A residue in the WEREWOLF transcription factor is critical for the proper balance of target gene expression and establishment of the regulatory network for patterning the Arabidopsis root epidermis.
Abstract
The Arabidopsis (Arabidopsis thaliana) root epidermis exhibits a position-dependent pattern of root-hair and nonhair cell types. A highly orchestrated network of gene regulatory interactions, including the R2R3-type MYB transcription factor WEREWOLF (WER), is responsible for generating this cell pattern during root development. In this study, we identified a novel wer mutant from a genetic enhancer screen, designated wer-4, that exhibits an abnormal pattern of root-hair and nonhair cells. We established that wer-4 bears a single-residue substitution (D105N) in the DNA-binding R3 MYB repeat of WER, which differentially affects the transcription of WER target genes, including GLABRA2, CAPRICE, TRIPTYCHON, and ENHANCER OF TRY AND CPC1. This modulation of the gene regulatory network leads to altered levels and distributions of cell fate regulators in the differentiating epidermal cells that ultimately generate the abnormal cell-type pattern. We also created several WER variants with substitutions at the Asp-105 position, and these exhibited a variety of gene expression and cell-type pattern alterations, further supporting the critical role of this residue. These findings provide insight into WER protein function and its importance in generating the proper balance of downstream transcriptional factors in the gene regulatory network that establishes root epidermal cell fate.
The Arabidopsis (Arabidopsis thaliana) root epidermis has been used extensively as a simple model for studying cell fate regulation in plants (Duckett et al., 1994; Schiefelbein et al., 2014; Huang and Schiefelbein, 2015). Only two cell types, root-hair cells and nonhair cells, are present in the Arabidopsis root epidermis, and the fate of a newly formed root epidermal cell is dependent on its relative position to underlying cortical cells. An epidermal cell located outside a cleft between two cortical cells (the H position) differentiates into a root-hair cell, whereas an epidermal cell located outside one cortical cell (the N position) differentiates into a mature nonhair cell (Berger et al., 1998). The obvious morphological differences between root-hair and nonhair cells, their consistent arrangement, and their early seedling phenotypes enable effective identification and characterization of mutant abnormalities. These features make the root epidermis a powerful system for studying cell specification using genetic and molecular tools.
A wealth of prior studies have uncovered a highly orchestrated network of transcriptional regulators responsible for establishing position-dependent gene expression leading to the two cell fates in the Arabidopsis root epidermis. The core component of this network is a MYB-bHLH-WD40 protein complex that preferentially accumulates in the N-position cells (Schiefelbein et al., 2014). In this complex, MYB is an R2R3-type MYB protein encoded by WEREWOLF (WER), the bHLH proteins are encoded by the functionally redundant GLABRA3 and ENHANCER OF GLABRA3 (GL3/EGL3), and the WD40 protein is encoded by TRANSPARENT TESTA GLABRA1 (TTG1; Galway et al., 1994; Lee and Schiefelbein, 1999; Walker et al., 1999; Bernhardt et al., 2003, 2005). The WER-GL3/EGL3-TTG1 complex directly promotes the transcription of GL2, leading to preferential GL2 accumulation in the N-position cells (Masucci et al., 1996; Song et al., 2011). GL2 encodes an HD-ZIP transcription factor that inhibits the expression of root-hair-promoting genes, thus causing the N-position cells to adopt the nonhair cell fate (Rerie et al., 1994; Bruex et al., 2012; Lin et al., 2015). Accordingly, null mutants of WER, GL3/EGL3, TTG1, and GL2 yield plants lacking nonhair cells and exhibiting a hairy-root phenotype.
In addition to promoting the nonhair cell fate, the WER-GL3/EGL3-TTG1 complex also influences root-hair cell fate through regulation of the single-repeat R3-type MYB genes CAPRICE (CPC), TRIPTYCHON (TRY), and ENHANCER OF TRY AND CPC1 (ETC1; Schellmann et al., 2002; Kirik et al., 2004; Simon et al., 2007). The CPC, TRY, and ETC1 genes are preferentially expressed in the N-position cells, but the proteins translocate to the adjacent H-position cells (Kurata et al., 2005), where they inhibit formation of the WER-GL3/EGL3-TTG1 complex through competitive binding to GL3/EGL3 (Wada et al., 2002; Song et al., 2011). As a consequence, the H-position cells express relatively low levels of GL2 and high levels of root-hair-promoting genes (Bruex et al., 2012; Lin et al., 2015). The CPC, TRY, and ETC1 proteins are largely functionally redundant, although the CPC gene is expressed most abundantly and plays the major role (Simon et al., 2007).
In addition to the CPC/TRY/ETC1 proteins, another factor influencing the accumulation pattern of the WER-GL3/EGL3-TTG1 complex is the preferential expression of the WER gene in the N-position cells (Lee and Schiefelbein, 1999; Ryu et al., 2005). This expression pattern is due to WER transcriptional repression in the H-position cells mediated by the receptor-like kinase SCRAMBLED (SCM; Kwak et al., 2005). In addition, WER-GL3/EGL3-TTG1 accumulation is influenced by GL3 and EGL3, which participate in negative transcriptional feedback loops and exhibit differential accumulation and mobility between N- and H-position cells as well as affecting CPC accumulation (Bernhardt et al., 2005; Kang et al., 2013). Collectively, these components and interactions of the gene regulatory network ultimately establish stable cell-type-specific gene expression in the H- and N-position cells.
To gain further insights into the mechanisms controlling cell-type patterning in the Arabidopsis root epidermis, we sought to identify additional mutants that alter the root-hair/nonhair cell distribution. Through an enhancer genetic screen using the cpc-1 mutant, we identified a novel mutant allele of WER that disrupts the position-dependent pattern of root-hair and nonhair cells. The WER protein encoded by the mutated WER gene possesses a single-residue substitution at position 105, which causes abnormal target gene transcription, disrupts the spatial distribution of cell fate regulators, and reduces the molecular distinction between H- and N-position cells. We further generated WER variants with additional substitutions at the same position, which also exhibit abnormalities in root epidermis gene expression and patterning. These findings highlight the critical role of WER transcriptional activity in root epidermal cell patterning, and they show how a single-nucleotide change can modulate a gene regulatory network to generate a new developmental phenotype.
RESULTS
Identification of the WER Mutant Allele wer-4
To discover further mutants affecting root epidermis patterning, we conducted an enhancer genetic screen in the cpc-1 GL2::GUS mutant background. The cpc-1 mutant produces fewer root-hair cells (approximately 40% of the wild-type number; Fig. 1, A and B) and exhibits a corresponding increase in ectopic GL2::GUS reporter expression in differentiating H-position cells (Fig. 1C), providing a sensitized background suitable for detecting subtle disruptions of the patterning mechanism. We mutagenized the cpc-1 GL2::GUS line using ethyl methanesulfonate and identified seedlings in subsequent generations that exhibited a more extreme reduced-hair phenotype. One of the resulting lines, ultimately designated as cpc-1 wer-4, produced very few root-hair cells (approximately 7% of the wild-type number; Fig. 1, A and B) and exhibited greater ectopic expression of GL2::GUS in differentiating H-position cells than cpc-1 (Fig. 1C), suggesting that the gene affected by this particular enhancer mutation acts upstream of GL2. We separated the wer-4 allele from cpc-1 genetically and discovered that the wer-4 single mutant produces an abnormal spatial distribution of epidermal cell types, including 13% nonhair cells in the H position (ectopic nonhair cells) and 28% root-hair cells in the N position (ectopic root-hair cells; Fig. 1B). We also showed that plants heterozygous for this mutation (wer-4/+) exhibited a normal root epidermis pattern (Fig. 1B). Thus, the wer-4 mutant possesses a recessive allele that affects cell-type patterning at an early stage during Arabidopsis root epidermis development.
Figure 1.
The wer-4 mutant allele enhances the cpc-1 phenotype and possesses a missense mutation in the WER gene. A, Seedling roots of the wild type (WT), cpc-1, cpc-1 wer-4, and wer-4 displaying their root-hair phenotypes. The arrows point to significantly shorter root hairs in the wer-4 root. Bar = 200 μm. B, Quantifications of root epidermis specification in seedling roots of various genetic backgrounds. WWGFP represents the WER::WER-GFP reporter. Error bars represent sd from three replicates. Statistical significance was determined by two-way ANOVA: ***, P < 0.001; ns, not significant. C, GL2::GUS reporter expression in seedling root tips of the wild type, cpc-1, and cpc-1 wer-4. Stars indicate H-position cell files. For each genotype, the left and right images show the same root under different magnifications. Bars = 50 μm. D, Alignment of the R2R3 domains of multiple Arabidopsis MYB proteins. The red arrow marks the position of the D105N residue substitution in the wer-4 mutant. The underlined residues indicate the three α-helices in each repeat. Stars indicate conserved Trp residues. Diamonds indicate conserved residues that directly associate with DNA bases in mammalian c-Myb. The green dotted frame indicates the conserved bHLH interaction domain.
To identify the mutated gene in wer-4, we performed genetic mapping with molecular markers and narrowed its location to a region on chromosome 5 near the marker nga151 (Bell and Ecker, 1994; see “Materials and Methods”). This region includes the root epidermis regulatory gene WER (Lee and Schiefelbein, 1999). Upon sequencing the WER gene in the wer-4 mutant, we identified a single G-to-A substitution within the open reading frame at position 4,764,045, which changes the Asp encoded by the 105th codon to Asn (D105N; Fig. 1D).
To determine whether the identified WER mutation is responsible for the wer-4 phenotype, we introduced the WER::WER-GFP transgene (which encodes a functional WER protein; Ryu et al., 2005) into the wer-4 mutant by crossing. The resulting WER::WER-GFP wer-4 plants exhibited a root epidermal cell-type pattern comparable to the WER::WER-GFP plants (Fig. 1B). This indicates that the single-nucleotide change in the WER gene in the wer-4 line is the cause of its abnormal cell-type pattern.
The wer-4 Mutant Alters Expression of WER Target Genes
The wer-4 mutant phenotype is distinct from previously described wer mutants, which all exhibit a strong hairy-root phenotype due to the loss of nonhair cells (Lee and Schiefelbein, 1999). The Asp-105 residue affected by the wer-4 mutation is located at the beginning of the third α-helix of the R3 domain, which is involved in DNA recognition (Ogata et al., 1994; Jia et al., 2004; Fig. 1D). Given this, we hypothesized that the wer-4 mutation causes abnormal regulation of WER target genes.
To investigate this possibility, we analyzed WER target gene expression in the wer-4 mutant. We observed strong effects of wer-4 on TRY and ETC1 gene expression. Using reverse transcription quantitative PCR (RT-qPCR), we found that these two genes exhibited a dramatic decrease in transcript abundance in wer-4 roots that was comparable to or even lower than that in the null wer-1 mutant (Fig. 2A). Consistent with this, the wer-4 mutant exhibited largely depleted ETC1::GUS signals in the root epidermis (Fig. 2B). These results suggest that the mutated WER protein in wer-4 is essentially unable to induce expression of these genes. The TRY and ETC1 genes encode CPC-like R3-type MYB proteins that are partially functionally redundant with CPC, and both etc1 and try mutants enhance the cpc-1 phenotype (Kirik et al., 2004; Simon et al., 2007). Considering this, the loss of TRY and ETC1 expression in wer-4 helps to explain its ability to enhance the cpc-1 mutant phenotype (Fig. 1, A and B).
Figure 2.
The wer-4 mutant affects the expression of WER target genes. A, Relative amounts of ETC1, TRY, GL2, and CPC transcripts in seedling root tips of the wild type (WT), wer-4, and wer-1 determined with RT-qPCR. Error bars represent sd from three replicates. Statistical significance was determined with one-way ANOVA: ***, P < 0.001; ns, not significant. B, ETC1::GUS transcriptional reporter expression in seedling root tips of the wild type and wer-4. Bar = 50 μm. C, Expression of GL2::GUS and GL2::GFP transcriptional reporters in wild-type and wer-4 seedling root tips. Stars indicate H-position cell files. In the fluorescence image, the red color represents propidium iodide and the green color represents GFP. Bars = 50 μm. D, Expression of CPC::GUS transcriptional reporter in wild-type and wer-4 seedling root tips. Stars indicate H-position cell files. Bar = 50 μm. E, Histograms of GL2::GFP signal levels in N- and H-position epidermal cells of wild-type and wer-4 seedling root tips (n = 170). Ten cells in H and N positions were analyzed per root, and 17 roots were analyzed for each genotype. F, Histograms of CPC::GUS signal levels in N- and H-position epidermal cells of wild-type and wer-4 seedlings root tips (n = 240). Ten cells in H and N positions were analyzed per root, and 24 roots were used for each genotype.
Although the try and etc1 mutants enhance cpc-1, by themselves the try, etc1, and try etc1 mutants do not substantially alter root epidermis development (Kirik et al., 2004). Thus, the abnormal cell-type pattern in the wer-4 mutant is not solely due to its effects on TRY and ETC1, implying that additional WER targets are affected in wer-4. Therefore, we studied the expression of two major players in root epidermal development that are known to be direct WER transcriptional targets: GL2 and CPC (Ryu et al., 2005; Song et al., 2011).
For GL2, we made use of the GL2::GUS and GL2::GFP transcriptional reporters and discovered that both reporters exhibited increased overall expression in developing wer-4 root epidermal cells, including some ectopic expression in H-position cells (Fig. 2C). To evaluate expression from individual cells, we quantified the fluorescence signals from differentiating epidermal cells in the N and H positions of the GL2::GFP line and plotted the signal distribution from both wild-type and wer-4 roots (Fig. 2E). Consistent with the visual phenotypes (Fig. 2C), a large proportion of the wer-4 cells exhibited greater GFP levels than wild-type cells in both the N and H positions (Fig. 2E). Furthermore, the wer-4 mutant possessed a wider distribution of GFP signal levels within cells in both the N and H positions, relative to the wild type (Fig. 2E). These results indicate that the wer-4 mutation leads to greater but more variable GL2 transcription in the developing root epidermis. Interestingly, the overall amount of GL2 transcripts in wer-4 roots was approximately 70% of that in the wild type (Fig. 2A), implying posttranscriptional regulation in wer-4.
To examine CPC gene expression, we used a CPC::GUS transcriptional reporter and discovered an overall decrease in the GUS staining level as well as greater cell-cell variation in the wer-4 root epidermis as compared with the wild type (Fig. 2D). By analyzing GUS signal levels from individual cells, we confirmed the greater cell-cell variation and the general reduction of GUS levels in the N-position cells as well as the greater variation of GUS levels in the H-position cells of wer-4 (Fig. 2F). This shows that the wer-4 mutant causes a general decrease in CPC transcription and reduces the establishment of distinct CPC expression within and between cells in the H and N positions. Consistently, the amount of CPC transcripts in wer-4 were significantly lower than in the wild type but still higher than in wer-1 (Fig. 2A), indicating that the CPC transcript amount is largely determined by its transcription level.
It is reported that ETC1, TRY, GL2, and CPC are also expressed in leaf trichome cells under the regulation of a parallel MYB-bHLH-WD40 protein complex containing the GL1 MYB protein instead of WER (Pattanaik et al., 2014). To determine whether the effects of wer-4 on ETC1, TRY, GL2, and CPC were root specific, we examined the expression of these genes in leaves. The ETC1::GUS, GL2::GUS, and CPC::GUS reporters exhibited comparable signals in the trichome cells of both wild-type and wer-4 leaves (Supplemental Fig. S1A). Additionally, the ETC1, TRY, GL2, and CPC transcript levels in leaves were not significantly affected by wer-4 (Supplemental Fig. S1B). This suggests that wer-4, like WER, does not participate in leaf trichome development.
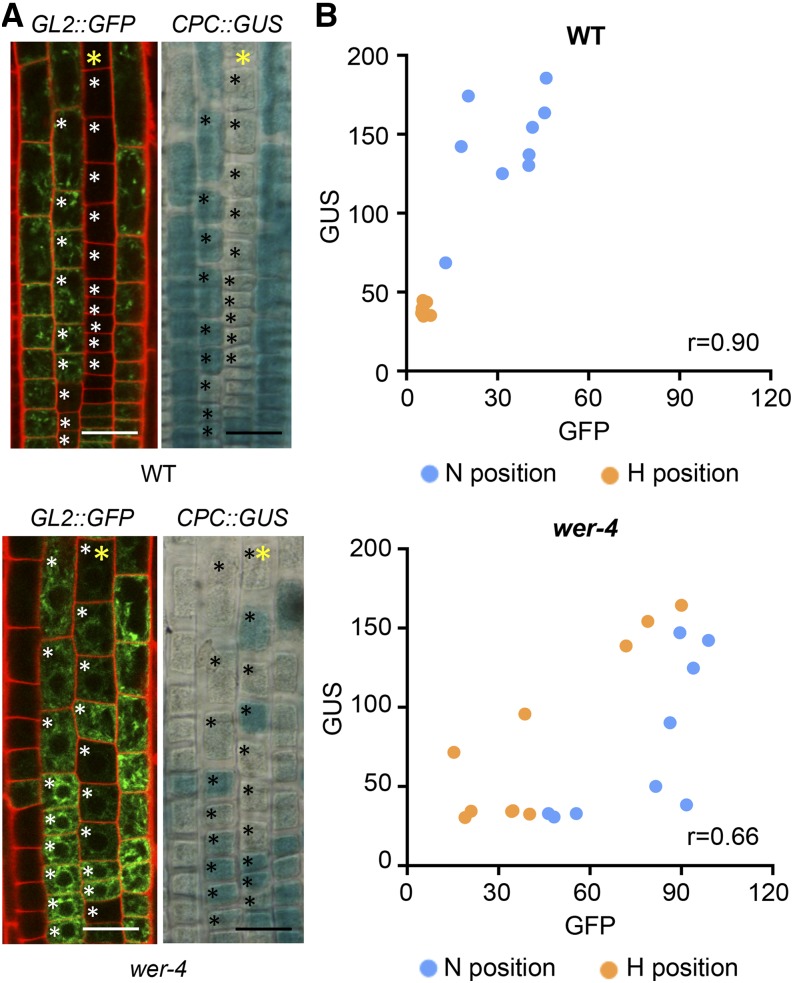
The abnormal distributions and various levels of GL2 and CPC transcription observed in the wer-4 root epidermis led us to examine whether wer-4 might disrupt the coordinated transcriptional regulation between GL2 and CPC. To simultaneously analyze the expression of both genes in individual cells, we generated wild-type and wer-4 plants bearing both the GL2::GFP and CPC::GUS reporters. In wild-type roots, GL2 and CPC are known to be coordinately regulated with preferential transcription of both genes in the differentiating N-position cells (Lee and Schiefelbein, 2002; Fig. 3; Supplemental Fig. S2A). In the wer-4 mutant, we also observed a general correlation in expression between the GL2::GFP and CPC::GUS reporters in both H- and N-position cells (Fig. 3; Supplemental Fig. S2B). Thus, despite the abnormal relative levels and lack of H/N-position cell specificity for GL2 and CPC expression in the wer-4 mutant, they largely remained under coordinated transcriptional regulation in individual cells. Given that coordinated regulation was maintained but the relative promoter activity of GL2 and CPC was altered (compare the GFP versus GUS reporter levels in the wild type and wer-4 in Fig. 2, E and F), we conclude that a primary effect of wer-4 is to alter the ratio of GL2 transcription to CPC transcription within individual cells relative to the wild type.
Figure 3.
Expression of the GL2 and CPC genes is coordinated in wer-4 root epidermal cells. A, Expression of the GL2::GFP and CPC::GUS reporters within a single root of wild-type (WT) and wer-4 seedlings. The yellow stars indicate H-position cell files. In the fluorescence images, the red color represents propidium iodide and green color represents GFP. Bars = 25 μm. B, Scatterplots of GFP and GUS signal levels in root epidermal cells of wild-type and wer-4 roots. Each data point represents one cell marked with black/white stars in A. r represents the Pearson correlation coefficient determined with all data points from both H and N positions in each plot.
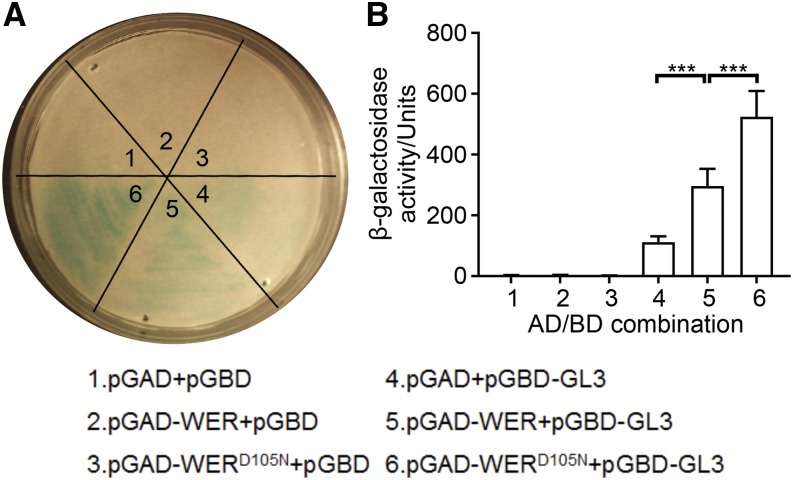
To understand the basis for altered WER target gene transcription by wer-4, we compared the function of wild-type WER and the wer-4 mutant protein (hereafter designated as WERD105N). As a component of the MYB-bHLH-WD40 complex, WER is reported to directly associate with the GL3/EGL3 bHLH proteins (Bernhardt et al., 2003). Using the yeast two-hybrid assay, we detected significant protein-protein interaction between wild-type WER and GL3 as well as WERD105N and GL3 (Fig. 4). Interestingly, WERD105N exhibited statistically stronger association with GL3 compared with WER in this assay (Fig. 4). These results indicate that the D105N substitution in the wer-4 mutant does not harm the association between WER and GL3. Considering this, we conclude that the alterations in downstream WER target gene expression in wer-4 are not likely to be caused by defective formation of the WER-GL3/EGL3-TTG1 complex.
Figure 4.
The WERD105N protein is able to associate with GL3. A, Yeast-two hybrid filter assays showing the β-galactosidase activities in yeast cultures expressing GL3 together with WER or WERD105N. The numbers in the image correspond to the numbered AD/BD combinations described at the bottom. B, Yeast-two hybrid liquid assays confirming the interaction between GL3 and WER or WERD105N. Error bars represent sd from three replicates. The numbers in the plot correspond to the numbered AD/BD combinations described at the bottom. Statistical significance was determined by one-way ANOVA: ***, P < 0.001.
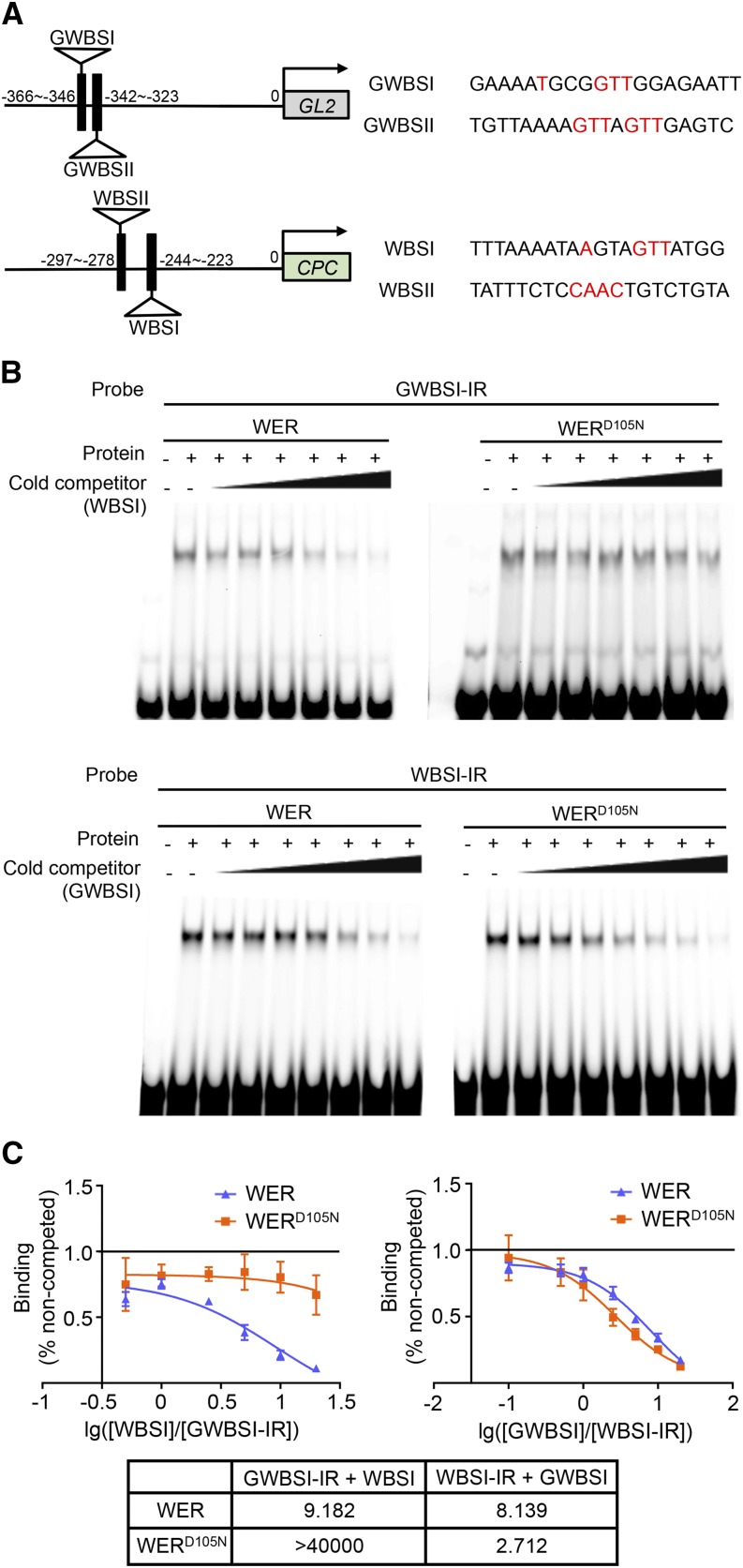
Next, we analyzed the affinities of WER and WERD105N proteins to their target gene promoters. Previous studies have defined two in vivo WER-binding sites within the GL2 promoter (elements GWBSI and GWBSII) and the CPC promoter (elements WBSI and WBSII; Fig. 5A; Ryu et al., 2005; Song et al., 2011). Using the electrophoretic mobility shift assay (EMSA), we observed that both WER and WERD105N exhibited detectable binding to three of these four promoter elements (Supplemental Fig. S3). To compare the relative binding of WER and WERD105N to the GL2 and CPC promoter elements, we performed competition EMSA using the CPC promoter element WBSI as an unlabeled competitor against the labeled GL2 promoter element GWBSI. We discovered that WERD105N remained bound to GWBSI at higher competitor concentrations than WER and resulted in a much higher half-maximal inhibitory concentration (IC50) value (Fig. 5, B and C), indicating that WERD105N has lower affinity for the CPC promoter element than for the GL2 promoter element, compared with wild-type WER protein. This result was supported by a reciprocal EMSA using GWBSI as an unlabeled competitor against the labeled WBSI, where WERD105N showed less resistance to the competitor than WER (Fig. 5, B and C). Interestingly, WERD105N also exhibited greater relative binding to GWBSI than to GWBSII compared with WER (Supplemental Fig. S4), implying that the GWBSI element may be primarily responsible for the differential effect of WERD105N. Together, these results indicate that the D105N substitution in the WER protein alters its relative affinity for its GL2 and CPC promoter-binding sites and therefore decreases the CPC/GL2 transcription ratio in the wer-4 root epidermal cells.
Figure 5.
The WERD105N protein exhibits altered affinities for WER-binding sites in the GL2 and CPC promoters. A, Schematic diagrams of previously identified WER-binding sites in the GL2 and CPC promoters. Numbers indicate the relative distance of each binding site from the transcription start site. On the right are the sequences of WER-binding sites. The nucleotides reported to be essential for WER recognition are colored in red. B, Competition EMSA between GWBSI and WBSI. The top section shows the result using GWBSI as the hot probe (labeled with infrared dye) and WBSI as the cold competitor. The concentrations of the unlabeled competitor are 0.5×, 1×, 2.5×, 5×, 10×, and 20× compared with the labeled probe in lanes 3 to 8 of the WER and WERD105N experiments. The bottom section shows the result using WBSI as the hot probe and GWBSI as the unlabeled competitor. The concentrations of the unlabeled competitor are 0.1×, 0.5×, 1×, 2.5×, 5×, 10×, and 20× compared with the labeled probe in lanes 3 to 9 of the WER and WERD105N experiments. C, Semilog plots of the competition EMSA results shown in B. Error bars indicate sd from three replicates. The one-site competitive binding curve model was used for nonlinear regression of each competition experiment. The calculated half-maximal inhibitory concentration (IC50) values (cold competitor/hot probe molar ratio) are listed in the table at bottom.
Effect of wer-4 on the Cell-Type Pattern
Next, we sought to understand how the altered regulation of WER target genes ultimately leads to the abnormal cell-type pattern in the wer-4 mutant. In the established model for epidermal cell patterning, the specification of root-hair/nonhair cell fates is the result of differential accumulation of the WER-GL3/EGL3-TTG1 complex in the H- and N-position cells (Schiefelbein et al., 2009, 2014). To determine whether this was altered in wer-4, we analyzed GL3 protein accumulation using the GL3::GL3-YFP reporter (Bernhardt et al., 2005). In contrast to the wild-type roots, where the GL3-YFP proteins exhibited preferential accumulation in the nuclei of N-position cells, the wer-4 roots showed relatively lower GL3-YFP accumulation in both H- and N-position cells (Fig. 6A). Quantification of the GL3-YFP signals revealed that the difference between N- and H-position cells in the wer-4 mutant was much less significant than in the wild type (Fig. 6E). Notably, GL3-YFP accumulation in the root apical meristem remained unchanged in the wer-4 mutant (Fig. 6A), indicating that the impact of wer-4 is specific to the root epidermis. The abnormal GL3 accumulation in the wer-4 root epidermal cells indicates that WERD105N is unable to establish differential accumulation of the WER-GL3/EGL3-TTG1 complex between the H and N positions.
Figure 6.
The wer-4 mutant disrupts root epidermal cell fate establishment. A, Accumulation of the GL3-YFP fusion protein in wild-type (WT) and wer-4 seedling roots bearing the GL3::GL3-YFP transgene. White stars mark the H-position cell files. The red color represents propidium iodide and the green color represents YFP. For each genotype, the left and right images show the same root focused on the epidermal and stele layers. Bar = 50 μm. B, Accumulation of the CPC-GFP fusion protein in wild-type and wer-4 seedling roots bearing the CPC::CPC-GFP transgene. White stars mark the H-position cell files. The red color represents propidium iodide and the green color represents GFP. For each genotype, the left and right images show the same root focused on the epidermal and stele layers. Bar = 50 μm. C, Quantifications of root epidermis specification in seedling roots of the wild type, scm-2, wer-4, and scm-2 wer-4. Error bars represent sd from three replicates. Statistical significance was determined by two-way ANOVA: *, P < 0.05. D, Quantifications of the CPC-GFP signals in epidermis of wild-type and wer-4 roots. For each root, the GFP signals within all measurable epidermal cells were measured and results are plotted using the average GFP signals per cell. A total of 20 roots were measured for each genotype. Statistical significance was determined with Student’s t test: ***, P < 0.001. E, Histograms of GL3::GL3-YFP signal levels in N- and H-position cells of wild-type and wer-4 seedling root tips (n = 150). Ten cells in each position were analyzed per root, and 15 roots were used for each genotype.
The CPC-mediated lateral inhibition pathway helps to generate the proper accumulation pattern of the WER-GL3/EGL3-TTG1 complex (Bernhardt et al., 2005; Schiefelbein et al., 2014). Therefore, it is likely that the reduced transcription of CPC in wer-4 (Fig. 2, D and F) is, at least in part, responsible for its altered WER-GL3/EGL3-TTG accumulation. To address whether reduced CPC transcription leads to reduced CPC protein production, we made use of the CPC::CPC-GFP reporter (Kurata et al., 2005). It has been reported that the GFP tag affects the mobility of CPC and traps CPC within the N-position cells (Kurata et al., 2005). Indeed, we observed the CPC-GFP signal in the nuclei of both H- and N-position cells in wild-type and wer-4 roots (Fig. 6B). Nevertheless, we observed much lower levels of nuclear GFP signal in the wer-4 mutant compared with the wild type (Fig. 6B), and quantification revealed a significant decrease in average GFP signal level in wer-4 (Fig. 6D). Notably, the CPC-GFP signal within the stele tissue showed no decrease in the wer-4 mutant (Fig. 6B). These results indicate that the wer-4 mutant produces less CPC in the root epidermis.
In addition to CPC, the SCM receptor-mediated signaling pathway is also involved in establishing the position-dependent accumulation of the WER-GL3/EGL3-TTG1 complex (Kwak et al., 2005; Kwak and Schiefelbein, 2007). To test the possible contribution of SCM to the wer-4 phenotype, we generated the scm-2 wer-4 double mutant and discovered that it exhibited more extreme disruption of the cell-type pattern than the wer-4 single mutant (Fig. 6C). This additive genetic effect implies that wer-4 does not generate its abnormal cell-type pattern through altering SCM (e.g. hypothetical feedback regulation of wer-4 on SCM). Thus, the negative effect of wer-4 on CPC is the more likely explanation for the misregulated accumulation of the WER-GL3/EGL3-TTG1 complex.
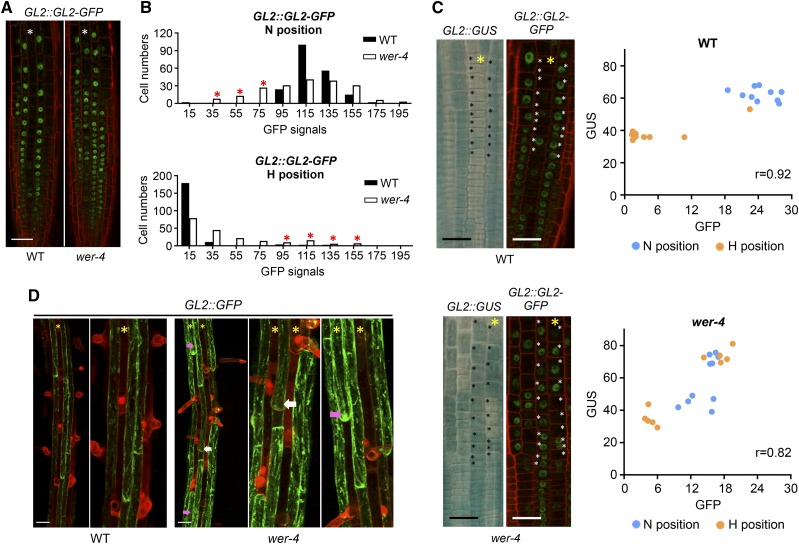
In wild-type roots, the differential accumulation of the WER-GL3/EGL3-TTG1 complex causes preferential GL2 gene expression and GL2 protein accumulation in N-position cells, which generates the nonhair cell fate (Galway et al., 1994; Masucci et al., 1996; Lee and Schiefelbein, 1999; Bernhardt et al., 2003; Lin et al., 2015). Thus, we analyzed whether GL2 protein accumulation is altered in the wer-4 mutant. Using a GL2::GL2-GFP translational fusion line, we found that wer-4 exhibited variable GL2-GFP accumulation in N-position cells as well as many H-position cells (i.e. ectopic GL2-accumulating cells; Fig. 7A). To examine the relationship between GL2 promoter activity and GL2 protein accumulation within individual cells, we created wild-type and wer-4 lines bearing both the GL2::GUS and GL2::GL2-GFP reporters. We observed a strong correlation between GL2::GUS and GL2::GL2-GFP across individual cells in both wild-type and wer-4 roots, indicating that GL2 promoter activity largely determines relative GL2 protein accumulation (Fig. 7C; Supplemental Fig. S5). We also quantified GL2-GFP levels in H- and N-position cells from the wild type and wer-4 bearing the GL2::GL2-GFP reporter and discovered that cells in both positions exhibited higher signal variations in wer-4 than in the wild type (Fig. 7B). Specifically, 25% of the N-position cells in wer-4 exhibited weaker GFP signal than the N-position cells in the wild type (marked with red stars in Fig. 7B, top), which roughly matches the percentage (28%) of root-hair cells in the N position of wer-4 (Fig. 1B). Approximately 20% of the H-position cells in wer-4 exhibited GFP signal that was comparable to or higher than the GFP signal in wild-type N-position cells (marked with red stars in Fig. 7B, bottom), which is comparable to the fraction (13%) of nonhair cells produced in the H position of wer-4 (Fig. 1B). These results suggest that epidermal cell fates in the wer-4 mutant are correlated with GL2 protein levels. To further address this possibility, we analyzed older epidermal cells (within the early maturation zone) in wild-type and wer-4 roots bearing GL2::GFP, which enabled us to assess both GL2 transcription (which is proportional to GL2 protein levels) and cell fate (i.e. whether a root hair is produced) in individual cells. We discovered that root-hair cells in the N position of wer-4 exhibited lower GFP signal than the adjacent nonhair cells in the same cell file, and nonhair cells in the H position of the wer-4 mutant showed higher GFP signal relative to their H-position neighbors and comparable signal to nonhair cells in the N position (Fig. 7D). These results support the hypothesis that, in wer-4, ectopic cell fates in the H and N positions are the result of abnormal GL2 protein accumulation.
Figure 7.
Ectopic cell fates in the wer-4 mutant are associated with abnormal GL2 protein accumulation. A, Accumulation of GL2-GFP fusion protein in the root epidermis of wild-type (WT) and wer-4 seedlings carrying the GL2::GL2-GFP reporter. Red color indicates propidium iodide and green color indicates GFP. Stars indicate H-position cell files. Bar = 50 μm. B, Histograms of quantified GL2::GL2-GFP signals in wild-type and wer-4 roots (n = 200). Ten cells from H and N positions in each root were measured, and 20 roots were used for each genotype. Red stars in the N-position graph indicate groups of wer-4 N-position cells with GFP signals lower than wild-type N-position cells. Red stars in the H-position graph indicate groups of wer-4 H-position cells with GFP signals comparable to or higher than wild-type N-position cells. C, Expression of GL2::GUS and GL2::GL2-GFP reporters in a single root of the wild type and wer-4. The yellow stars indicate H-position cell files. The scatterplots at right show the GFP and GUS signal levels of cells marked with black/white stars in the wild-type and wer-4 images. r represents the Pearson correlation coefficient determined with all data points from both H and N positions from each plot. Bars = 25 μm. D, Expression of GL2::GFP in the differentiation zone (where root hairs are visible) of wild-type and wer-4 seedling roots. Stars indicate H-position cell files. For both wild-type and wer-4 images, particular regions are zoomed in on at right. White arrows point to ectopic root-hair cells in N-cell positions, and pink arrows point to ectopic nonhair cells in H-cell positions in wer-4. Bars = 50 μm.
Finally, we examined the possible effect of wer-4 on the ability of root epidermal cells to differentiate properly. In particular, we hypothesized that the ectopic root-hair cells that arise in the N position may not fully differentiate like authentic root hair cells, due to the substantial amounts of GL2 protein they possess (Fig. 7B). Indeed, we discovered that the length of root hairs formed by the N-position cells of the wer-4 mutant are much shorter than root hairs formed on the H-position cells of the wild type or wer-4 (Fig. 8). This difference was also apparent from visual observation of the wer-4 mutant roots (Fig. 1A).
Figure 8.
Histogram of root-hair length from wild-type (WT) and wer-4 root epidermal cells (n = 150). For wild-type roots, only root hairs from H-position cells were measured. For wer-4 roots, root hairs from cells in the H and N positions were measured separately. For each genotype and position, 15 roots were analyzed. For each root, 10 root hairs from fully mature cells were measured.
In summary, the wer-4 mutation alters cell fate patterning as well as cell differentiation, likely due to inappropriate establishment of cell-type-specific accumulation of the WER-GL3/EGL3-TTG1 complex, which leads to variable levels of GL2 expression and GL2 protein accumulation.
WER Protein Function Is Altered by Manipulating Its Asp-105 Residue
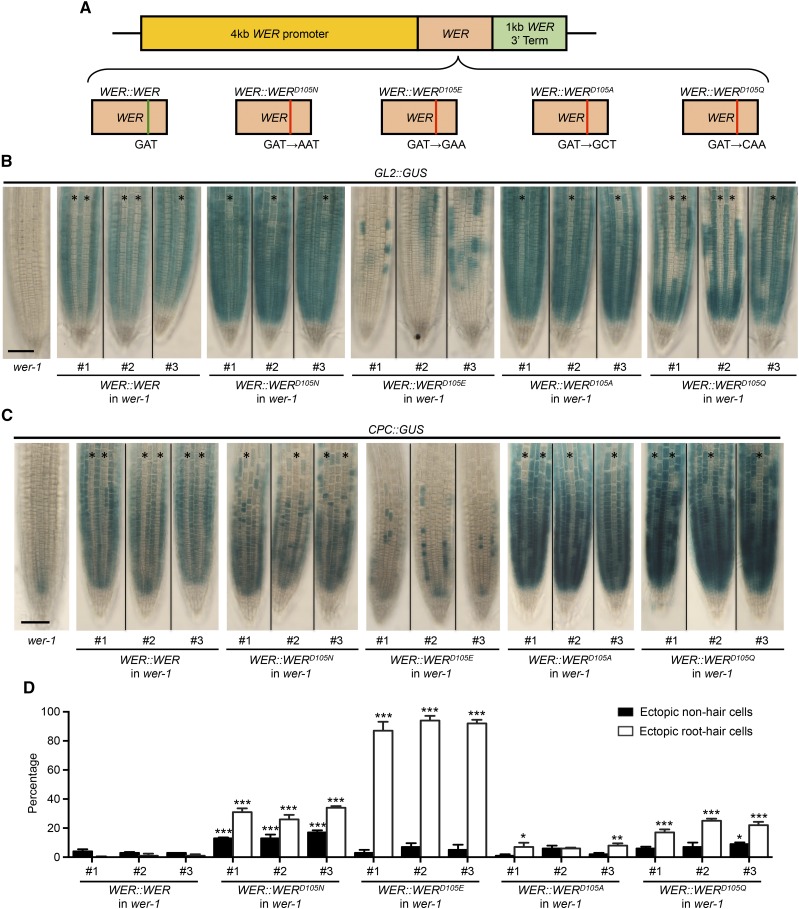
Our analysis of the wer-4 mutant reveals that the Asp-105 residue is important for proper WER protein function, and a substitution from Asp to Asn alters its transcriptional regulatory activity. To further analyze this residue and its importance in root epidermal patterning, we engineered additional substitutions at this position, including Glu (E), with an R group similar to Asp, Gln (Q), the amide derivative of Asn, and Ala (A), with the smallest and uncharged R group. We constructed each transgene using an identical WER genomic DNA fragment, including 4-kb 5′ flanking sequence and 1-kb 3′ flanking sequence, each differing only in nucleotides affecting codon 105 (Fig. 9A). As controls, transgenes encoding WER and WERD105N were also constructed. To monitor the effect of each WER transgene on target gene transcription, we transformed each construct into wer-1 GL2::GUS or wer-1 CPC::GUS plants. At least three independent homozygous single-insertion T3 lines were analyzed for each transformation experiment.
Figure 9.
Substitutions of the WER Asp-105 residue alter root epidermal cell-type pattern. A, Schematic drawings illustrate WER::WER transgenes with different residue substitutions at position 105. B, Expression of the GL2::GUS transcriptional reporter in the wer-1 mutant and wer-1 mutants bearing different WER::WER transgenes. For each transgene, representative roots from three independent single-insertion lines are shown. Stars indicate H-position cell files. Bar = 50 μm. C, Expression of the CPC::GUS reporter in the wer-1 mutant and wer-1 mutants bearing different WER::WER transgenes. For each transgene, representative roots from three independent single-insertion lines are shown. Stars indicate H-position cell files. Bar = 50 μm. D, Quantifications of root epidermis specification in the wer-1 mutants bearing different WER::WER transgenes. Error bars represent sd from three replicates. Two-way ANOVA was used to determine the differences among different transgenic lines using the #1 line of the WER::WER transgene as the control. All transgenic lines showing significant differences in H and/or N positions from the control are marked: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
The wer-1 plants carrying the WER::WER transgene exhibited a wild-type cell-type pattern and preferential expression of both GL2::GUS and CPC::GUS reporters in N-position cells (Fig. 9, B–D). The wer-1 plants bearing the WER::WERD105N transgene exhibited a phenotype similar to wer-4, including a distorted cell-type pattern, overall elevated and ectopic GL2::GUS expression, and abnormal CPC::GUS expression (Fig. 9, B–D). These results indicate that the control transgenes successfully replicate the WER functions in wild-type and wer-4 plants.
Each of the three additional substitutions of the Asp-105 residue alters WER protein function in a different way. The WER::WERD105E transgene exhibited the least effective WER protein function. In these lines, only 10% of N-position cells were able to differentiate as nonhair cells (Fig. 9D). Accordingly, the expression of the GL2::GUS and CPC::GUS reporters occurred in a small fraction of the differentiating epidermal cells (Fig. 9, B and C). These results indicate that the D105E substitution substantially impairs WER’s ability to induce GL2 and CPC transcription, and ultimately, nonhair cell specification.
The WER::WERD105A transgene largely restored the wild-type root epidermal cell pattern to the wer-1 mutant, although two of the three independent transgenic lines exhibited a small increase in ectopic root-hair cells (around 6%; Fig. 9D). The preferential expression of both GL2::GUS and CPC::GUS reporters in N-position cells was also restored, but both reporters were expressed at higher overall levels than in the wild type (Fig. 9, B and C), suggesting that the D105A residue substitution enhances WER’s ability to promote transcription from the GL2 and CPC promoters without significantly disrupting the cell fate network.
The WER::WERD105Q wer-1 plants produced approximately 20% ectopic root-hair cells in the N position (Fig. 9D). The proportion of cells expressing GL2::GUS and CPC::GUS in the N positions were both reduced (Fig. 9, B and C), and interestingly, the overall expression level of each reporter was increased compared with the WER::WER wer-1 lines. These results indicate that the D105Q substitution enhances WER’s ability to promote GL2 and CPC transcription, but the balance of these and/or other regulators within the cell fate network is disrupted to cause abnormal cell specification.
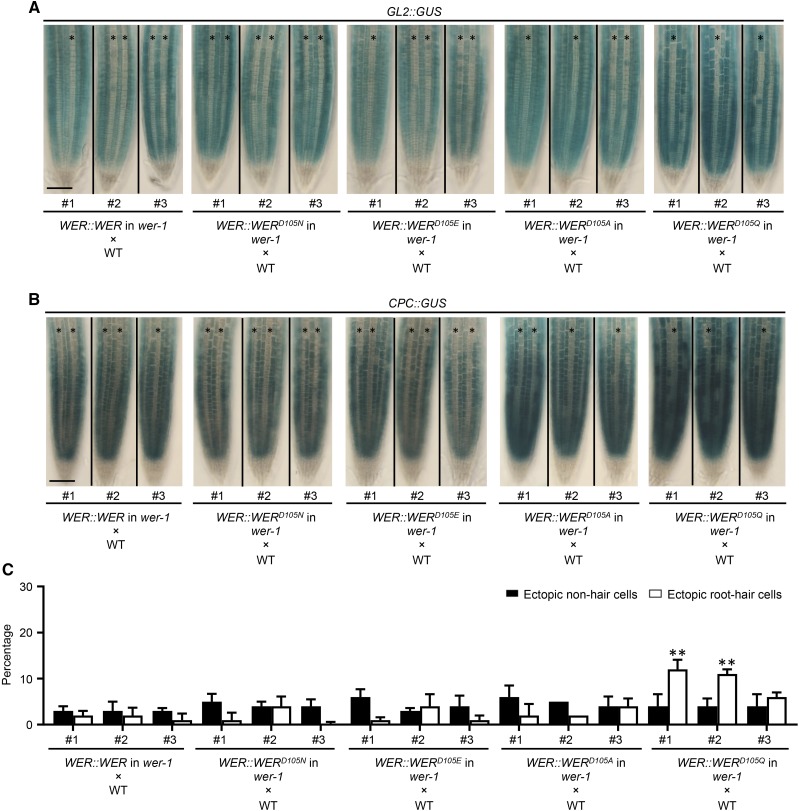
To functionally compare different WER variants with the wild-type WER, we crossed the homozygous single-insertion T3 plants carrying various WER::WER transgenes with the wild type and analyzed the F1 plants. For each transgene, three independent T3 lines were used to generate F1 plants.
The F1 plants from the WER::WERD105N × wild type crosses exhibited wild-type GL2::GUS and CPC::GUS expression and a normal root epidermis pattern (Fig. 10), which is consistent with our earlier analysis of wer-4/+ plants (Fig. 1B). Similarly, the F1 plants from the WER::WERD105E × wild type crosses exhibited wild-type GL2::GUS and CPC::GUS expression and root epidermis pattern (Fig. 10). Thus, a single copy of the wild-type WER is sufficient to complement the defective WERD105E function. The F1 plants from the WER::WERD105A × wild type crosses also exhibited wild-type GL2::GUS and CPC::GUS expression and root epidermis pattern (Fig. 10), similar to the phenotypes of the WER::WERD105A T3 plants (Fig. 9, B–D). Interestingly, these F1 plants showed higher CPC::GUS expression levels compared with the control (Fig. 10B), suggesting that WERD105A may outcompete WER in regulating CPC. The F1 plants from the WER::WERD105Q × wild type crosses showed significantly increased CPC::GUS expression levels (Fig. 10B), occasional loss of GL2::GUS/CPC::GUS expression in N-position cells (Fig. 10, A and B), and significant increases in ectopic root-hair cells in two of the three F1 populations (Fig. 10C). This suggests that WERD105Q outcompetes WER in regulating CPC and disrupts nonhair cell fate establishment despite the presence of wild-type WER.
Figure 10.
Functional comparison between WER and WER variants. A, Expression of the GL2::GUS transcriptional reporter in the F1 seedling roots from crosses between wild-type (WT) plants and wer-1 mutants bearing different WER::WER transgenes. For each transgene, representative F1 roots from crosses using three independent single-insertion lines are shown. Stars indicate H-position cell files. Bar = 50 μm. B, Expression of the CPC::GUS transcriptional reporter in the F1 seedling roots from crosses between wild-type plants and wer-1 mutants bearing different WER::WER transgenes. For each transgene, representative F1 roots from crosses using three independent single-insertion lines are shown. Stars indicate H-position cell files. Bar = 50 μm. C, Quantifications of root epidermis specification in the F1 seedling roots from crosses between wild-type plants and wer-1 mutants bearing different WER::WER transgenes. Error bars represent sd from three replicates. Two-way ANOVA was used to determine the differences among different transgenic lines using the #1 F1 population of the WER::WER transgene as the control. All F1 populations showing significant differences in H and/or N positions from the control are marked: **, P < 0.01.
In summary, the D105E, D105A, and D105Q substitutions alter WER’s ability to properly regulate the GL2 and CPC genes. The D105E substitution leads to defective WER function that can be rescued by wild-type WER; the D105A and D105Q substitutions enhance WER’s ability to regulate GL2 and CPC, with relatively stronger impact on the CPC gene. In the case of the D105Q substitution, this impact is significant enough to disrupt nonhair cell fate establishment.
DISCUSSION
In this report, we demonstrated the significance of a specific residue (Asp-105) in the WER transcription factor for appropriate regulation of root epidermal patterning. It is particularly interesting that substitutions of this residue did not abolish WER function, but rather they altered the ability of WER to properly regulate downstream genes and caused a variety of cell-type pattern phenotypes. The importance of this residue was first recognized through the identification and characterization of the wer-4 mutant, which exhibited a novel cell-type pattern in the root epidermis. We showed that the D105N substitution in wer-4 caused differential effects on WER target promoter binding, generated an imbalance in the levels of downstream gene expression, and reduced the molecular distinctions that normally exist between differentiating H- and N-position epidermal cells. We created three additional WER variants with substitutions at Asp-105, and each exhibited abnormalities in WER function and/or cell-type pattern. Altogether, these results reinforce the central role of WER in defining the epidermal cell-type pattern, and they reveal the specific importance of the Asp-105 residue for the transcriptional regulatory activity of WER.
Role of the Asp-105 Residue for WER Protein Function
Plant R2R3-type MYB proteins are defined by their similarities to the mammalian c-Myb protein, of which the R2 and R3 repeats comprise the minimum DNA-binding domains (Paz-Ares et al., 1987; Sakura et al., 1989; Kanei-Ishii et al., 1990). The solution structure of the mouse c-Myb R2 and R3 domains revealed that each domain consists of three α-helices with a Trp-formed hydrophobic core, and the third helices of each domain are DNA recognition helices containing several amino acids that directly associate with DNA bases (Ogata et al., 1994). The R2 and R3 repeats of the WER protein resemble those in the mammalian c-Myb protein (Jin and Martin, 1999), with each repeat containing three α-helices with appropriately spaced Trp residues and the same DNA-associating amino acids at the same relative positions (Tombuloglu et al., 2013; Wang et al., 2015; Fig. 1D). Furthermore, the previously defined in vivo DNA-binding sites of WER show substantial similarity to the DNA-binding consensus sequence for the mammalian c-Myb (Ryu et al., 2005; Kang et al., 2009; Song et al., 2011). Thus, it is likely that WER recognizes DNA in a comparable manner with its well-studied mammalian homolog, although WER itself has not been analyzed biochemically in the same detail as c-Myb (Saikumar et al., 1990; Gabrielsen et al., 1991).
The Asp-105 residue in the WER protein is conserved in more than 90% of all R2R3-type MYB proteins in Arabidopsis, and it is also present in the mammalian c-Myb (Ogata et al., 1994; Lin-Wang et al., 2010). Although this residue is not one of the c-Myb residues shown to directly associate with DNA, it is located near these residues and within the same DNA-recognizing helix of the R3 domain (Fig. 1D). Intriguingly, the solution structure of mouse c-Myb suggests that this Asp residue may be involved in the formation of a salt bridge that aids interaction between the R2 and R3 domains, which is essential for DNA binding (Ogata et al., 1994). However, to our knowledge, no studies have directly analyzed the functional importance of this amino acid.
In our study, we discovered that substitutions of the Asp-105 residue affected WER’s ability to promote transcription of its target genes. Specifically, the wer-4 mutant (encoding the WERD105N protein) exhibited a dramatic reduction in TRY and ETC1 gene expression, a mild reduction in CPC gene expression, and a slightly elevated level of GL2 gene expression in the developing root epidermis. In addition, our EMSA experiments showed that the WERD105N protein has an altered relative affinity for its target promoters, with a greater preference for the GL2 promoter over the CPC promoter, compared with the wild-type WER. Given that the Asp-105 residue is located in the putative DNA recognition helix, we conclude that the role of the Asp-105 residue is to aid DNA recognition, and the abnormal transcriptional regulation of WER target genes in wer-4 is due to the differential effect of the D105N substitution on WER’s affinity for individual target gene promoters.
We also made use of the yeast two-hybrid assays to show that the D105N substitution in WER does not reduce, and may actually enhance, the interaction between WER and the GL3 bHLH protein. Consistent with this, the Asp-105 residue is not located within the conserved bHLH-binding motif of WER (Zimmermann et al., 2004; Fig. 1D), although neighboring residues like Asp-105 may partly influence the protein interactions. The potentially enhanced association between WERD105N and GL3 is not likely to explain the differential effects of wer-4 on various target gene transcription because such an interaction change is expected to equally impact WER target genes.
In addition to the wer-4 mutant, we generated and analyzed other Asp-105 substitutions of WER. The D105E substitution essentially destroys WER activity and results in a phenotype resembling the wer-1 null mutant. The D105A and D105Q substitutions cause similar effects on WER function, although the D105Q substitution leads to a more severe and semidominant disruption of nonhair cell fate establishment. A possible explanation is that both the D105A and D105Q substitutions increase CPC production, which alters lateral inhibition and indirectly disturbs WER action, but D105Q has a greater effect on CPC than D105A. It is notable that, like wer-4, both the D105A and D105Q substitutions exhibit a relatively stronger effect on CPC than GL2, further supporting the importance of the Asp-105 residue in balancing the activity of WER among its various target gene promoter regions.
A Model for the Abnormal Pattern Formation in the wer-4 Mutant
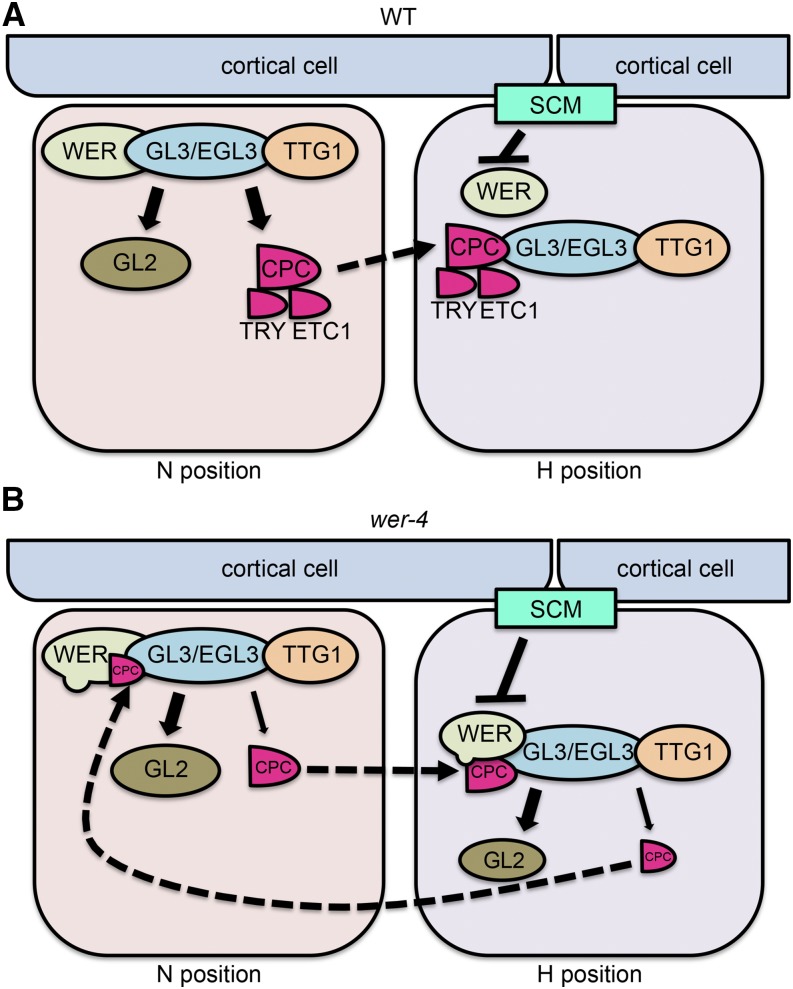
The wer-4 mutant exhibits a novel cell-type distribution in the root epidermis, with root-hair cells and nonhair cells produced in both the H and N positions rather than strictly position-dependent cell fate specification. Based on our results, we propose the following explanation for this abnormal pattern (Fig. 11). The WERD105N protein encoded by wer-4 has altered relative affinities for various WER target gene promoters, including relatively weak affinity for the CPC promoter and very weak affinity for the TRY and ETC1 promoters. This results in relatively low production of R3-type MYB competitors (CPC, TRY, and ETC1) in the developing root epidermis, which allows for abnormal accumulation of the WER-GL3/EGL3-TTG1 complex in the H-position cells. As a result, abnormally high levels of CPC are produced in the H-position cells, relative to the N-position cells, and this leads to abnormal movement of H-cell-produced CPC to N-position cells. Thus, rather than mediating unidirectional lateral inhibition (from N-position cells to H-position cells), as in wild-type roots, the CPC in wer-4 tends to mediate mutual disruption of the WER-GL3/EGL3-TTG complex in both H- and N-position cells. This weakens the position-dependent accumulation of the WER-GL3/EGL3-TTG1 complex that normally occurs in N-position cells, although the intact SCM signaling pathway in wer-4 ensures that N-position cells still tend to accumulate higher complex levels than H-position cells. Overall, the weakened and bidirectional lateral inhibition causes epidermal cells to accumulate variable amounts of the WER-GL3/EGL3-TTG complex in both H- and N-position cells, which directly leads to variable and ectopic expression of GL2 and accumulation of GL2 protein. The final fate of each individual cell depends on its GL2 protein level; epidermal cells that accumulate no, or low levels of, GL2 protein differentiate into root-hair cells, whereas cells that accumulate higher GL2 protein differentiate into nonhair cells (Fig. 7, B and D). Thus, the abnormal WER target gene expression in wer-4 ultimately leads to a mixture of both root-hair and nonhair cell types in the H- and N-position cells.
Figure 11.
Models for epidermal cell fate regulation in wild-type and wer-4 roots. The solid arrows (sharp or blunt) indicate transcriptional regulation. The dashed arrows indicate protein movement. A, In the wild-type (WT) root epidermis, the WER-GL3/EGL3-TTG1 complex preferentially accumulates in N-position cells and promotes the expression of GL2, CPC, TRY, and ETC1. The GL2 protein remains in N-position cells and inhibits root hair formation. The CPC, TRY, and ETC1 proteins move to adjacent H-position cells and compete with WER for GL3/EGL3 binding, allowing root hair formation. B, In the wer-4 mutant, the D105N residue substitution disrupts WER target gene transcription, largely abolishing TRY and ETC1 expression and reducing the expression of CPC relative to that of GL2. As a consequence, there is reduced competition for GL3/EGL3 binding and enhanced formation of the WER-GL3/EGL3-TTG1 complex in H-position cells. This triggers abnormal expression of GL2 and CPC in the H-position cells, leading to inappropriate CPC movement and accumulation in N-position cells as well as reduction of the WER-GL3/EGL3-TTG1 complex. Therefore, in the wer-4 root epidermis, the WER-GL3/EGL3-TTG1 complex accumulates abnormally in both H and N positions, leading to misspecification of cells in both positions.
In addition to an effect on cell fate specification, we have shown that wer-4 also affects root hair morphogenesis. Specifically, many of the ectopic root-hair cells in wer-4 produced shorter root hairs than the wild type (Fig. 8), suggesting that the small yet significant amounts of GL2 present in these N-position cells are able to partially inhibit root hair growth but are insufficient to induce the nonhair cell fate. This suggests that GL2, as a well-known positive regulator of nonhair genes and a negative regulator of root-hair genes (Lin et al., 2015), functions in a concentration-dependent manner in root-hair cell differentiation.
Notably, our study revealed a discrepancy between the relative levels of GL2 gene transcription and GL2 protein in wer-4. Although wer-4 has an elevated level of GL2::GFP transcriptional reporter expression relative to the wild type (Fig. 2E), the overall level of its GL2::GL2-GFP translational fusion reporter signal is comparable to the wild type (Fig. 7B). Given that these two reporter constructs contain the same 5′-GL2 promoter region (2-kb fragment; see “Materials and Methods”), this discrepancy suggests the existence of a posttranscriptional mechanism regulating GL2. This hypothesis is also consistent with our RT-qPCR result showing a slight decrease in GL2 transcript level in the wer-4 mutant (Fig. 2A). It is notable that overexpressing GL2 with the CaMV35S promoter has been reported to induce GL2 self-inhibition and perhaps cell toxicity (Ohashi et al., 2002).
Evolutionary Implications of the WER Asp-105 Substitutions
A central issue in evolutionary developmental biology is to understand how transcriptional regulatory networks might evolve to generate new developmental phenotypes (Nocedal and Johnson, 2015). Our study of the effects of altering a single residue in the WER protein on the root epidermal network provides some insight into this issue. We found that WER transcriptional activity is affected in different ways by substitutions of the Asp-105 residue. In particular, our data show that the D105N substitution modifies the affinities of WER for its target gene promoters, implying that this residue is important for modulating the relative activity of WER on its targets. From an evolutionary view, this opens the possibility that substitutions of this residue may provide a way to modulate or rewire the network and generate new phenotypic variations. Indeed, it may be argued that WERD105N, WERD105Q, and WERD105E represent examples of novel regulatory networks, because these changes in WER altered the spatial distribution of gene expression programs and yielded new root epidermal cell-type patterns.
Furthermore, it is notable that two of the Asp-105 substitutions of WER that we generated and analyzed in our study, D105A and D105E, occur naturally in some members of the R2R3-type MYB protein family of Arabidopsis (Fig. 1D; Stracke et al., 2001). Among the 125 R2R3-type MYB proteins (Stracke et al., 2001), almost 95% possess an Asp residue (D) in the corresponding position of Asp-105 in WER (i.e. d-type), whereas five members have Ala (A-type) and four members have Glu (E-type; Lin-Wang et al., 2010). Interestingly, four of the A-type MYB proteins (MYB75, MYB90, MYB113, and MYB114) are involved in anthocyanin biosynthesis (Gonzalez et al., 2008; Lin-Wang et al., 2010); two of the E-type MYB proteins (MYB115 and MYB118) are involved in glucosinolate and ω-7 biosynthesis (Zhang et al., 2015; Troncoso-Ponce et al., 2016); and another two E-type MYB proteins (MYB64 and MYB119) are involved in female gametogenesis (Rabiger and Drews, 2013). Thus, groups of R2R3-type MYB proteins carrying variations of WER Asp-105 tend to participate in particular biological processes. This implies that changes in this residue may help R2R3-type MYBs to evolve new target gene specificities permitting the MYBs and their associated regulatory gene networks to generate new phenotypes.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Most of the mutant and transgenic lines have been previously described: cpc-1 (Wada et al., 1997), wer-1 (Lee and Schiefelbein, 1999), GL2::GUS (Masucci et al., 1996), GL2::GFP (Lin and Schiefelbein, 2001), CPC::GUS (Wada et al., 2002), ETC1::GUS (Kirik et al., 2004), GL3::GL3-YFP (Bernhardt et al., 2005), and CPC::CPC-GFP (Kurata et al., 2005). The GL2::GL2-GFP transgenic line was a kind gift from Dr. Lijun An and Dr. Fei Yu (Northwest Agriculture and Forestry University, China). The GL2::GUS reporter includes a 4-kb promoter region of GL2 gene upstream of the translational start site (ATG). The GL2::GFP and GL2::GL2-GFP reporters both include a 2-kb promoter region of GL2 gene.
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized with 30% (v/v) bleach and 0.02% (v/v) Triton X-100. Seeds were sown on mineral nutrient mix medium solidified with 0.3% (w/v) Gelrite (Schiefelbein and Somerville, 1990). Plates were then incubated at 23°C under continuous light and 4-d-old seedlings were used for experiments. Mature plants were generated by transplanting seedlings to soil and grown in growth chambers under a long-day light cycle at 23°C (day) to 18°C (night).
Genetic Screening and Positional Mapping
Mutagenesis of the cpc-1 GL2::GUS line (Wassilewskija ecotype) with ethyl methanesulfonate was performed as previously reported (Estelle and Somerville, 1987). The cpc-1 wer-4 mutant was identified from the M2 population through a visual screen for root hair density with a dissection microscope. The F2 and F3 offspring from a cross between cpc-1 wer-4 and a Columbia wild-type plant were analyzed using multiple simple sequence length polymorphism markers (Bell and Ecker, 1994), and strong linkage was identified with marker nga151 (position 4.67 Mb on chromosome 5), near the WER gene (position 4.76 Mb on chromosome 5).
The derived cleaved-amplified polymorphic sequences (Neff et al., 2002) technique was used for wer-4 genotyping, using primers listed in Supplemental Table S1.
Transgene Construction and Plant Transformation
The WER genomic segment including 4-kb 5′ promoter sequence, 1-kb genomic sequence, and 1-kb 3′ terminal sequence was cloned using Phusion (NEB) and integrated into the pCB302 binary vector (digested with SpeI and BamHI; Xiang et al., 1999) using the HiFi assembly system (NEB). For WER::WER transgenes carrying different substitutions of Asp-105, the same 6-kb WER genomic segment was cloned into two pieces separated at the mutation site using primers carrying corresponding nucleotide changes. The two WER genomic sequence fragments (4.7 and 1.3 kb) bearing the preferred mutations were then combined into the pCB302 vector using the HiFi assembly system. Cloning primers are listed in Supplemental Table S1. Verified constructs were then transformed into wer-1 plants carrying either the CPC::GUS or GL2::GUS reporter through floral dipping as described (Clough and Bent, 1998).
After plant transformation, T0 plants were grown and T1 seeds were harvested and subjected to glufosinate-ammonium (PESTANAL, Sigma-Aldrich) selection. Resistant T1 seedlings were then grown for T2 seeds, and the segregation rate of individual T2 populations for resistance and root-hair pattern was used to identify single-insertion lines. For each transformation experiment, homozygous T3 populations from at least three independent single-insertion lines were used for further experiments.
Microscopy and Image Analysis
The quantification of root epidermal cell types was performed using a bright-field compound microscope, following brief staining with Toluidine Blue. Cell positions were determined according to underlying cortical cells, and hair cells were scored by visible protrusion as root hairs regardless of root hair length. For each genotype, three independent replicates were performed. For each replicate, up to 10 seedlings were used, and 10 cells in both H and N positions were scored in each seedling (total of 100 cells).
Histochemical analysis of GUS fusion reporter lines was performed as described (Masucci et al., 1996). Specifically, 10 μL mL−1 X-Gluc (Gold Biotechnology) substrates was used for GL2::GUS (20 min at 37°C) and 20 μL mL−1 X-Gluc substrates was used for CPC::GUS (90 min at 37°C) and ETC1::GUS (3 h at 37°C). GUS signal quantification was performed using ImageJ as described (Béziat et al., 2017). To generate histograms for CPC::GUS signal distribution, wild-type and wer-4 roots were stained and photographed under the same conditions. For each root, 10 continuous cells in one file were analyzed from the oldest cell prior to rapid elongation (i.e. the cell’s length exceeds its width) down toward the root tip. For all cells, the GUS signal was measured using the same region of interest (ROI) frame, and the mean values were plotted into histograms.
To analyze GUS reporter expression in leaves, the first pair of true leaves from 14-d-old plants was collected and incubated in staining buffer containing 20 μL mL−1 X-Gluc substrate at 37°C for 2 h (for GL2::GUS) or 4 h (for CPC::GUS and ETC1::GUS). The stained leaves were then cleared with ethanol:acetic acid as described (Béziat et al., 2017) to remove chlorophyll prior to imaging.
Fluorescence imaging was performed using a TCS SP5 DM6000B broadband confocal microscope (Leica) with 20× or 40× dry lens. Seedling roots were briefly stained in propidium iodide for cell wall visualization. Default excitement and emission settings for GFP, YFP, and propidium iodide signals were used for imaging. To generate histograms for GL2::GFP signal distribution, wild-type and wer-4 root images were captured using the same settings. Care was taken to ensure that each root was imaged on similar Z-axis positions marked by the maximum nucleus size. GFP quantification was performed using ImageJ under Red-Green-Blue separate channels, and only green channels were quantified. Ten continuous cells in each H- and N-position cell file were quantified using similar criteria as for GUS quantification. The GFP signal for the entire group of cells was measured using free-shape ROIs, and the mean values were plotted into histograms. Similarly, for GL2::GL2-GFP and GL3::GL3-YFP signal histograms, 10 continuous cells in each H- and N-position cell file were analyzed from wild-type and wer-4 roots. For each root, GFP signal in the nuclei was specifically collected using round-shape ROIs, and the mean values from each cell were used for plotting histograms. For CPC::CPC-GFP analysis, total signal levels in all cells within the meristematic zone (from the first measurable cell to the last cell before rapid elongation) from both H and N positions were measured with free-shape ROIs and divided by the cell number to generate an average GFP signal for each root. For each genotype, 20 roots were measured.
To examine the expression of fluorescence reporters and GUS reporters within cells of the same root, seedling roots were first imaged with a confocal microscope, and then the roots were removed from the microscope slides and stained for GUS signal. The stained roots were then imaged with the bright-field microscope. Special care was taken to ensure that the roots were placed in a similar posture on the slide as for the confocal imaging according to the cotyledons, and the same groups of cells was chosen for imaging based on the landmarks of the root epidermis. For GL2::GUS signal quantification, due to the significant difference in signal levels between wild-type and wer-4 roots, staining time was set differently for the wild type and wer-4 to ensure that signals from both genotypes were within measurable ranges.
To measure root hair length, photographs of root hairs were obtained using a light compound microscope, and measurement was performed using ImageJ. For each wild-type root, 10 root hairs on cells located in the H position were measured in the full maturation zone, which is approximately 3 to 5 mm from root tips and marked by an overall stable length of root hairs. For each wer-4 root, 10 root hairs on cells located in H and N positions were measured separately within the full maturation zone.
RNA Extraction and RT-qPCR
Total RNA was extracted from root tips (including meristematic zone, elongation zone, and early maturation zone) as described (Huang et al., 2015; RNeasy Plant Mini kit, Qiagen). The same method was used to extract RNA from the first pair of true leaves from 14-d-old plants. RNA was treated with the RQ1 DNase (Promega). cDNA was synthesized with the SuperScript First-Strand Synthesis System (Invitrogen). qPCR was set up using the Radiant Green Hi-ROX qPCR Kit (Alkali Scientific) and conducted using the StepOnePlus real-time PCR system (Applied Biosystems). The Delta-Delta-Ct method (Livak and Schmittgen, 2001) was used to determine the relative transcript amounts. The GAPCP2 gene (AT1G16300, encoding a GAPDH isoform) was used as the internal reference gene. Primers used for RT-qPCR are listed in Supplemental Table S1.
Yeast Two-Hybrid Assays
The yeast two-hybrid assays were conducted as previously described (Lee and Schiefelbein, 1999; Bernhardt et al., 2003). The pGBT9 construct containing the BD-GL3 fusion and the pGAD424 construct containing the AD-WER fusion were the same as previously used (Bernhardt et al., 2003). The AD-WERD105N construct was generated through replacing the wild-type WER coding sequence with the wer-4 WER coding sequence. After transformation into the HF7c yeast strain, the β-galactosidase assays were conducted using at least three individual transformants for each AD/BD combination, and analysis of each transformant was repeated three times.
EMSA
The EMSA was performed as described (Ryu et al., 2005) with purified WER and WERD105N proteins. For EMSA experiments with GWBSI/II or WBSI/II, and competition EMSA experiment between GWBSI and GWBSII, probe labeling was carried out with T4 polynucleotide kinase and [γ-32P]ATP (Ryu et al., 2005). For the competition EMSA experiment between GWBSI and WBSI, commercial hot probes with infrared labeling were used (Integrated DNA Technologies). Each EMSA experiment was repeated at least three times. Probe sequences are listed in Supplemental Table S1. The EMSA binding signals were quantified using ImageJ and nonlinear regression analysis. Given the different affinities of WER and WERD105N to GWBSI and WBSI, in both competition assays, the relative amounts of WER and WERD105N were adjusted to ensure that binding signals for both proteins are within measurable ranges.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: WER (AT5G14750), MYB23 (AT5G40330), GL1 (AT3G27920), MYB113 (AT1G66370), MYB115 (AT5G40360), GL2 (AT1G79840), CPC (AT2G46410), ETC1 (AT1G01380), TRY (AT5G53200), GL3 (AT5G41315), and SCM (AT1G11130).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Expression of WER target genes in leaves is not affected by wer-4.
Supplemental Figure S2. Correlation between GL2 and CPC gene expression is largely maintained in the wer-4 mutant.
Supplemental Figure S3. The WER and WERD105N proteins are able to bind to GL2 and CPC promoter regions.
Supplemental Figure S4. The WERD105N protein exhibits unbalanced affinities between binding sites in GL2 promoter regions compared with WER.
Supplemental Figure S5. Cell-to-cell variation in GL2 protein level in wer-4 is largely determined by GL2 transcription.
Supplemental Table S1. Primers used in genotyping, cloning, RT-qPCR, and EMSA.
ACKNOWLEDGMENTS
We thank Dr. Lijun An (Dr. Fei Yu’s laboratory, Northwest Agriculture and Forestry University, China) for the kind gift of the GL2::GL2-GFP transgenic line. We thank Dr. Erik Nielsen (University of Michigan), Dr. Andrzej Wierzbicki (University of Michigan), and Dr. Jairam Menon (University of Michigan) for suggestions and informative discussion. We thank Gregg Soboccinski (University of Michigan) for technical assistance with microscopy. We thank Dr. Chen Zhang (University of Michigan) and Dr. Kenneth Cadigan (University of Michigan) for helpful suggestions on the EMSA experiments.
Footnotes
This work was supported by a grant from the National Science Foundation (IOS-1444400).
Articles can be viewed without a subscription.
References
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144 [DOI] [PubMed] [Google Scholar]
- Berger F, Haseloff J, Schiefelbein J, Dolan L (1998) Positional information in root epidermis is defined during embryogenesis and acts in domains with strict boundaries. Curr Biol 8: 421–430 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, Schiefelbein J (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439 [DOI] [PubMed] [Google Scholar]
- Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J (2005) The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 132: 291–298 [DOI] [PubMed] [Google Scholar]
- Béziat C, Kleine-Vehn J, Feraru E (2017) Histochemical staining of β-glucuronidase and its spatial quantification. Methods Mol Biol 1497: 73–80 [DOI] [PubMed] [Google Scholar]
- Bruex A, Kainkaryam RM, Wieckowski Y, Kang YH, Bernhardt C, Xia Y, Zheng X, Wang JY, Lee MM, Benfey P, et al. (2012) A gene regulatory network for root epidermis cell differentiation in Arabidopsis. PLoS Genet 8: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Duckett CM, Grierson C, Linstead P, Schneider K, Lawson E, Dean C, Poethig S, Roberts K (1994) Clonal relationships and cell patterning in the root epidermis of Arabidopsis. Development 120: 2465 [Google Scholar]
- Estelle MA, Somerville C (1987) Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet 206: 200–206 [Google Scholar]
- Gabrielsen OS, Sentenac A, Fromageot P (1991) Specific DNA binding by c-Myb: Evidence for a double helix-turn-helix-related motif. Science 253: 1140–1143 [DOI] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166: 740–754 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53: 814–827 [DOI] [PubMed] [Google Scholar]
- Huang L, Schiefelbein J (2015) Conserved gene expression programs in developing roots from diverse plants. Plant Cell 27: 2119–2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Clegg MT, Jiang T (2004) Evolutionary dynamics of the DNA-binding domains in putative R2R3-MYB genes identified from rice subspecies indica and japonica genomes. Plant Physiol 134: 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Martin C (1999) Multifunctionality and diversity within the plant MYB-gene family. Plant Mol Biol 41: 577–585 [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, Sarai A, Sawazaki T, Nakagoshi H, He DN, Ogata K, Nishimura Y, Ishii S (1990) The tryptophan cluster: A hypothetical structure of the DNA-binding domain of the myb protooncogene product. J Biol Chem 265: 19990–19995 [PubMed] [Google Scholar]
- Kang YH, Kirik V, Hulskamp M, Nam KH, Hagely K, Lee MM, Schiefelbein J (2009) The MYB23 gene provides a positive feedback loop for cell fate specification in the Arabidopsis root epidermis. Plant Cell 21: 1080–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YH, Song SK, Schiefelbein J, Lee MM (2013) Nuclear trapping controls the position-dependent localization of CAPRICE in the root epidermis of Arabidopsis. Plant Physiol 163: 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Simon M, Huelskamp M, Schiefelbein J (2004) The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol 268: 506–513 [DOI] [PubMed] [Google Scholar]
- Kurata T, Ishida T, Kawabata-Awai C, Noguchi M, Hattori S, Sano R, Nagasaka R, Tominaga R, Koshino-Kimura Y, Kato T, et al. (2005) Cell-to-cell movement of the CAPRICE protein in Arabidopsis root epidermal cell differentiation. Development 132: 5387–5398 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Schiefelbein J (2007) The role of the SCRAMBLED receptor-like kinase in patterning the Arabidopsis root epidermis. Dev Biol 302: 118–131 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Shen R, Schiefelbein J (2005) Positional signaling mediated by a receptor-like kinase in Arabidopsis. Science 307: 1111–1113 [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J (1999) WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99: 473–483 [DOI] [PubMed] [Google Scholar]
- Lee MM, Schiefelbein J (2002) Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback. Plant Cell 14: 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Ohashi Y, Kato M, Tsuge T, Gu H, Qu LJ, Aoyama T (2015) GLABRA2 directly suppresses basic helix-loop-helix transcription factor genes with diverse functions in root hair development. Plant Cell 27: 2894–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Schiefelbein J (2001) Embryonic control of epidermal cell patterning in the root and hypocotyl of Arabidopsis. Development 128: 3697–3705 [DOI] [PubMed] [Google Scholar]
- Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC (2010) An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18: 613–615 [DOI] [PubMed] [Google Scholar]
- Nocedal I, Johnson AD (2015) How transcription networks evolve and produce biological novelty. Cold Spring Harb Symp Quant Biol 80: 265–274 [DOI] [PubMed] [Google Scholar]
- Ogata K, Morikawa S, Nakamura H, Sekikawa A, Inoue T, Kanai H, Sarai A, Ishii S, Nishimura Y (1994) Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell 79: 639–648 [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Ruberti I, Morelli G, Aoyama T (2002) Entopically additive expression of GLABRA2 alters the frequency and spacing of trichome initiation. Plant J 29: 359–369 [DOI] [PubMed] [Google Scholar]
- Pattanaik S, Patra B, Singh SK, Yuan L (2014) An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Front Plant Sci 5: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H (1987) The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J 6: 3553–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiger DS, Drews GN (2013) MYB64 and MYB119 are required for cellularization and differentiation during female gametogenesis in Arabidopsis thaliana. PLoS Genet 9: e1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev 8: 1388–1399 [DOI] [PubMed] [Google Scholar]
- Ryu KH, Kang YH, Park YH, Hwang I, Schiefelbein J, Lee MM (2005) The WEREWOLF MYB protein directly regulates CAPRICE transcription during cell fate specification in the Arabidopsis root epidermis. Development 132: 4765–4775 [DOI] [PubMed] [Google Scholar]
- Saikumar P, Murali R, Reddy EP (1990) Role of tryptophan repeats and flanking amino acids in Myb-DNA interactions. Proc Natl Acad Sci USA 87: 8452–8456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H, Kanei-Ishii C, Nagase T, Nakagoshi H, Gonda TJ, Ishii S (1989) Delineation of three functional domains of the transcriptional activator encoded by the c-myb protooncogene. Proc Natl Acad Sci USA 86: 5758–5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J 21: 5036–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Huang L, Zheng X (2014) Regulation of epidermal cell fate in Arabidopsis roots: The importance of multiple feedback loops. Front Plant Sci 5: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J, Kwak SH, Wieckowski Y, Barron C, Bruex A (2009) The gene regulatory network for root epidermal cell-type pattern formation in Arabidopsis. J Exp Bot 60: 1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Somerville C (1990) Genetic control of root hair development in Arabidopsis thaliana. Plant Cell 2: 235–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Lee MM, Lin Y, Gish L, Schiefelbein J (2007) Distinct and overlapping roles of single-repeat MYB genes in root epidermal patterning. Dev Biol 311: 566–578 [DOI] [PubMed] [Google Scholar]
- Song SK, Ryu KH, Kang YH, Song JH, Cho YH, Yoo SD, Schiefelbein J, Lee MM (2011) Cell fate in the Arabidopsis root epidermis is determined by competition between WEREWOLF and CAPRICE. Plant Physiol 157: 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4: 447–456 [DOI] [PubMed] [Google Scholar]
- Tombuloglu H, Kekec G, Sakcali MS, Unver T (2013) Transcriptome-wide identification of R2R3-MYB transcription factors in barley with their boron responsive expression analysis. Mol Genet Genomics 288: 141–155 [DOI] [PubMed] [Google Scholar]
- Troncoso-Ponce MA, Barthole G, Tremblais G, To A, Miquel M, Lepiniec L, Baud S (2016) Transcriptional activation of two delta-9 palmitoyl-ACP desaturase genes by MYB115 and MYB118 is critical for biosynthesis of omega-7 monounsaturated fatty acids in the endosperm of Arabidopsis seeds. Plant Cell 28: 2666–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, Goto K, Marks MD, Shimura Y, Okada K (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129: 5409–5419 [DOI] [PubMed] [Google Scholar]
- Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tang J, Hu R, Wu P, Hou XL, Song XM, Xiong AS (2015) Genome-wide analysis of the R2R3-MYB transcription factor genes in Chinese cabbage (Brassica rapa ssp. pekinensis) reveals their stress and hormone responsive patterns. BMC Genomics 16: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40: 711–717 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li B, Huai D, Zhou Y, Kliebenstein DJ (2015) The conserved transcription factors, MYB115 and MYB118, control expression of the newly evolved benzoyloxy glucosinolate pathway in Arabidopsis thaliana. Front Plant Sci 6: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J 40: 22–34 [DOI] [PubMed] [Google Scholar]