A plant virus-based system enables transient gain- and loss-of-function studies in pepper, a highly recalcitrant species for genetic transformation.
Abstract
While pepper (Capsicum annuum) is a highly recalcitrant species for genetic transformation studies, plant virus-based vectors can provide alternative and powerful tools for transient regulation and functional analysis of genes of interest in pepper. In this study, we established an effective virus-based vector system applicable for transient gain- and loss-of-function studies in pepper using Broad bean wilt virus2 (BBWV2). We engineered BBWV2 as a dual gene expression vector for simultaneous expression of two recombinant proteins in pepper cells. In addition, we established enhanced and stable expression of recombinant proteins from the BBWV2-based dual vector via coexpression of a heterologous viral suppressor of RNA silencing. We also developed a BBWV2-based virus-induced gene silencing (VIGS) vector, and we successfully silenced the phytoene desaturase gene (PDS) using the BBWV2-based VIGS vector in various pepper cultivars. Additionally, we optimized the BBWV2-based VIGS system in pepper by testing the efficiency of PDS gene silencing under different conditions. This BBWV2-based vector system represents a convenient approach for rapid and simple analysis of gene functions in pepper.
Pepper (Capsicum annuum), a member of the family Solanaceae, is an important crop grown worldwide. Recently, intensive high-throughput genomic sequencing studies have provided extensive data on transcriptomic regulation in pepper and increased the need for rapid and simple gene function analysis systems (Liu et al., 2013; Kim et al., 2014; Qin et al., 2014; Dubey et al., 2019). However, limited progress has been made in terms of functional studies of pepper genes owing to the lack of efficient genetic transformation methods for pepper (Lee et al., 2004; Bulle et al., 2016). Currently, the recalcitrance of in vitro regeneration and genetic transformation of pepper is still a major drawback for functional characterization of candidate genes via gain- and loss-of-function studies in pepper (Lee et al., 2004; Aarrouf et al., 2012). On the contrary, transient gene expression approaches, including Agrobacterium tumefaciens-mediated gene delivery (agroinfiltration) and protoplast transformation, have been performed for ectopic expression of genes of interest and examination of their functions at the cellular level in pepper (Ruffel et al., 2005; Sohn et al., 2006; Jeon et al., 2007). However, these approaches cannot be used to systemically explore the function of a gene at the whole-plant level.
Plant virus-mediated gene delivery systems can be employed as an alternative approach for transient gain- and loss-of-function studies, particularly in plant species recalcitrant for genetic transformation (Zhang and Ghabrial, 2006; Seo et al., 2016; Ding et al., 2018). Since plant virus-based vectors systemically infect their host plants, genes of interest can be delivered into the plants at the whole-plant level within a short period by simple cloning and inoculation (Gleba et al., 2007). Regarding the expression of genes of interest in plants, virus vector systems are superior to the transgenic approach because viruses can replicate to high titers in the infected cells (Gleba et al., 2007). For transient loss-of-function studies, plant viruses can be engineered as virus-induced gene silencing (VIGS) vectors (Robertson, 2004). In the last two decades, VIGS systems have been widely used as fast and efficient reverse genetic tools for successful characterization of numerous plant genes involved in plant development, metabolic regulation, and disease resistance in a variety of plant species (Purkayastha and Dasgupta, 2009; Ramegowda et al., 2014).
In pepper, Tobacco rattle virus (TRV) has been used as a VIGS vector (Chung et al., 2004). However, the pTRV empty VIGS vector can induce systemic necrosis in various plant species, including tomato (Solanum lycopersicum) and pepper, as well as Nicotiana benthamiana (Hartl et al., 2008; Wu et al., 2011; Tran et al., 2016). Furthermore, TRV VIGS vectors carrying host-derived sequence inserts can induce systemic necrosis in solanaceous plants when silencing of the target genes is not successfully triggered, making it difficult to characterize the defense and cell death response genes (Hartl et al., 2008; Wu et al., 2011). A few viruses that can infect pepper, including Potato virus X (PVX), Pepper mottle virus, and Tobacco mosaic virus (TMV), have been developed as viral vectors for systemic gene expression in plants (Gleba et al., 2007; Song and Ryu, 2017). However, these viral vectors could cause severe viral symptoms, such as necrosis, yellowing, stunting, and/or leaf malformation, in pepper; thus, they are not suitable for systemic gene function analysis in pepper (Scholthof et al., 1996; Ruffel et al., 2002; Gleba et al., 2007).
Broad bean wilt virus2 (BBWV2), which belongs to the genus Fabavirus in the family Secoviridae, has a wide host range, including many economically important crops, such as pepper, cucumber (Cucumis sativus), broad bean (Vicia faba), and sesame (Sesamum indicum; Lisa and Boccardo, 1996; Kwak et al., 2013; Seo et al., 2017). The BBWV2 genome is composed of two single-stranded positive-sense RNA molecules, RNA1 and RNA2, which are approximately 5,960 and 3,600 nucleotides in length, respectively (Kwak et al., 2016). Each RNA segment contains a single open reading frame (ORF) that is translated into a single polyprotein precursor (Kwak et al., 2016). The polyprotein precursor encoded by RNA1 yields five mature proteins by proteolytic cleavage: protease cofactor, NTP-binding motif, VPg, protease, and RNA-dependent RNA polymerase. The polyprotein precursor encoded by RNA2 yields three mature proteins: movement protein (MP), large coat protein (LCP), and small coat protein (SCP). In our previous studies, we generated infectious cDNA clones of two distinct strains of BBWV2, pBBWV2-PAP1 (a severe strain) and pBBWV2-RP1 (a mild strain), and identified MP as the responsible symptom severity determinant (Kwak et al., 2016; Seo et al., 2017).
In this study, we engineered pBBWV2-RP1, which causes systemic infection with no obvious symptoms in pepper, as an effective virus-based vector system applicable for transient gain- and loss-of-function studies. Genetic engineering of a dual gene insertion cassette between the MP and LCP cistrons in BBWV2 RNA2 was successful in expressing two genes simultaneously in the same cells. Stable expression of recombinant proteins was further enhanced via coexpression of heterologous viral suppressors of RNA silencing (VSRs) from the BBWV2-based dual vector. We also engineered pBBWV2-RP1 as a VIGS vector that works successfully in pepper. We suggest that the developed BBWV2-based vector system could provide a useful tool to the research community for functional studies of pepper genes.
RESULTS AND DISCUSSION
Engineering of BBWV2 as a Viral Gene Expression Vector
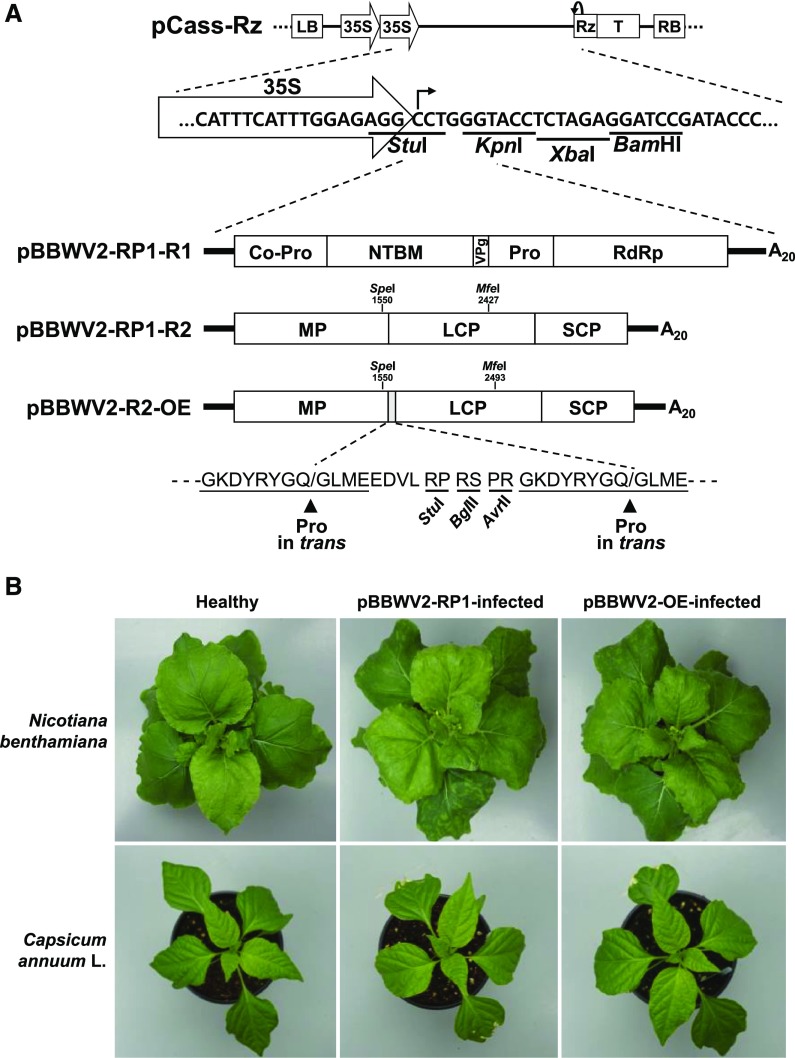
In our previous study, we constructed infectious cDNA clones of two BBWV2 strains (Kwak et al., 2016). pBBWV2-RP1 caused very mild symptoms, whereas pBBWV2-PAP1 caused severe symptoms, including stunting and leaf distortion (Kwak et al., 2016). The symptomatic characteristics of a viral vector are important for its use in functional studies of genes of interest because the obtained phenotypes can be superimposed on, and sometimes complicated by, viral symptoms. Therefore, pBBWV2-RP1 is advantageous for use in engineering as a viral vector. To develop pBBWV2-RP1 as a viral vector for the expression of genes of interest in pepper, an additional protease cleavage site (GKDYRYGQ/GLME) of BBWV2 and cloning sites (StuI, BglII, and AvrII) were engineered into the polyprotein ORF between the MP and LCP cistrons in pBBWV2-RP1-R2 (Fig. 1A). To minimize the probability of homologous recombination between duplicated cleavage sites and the subsequent instability, the nucleotide sequence of the inserted protease cleavage site was synonymously modified based on the codon usage frequency for BBWV2. The resultant plasmid, pBBWV2-R2-OE, was infectious when agroinfiltrated together with pBBWV2-RP1-R1 and induced very mild symptoms in N. benthamiana and no obvious symptoms in pepper plants (‘Sinhong’), similar to the actions of its parental clone, pBBWV-RP1-R2 (Fig. 1B). To examine infection efficiency of pBBWV2-OE, 15 plants of each species, including N. benthamiana, pepper, Perilla frutescens, and Arabidopsis (Arabidopsis thaliana), were inoculated by agroinfiltration. Reverse transcription (RT)-PCR analysis confirmed that all inoculated plants were systemically infected (Supplemental Table S1). In addition, there was no obvious difference in the accumulation of viral RNA in N. benthamiana plants infected with pBBWV2-OE compared with that in plants infected with pBBWV-RP1 (Supplemental Fig. S1). The results indicated that engineering the gene expression cassette into the viral genome did not affect virus replication competence. A desired gene can be cloned into pBBWV2-R2-OE using the cloning sites to create an in-frame translational fusion. During viral infection, the cloned gene will be expressed as a part of the viral polyprotein and processed by protease in trans to yield mature proteins containing 10 additional amino acids (GLMEEDVLRP) at the N terminus and 14 amino acids (RPRSPRGKDYRYGQ) at the C terminus when cloned using the BglII cloning site (Fig. 1A).
Figure 1.
Engineering of BBWV2 as a viral gene expression vector. A, Schematic representation of the construction of the pBBWV2-OE vector. The binary vector, pCass-Rz, contains a left border of T-DNA (LB), a double 35S promoter (35S), multiple cloning sites, a cis-cleaving ribozyme sequence (Rz), a NOS terminator (T), and a right border of T-DNA (RB) in sequential order. pBBWV2-R2-OE contains a single gene expression cassette between the MP and LCP cistrons. The gene insertion cassette contains one additional peptide cleavage site and three unique cloning sites (StuI, BglII, and AvrII) as indicated. Amino acid sequences of the peptide cleavage sites recognized by Pro viral protease are underlined, and arrowheads indicate the locations of the cleaved peptide bonds. Co-Pro, Protease cofactor; NTB, NTP-binding motif; RdRp, RNA-dependent RNA polymerase. B, Symptoms observed in N. benthamiana and pepper (‘Sinhong’) infected with pBBWW2-RP1 or pBBWV2-OE. Both BBWV2 constructs induced only mild mosaic symptoms in N. benthamiana and no obvious symptoms in pepper.
To verify the expression of recombinant proteins using the BBWV2 vector in pepper, the GFP gene (gfp) was initially selected and cloned into pBBWV2-R2-OE using the BglII and AvrII cloning sites, yielding a construct designated as pBBWV2-R2-OE-GFP (Fig. 2A). N. benthamiana and pepper (‘Sinhong’) were infiltrated with a mixture of A. tumefaciens cultures containing pBBWV2-RP1-R1 and pBBWV2-R2-OE-GFP. At 6 d postinoculation (dpi), the infiltrated plants were observed under UV light and strong and systemic expression of GFP was detected in the upper leaves of the infiltrated plants (Fig. 2B). pBBWV2-OE-GFP was also infectious in other hosts, including P. frutescens and Arabidopsis, and competent for replication and systemic GFP expression in these host plants (Fig. 2B; Supplemental Table S1).
Figure 2.
GFP expression using the BBWV2-based gene expression vector in various plant species. A, Schematic representation of pBBWV2-R2-OE-GFP. The gfp gene was cloned into pBBWV2-R2-OE using the BglII and AvrII sites. B, Detection of fluorescence signals in the leaves of N. benthamiana, pepper (‘Sinhong’), P. frutescens, and Arabidopsis after infection with pBBWV2-OE-GFP. Whole-leaf fluorescence signals were observed using an in vivo fluorescence imaging system.
Engineering of BBWV2 as a Dual Gene Expression Vector
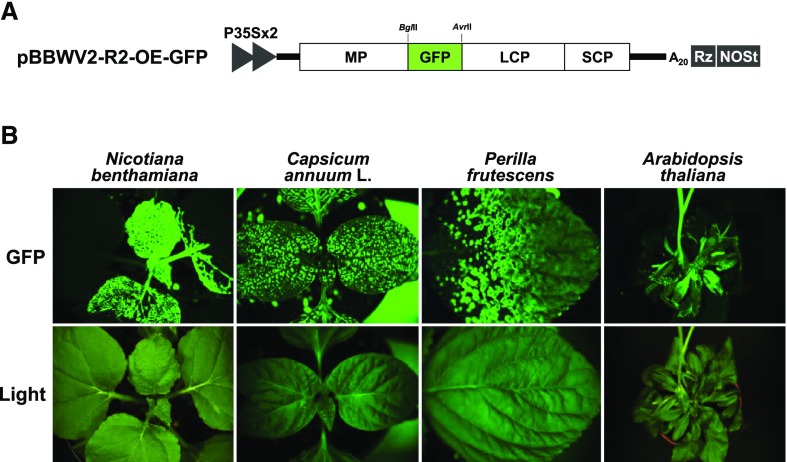
For simultaneous delivery of two genes into the same cells, we modified pBBWV-R2-OE by engineering a dual gene expression cassette between the MP and LCP cistrons. The dual gene expression cassette was composed of two additional protease cleavage sites (the additional protease cleavage sites were synonymously modified to minimize the probability of homologous recombination with each other) and two restriction enzyme sites (BglII and AvrII; Fig. 3A). The resulting dual gene expression vector was designated as pBBWV2-OEx2 (Fig. 3A). Two different genes could be expressed simultaneously in the same cells by the pBBWV2-OEx2 vector upon utilizing two independent gene insertion cassettes via viral polyprotein processing. The infectivity of pBBWV2-OEx2 was confirmed by agroinfiltration in N. benthamiana and pepper, and pBBWV2-OEx2 induced symptoms identical to those induced by pBBWV2-RP1 in the host plants. In addition, the insertion of the dual gene expression cassette into the viral genome did not affect virus replication competence (Supplemental Fig. S1).
Figure 3.
Simultaneous expression of two recombinant proteins using the BBWV2-based dual gene expression vector. A, Schematic representation of the construction of pBBWV2-R2-OEx2. pBBWV2-R2-OEx2 contains a dual gene expression cassette between the MP and LCP cistrons. The dual gene insertion cassette contains two additional peptide cleavage sites and two unique cloning sites (BglII and AvrII) as indicated. Amino acid sequences of the peptide cleavage sites recognized by Pro viral protease are underlined, and arrowheads indicate the locations of the cleaved peptide bonds. B, Schematic representation of pBBWV2-R2-OEx2-GFP/nCFP. The gfp and NLS-tagged cfp genes were cloned into pBBWV2-R2-OEx2 using the BglII and AvrII sites, respectively. C, Confocal images showing subcellular distribution of GFP and NLS-tagged CFP expressed by the BBWV2-based dual gene expression vector in N. benthamiana and pepper cells. No fluorescence was detected in the cells infected with pBBWV2-OEx2. Bars = 50 μm.
To verify the simultaneous expression of two different proteins from the pBBWV2-OEx2 vector, we used two fluorescence reporter genes, gfp and the cyan fluorescent protein gene (cfp). To separately detect the expression of the resulting fluorescent proteins (i.e. GFP and CFP) in the same cells, CFP was tagged with the SV40 T antigen nuclear localization signal (NLS). The gfp and NLS-tagged cfp genes were cloned into pBBWV2-R2-OEx2 using the restriction enzyme sites BglII and AvrII, respectively, in the dual gene expression cassette, resulting in a construct designated as pBBWV2-R2-OEx2-GFP/nCFP (Fig. 3B). N. benthamiana and pepper (‘Sinhong’) were agroinfiltrated with pBBWV2-OEx2-GFP/nCFP. At 6 dpi, systemic leaves of N. benthamiana and pepper plants agroinfiltrated with pBBWV2-OEx2-GFP/nCFP were subjected to confocal microscopy to observe fluorescent signals. As expected, GFP signals were detected throughout the cytoplasm as well as in the nucleoplasm, whereas CFP signals were strongly observed in the nucleus (Fig. 3C), showing that two different genes could be successfully delivered into the same cells of N. benthamiana and pepper plants via the pBBWV2-OEx2 vector. As negative controls, no fluorescent signals were evident in the systemic leaves of the plants agroinfiltrated with pBBWV2-OEx2 (empty vector; Fig. 3C).
Heterologous VSRs Could Enhance Stable Gene Expression from the BBWV2-Based Vector
A previous study has shown the BBWV2 MP and LCP function as relatively weak VSRs that can suppress single-stranded RNA-induced RNA silencing; however, they cannot suppress double-stranded RNA (dsRNA)-induced RNA silencing (Kong et al., 2014). This suggests that dsRNA replication intermediates of BBWV2 can be targeted by host gene-silencing machineries, resulting in a decline in virus replication as the infection progresses and persists at a low level. Thus, we aimed to examine how long GFP expression from the BBWV2-based vector could be maintained in the infected plants. Time-course observation under UV light showed high GFP expression levels at the early infection stage that gradually decreased as viral infection progressed systemically (Fig. 4C).
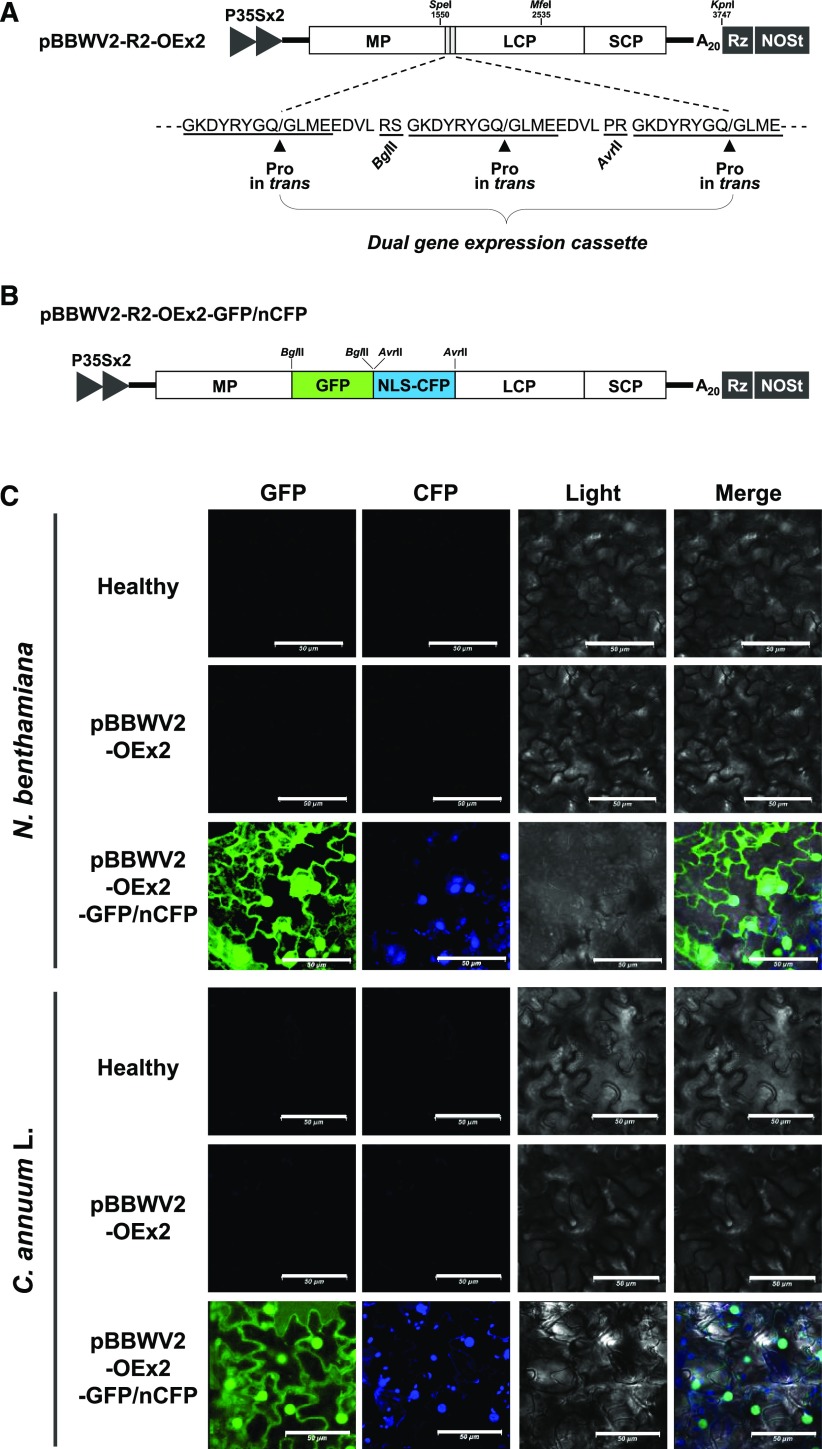
Figure 4.
Effects of heterologous VSRs on gene expression by the BBWV2-based vector. A, Schematic representation of the BBWV2-based dual gene expression constructs simultaneously harboring the gfp gene and either CMV 2b (333 bp), FHV B2 (321 bp), or TBSV P19 (519 bp). B, Symptoms in N. benthamiana infected with pBBWW2-OEx2 (empty vector), pBBWV2-OEx2-GFP, -GFP/2b, -GFP/B2, or -GFP/P19. C, Time-course observation of the effects of heterologous VSRs on GFP expression by the BBWV2-based vector. After infection with the indicated recombinant BBWV2 constructs, whole-leaf fluorescence signals were observed using an in vivo fluorescence imaging system in a time-course manner.
To overcome this phenomenon, we aimed to examine whether coexpression of heterologous VSRs could improve foreign gene expression efficiency of the BBWV2-based vector system. Therefore, the pBBWV2-OEx2 vector was used to simultaneously express heterologous VSRs together with GFP in the same cells. First, the gfp gene was cloned into pBBWV2-R2-OEx2 using the BglII site to generate pBBWV2-R2-OEx2-GFP. Then, three heterologous VSRs, including the Cucumber mosaic virus (CMV) 2b, Tomato bushy stunt virus (TBSV) p19, and Flock house virus (FHV) B2 (an insect viral RNA-silencing suppressor but active in plants; Seo et al., 2012), were cloned into pBBWV2-R2-OEX2-GFP using the AvrII site. The resulting clones were designated as pBBWV2-R2-OEx2-GFP/2b, -GFP/P19, and -GFP/B2, respectively (Fig. 4A).
The expression of heterologous VSRs using a virus-based vector could increase symptom severity (Zhang and Ghabrial, 2006; Seo et al., 2009b). N. benthamiana plants were agroinfiltrated with pBBWV2-OEx2-GFP/2b, -GFP/B2, or -GFP/P19, and symptom development was monitored for 4 weeks postinoculation. Interestingly, FHV B2 minimally increased symptom severity, whereas CMV 2b and TBSV P19 caused significant symptom enhancement (Fig. 4B). For example, plants infected with pBBWV2-OEx2-GFP/P19 showed severe stunting and curling in the systemic leaves, and pBBWV2-OEx2-GFP/2b caused leaf size reduction and mosaic symptoms in the systemic leaves. However, only mild mosaic symptoms were observed in the plants infected with pBBWV2-OEx2-GFP/B2 (Fig. 4B).
Next, to investigate the effects of heterologous VSRs on enhancing gene expression from the BBWV2-based vector, N. benthamiana plants infected with pBBWV2-OEx2-GFP/2b, -GFP/B2, or -GFP/P19 were observed under UV light for different durations (Fig. 4C). The results showed that GFP expression from the BBWV2-based vector was dramatically enhanced by B2, whereas 2b exhibited only marginal effects on enhancing GFP expression. P19 also increased GFP expression; however, the associated increase in symptom severity made it unfavorable for use in the BBWV2-based vector system. Reverse transcription quantitative PCR (RT-qPCR) analysis revealed that heterologous VSRs increased the accumulation levels of viral RNA in association with the degree of GFP expression (Supplemental Fig. S2). To examine whether plant growth temperature affects gene expression level from the BBWV2-based vector, N. benthamiana plants were infected with pBBWV2-OEx2-GFP/B2 and grown in plant growth chambers at different temperatures (20°C, 25°C, and 30°C). Time-course observation of GFP signals showed reduced and delayed GFP expression in the plants grown at 20°C (Supplemental Fig. S3).
Application of the BBWV2-Based Gene Expression Vector for Transient Gain-of-Function Studies in Pepper
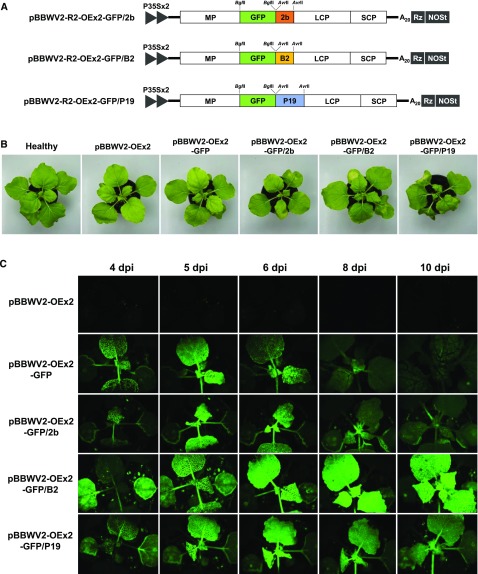
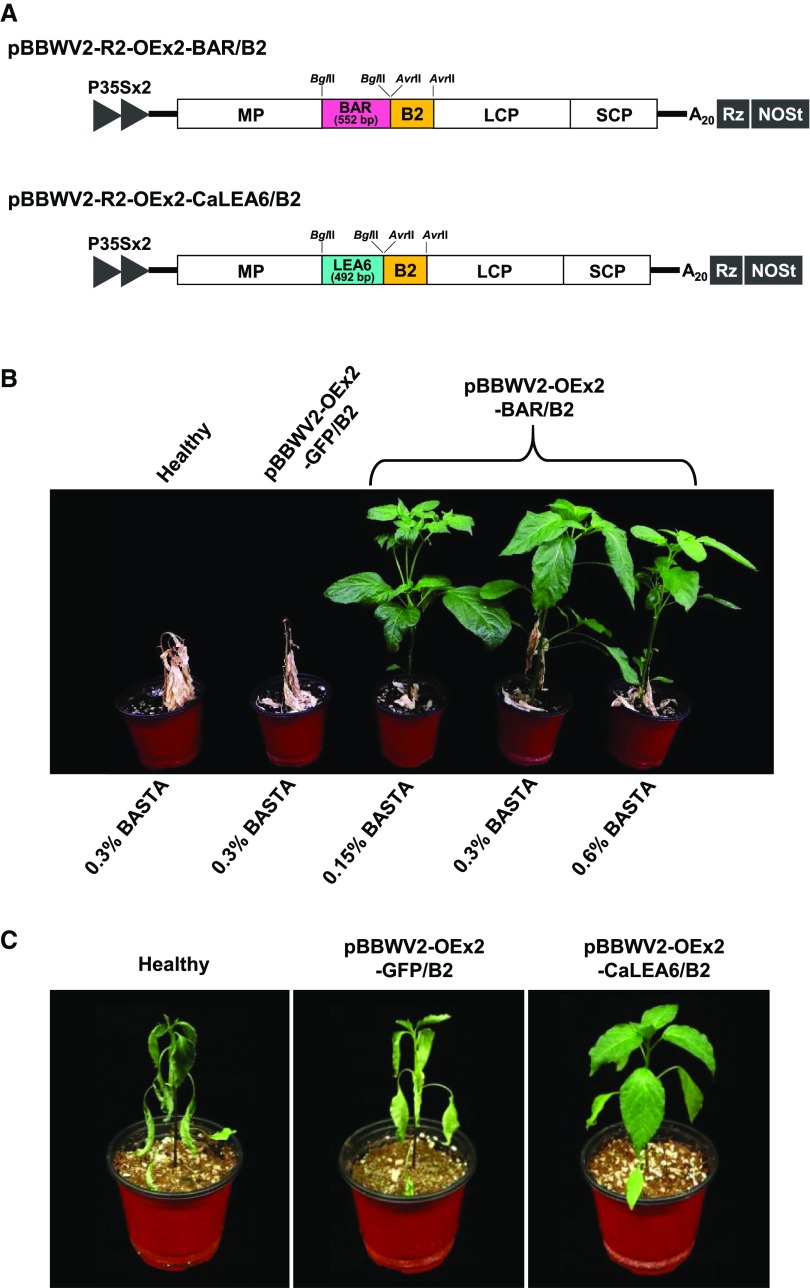
Next, we investigated whether the gene products expressed by the BBWV2-based vectors could retain their own biological activities. We selected and cloned the herbicide resistance bar gene (coding for phosphinothricin acetyltransferase) and CaLEA6 gene (coding for a late embryogenesis abundant-like protein that belongs to the hydrophobic LEA group 6; Kim et al., 2005) into pBBWV2-R2-OEx2-B2 using the BglII site to generate pBBWV2-R2-OEx2-BAR/B2 and pBBWV2-R2-OEx2-CaLEA6/B2, respectively (Fig. 5A).
Figure 5.
Application of the BBWV2-based gene expression vector for transient gain-of-function studies in pepper. A, Schematic representation of the BBWV2-based dual gene expression constructs simultaneously harboring either the bar gene (552 bp) or the CaLEA6 gene (492 bp) and FHV B2. B, Conferment of herbicide resistance in pepper by infection with the BBWV2-based vector expressing the bar gene. Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OEx2-GFP/B2 or -BAR/B2. At 8 dpi, herbicide treatment was applied to the plants as indicated. Photographs were taken 4 weeks after herbicide treatment. C, Conferment of drought tolerance in pepper by infection with the BBWV2-based vector expressing the CaLEA6 gene. Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OEx2-GFP/B2 or -CaLEA6/B2. After inoculation, drought stress was simultaneously applied to the plants by withholding watering. The plants were photographed 17 d after drought treatment.
The bar gene has been widely used as an effective selectable marker since it provides tolerance to glufosinate ammonium (Gordon-Kamm et al., 1990; Cao et al., 1992). To examine if the BBWV2-based vector systemically delivers the functionally active bar gene in a plant, 2-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OEx2-BAR/B2. At 8 dpi, the infected plants were sprayed with the herbicide BASTA, containing glufosinate ammonium as an active ingredient. All plants infected with pBBWV2-OEx2-BAR/B2 were resistant to BASTA when applied as a 0.15%, 0.3%, or 0.6% (v/v) solution in deionized water (Fig. 5B). In contrast, the healthy plants and pBBWV2-OEx2-GFP/B2-infected plants died within 2 weeks after herbicide treatment (Fig. 5B).
LEA proteins play a protective role under drought conditions by sequestering ions and maintaining minimum cellular water requirements (Hand et al., 2011). The CaLEA6 gene has been isolated from pepper (Kim et al., 2005), and transgenic tobacco (Nicotiana tabacum) plants that overexpress CaLEA6 show tolerance to drought and salt stresses (Kim et al., 2005). Thus, we examined if the CaLEA6 gene delivered by the BBWV2-based vector could confer systemic drought tolerance in pepper. Two-week-old pepper seedlings (‘Sinhong’) were agroinfiltrated with pBBWV2-OEx2-CaLEA6/B2. First, to investigate the expression levels of CaLEA6, plants were divided into two groups after inoculation; one group was maintained under normal watering conditions, and the second group was grown under drought conditions by withholding watering. RT-qPCR showed that CaLEA6 transcript levels were dramatically up-regulated by pBBWV2-OEx2-CaLEA6/B2 infection relative to that by pBBWV2-OEx2-GFP/B2 or in healthy plants (Supplemental Fig. S4). As shown previously (Kim et al., 2005), CaLEA6 transcripts accumulated to no detectable level in healthy plants under normal conditions (Supplemental Fig. S4). After 17 d under drought conditions, the healthy plants and pBBWV2-OEx2-GFP/B2-infected plants withered severely, while plants infected with pBBWV2-OEx2-CaLEA6/B2 maintained their leaf turgidity and exhibited tolerance to dehydration (Fig. 5C), indicating that systemic overexpression of CaLEA6 using the BBWV2-based vector increased drought tolerance in pepper.
While pepper is an important vegetable crop grown worldwide and a variety of cultivars have been bred, it is still highly desirable to increase pepper resistance to various diseases, pests, and environmental stresses. As exemplified by the systemic delivery of the bar and CaLEA6 genes, the BBWV2-based gene expression vector could be easily employed for rapid evaluation of candidate genes with antimicrobial or insecticidal activities as well as other traits that might enhance the commercial value of pepper.
Stability of the Heterologous Gene Expressed from the BBWV2-Based Vector
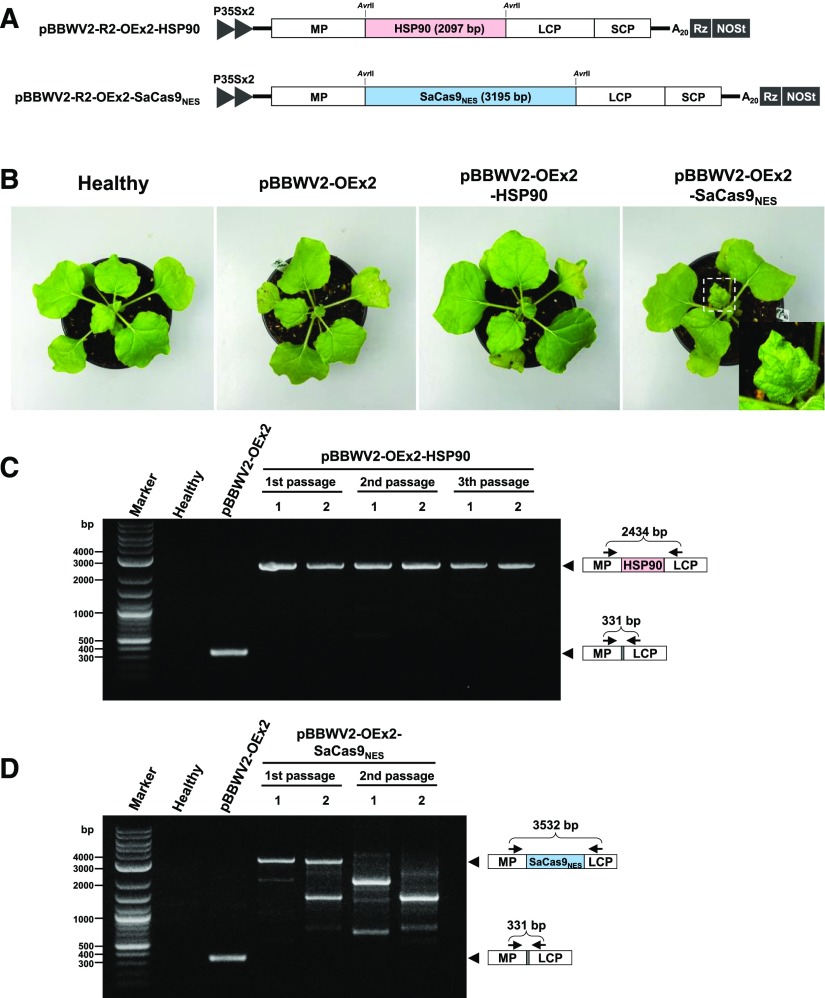
To test the stability and upper size limit of heterologous gene insertion in the BBWV2-based vector, two large genes, Hsp90 (2,097 bp) and Staphylococcus aureus Cas9 tagged with a nuclear export signal sequence (SaCas9NES; 3,195 bp), were cloned into pBBWV2-R2-OEx2 using the AvrII site to generate pBBWV2-R2-OEx2-HSP90 and pBBWV2-R2-OEx2-SaCas9NES, respectively (Fig. 6A). N. benthamiana plants were agroinfiltrated with pBBWV2-OEx2-HSP90 and -SaCas9NES, and symptom appearance was monitored for 2 weeks postinoculation. While HSP90 had no visible effect on symptom severity, SaCas9NES caused necrotic malformation on the upper systemic leaves (Fig. 6B), suggesting that ectopically overexpressed SaCas9NES may have off-target effects on plant cells. To examine stable insertion of the Hsp90 and SaCas9NES genes in the viral genome, we analyzed total RNA extracted from the upper symptomatic leaves by RT-PCR using a primer pair spanning the gene insertion cassettes (Fig. 6, C and D). Hsp90 remained intact in the viral genome, while some truncated variant progeny were detected in the samples inoculated with pBBWV2-OEx2-SaCas9NES (Fig. 6, C and D). Next, the progeny viruses were transferred between plants by mechanical sap inoculation. Each inoculated plant was analyzed by RT-PCR to examine the stability of heterologous gene insertion in the viral genome. Hsp90 remained stable in the viral genome during virus replication even after three serial passages (Fig. 6C). However, only truncated variants were detected from the second passage of progeny of pBBWV2-OEx2-SaCas9NES (Fig. 6D), indicating that SaCas9NES is unstable in the BBWV2 genome, probably because of the genome capacity of BBWV2.
Figure 6.
Analysis of the stability of heterologous gene insertions in the BBWV2 genome. A, Schematic representation of pBBWV2-R2-OEx2-HSP90 and -SaCas9NES. The Hsp90 gene (2,097 bp) or SaCas9NES (3,195 bp) was cloned into pBBWV2-R2-OEx2 using the AvrII site. B, Symptoms in N. benthamiana infected with pBBWW2-OEx2 (empty vector), pBBWV2-OEx2-HSP90, or -SaCas9NES. Images were captured at 8 d after agroinfiltration. C and D, RT-PCR analyses of the stability of heterologous genes in the BBWV2-based vector during serial passages. Total RNA was isolated from pBBWV2-OEx2-infected, pBBWV2-OEx2-HSP90-infected, or pBBWV2-OEx2-SaCas9NES-infected leaves of each pepper plant and subjected to RT-PCR analysis. The progeny viruses were transferred from plant to plant by mechanical sap inoculation. The numbered lanes indicate two individual plants tested in each passage. The arrowheads point at the RT-PCR products of the expected sizes as shown by schematic maps of BBWV2 MP/LCP, MP/HSP90/LCP, and MP/SaCas9NES/LCP regions (arrows indicate the regions that have been amplified).
Engineering of BBWV2 as a VIGS Vector
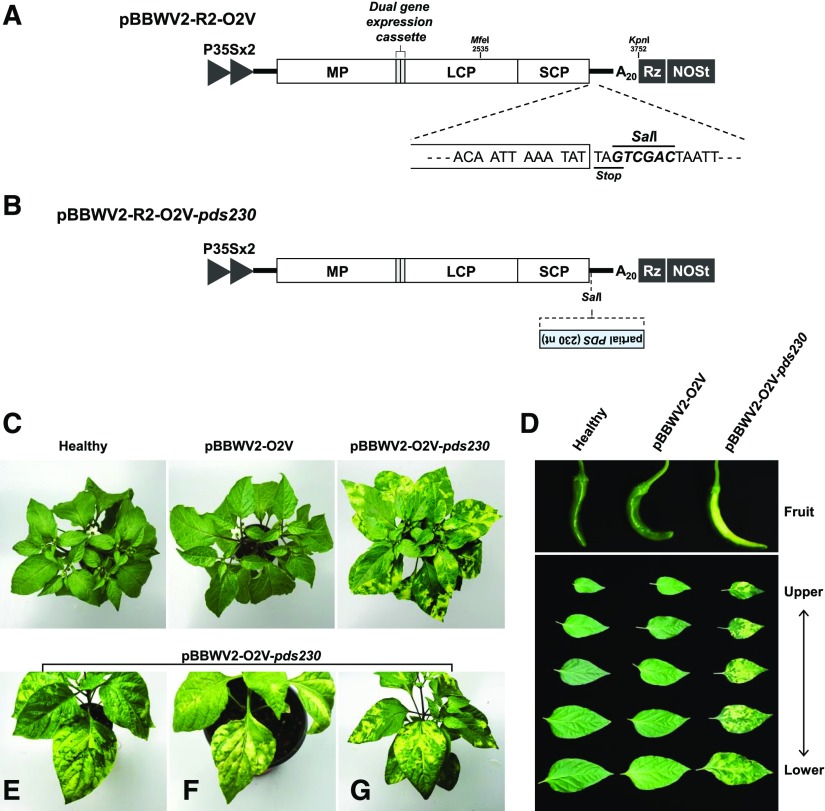
VIGS is an attractive tool for fast evaluation of transient loss-of-function phenotypes in plants (Robertson, 2004; Becker and Lange, 2010). BBWV2 seemed to encode relatively weak VSRs; however, since it persisted systemically with mild symptoms in the infected plants, we hypothesized that BBWV2 could be developed as a useful VIGS vector in pepper. To develop BBWV2 as a VIGS vector, we engineered an additional cloning site (SalI) immediately after the stop codon of RNA2 ORF in the RNA2 3′ untranslated region (UTR) of pBBWV2-R2-OEx2, and the resulting clone was designated as pBBWV2-R2-O2V (Fig. 7A). We initially assessed the potential of BBWV2 as a VIGS vector to inhibit endogenous PDS in pepper. PDS is essential for carotenoid production in plants (Norris et al., 1995), and inhibition of endogenous PDS by VIGS can induce photobleaching (Ratcliff et al., 2001). A 230-bp partial fragment of pepper PDS (GenBank accession no. NM_001324813.1) was inserted in reverse orientation into pBBWV2-R2-O2V using the SalI site, resulting in a construct designated as pBBWV2-R2-O2V-pds230 (Fig. 7B).
Figure 7.
Engineering of BBWV2 as a VIGS vector. A, Schematic representation of pBBWV2-R2-O2V. pBBWV2-R2-O2V contains an additional cloning site (SalI) engineered right after the stop codon of RNA2 ORF as well as a dual gene expression cassette between the MP and LCP cistrons. B, Schematic representation of pBBWV2-R2-O2V-pds230. A 230-bp partial PDS gene was cloned into pBBWV2-R2-O2V in reverse orientation using the SalI site. C, Silencing of pepper PDS using the BBWV2-based VIGS vector. Phenotypes of pepper (‘Sinhong’) were observed 4 weeks after inoculation with the BBWV2-based VIGS vector carrying a fragment of pepper PDS gene (pBBWV-O2V-pds230) and empty vector control (pBBWV-O2V). D, Representative photobleaching phenotypes in the different-aged leaves and fruit induced by silencing of PDS using the BBWV2-based VIGS. E to G, Photobleaching phenotypes induced by infection with pBBWV2-O2V-pds230 in different pepper cultivars: ‘Quarri’ (E), ‘Titanic’ (F), and ‘Wellbeing’ (G).
Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OV2-pds230 and grown in a growth chamber at 25°C. At 2 weeks after inoculation, the infected plants developed photobleached leaves, indicating that PDS was successfully silenced by the BBWV2-based VIGS vector (Fig. 7C). PDS silencing was maintained and progressed to the upper leaves as the plant grew, and photobleaching was also observed in some pepper fruits (Fig. 7D).
Optimization of the BBWV2-Based VIGS in Pepper
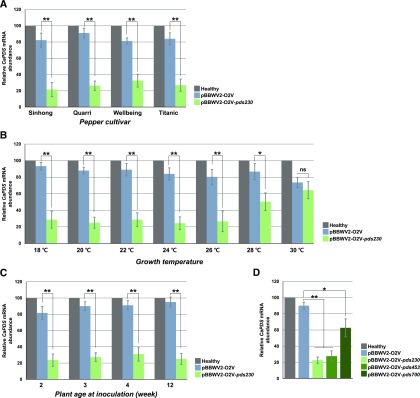
Different pepper cultivars could vary in their susceptibility to BBWV2 and, thereby, in VIGS efficiency. When tested using pBBWV2-O2V-pds230, silencing of PDS was visually evident in other tested pepper cultivars, including ‘Quarri’, ‘Titanic’, and ‘Wellbeing’ (Fig. 7, E–G, respectively). To estimate the efficiency of BBWV2-based VIGS at the molecular level, we analyzed PDS mRNA levels by RT-qPCR. Although VIGS efficiency varied among pepper cultivars (approximately 67%–79% decrease), ‘Sinhong’ exhibited the most efficient PDS gene silencing (Fig. 8A). It is noteworthy that a decrease in PDS mRNA level was detected in the control plants infected with pBBWV2-O2V (empty vector) with no visible photobleaching, indicating that BBWV2 infection itself caused down-regulation of PDS in pepper (Fig. 8A).
Figure 8.
Optimization of BBWV2-based VIGS in pepper. A, Effects of pepper cultivar on BBWV2-based VIGS efficiency. Two-week-old pepper plants from cultivars ‘Sinhong,’ ‘Quarri,’ ‘Titanic,’ and ‘Wellbeing’ were infected with pBBWV2-O2V (empty vector) or pBBWV2-O2V-pds230 and grown in a growth chamber at 25°C. B, Effects of growth temperature on BBWV2-based VIGS efficiency. Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-O2V (empty vector) or pBBWV2-O2V-pds230 and grown in plant growth chambers at different temperatures as indicated. C, Effects of plant age on BBWV2-based VIGS efficiency. Pepper seedlings (‘Sinhong’) at different ages (2-, 3-, 4-, or 12-week-old plants) were infected with pBBWV2-O2V (empty vector) or pBBWV2-O2V-pds230 and grown in a growth chamber at 24°C. D, Effects of insert size on BBWV2-based VIGS efficiency. Two-week-old pepper seedlings (‘Sinhong’) were infected with the BBWV2-based VIGS vectors carrying different sizes (230, 453, or 700 bp) of partial PDS gene and grown in a growth chamber at 24°C. In each experiment, total RNA was extracted from the photobleached leaves of three individual plants 4 weeks after inoculation, and relative accumulation levels of PDS transcripts were quantified by RT-qPCR. Means ± se of three replications are shown, and each column represents one group with nine plants. Significant difference was analyzed using a paired Student’s t test: *, P < 0.05; **, P < 0.01; ns, not significant.
Plant growth temperature is a key factor that affects VIGS efficiency (Robertson, 2004; Hartl et al., 2008). To assess the effect of plant growth temperature on BBWV2-based VIGS, 2-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-O2V-pds230 and grown in plant growth chambers at different temperatures (18°C, 20°C, 22°C, 24°C, 26°C, 28°C, and 30°C). Although little photobleaching was observed in the plants grown at 28°C and 30°C, the plants grown at other temperatures showed similar photobleached phenotypes. Correspondingly, RT-qPCR analysis revealed that the efficiency of PDS VIGS at various temperatures was associated with the degree of photobleaching (Fig. 8B). In addition, the earliest photobleaching was observed in the plants grown at 24°C and 26°C, whereas photobleaching was delayed for 2 to 7 d in the plants grown at 18°C, 20°C, and 22°C, since plant growth was inhibited. Therefore, 24°C was the optimal temperature for BBWV2-based VIGS in pepper.
The developmental stage of a plant at the time of inoculation might affect virus replication and spread in the plant, and thus could influence VIGS efficiency (Hileman et al., 2005; Deng et al., 2012). To test this possibility in the BBWV2-based VIGS system, pepper seedlings (‘Sinhong’) at different ages (2, 3, and 4 weeks old) were infected with pBBWV2-O2V-pds230 and grown in a plant growth chamber at 24°C. All tested plants exhibited photobleaching around 2 weeks postinoculation regardless of age, and there were no significant differences in reduction of the PDS mRNA level among plants at different ages (Fig. 8C). We also examined whether BBWV2-VIGS works efficiently in pepper plants at a late developmental stage. Twelve-week-old pepper plants (‘Sinhong’) were infected with pBBWV2-OV2-pds230 and grown at 24°C. At 4 weeks after inoculation, photobleaching occurred in upper systemic leaves and significant reduction of PDS mRNA levels was evident by RT-qPCR (Fig. 8C; Supplemental Fig. S5).
Finally, we examined whether the size of the host-derived sequence insert affects BBWV2-based VIGS efficiency. To this end, the BBWV2-based VIGS constructs carrying partial PDS genes of different sizes (230, 453, and 700 bp), which were named pBBWV2-O2V-pds230, -pds453, and -pds700, respectively, were used to inoculate 2-week-old pepper seedlings (‘Sinhong’), and the inoculated plants were grown at 24°C and monitored for 6 weeks. The plants infected with pBBWV2-O2V-pds230 or -pds453 showed similar photobleached phenotypes, whereas little or delayed photobleaching was observed in plants infected with pBBWV2-O2V-pds700 (Supplemental Fig. S6). RT-qPCR analysis confirmed the association of the degree of photobleaching with the PDS VIGS efficiency by the size of the partial insert (Fig. 8D).
Considering the importance of pepper as a vegetable crop, it is highly desirable to identify and evaluate valuable traits to improve the productivity, disease and environmental resistance, and commercial value of pepper. The whole genome of pepper has been sequenced (Kim et al., 2014; Qin et al., 2014), and high-throughput transcriptome studies have identified a large number of genes associated with various biological processes (Liu et al., 2013; Dubey et al., 2019). However, most of the identified pepper genes remain to be elucidated because of the lack of efficient methods for gain- and loss-of-function studies in pepper.
Plant virus-based vectors are attractive and powerful tools for rapid and simple analysis of gene function by systemically overexpressing or silencing desirable genes in plants (Robertson, 2004; Gleba et al., 2007). Various RNA viruses were engineered as viral vectors for expression of the recombinant proteins based on two different approaches according to their viral genome organization. In the first approach, viral vectors derived from the α-like superfamily of viruses, including PVX and TMV, have been engineered to contain additional subgenomic promoters, and a gene of interest can be inserted downstream of a duplicated subgenomic promoter (Donson et al., 1991; Chapman et al., 1992). The second approach is to apply viral polyprotein processing strategies. Mainly, potyviruses have been engineered to express a gene of interest by in-frame insertion into the viral polyprotein ORF (Masuta et al., 2000; Arazi et al., 2001; Seo et al., 2016). Potyvirus-based vectors appear to have a relatively flexible capacity to carry and express large heterologous genes (up to 4 kb; Seo et al., 2016). In contrast, viral vectors derived from the viruses having segmented genomes, such as Bean pod mottle virus (BPMV), have some restrictions in carrying heterologous genes because the estimated maximum gene size that can be inserted into the BPMV genome is approximately 1.8 kb (Zhang et al., 2006). In this study, we showed the successful application of a proteolytic polyprotein processing strategy to engineer BBWV2 as a dual gene expression vector that could deliver two genes simultaneously in pepper (Fig. 3). The same approach can be broadly used in other viruses belonging to the family Secoviridae, which exhibit similar genetic structures to BBWV2. RNA viruses have their optimal genome capacity for efficient replication and virion assembly. Therefore, virus-based vectors have restrictions in carrying and expressing heterologous sequences according to their genome capacity. We tested two large genes, Hsp90 (2,097 bp) and SaCas9NES (3,195 bp), to examine the upper size limit of genome capacity of the BBWV2-based vector for stable expression of a heterologous gene. Because Hsp90, but not SaCas9NES, was stably maintained in the viral genome during serial passages (Fig. 6), the estimated maximum insert size that can be stably maintained in the BBWV2 genome appears to be up to 2 kb.
We showed that coexpression of heterologous VSRs could enhance the stable expression of recombinant proteins by the BBWV2-based vector. In particular, FHV B2 was suitable as a heterologous VSR that could enhance gene expression by the BBWV2-based vector because it did not enhance BBWV2-induced symptoms in pepper (Fig. 4). RNA viruses have evolved VSRs that use various strategies to overcome RNA silencing-based host immunity (Ruiz-Ferrer and Voinnet, 2009; Ding, 2010). While many VSRs encoded by plant and animal viruses have been identified, each VSR has a specific mode of action in suppressing RNA silencing (Ding, 2010; Csorba et al., 2015). TBSV P19 binds to 21-nucleotide duplex small interfering RNAs with high affinity and thereby prevents the incorporation of small interfering RNAs into the RNA-induced silencing complexes (Vargason et al., 2003; Lakatos et al., 2004). CMV 2b prevents the spread of silencing signals and inhibits the slicing activity of AGO1 (Zhang et al., 2006). On the contrary, FHV B2 has dsRNA-binding activity, which can protect viral dsRNA intermediates from Dicer cleavage (Lu et al., 2005; Seo et al., 2012). It has been suggested that VSRs with dsRNA-binding activity can suppress RNA silencing in both animals and plants (Ruiz-Ferrer and Voinnet, 2009; Ding, 2010). As mentioned above, the BBWV2-encoded VSRs do not inhibit dsRNA-induced RNA silencing. Therefore, the dsRNA-protecting activity of B2 might have synergistic effects on enhancing BBWV2 replication and foreign gene expression.
We also reported the ability of the BBWV2-based gene expression vector to deliver and evaluate desired genes, including the herbicide resistance bar and drought tolerance-associated CaLEA6, in pepper (Fig. 5). As shown in our examples, the BBWV2-based gene expression vector could be used as a simple and rapid tool for transient gain-of-function studies at the whole-plant level in pepper. Using the BBWV2-based vector, we are currently characterizing the gain-of-function effects of some genes identified by RNA sequencing to be expressed differentially in susceptible and resistant responses against CMV in pepper.
VIGS is a simple and powerful method used in transient loss-of-function studies (Robertson, 2004; Ramegowda et al., 2014). This reverse genetic approach is greatly useful when desired knockout plants are unavailable. Because of the lack of efficient methods for genetic transformation in pepper, generating a knockout plant to examine the loss-of-function effects of a gene is still a very difficult challenge in pepper. Therefore, VIGS is an important alternative approach to investigate gene functions in pepper. Currently, the TRV-based VIGS system has been used in pepper (Chung et al., 2004). Although TRV-based VIGS has been widely used in various plant species, it can induce necrotic responses in pepper when silencing of the target genes is not successfully triggered (Hartl et al., 2008; Wu et al., 2011). In this study, we engineered a BBWV2 strain that causes no visible symptoms in pepper as a VIGS vector. BBWV2-based VIGS worked successfully in pepper at the whole-plant level with little effects on plant growth (Fig. 7). BBWV2 has a reported host range of over 177 species in 39 families, including many economically important crops, such as pepper, cucumber, broad bean, pea (Pisum sativum), spinach (Spinacia oleracea), and sesame (Lisa and Boccardo, 1996). Therefore, BBWV2-based VIGS could be tested in a wide range of important crop plants.
MATERIALS AND METHODS
Construction of the BBWV2-Based Vectors
pBBWV2-R2-OE was constructed by engineering a single gene expression cassette between the MP and LCP cistrons of pBBWV2-RP1-R2 (Kwak et al., 2016). A 949-bp DNA fragment consisting of the C terminus of MP (from the SpeI site), a single gene expression cassette sequence (5′-GGATTAATGGAAGAGGACGTACTCAGGCCTAGATCTCCTAGGGGGAAGGACTACCGTTACGGGCAG-3′; StuI, BglII, and AvrII sites are shown in boldface, and the nucleotide sequence for the protease cleavage site is underlined), and the N-terminal half of LCP (to the MfeI site) was synthesized (Bioneer) and inserted into pBBWV2-RP1-R2, which was opened with SpeI and MfeI (Fig. 1A). The resulting construct was designated as pBBWV2-R2-OE.
Similarly, pBBWV2-R2-OEx2 was constructed by engineering a dual gene expression cassette between the MP and LCP cistrons of pBBWV2-RP1-R2. A 991-bp DNA fragment consisting of the C terminus of MP (from the SpeI site), a dual gene insertion cassette sequence (5′-GGGCTCATGGAGGAGGATGTGCTAAGATCTGGTAAGGATTACAGGTACGGACAGGGATTAATGGAAGAGGACGTACTCCCTAGGGGGAAGGACTACCGTTACGGGCAG-3′; BglII and AvrII sites are shown in boldface, and the nucleotide sequence for the protease cleavage site is underlined), and the N-terminal half of LCP (to the MfeI site) was synthesized (Bioneer) and inserted into pBBWV2-RP1-R2, which was opened with SpeI and MfeI (Fig. 3A). The resulting construct was designated as pBBWV2-R2-OEx2.
BBWV2 was engineered as a VIGS vector by introducing an additional cloning site (SalI) in the RNA2 3′ UTR (right after the stop codon of the RNA2 ORF) of pBBWV2-R2-OEx2. A 1,218-bp DNA fragment corresponding to the 3′ half of BBWV2 RNA2 with five additional nucleotides (TCGAC) in the 3′ UTR (from the MfeI site in LCP to the KpnI site at the 3′ end of RNA2) was synthesized (Bioneer) and inserted into pBBWV2-R2-OEx2, which was opened with MfeI and KpnI (Fig. 7A). The resulting construct was designated as pBBWV2-R2-O2V.
Insertion of Heterologous Genes into the pBBWV2-Based Vector
The gfp gene was amplified using a primer pair (5′-GAAGATCTATGGTGAGCAAGGGCGAG-3′ and 5′-GATCCTAGGGAGGATCCCCTTGTACAGCT-3′; BglII and AvrII sites are shown in boldface), then digested with BglII and AvrII and cloned into pBBWV2-R2-OE to generate pBBWV2-R2-OE-GFP (Fig. 2A). The gfp gene was amplified using a primer pair harboring BglII sites (5′-GAAGATCTATGGTGAGCAAGGGCGAG-3′ and 5′-GAAGATCTGAGGATCCCCTTGTACAGCT-3′; BglII sites are shown in boldface). The NLS-tagged cfp gene was amplified using a primer pair harboring AvrII sites (5′-GATCCTAGGATGGTGAGCAAGGGCGAGG-3′ and 5′-GATCCTAGGGACCTTTCTCTTCTTCTTTGGA-3′; AvrII sites are shown in boldface). The resulting amplicons were digested with BglII and AvrII and cloned into the first (BglII) and second (AvrII) gene insertion sites of pBBWV2-R2-OEx2, respectively, for simultaneous expression of the two genes. The resulting clone was designated as pBBWV2-R2-OEx2-GFP/nCFP (Fig. 3B). A similar cloning strategy was used for cloning of CMV 2b (any strain in subgroup I; Seo et al., 2009a), FHV B2 (Lu et al., 2005), TBSV P19 (Qu et al., 2003), bar, CaLEA6, Hsp90, and SaCas9NES into the first or second gene insertion site of pBBWV2-R2-OEx2. The list of primers used to amplify CMV 2b, FHV B2, TBSV P19, bar, CaLEA6, Hsp90, and SaCas9NES is available on request. Partial fragments of pepper PDS were amplified using primer pairs harboring SalI sites (PDS-Fw, 5′-TACGCGTCGACATGCCCCAAATTGGACTTGTTT-3′, and PDS-230-Rv, 5′-TACGCGTCGACCAAACAACCTTTAAAGGCCGGA-3′, for a 230-bp partial PDS fragment; PDS-Fw and PDS-453-Rv, 5′-TACGCGTCGACTGCAGCTACCTTTCCACCTAGA-3′, for a 453-bp partial PDS fragment; PDS-Fw and PDS-700-Rv, 5′-TACGCGTCGACGCCATGTAAGCATTTCATTGTTC-3′, for a 700-bp partial PDS fragment; SalI sites are shown in boldface) and cloned into pBBWV2-R2-O2V in reverse orientation to generate pBBWV2-R2-O2V-pds230, -pds453, and -psd700, respectively.
Plant Growth and Inoculation
Pepper (Capsicum annuum) plants were grown in a growth chamber at 25°C under a 16/8-h photoperiod. To optimize VIGS efficiency, plants were grown in plant growth chambers at different temperatures (18°C, 20°C, 22°C, 24°C, 26°C, 28°C, or 30°C) after inoculation. Pepper seedlings at different ages (2, 3, and 4 weeks old) were selected for inoculation. For Agrobacterium tumefaciens-mediated inoculation of recombinant BBWV2 clones, plasmid DNA of each BBWV2 construct was transformed into A. tumefaciens strain EHA105, and agroinfiltration was performed as described previously (Seo et al., 2009a; Kwak et al., 2013). To detect virus infection in the inoculated plants, RT-PCR was performed using a BBWV2-specific primer pair (5′-CAGAGAAGTGGTTGGTCCCGTG-3′ and 5′-ATGGGAGGCTAGTGACCTACG-3′) as described previously (Kwak et al., 2013).
Fluorescence Observation and Confocal Microscopy
Whole-leaf fluorescence signals were observed using an in vivo fluorescence imaging system (FOBI; NeoScience) according to the manufacturer’s instructions. Fluorescence signals emitted by GFP and CFP in plant leaves were observed using a Leica SP8 laser-scanning confocal microscope equipped with a specific laser/filter combination to detect CFP and GFP (excitation at 458 and 488 nm, respectively).
Herbicide Treatment
Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OEx2-BAR/B2. At 8 dpi, the infected plants were sprayed with the herbicide BASTA (Bayer CropScience) containing glufosinate ammonium as an active ingredient as a 0.15%, 0.3%, or 0.6% (v/v) solution in deionized water. Pepper plants were photographed 4 weeks after herbicide treatment.
Drought Stress Treatment
Two-week-old pepper seedlings (‘Sinhong’) were infected with pBBWV2-OEx2-CaLEA6/B2. After inoculation, drought stress was applied to the plants by withholding watering. The plants were photographed 17 d after drought treatment.
Total RNA Extraction, RT-PCR, and RT-qPCR
Total RNA was extracted using a PureLink RNA Mini kit (Ambion) and further treated with TURBO DNA-free (Ambion) according to the manufacturer’s instructions. RT-PCR was performed using a primer pair spanning the gene insertion cassette (5′-GGAACATTGGCTCTCAGAG-3′ and 5′-GGAATGCTTGCATATCCAC-3′) to analyze stable insertion of heterologous genes in the BBWV2-based vector during viral infection. RT-qPCR was performed using 2X SYBR Green Real-Time PCR Smart mix (Solgent) and the AriaMx Real-Time PCR system (Agilent). Primers were designed using Primer Express 3.0 software (Applied Biosystems) as follows: CaPDS-198-Fw (5′-GGCTAAGGATTTCCGGCCTT-3′) and CaPDS-427-Rv (5′-CCCTTGCCTCCAGCAGTATT-3′) for pepper PDS mRNA detection, CaLEA6-174-Fw (5′-GCAAATCTCTTACGCCCTC-3′) and CaLEA6-346-Rv (5′-AGTCAATGTCCCAATCTTTACC-3′) for CaLEA6 transcript detection, CaACT7-1335-Fw (5′-GGGATGGAGAAGTTTGGTGGTGG-3′) and CaACT7-1502-Rv (5′-GCTGAAACTAGTTCCCTACCAC-3′) for pepper actin mRNA detection, BBWV2-R2-3196-Fw (5′-CCAGAGAAGTGGTTGGTCCC-3′) and BBWV2-R2-3385-Rv (5′-TCCAACAGGTAATGCCCACC-3′) for BBWV2 RNA2 detection, and Nb-actin-qRT-Fw (5′-CGAGGAGCATCCAGTCCTCT-3′) and Nb-actin-qRT-Rv (5′-GTGGCTGACACCATCACCAG-3′) for actin mRNA detection in Nicotiana benthamiana. Actin was used as a housekeeping gene to standardize different samples.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: KT380022.1 (BBWV2 isolate RP1 segment RNA1), KT380023.1 (BBWV2 isolate RP1 segment RNA2), D00355.1 (CMV 2b), M21958.1 (TBSV p19), X77156.1 (FHV B2), KF780168.1 (bar), AF168168.1 (CaLEA6), and NM_001324813.1 (pepper PDS).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Replication competence of recombinant BBWV2 vectors in N. benthamiana.
Supplemental Figure S2. Accumulation of recombinant BBWV2 carrying foreign genes in N. benthamiana.
Supplemental Figure S3. Time-course observation of the effects of growth temperature on GFP expression by the BBWV2-based vector.
Supplemental Figure S4. Overexpression of CaLEA6 transcripts using the BBWV2-based vector in pepper.
Supplemental Figure S5. Silencing of pepper PDS gene at a late developmental stage using the BBWV2-based VIGS vector.
Supplemental Figure S6. Effects of insert size on BBWV2-based VIGS efficiency.
Supplemental Table S1. Infection efficiency of pBBWV2-based vector constructs in various host plants upon agroinfiltration.
ACKNOWLEDGMENTS
We thank Dr. A.L.N. Rao (University of California, Riverside) for kindly providing the binary plasmid pCass-RZ. We also thank Dr. Kook-Hyung Kim (Seoul National University) and Dr. Hong-Soo Choi (National Institute of Agricultural Sciences) for providing the A. tumefaciens strain EHA105 and seeds of various pepper cultivars, respectively. We are grateful to Dr. Jin-Ho Kang (Seoul National University) for fruitful discussions.
Footnotes
This work was supported in part by grants from the Next-Generation BioGreen 21 Program (PJ013129) funded by the Rural Development Administration of Korea and the Young Researcher Program (2018R1C1B5029927) funded by the National Research Foundation of Korea.
References
- Aarrouf J, Castro-Quezada P, Mallard S, Caromel B, Lizzi Y, Lefebvre V (2012) Agrobacterium rhizogenes-dependent production of transformed roots from foliar explants of pepper (Capsicum annuum): A new and efficient tool for functional analysis of genes. Plant Cell Rep 31: 391–401 [DOI] [PubMed] [Google Scholar]
- Arazi T, Slutsky SG, Shiboleth YM, Wang Y, Rubinstein M, Barak S, Yang J, Gal-On A (2001) Engineering zucchini yellow mosaic potyvirus as a non-pathogenic vector for expression of heterologous proteins in cucurbits. J Biotechnol 87: 67–82 [DOI] [PubMed] [Google Scholar]
- Becker A, Lange M (2010) VIGS: Genomics goes functional. Trends Plant Sci 15: 1–4 [DOI] [PubMed] [Google Scholar]
- Bulle M, Yarra R, Abbagani S (2016) Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed 36: 36 [Google Scholar]
- Cao J, Duan X, McEiroy D, Wu R (1992) Regeneration of herbicide resistant transgenic rice plants following microprojectile-mediated transformation of suspension culture cells. Plant Cell Rep 11: 586–591 [DOI] [PubMed] [Google Scholar]
- Chapman S, Kavanagh T, Baulcombe D (1992) Potato virus X as a vector for gene expression in plants. Plant J 2: 549–557 [DOI] [PubMed] [Google Scholar]
- Chung E, Seong E, Kim YC, Chung EJ, Oh SK, Lee S, Park JM, Joung YH, Choi D (2004) A method of high frequency virus-induced gene silencing in chili pepper (Capsicum annuum L. cv. Bukang). Mol Cells 17: 377–380 [PubMed] [Google Scholar]
- Csorba T, Kontra L, Burgyán J (2015) Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 479–480: 85–103 [DOI] [PubMed] [Google Scholar]
- Deng X, Elomaa P, Nguyen CX, Hytönen T, Valkonen JPT, Teeri TH (2012) Virus-induced gene silencing for Asteraceae—a reverse genetics approach for functional genomics in Gerbera hybrida. Plant Biotechnol J 10: 970–978 [DOI] [PubMed] [Google Scholar]
- Ding SW. (2010) RNA-based antiviral immunity. Nat Rev Immunol 10: 632–644 [DOI] [PubMed] [Google Scholar]
- Ding XS, Mannas SW, Bishop BA, Rao X, Lecoultre M, Kwon S, Nelson RS (2018) An improved Brome mosaic virus silencing vector: Greater insert stability and more extensive VIGS. Plant Physiol 176: 496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J, Kearney CM, Hilf ME, Dawson WO (1991) Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc Natl Acad Sci USA 88: 7204–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey M, Jaiswal V, Rawoof A, Kumar A, Nitin M, Chhapekar SS, Kumar N, Ahmad I, Islam K, Brahma V, et al. (2019) Identification of genes involved in fruit development/ripening in Capsicum and development of functional markers. Genomics doi:10.1016/j.ygeno.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18: 134–141 [DOI] [PubMed] [Google Scholar]
- Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, Start WG, O’Brien JV, Chambers SA, Adams WR Jr., Willetts NG, et al. (1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2: 603–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand SC, Menze MA, Toner M, Boswell L, Moore D (2011) LEA proteins during water stress: Not just for plants anymore. Annu Rev Physiol 73: 115–134 [DOI] [PubMed] [Google Scholar]
- Hartl M, Merker H, Schmidt DD, Baldwin IT (2008) Optimized virus-induced gene silencing in Solanum nigrum reveals the defensive function of leucine aminopeptidase against herbivores and the shortcomings of empty vector controls. New Phytol 179: 356–365 [DOI] [PubMed] [Google Scholar]
- Hileman LC, Drea S, Martino G, Litt A, Irish VF (2005) Virus-induced gene silencing is an effective tool for assaying gene function in the basal eudicot species Papaver somniferum (opium poppy). Plant J 44: 334–341 [DOI] [PubMed] [Google Scholar]
- Jeon JM, Ahn NY, Son BH, Kim CY, Han CD, Kim GD, Gal SW, Lee SH (2007) Efficient transient expression and transformation of PEG-mediated gene uptake into mesophyll protoplasts of pepper (Capsicum annuum L.). Plant Cell Tiss Organ Cult 88: 225–232 [Google Scholar]
- Kim HS, Lee JH, Kim JJ, Kim CH, Jun SS, Hong YN (2005) Molecular and functional characterization of CaLEA6, the gene for a hydrophobic LEA protein from Capsicum annuum. Gene 344: 115–123 [DOI] [PubMed] [Google Scholar]
- Kim S, Park M, Yeom SI, Kim YM, Lee JM, Lee HA, Seo E, Choi J, Cheong K, Kim KT, et al. (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet 46: 270–278 [DOI] [PubMed] [Google Scholar]
- Kong L, Wang Y, Yang X, Sunter G, Zhou X (2014) Broad bean wilt virus 2 encoded VP53, VP37 and large capsid protein orchestrate suppression of RNA silencing in plant. Virus Res 192: 62–73 [DOI] [PubMed] [Google Scholar]
- Kwak HR, Kim MK, Lee YJ, Seo JK, Kim JS, Kim KH, Cha B, Choi HS (2013) Molecular characterization and variation of the broad bean wilt virus 2 isolates based on analyses of complete genome sequences. Plant Pathol J 29: 397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HR, Lee YJ, Kim J, Kim MK, Kim JS, Choi HS, Seo JK (2016) A determinant of disease symptom severity is located in RNA2 of broad bean wilt virus 2. Virus Res 211: 25–28 [DOI] [PubMed] [Google Scholar]
- Lakatos L, Szittya G, Silhavy D, Burgyán J (2004) Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J 23: 876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Kim HS, Kim JY, Jung M, Park YS, Lee JS, Choi SH, Her NH, Lee JH, Hyung NI, et al. (2004) A new selection method for pepper transformation: Callus-mediated shoot formation. Plant Cell Rep 23: 50–58 [DOI] [PubMed] [Google Scholar]
- Lisa V, Boccardo G (1996) Fabaviruses: Broad bean wilt and allied viruses In Harrison BD, and Murant AF, eds, The Plant Viruses, Vol Vol 5 Springer, Boston, pp 229–250 [Google Scholar]
- Liu S, Li W, Wu Y, Chen C, Lei J (2013) De novo transcriptome assembly in chili pepper (Capsicum frutescens) to identify genes involved in the biosynthesis of capsaicinoids. PLoS ONE 8: e48156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Maduro M, Li F, Li HW, Broitman-Maduro G, Li WX, Ding SW (2005) Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436: 1040–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuta C, Yamana T, Tacahashi Y, Uyeda I, Sato M, Ueda S, Matsumura T (2000) Development of clover yellow vein virus as an efficient, stable gene-expression system for legume species. Plant J 23: 539–546 [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkayastha A, Dasgupta I (2009) Virus-induced gene silencing: A versatile tool for discovery of gene functions in plants. Plant Physiol Biochem 47: 967–976 [DOI] [PubMed] [Google Scholar]
- Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, et al. (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc Natl Acad Sci USA 111: 5135–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu F, Ren T, Morris TJ (2003) The coat protein of turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J Virol 77: 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramegowda V, Mysore KS, Senthil-Kumar M (2014) Virus-induced gene silencing is a versatile tool for unraveling the functional relevance of multiple abiotic-stress-responsive genes in crop plants. Front Plant Sci 5: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff F, Martin-Hernandez AM, Baulcombe DC (2001) Technical Advance. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J 25: 237–245 [DOI] [PubMed] [Google Scholar]
- Robertson D. (2004) VIGS vectors for gene silencing: Many targets, many tools. Annu Rev Plant Biol 55: 495–519 [DOI] [PubMed] [Google Scholar]
- Ruffel S, Dussault MH, Palloix A, Moury B, Bendahmane A, Robaglia C, Caranta C (2002) A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J 32: 1067–1075 [DOI] [PubMed] [Google Scholar]
- Ruffel S, Gallois JL, Lesage ML, Caranta C (2005) The recessive potyvirus resistance gene pot-1 is the tomato orthologue of the pepper pvr2-eIF4E gene. Mol Genet Genomics 274: 346–353 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60: 485–510 [DOI] [PubMed] [Google Scholar]
- Scholthof HB, Scholthof KBG, Jackson AO (1996) Plant virus gene vectors for transient expression of foreign proteins in plants. Annu Rev Phytopathol 34: 299–323 [DOI] [PubMed] [Google Scholar]
- Seo JK, Choi HS, Kim KH (2016) Engineering of soybean mosaic virus as a versatile tool for studying protein-protein interactions in soybean. Sci Rep 6: 22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JK, Kwak HR, Choi B, Han SJ, Kim MK, Choi HS (2017) Movement protein of broad bean wilt virus 2 serves as a determinant of symptom severity in pepper. Virus Res 242: 141–145 [DOI] [PubMed] [Google Scholar]
- Seo JK, Kwon SJ, Choi HS, Kim KH (2009a) Evidence for alternate states of Cucumber mosaic virus replicase assembly in positive- and negative-strand RNA synthesis. Virology 383: 248–260 [DOI] [PubMed] [Google Scholar]
- Seo JK, Kwon SJ, Rao ALN (2012) Molecular dissection of Flock house virus protein B2 reveals that electrostatic interactions between N-terminal domains of B2 monomers are critical for dimerization. Virology 432: 296–305 [DOI] [PubMed] [Google Scholar]
- Seo JK, Lee HG, Kim KH (2009b) Systemic gene delivery into soybean by simple rub-inoculation with plasmid DNA of a Soybean mosaic virus-based vector. Arch Virol 154: 87–99 [DOI] [PubMed] [Google Scholar]
- Sohn KH, Lee SC, Jung HW, Hong JK, Hwang BK (2006) Expression and functional roles of the pepper pathogen-induced transcription factor RAV1 in bacterial disease resistance, and drought and salt stress tolerance. Plant Mol Biol 61: 897–915 [DOI] [PubMed] [Google Scholar]
- Song EG, Ryu KH (2017) A pepper mottle virus-based vector enables systemic expression of endoglucanase D in non-transgenic plants. Arch Virol 162: 3717–3726 [DOI] [PubMed] [Google Scholar]
- Tran PT, Choi H, Choi D, Kim KH (2016) Virus-induced gene silencing reveals signal transduction components required for the Pvr9-mediated hypersensitive response in Nicotiana benthamiana. Virology 495: 167–172 [DOI] [PubMed] [Google Scholar]
- Vargason JM, Szittya G, Burgyán J, Hall TMT (2003) Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115: 799–811 [DOI] [PubMed] [Google Scholar]
- Wu C, Jia L, Goggin F (2011) The reliability of virus-induced gene silencing experiments using tobacco rattle virus in tomato is influenced by the size of the vector control. Mol Plant Pathol 12: 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ghabrial SA (2006) Development of Bean pod mottle virus-based vectors for stable protein expression and sequence-specific virus-induced gene silencing in soybean. Virology 344: 401–411 [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH (2006) Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20: 3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]