Abstract
Background:
The Phase III OlympiAD study () showed a statistically significant progression-free survival benefit with olaparib versus chemotherapy treatment of physician’s choice (TPC) in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. From this study, we report the effect of olaparib on health-related quality of life (HRQoL).
Methods:
Patients were randomized 2:1 to olaparib monotherapy (300 mg bid) or single-agent TPC. The primary HRQoL endpoint was mean change from baseline in the two-item global health status/QoL score determined from patient-completed EORTC QLQ-C30 questionnaires and assessed using a mixed model for repeated measures. Symptoms and functioning domains, best overall response and time to deterioration of QoL were also evaluated.
Results:
Overall questionnaire compliance rates were 93.2% for olaparib and 76.3% for TPC. Between-treatment global health status/QoL comparison showed a significant improvement in the olaparib arm versus the TPC arm, with mean change 3.9 (SD 1.2) versus −3.6 (2.2), a difference of 7.5 points (95% CI 2.48, 12.44; P=0.0035). A higher proportion of patients in the olaparib arm showed a best overall response of ‘improvement’ in global health status/QoL (33.7% vs 13.4%). Median time to global health status/QoL deterioration was not reached in olaparib patients and was 15.3 months for TPC patients (hazard ratio 0.44 [95% CI 0.25, 0.77]; P=0.004). For QLQ-C30 symptoms and functioning subscales, only nausea/vomiting symptom score was worse in the olaparib arm compared with TPC (across all visits compared with baseline).
Conclusion:
HRQoL was consistently improved for patients treated with olaparib, compared with chemotherapy TPC.
Keywords: Olaparib, OlympiAD, health-related quality of life, EORTC QLQ-C30, breast cancer, BRCA
Introduction
The management of metastatic breast cancer (mBC) is an ongoing challenge, characterized by low median overall survival of 2–3 years and 5-year survival of ~25%.1 Patients with metastatic, triple-negative breast cancer represent an even greater treatment challenge, with median overall survival of ~1 year.2 It is now recognized that antitumour therapy for patients with mBC should be implemented with the dual goals of prolonging patient survival and optimizing their quality of life (QoL).1 Additionally, routine care of cancer patients should also include readiness of healthcare professionals to modify treatment strategies based on treatment efficacy, disease characteristics, adverse drug reactions, and patient-reported outcomes (PROs; symptoms, functioning, and health-related quality of life [HRQoL]).1
For patients with functional deficiency in BRCA1 and/or BRCA2 (BRCAm) and human epidermal growth factor receptor 2 (HER2)-negative mBC, chemotherapy may help reduce disease symptoms and prolong survival, but it is also associated with substantial toxicity that can detrimentally impact on patients’ QoL.3,4 Poly(ADP-ribose) polymerase (PARP) inhibitors have recently emerged as new treatment options for patients with BRCAm HER2-negative mBC.5,6 The Phase III OlympiAD study in patients with germline BRCAm (gBRCAm) HER2-negative mBC showed that olaparib monotherapy resulted in a statistically significant and clinically meaningful progression-free survival (PFS) benefit versus chemotherapy treatment of physician’s choice (TPC): 7.0 versus 4.2 months; hazard ratio (HR) 0.58; 95% confidence interval (CI) 0.43–0.80; P<0.001.5 Furthermore, olaparib-treated patients had a lower incidence of grade ≥3 adverse events versus TPC (36.6% vs 50.5%). Based on these results, olaparib became the first approved targeted therapy for patients with gBRCAm HER2-negative mBC who were previously treated with chemotherapy.7
A pre-specified secondary objective of the OlympiAD study was to assess the effect of olaparib on HRQoL using the European Organisation for Research and Treatment of Cancer Quality-of-Life Questionnaire Core 30-item module (EORTC QLQ-C30) global QoL scale. We report full results regarding these longitudinal HRQoL assessments in the OlympiAD trial.
Methods
Study design and patient population
The OlympiAD study design and patient population have been fully reported in the primary analyses.5 Briefly, OlympiAD was a Phase III, international, randomized, open-label study () of patients aged ≥18 years with gBRCAm HER2-negative mBC (triple negative or hormone receptor positive) who had received ≤2 previous chemotherapy regimens. Patients were randomized 2:1 using an interactive voice or web response system to olaparib tablets (300 mg twice daily) or single-agent, pre-defined, chemotherapy TPC (capecitabine, eribulin or vinorelbine - see Supplementary Materials for dosage).
Study outcome measures
Primary and secondary efficacy and safety outcomes are described fully in the primary manuscript.5 The pre-specified PROs reported here were assessed using the paper based 30-item EORTC QLQ-C30 questionnaire completed by the patient at baseline (before randomization once eligibility was confirmed) and every 6 weeks until investigator-assessed objective disease progression. Patients who discontinued treatment because of toxicity continued PRO assessments until disease progression. The EORTC QLQ-C30 includes: a two-item global QoL scale (global health status/QoL score); five multi-item functional scales (physical, role, emotional, cognitive, social); three multi-item symptom scales (fatigue, pain, nausea/vomiting); five single items assessing common cancer symptoms (dyspnoea, insomnia, appetite loss, constipation, diarrhoea); and a single item addressing the financial impact of disease.8 Scores for the QLQ-C30 range from 0 to 100. For global health status/QoL score and functional scales, higher scores indicate better HRQoL and level of functioning. For symptom scales, higher scores indicate greater severity of symptoms. The impact of olaparib on symptoms and HRQoL as assessed by multi-item functional and symptoms scales and common cancer symptoms were exploratory analyses.
The primary HRQoL endpoint compared mean change from baseline between treatment arms in the two-item global health status/QoL score across all visits. Secondarily, we assessed the proportion of patients in each arm who experienced a clinically meaningful increase (improvement) or decrease (deterioration) in global health status/QoL score (defined as ≥10-point change from baseline).9 Best overall HRQoL response was defined as the best HRQoL response the patient achieved from randomization to disease progression. Best HRQoL response was categorized as ‘improved’, ‘no change’, ‘deterioration’, or ‘other’ according to the following criteria: ‘improved’ – two visit responses of ‘improved’ sustained for ≥21 days with no intervening response of ‘deterioration’; ‘no change’ – two visit responses of either ‘no change’ or ‘improved’ and ‘no change’ ≥21 days apart with no intervening response of ‘deterioration’; ‘deterioration’ – a visit response of ‘deterioration’ without a response of ‘improved’ or ‘no change’ within 21 days; ‘other’ – patients who met the criteria for ‘improved’, ‘no change’ or ‘deterioration’. For additional information on continued treatment effectiveness, an analysis of the number of patients remaining on treatment for ≥6 months and time to subsequent therapies are shown in Supplementary Materials.
Statistical analysis
Sample-size determination has been reported in the primary manuscript.5 Questionnaire compliance and completion data were analysed overall and by study visit and summarized by treatment. All PRO data were analysed using the full analysis set on an intention-to-treat basis that included all randomized patients with a baseline and ≥1 post-baseline assessment. For the primary HRQoL analysis, a linear mixed-model repeated-measures (MMRM) analysis adjusting for score at baseline, time, and treatment-by-time interaction was used to estimate the cumulative effect of olaparib versus TPC on global health status/QoL. MMRM methodology uses observed data to implicitly impute unobserved data; by including covariates in the model, the analysis assumes that patients with missing data would behave similarly to other patients with similar values for these covariates (eg treatment group) had they not missed the assessment. The analysis included all post-baseline visits up to the last scheduled visit in which ≥20 patients in each treatment arm had an evaluable score. Differences between treatment groups were compared using adjusted mean estimates per treatment group and corresponding 95% CIs and P values.
Best overall global health status/QoL response rates (improvement, no change or deterioration) were summarized descriptively for patients. Multi-item functional and symptom subscales and single-item cancer symptoms were exploratory variables. For global health status/QoL and multi-item functional subscales, best overall response was evaluated in patients with baseline score ≥10. For multi-item symptom subscales and single-item symptom scales, best overall response was evaluated in patients with baseline score ≤90.
Time to deterioration (TTD) of global health status/QoL (planned analysis) and functional and symptom scales (post hoc analysis) was defined as time from randomization until the date of a clinically important deterioration in the global health status/QoL score or functional and symptom scores. For multi-item functional and global health status/QoL subscales, deterioration included patients with baseline score ≥10; for symptom subscales/single items, deterioration included patients with baseline score ≤90. Clinically important deterioration was defined as a decrease from baseline of ≥10 points (or an increase from baseline of ≥10 points for the symptom scales) that was sustained at the next scheduled visit. Patients who did not experience deterioration by the time of progression (PRO data beyond progression were not collected) were censored at the time of the last-available PRO assessment. Data were analysed using a log-rank test, with HR and 95% CI generated from the log-rank test statistics. The Kaplan–Meier method was used to estimate medians.
Results
Study population characteristics
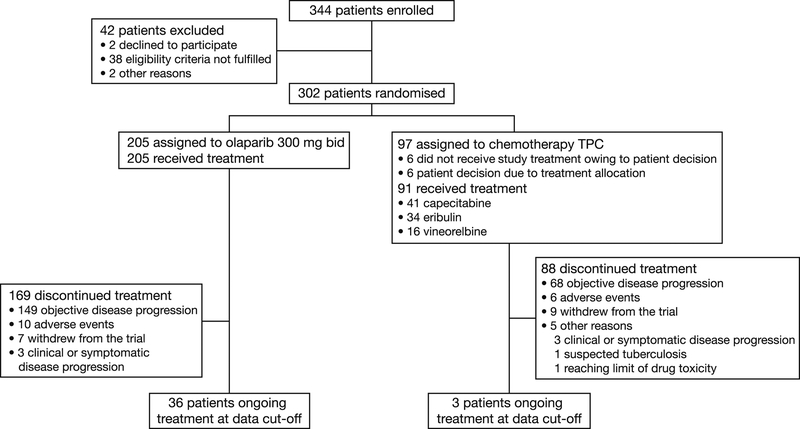
In total, 302 patients (intention-to-treat population) were randomly assigned to treatment (olaparib, n=205; TPC, n=97) between 7 April 2014 and 27 November 2015. Of the 97 TPC patients, 91 received study treatment (capecitabine, n=41; eribulin, n=34; vinorelbine, n=16). A participant flow diagram (Figure 1) and baseline demographic characteristics, which were well balanced between the two treatment groups, have been described.5 Mean (standard deviation [SD]) global health status/QoL score (scale range 0–100; higher scores indicate higher levels of function and QoL) at baseline was 63.2 (21.0) for the olaparib treatment arm and 63.3 (21.2) for TPC (capecitabine 62.3 [19.6], eribulin 59.1 [22.1], vinorelbine 67.7 [21.5]).5 Multi-item symptom scores at baseline in both treatment arms were similar to those cited for recurrent or mBC in the EORTC QLQ-C30 reference values manual.10
Fig. 1.
Participant flow diagram. bid, twice daily; TPC, treatment of physician's choice.
Questionnaire compliance/completion
Compliance and completion rates for EORTC QLQ-C30 at baseline were >95% in both arms (Table 1). Completion rates declined faster in the TPC arm.
Table 1.
Compliance and completion rates for the EORTC QLQ-C30 questionnaire
| Time point | Expected forms, n | Compliance rate,a % | Completion rate,b % | |||
|---|---|---|---|---|---|---|
| Olaparib | TPC | Olaparib (n=205) | TPC (n=97) | Olaparib (n=205) | TPC (n=97) | |
| Baseline | 205 | 97 | 99.0 | 95.9 | 99.0 | 95.9 |
| Visit 6 (week 6) | 205 | 96 | 87.3 | 71.9 | 87.3 | 71.1 |
| Visit 8 (week 12) | 184 | 73 | 87.0 | 71.2 | 78.0 | 53.6 |
| Visit 10 (week 18) | 167 | 50 | 84.4 | 70.0 | 68.8 | 36.1 |
| Visit 12 (week 24) | 136 | 36 | 84.6 | 63.9 | 56.1 | 23.7 |
| Visit 14 (week 30) | 115 | 30 | 84.4 | 63.3 | 47.3 | 19.6 |
| All visits (overall)c | 205 | 97 | 93.2 | 76.3 | 93.2 | 76.3 |
EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module; PRO, patient-reported outcome; TPC, treatment of physician’s choice.
Note that 19 assessments are included whereby the questionnaire was administered to a caregiver, relative, or unspecified person, rather than directly to the patient.
Compliance rate for each treatment arm was defined as (number of evaluable questionnaires [defined as a questionnaire with enough responses to score at least one scale/domain])/(number of patients expected to complete questionnaires at each visit [ie patients still under PRO follow-up]) × 100%.
Completion rate for each treatment arm was defined as (number of received questionnaires)/(PRO analysis population) × 100%.
For overall, patients were counted as received/evaluable if they had a received/evaluable baseline and at least one received/evaluable post-baseline form.
Global health status/QoL
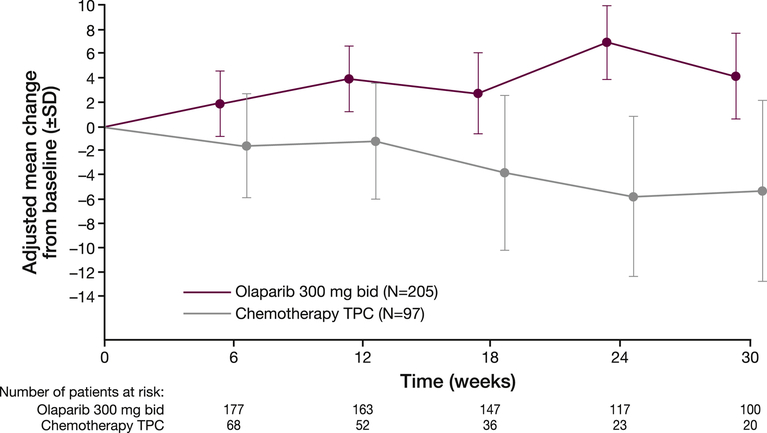
The between-treatment comparison (average over time) based on an MMRM model showed a clinically significant improvement from baseline in mean (SD) global health status/QoL score for olaparib versus TPC (3.9 [1.2] vs −3.6 [2.2]; difference 7.5 [95% CI 2.48–12.44]; P=0.0035).5 The adjusted mean change from baseline over time (Figure 2) showed that, for each visit, patients in the olaparib arm had an improvement in mean global health status/QoL score, whereas patients in the TPC arm had a decline.
Fig. 2.
Adjusted mean (SD) change from baseline in EORTC QLQ-C30 global health status/quality of life score across time points in patients in the olaparib and TPC arms. bid, twice daily; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module; SD, standard deviation; TPC, treatment of physician's choice. A higher score represents better overall health-related quality of life. Note that data are restricted to visits with at least 20 patients in each treatment arm.
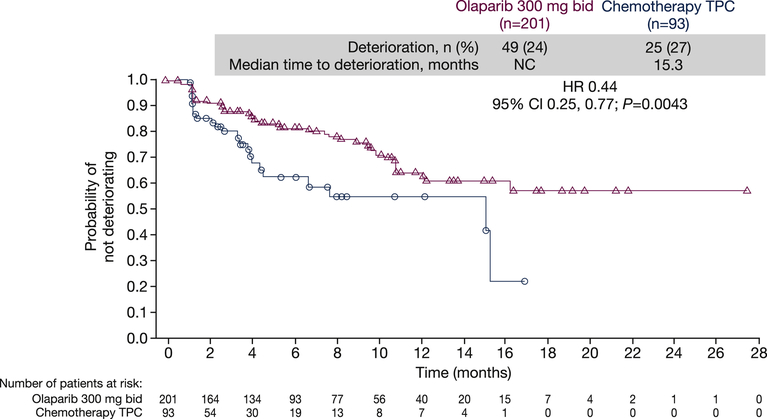
Median TTD in global health status/QoL (≥10 points) was not reached in the olaparib arm and was 15.3 months in the TPC arm (HR 0.44; 95% CI 0.25–0.77; P=0.004; Figure 3).5 Global scores had deteriorated in fewer patients in the olaparib arm versus TPC at 6 months (18.5% vs 38.8%) and 12 months (36.0% vs 46.5%). Censoring of the majority of olaparib-treated patients because they did not meet the 10-point threshold criterion for deterioration means that these TTD data should be interpreted with caution (see Discussion).
Fig. 3.
Kaplan–Meier plot of time to deterioration in EORTC QLQ-C30 global health status/quality of life at progression. bid, twice daily; CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module; HR, hazard ratio; NC, not calculable; QoL, quality of life; TPC, treatment of physician's choice. Deterioration was defined as the onset of a ≥10-point decrease in global health status/QoL score from baseline. Patients whose global health status/QoL score did not show a clinically important deterioration and who were alive at the time of analysis, or who experienced deterioration in global health status/QoL or death after two or more missed assessments, were censored at the latest evaluable EORTC QLQ-C30 assessment.
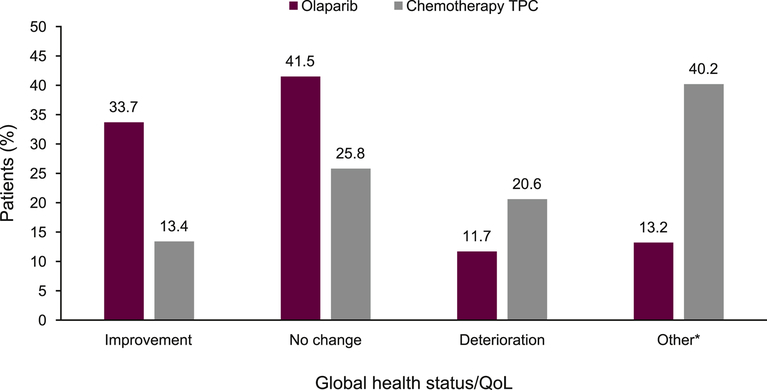
More patients in the olaparib treatment arm showed an improvement in best overall response rates for global health status/QoL versus TPC (33.7% vs 13.4%), and more patients in the TPC arm showed a deterioration in global health status/QoL versus olaparib (20.6% vs 11.7%) (Figure 4).
Fig. 4.
Best overall response (improvement, no change and deterioration) rates for EORTC QLQ-C30 global health status/quality of life. QoL, quality of life; TPC, treatment of physician's choice. Best overall response of improvement and deterioration were defined as a ≥10-point change from baseline and sustained for at least 21 days (for ‘improvement’, without an intervening visit response of ‘deterioration’; for ‘deterioration’, without an intervening response of ‘improved’ or ‘no change’). *‘Other’ includes patients with a best response of ‘other’, defined as patients who did not demonstrate improvement, no change or deterioration, and those with missing follow-up data or non-evaluable patients (owing to baseline scores being outside the limit). Patients typically classed as ‘other’, for example, did not have a sustained response observed at the next visit but had one visit response of improvement, followed by a deterioration, then a visit with no change.
Functional subscales
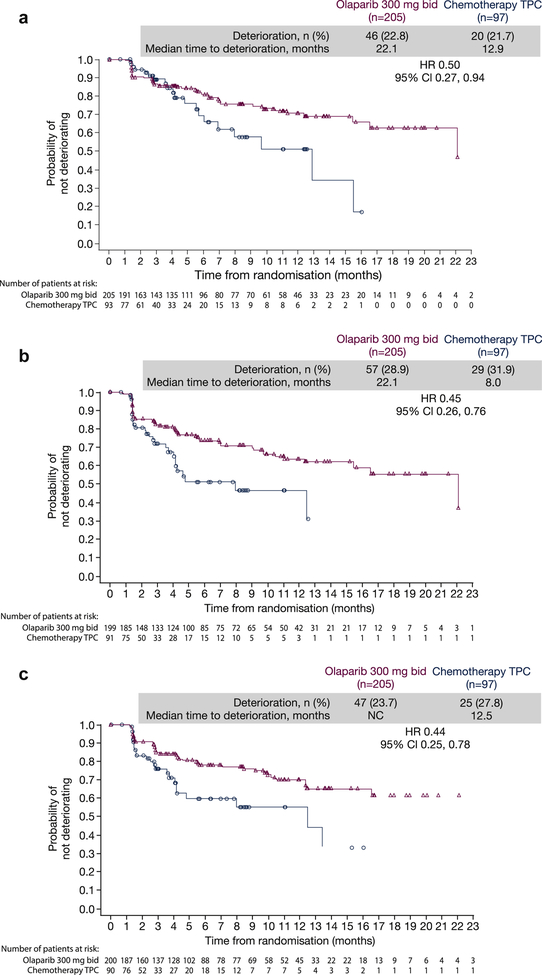
Kaplan–Meier estimates of TTD (before or at progression) showed that olaparib delayed TTD versus TPC for each of the five functional subscales (Figure 5).
Fig. 5.
Kaplan–Meier estimates of time to deterioration for EORTC QLQ-C30 functional subscales before or at progression. (a) Physical; (b) role; (c) social; (d) cognitive and (e) emotional. bid, twice daily; CI, confidence interval; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module; HR, hazard ratio; NC, not calculable; TPC, treatment of physician's choice.
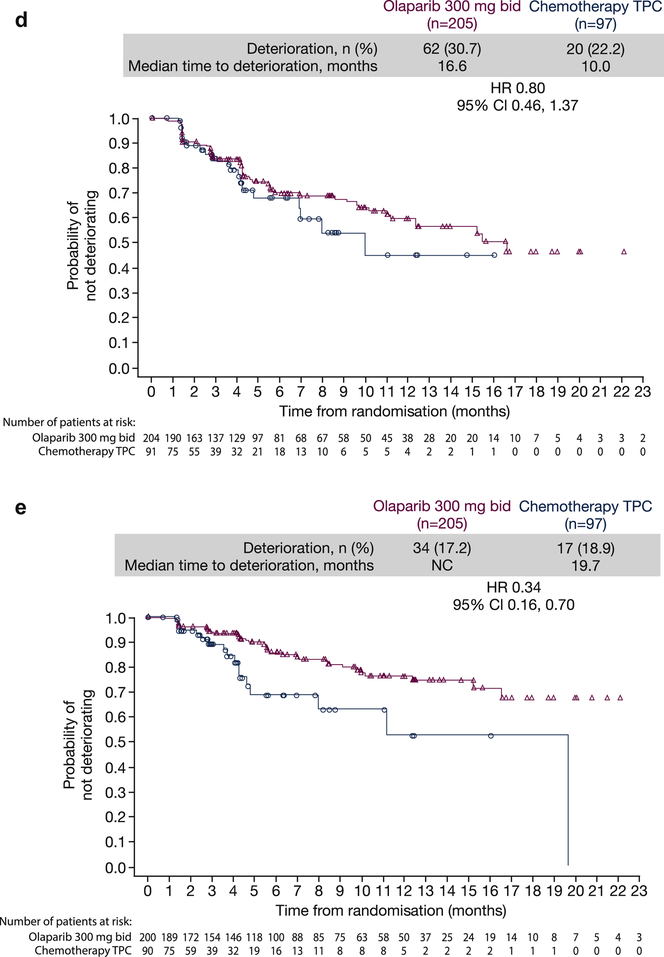
The best overall response data for the functional subscales support the results for global health status/QoL and highlight that, across all five functional subscales, more patients experienced an improvement in functional subscales in the olaparib treatment arm versus TPC (Supplementary Figure 1A); concordantly, more patients experienced a deterioration in functional subscales in the TPC arm versus olaparib.
Symptom subscales
For fatigue, pain, and nausea/vomiting multi-item symptom scales (decrease in score indicates improvement in symptoms), as well as single-item symptom scales, a higher proportion of patients in the olaparib treatment arm experienced improvements versus TPC (Supplementary Figure 1B and 1C).
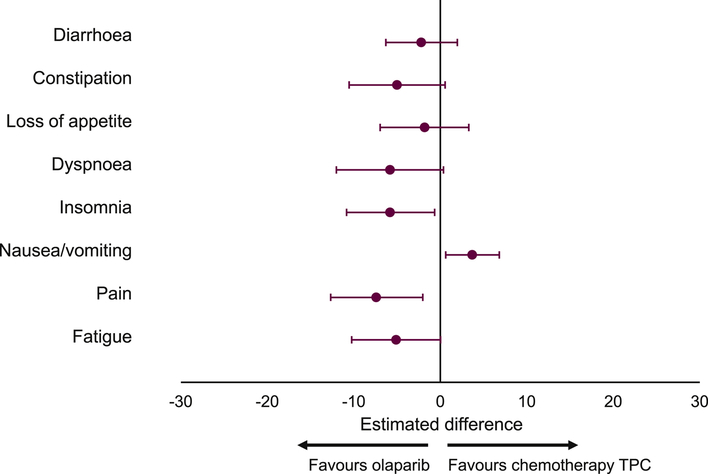
Adjusted mean changes from baseline in symptom scores across all visits (Figure 6) showed improvements in fatigue, pain, dyspnoea, insomnia, loss of appetite, constipation, and diarrhoea scores among patients receiving olaparib versus patients receiving TPC. Only mean nausea/vomiting scores were better (relative to baseline) in the TPC arm versus olaparib. Apart from nausea/vomiting, the HR for TTD favoured olaparib versus TPC across all symptom scales (Supplementary Figure 2).
Fig. 6.
Estimated difference (olaparib vs TPC) in adjusted mean change from baseline in EORTC QLQ-C30 symptom subscale scores (all visits). EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module; TPC, treatment of physician's choice.
Time to subsequent therapies
Results of the analyses of time to subsequent therapies are presented in Supplementary Materials.
Discussion
Optimizing HRQoL is a key component in the guidelines for treatment and management of mBC as treatment is usually not curative but palliative.1 Our findings show that the efficacy benefits reported with olaparib among patients with gBRCAm HER2-negative mBC are accompanied by improvements in symptoms, functioning, and HRQoL assessed using EORTC QLQ-C30.
Adjusted mean change from baseline in global health status/QoL score across all visits revealed greater improvements with olaparib versus TPC. Overall, HRQoL in patients receiving olaparib improved during time on treatment versus patients receiving TPC, in whom a deterioration in HRQoL over time was observed. The difference between arms is consistent with a small, clinically significant improvement (7.5-point difference lies within the 5- to 10-point change designated as small [moderate, 10–20; large, >20]) on a group-level assessment.9,11 TTD was also extended in the olaparib arm versus TPC. However, the median TTD findings should be interpreted cautiously as PRO data were collected only until disease progression, and most patients had not reached the 10-point threshold criterion for deterioration at this time. Patients who did not meet the deterioration criteria were censored at the time of their last global health status/QoL assessment. Given these limitations, the best overall response represents a more instructive assessment to determine the impact of treatments on HRQoL. The findings of best overall response showed that olaparib-treated patients were almost three times as likely as those receiving TPC to report a clinically significant improvement in global health status/QoL.
EORTC QLQ-C30 functional and symptom subscale scores were generally improved in the olaparib arm versus TPC. Marked improvements in functioning favoured olaparib over TPC, as measured on the physical, cognitive, and emotional subscales. For each symptom subscale, more patients in the olaparib group showed a best overall response of a clinically meaningful improvement in symptom score versus TPC. Single symptoms included in the scale are considered as being important to patients with breast cancer. Our findings showed particular benefits of olaparib over TPC in terms of improved pain and dyspnoea. For patients who experienced a deterioration in symptoms, this was typically greater for those receiving TPC versus olaparib.
Differences in adjusted mean change from baseline in symptom scores for nausea/vomiting favoured TPC. This is a known effect of olaparib that has been shown to be greater during treatment initiation.12 Indeed, a high proportion of patients receiving olaparib reported an improvement in the nausea/vomiting subscale over time. Previously reported safety data from the OlympiAD trial showed that more patients experienced nausea/vomiting with olaparib versus TPC, but no grade ≥3 events were reported and discontinuation rates were low.5,13 We found that, although a higher proportion of olaparib-treated patients reported a best overall response of improvement in nausea/vomiting, differences in adjusted mean change from baseline in symptom scores for nausea/vomiting favoured TPC. These data are hard to reconcile but suggest that patients whose symptoms do not improve experience a greater decrease in score. In clinical practice, supportive measures such as early antiemetic administration may be appropriate to manage events of nausea and vomiting, thus minimizing olaparib dose adjustments/discontinuations. The improvements in HRQoL in the olaparib arm versus TPC are consistent with the lower rate of discontinuations due to adverse events (4.9% vs 7.7%) and the longer median treatment duration (8.2 [range 0.5–28.7] vs 3.4 [0.7–23.0] months) reported previously in the OlympiAD trial.5 The supportive analyses of time to subsequent therapies (see Supplementary Materials) further expand on these data, showing that more patients in the olaparib arm remained on treatment for ≥6 months versus TPC (60.0% vs 27.5%), and median time to first and second subsequent therapy were longer with olaparib versus TPC (9.4 vs 4.2 months and 14.3 vs 10.5 months, respectively); time on assigned treatment has been recognized as a meaningful clinical-trial endpoint in settings whereby a disease course involves multiple rounds of subsequent treatment after first progression.14
Limitations of the study include the open-label trial design, which has the potential to introduce patient biases. In addition, the absence of post-progression PRO data collection may have underestimated the impact of olaparib on HRQoL given that patients receiving TPC had disease progression earlier than those receiving olaparib, and progression would be expected to result in a further decrease in HRQoL. However, this would also be dependent on the HRQoL impact of post-progression treatment. Questionnaire compliance and completion rates were lower in the TPC arm versus olaparib (76.3% vs 93.2%), and completion rates also declined faster in the TPC arm because of earlier progression and cessation of EORTC QLQ-C30 data collection. This higher rate of missing data may also have biased the results of the analysis. The direction of any bias on the effect of treatment is unclear as it depends on whether missing data would have improved or worsened PRO measures for the TPC arm. However, the selective aspect of completing EORTC QLQ-C30 data collection is likely to be largely explained by observed covariates included in the MMRM analysis, ie treatment arm and baseline HRQoL score, which assumes that patients with missing data would behave similarly to other patients in the same treatment group, and with similar covariate values, had they not missed the assessment. Studies have shown that, when such observed covariates are included, the adjusted estimates are robust even when data are not missing completely at random.15
Favourable PROs have also been reported with another PARP inhibitor among patients with gBRCAm advanced breast cancer.6 Improvement in global health score from baseline with talazoparib in the EMBRACA trial (3.0) was similar to that reported here (3.9). Our findings and those of EMBRACA provide further support of the beneficial effects of PARP inhibitors on HRQoL versus chemotherapy in patients with mBC. Based on these data, recently updated advanced breast cancer (ABC4) and National Comprehensive Cancer Network guidelines recommend PARP inhibitors as a treatment option for BRCA-associated HER2-negative mBC treated with an anthracycline with or without a taxane in the adjuvant and/or metastatic setting.16,17
Conclusions
The planned and post hoc analyses of data we report from the Phase III OlympiAD study suggest that olaparib treatment can lead to improvements in the symptoms, functioning, and HRQoL of patients with gBRCAm HER2-negative mBC versus chemotherapy TPC. PRO results will be valuable to both physicians and patients when considering the clinical benefits of PARP inhibitor treatment in mBC, versus chemotherapy.
Supplementary Material
Highlights.
Quality of life (QoL) during treatment was assessed by EORTC QLQ-C30 questionnaire
Significant improvement from baseline in QoL score for olaparib versus chemotherapy
More patients receiving olaparib showed an improvement in functional subscales
Only nausea/vomiting symptom score was worse with olaparib versus chemotherapy
Acknowledgements:
Medical writing assistance was provided by Debbi Gorman, PhD, and Martin Goulding, DPhil, from Mudskipper Business Ltd, funded by AstraZeneca and MSD.
Funding: This study was supported by AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA (MSD), who are co-developing olaparib.
Role of the funding source: The study sponsor, AstraZeneca, was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data access, responsibility and analysis
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Mark Robson, Wendy Bannister and Carsten Goessl had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Wendy Bannister is the statistical author who has responsibility for the statistical analysis.
Conflict of interest statement
MR reports receiving honoraria (advisory) from AstraZeneca, and has acted in a consulting or advisory role for AstraZeneca, Daiichi-Sankyo (uncompensated), McKesson, Merck (uncompensated) and Pfizer (uncompensated). Research funding has been received from AbbVie (institution), AstraZeneca (Institution), Invitae (Institution, in-kind), Medivation (Institution), Myriad (Institution, in-kind), Pfizer (institution) and Tesaro (institution). Travel or accommodation expenses have been received from AstraZeneca and other transfer of value items are from AstraZeneca (editorial services) and Pfizer (editorial services). KJR reports intellectual property interests for herself and her spouse regarding a discovery or technology relating to health or medicine. S-AI reports consultancy for AstraZeneca, Novartis, Spectrum, Hanmi, Pfizer, and Roche and research funding from AstraZeneca. ES reports honoraria from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Clinigen, Egis, Eli Lilly, Janssen, Novartis, Pfizer, Pierre Fabre, prIME, Roche, and Teva, travel support from Amgen, AstraZeneca, Egis, Novartis, Pfizer, and Roche, and research funding from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, and Samsung. BX reports no conflicts of interest. SMD reports honoraria from AstraZeneca, Clovis Oncology, and Bristol-Myers Squibb and research funding to the University of Pennsylvania from AstraZeneca and Clovis Oncology. NM reports honoraria from Chugai Pharma, AstraZeneca, Pfizer, and Takeda and institutional research funding from Chugai Pharma, AstraZeneca, Kyowa Hakka Kirin, MSD, Novartis, Pfizer, Eli-Lilly, and Daiichi Sankyo. WL reports no conflicts of interest. NT reports consulting for AstraZeneca and receiving grant support from Myriad Genetics and Ambry Genetics. AA reports receiving fees for serving on an advisory board from Roche and Syndax Pharmaceuticals and her spouse holding stock options in AstraZeneca. SD reports honoraria from Novartis, Roche, AstraZeneca, Pfizer, GE Healthcare, and Puma Biotechnology, consulting for Novartis, Roche, Pfizer, AstraZeneca, and Puma Biotechnology, research funding from Novartis, Roche, AstraZeneca, Pfizer, and Puma Biotechnology, and travel, expenses, and accommodations from Pfizer, AstraZeneca, and Novartis. WB, CG, AD, and RH are employees of AstraZeneca. PFC reports speakers’ bureaus with Roche, Novartis, AstraZeneca, and GlaxoSmithKline, travel, accommodations, and expenses from Novartis, GlaxoSmithKline, and Celgene, and research funding from Roche, Novartis, and Merck Serono.
AUTHOR DECLARATION
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work
Mark Robson reports receiving honoraria (advisory) from AstraZeneca, and has acted in a consulting or advisory role for AstraZeneca, Daiichi-Sankyo (uncompensated), McKesson, Merck (uncompensated) and Pfizer (uncompensated). Research funding has been received from AbbVie (institution), AstraZeneca (Institution), Invitae (Institution, in-kind), Medivation (Institution), Myriad (Institution, in-kind), Pfizer (institution) and Tesaro (institution). Travel or accommodation expenses have been received from AstraZeneca and other transfer of value items are from AstraZeneca (editorial services) and Pfizer (editorial services)
Kathryn J Ruddy reports intellectual property interests for herself and her spouse regarding a discovery or technology relating to health or medicine.
Seock-Ah Im reports consultancy for AstraZeneca, Novartis, Spectrum, Hanmi, Pfizer, and Roche and research funding from AstraZeneca.
Elzbiete Senkus reports honoraria from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Clinigen, Egis, Eli Lilly, Janssen, Novartis, Pfizer, Pierre Fabre, prIME, Roche, and Teva, travel support from Amgen, AstraZeneca, Egis, Novartis, Pfizer, and Roche, and research funding from Amgen, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, and Samsung.
Binghe Xu reports no conflicts of interest.
Susan M Domchek reports honoraria from AstraZeneca, Clovis Oncology, and Bristol-Myers Squibb and research funding to the University of Pennsylvania from AstraZeneca and Clovis Oncology.
Norikazu Masuda reports honoraria from Chugai Pharma, AstraZeneca, Pfizer, and Takeda and institutional research funding from Chugai Pharma, AstraZeneca, Kyowa Hakka Kirin, MSD, Novartis, Pfizer, Eli-Lilly, and Daiichi Sankyo.
Wei Li reports no conflicts of interest. NT reports consulting for AstraZeneca and receiving grant support from Myriad Genetics and Ambry Genetics.
Anne Armstrong reports receiving fees for serving on an advisory board from Roche and Syndax Pharmaceuticals and her spouse holding stock options in AstraZeneca.
Suzette Delaloge reports honoraria from Novartis, Roche, AstraZeneca, Pfizer, GE Healthcare, and Puma Biotechnology, consulting for Novartis, Roche, Pfizer, AstraZeneca, and Puma Biotechnology, research funding from Novartis, Roche, AstraZeneca, Pfizer, and Puma Biotechnology, and travel, expenses, and accommodations from Pfizer, AstraZeneca, and Novartis.
Wendy Bannister, Carsten Goessl, Arnold Degboe, and Robert Hettle are employees of AstraZeneca.
Pierrefranco Conte reports speakers’ bureaus with Roche, Novartis, AstraZeneca, and GlaxoSmithKline, travel, accommodations, and expenses from Novartis, GlaxoSmithKline, and Celgene, and research funding from Roche, Novartis, and Merck Serono.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from robsonm@MSKCC.ORG
References
- 1.Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 3). Breast 2017;31:244–59. [DOI] [PubMed] [Google Scholar]
- 2.Khosravi-Shahi P, Cabezon-Gutierrez L, Custodio-Cabello S. Metastatic triple negative breast cancer: optimizing treatment options, new and emerging targeted therapies. Asia Pac J Clin Oncol 2018;14:32–9. [DOI] [PubMed] [Google Scholar]
- 3.Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psychooncology 2004;13:295–308. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother 2003;4:889–901. [DOI] [PubMed] [Google Scholar]
- 5.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 6.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA. LYNPARZA (olaparib) tablets, for oral use. Prescribing information. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s001lbl.pdf.
- 8.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- 9.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44. [DOI] [PubMed] [Google Scholar]
- 10.Scott NW, Fayers PM, Aaronson NK et al. EORTC QLQ-C30 reference values. 2008. Available at: http://groups.eortc.be/qol/sites/default/files/img/newsletter/reference_values_manual2008.pdf.
- 11.Cocks K, King MT, Velikova G, de Castro G. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer 2012;48:1713–21. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Ledermann J, Matulonis U, et al. Management of nausea and vomiting during treatment with the capsule (CAP) and tablet (TAB) formulations of the PARP inhibitor olaparib. European Cancer Congress; Vienna, Austria, 25–29 September 2015;abst 2759. [Google Scholar]
- 13.Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 2019;doi: 10.1093/annonc/mdz012:[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matulonis UA, Oza AM, Ho TW, Ledermann JA. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015;121:1737–46. [DOI] [PubMed] [Google Scholar]
- 15.Sloan JA, Dueck AC, Erickson PA, Guess H, Revicki DA, Santanello NC. Analysis and interpretation of results based on patient-reported outcomes. Value Health 2007;10(Suppl 2):S106–15. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso F, Senkus E, Costa A, Papadopoulos E. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 2018;29:1634–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. NCCN guidelines for the treatment of cancer by site. 2018. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.