dejnirattisai W, et al. Longitudinal analysis of antibody cross-neutralization following Zika virus and dengue virus infection in Asia and the Americas. J Infect Dis. 2018;218(4):536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chew CH, Woon YL, Amin F, et al. Rural-urban comparisons of dengue seroprevalence in Malaysia. BMC Public Health. 2016;16:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Musso D, Lanteri MC. Zika virus in Singapore: unanswered questions. Lancet Infect Dis. 2017;17(8):782–3. [DOI] [PubMed] [Google Scholar]
- 20. Bhardwaj S, Gokhale MD, Mourya DT. Zika virus: current concerns in India. Indian J Med Res. 2017;146(5):572–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khongwichit S, Wikan N, Auewarakul P, et al. Zika virus in Thailand. Microbes Infect. 2018;20(11–12):670–5. [DOI] [PubMed] [Google Scholar]
- 22. Duong V, Ong S, Leang R, et al. Low circulation of Zika virus, Cambodia, 2007–2016. Emerg Infect Dis. 2017;23(2):296–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Institut Pasteur du Laos Arbovirus surveillance. 2016. http://www.pasteur.la/project-carried-on-in-the-lab-7/arbovirus-surveillance/ [accessed 20 June 2019].
- 24. Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis. 2015;21(4):722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quyen NTH, Kien DTH, Rabaa M, et al. Chikungunya and Zika virus cases detected against a backdrop of endemic dengue transmission in Vietnam. Am J Trop Med Hyg. 2017;97(1):146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruchusatsawat K, Wongjaroen P, Posanacharoen A, et al. Long-term circulation of Zika virus in Thailand: an observational study. Lancet Infect Dis. 2019;19(4):439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chouin-Carneiro T, Vega-Rua A, Vazeille M, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis. 2016;10(3):e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roundy CM, Azar SR, Rossi SL, et al. Variation in Aedes aegypti mosquito competence for Zika virus transmission. Emerg Infect Dis. 2017;23(4):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y, Liu J, Du S, et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature. 2017;545(7655):482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ministry of Health Malaysia Press statement of the Director-General of Health Malaysia: current situation for Zika in Malaysia (22 September 2016). Putrajaya, Malaysia: Ministry of Health; 2016. http://www.moh.gov.my/index.php/database_stores/store_view_page/21/792 [accessed 20 June 2019].
- 31. Dony JJF, Ibrahim MY, Jeffree MS, et al. The first outbreak of autochthonous Zika virus in Sabah, Malaysian Borneo. PLOS Curr Outbreaks. Edition 1. http://currents.plos.org/outbreaks/index.html%3Fp=77803.html [accessed 20 June 2019]. [Google Scholar]
- 32. Wong PS, Li MZ, Chong CS, et al. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis. 2013;7(8):e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pastorino B, Sengvilaipaseuth O, Chanthongthip A, et al. Low Zika virus seroprevalence in Vientiane, Laos, 2003–2015. Am J Trop Med Hyg. 2019;100(3):639–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perng GC, Ho TC, Shih HI, et al. Seroprevalence of Zika and dengue virus antibodies among migrant workers, Taiwan, 2017. Emerg Infect Dis. 2019;25(4):814–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. San K, Rajadhan V. Seroprevalence of Zika virus in Cambodia: a preliminary report. Adv Lab Med Int. 2016;6:37–40. https://advlabmedint.simdif.com/page-26427321.html [accessed 20 June 2019]. [Google Scholar]
- 36. Sornjai W, Jaratsittisin J, Auewarakul P, et al. Analysis of Zika virus neutralizing antibodies in normal healthy Thais. Sci Rep. 2018;8(1):17193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sasmono R, Dhenni R, Yohan B, et al. Zika virus seropositivity in 1–4-year-old children, Indonesia, 2014. Emerg Infect Dis. 2018;24(9):1740–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siraj AS, Perkins TA. Assessing the population at risk of Zika virus in Asia—is the emergency really over? BMJ Glob Health. 2017;2:e000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saifur RG, Hassan AA, Dieng H, et al. Update on temporal and spatial abundance of dengue vectors in Penang, Malaysia. J Am Mosq Control Assoc. 2012;28(2):84–92. [DOI] [PubMed] [Google Scholar]
- 40. Nurtop E, Villarroel PMS, Pastorino B, et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J. 2018;15:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.