Abstract
Treatment of neuroendocrine tumors with 177Lu-octreotate results in prolonged survival and improved quality of life for the patient. However, the treatment is today limited by side effects on kidney and bone marrow, and complete tumor remission is rarely seen. A possible way to minimize dose-limiting toxicity and to optimize this treatment method is to use radioprotectors in conjunction with radiotherapy. A recombinant form of α1-microglobulin (rA1M) was recently shown to preserve kidney structure and function after 177Lu-octreotate injection in mice and was suggested as a radioprotector in peptide receptor radionuclide therapy. The aims of this work were to investigate the influence of rA1M on the in vivo biokinetics of 177Lu-octreotate, with a focus on tumor tissue, and to study the impact of rA1M on the therapeutic response in tumor tissue subjected to 177Lu-octreotate treatment. Methods: The biodistribution of 177Lu-octreotate was examined in BALB/c nude mice with GOT2 tumors 1–168 h after injection with either 177Lu-octreotate or coadministration of 177Lu-octreotate and rA1M. The effects of rA1M on the tumor response after 177Lu-octreotate treatment were studied in BALB/c nude mice with GOT1 tumors. Three groups of mice were administered rA1M, 177Lu-octreotate, or both. Another group served as untreated controls. Tumor volume was measured to follow the treatment effects. Results: No statistically significant difference in biodistribution of 177Lu was observed between the groups receiving 177Lu-octreotate or coinjection of 177Lu-octreotate and rA1M. The therapy study showed a decrease in mean tumor volume during the first 2 wk for both the 177Lu-octreotate group and the coadministration group, followed by tumor regrowth. No statistically significant difference between the groups was found. Conclusion: rA1M did not negatively impact absorbed dose to tumor or therapeutic response in combination with 177Lu-octreotate and may be a promising kidney protector during 177Lu-octreotate treatment of patients with neuroendocrine tumors.
Keywords: α1-microglobulin, somatostatin receptors, GOT1, GOT2, radioprotector
Targeted radionuclide therapy with somatostatin analogs has been used for more than 2 decades to treat patients with metastasized neuroendocrine tumors (NETs). Currently, the β-emitters 90Y (energy, 0.933 MeV/nuclear transition (1)) and 177Lu (energy, 0.148 MeV/nuclear transition (1)) are used, of which 177Lu has been found to have superior dosimetric properties for systemic therapy (2). Clinical studies with both 177Lu-octreotate and 90Y-octreotate have shown promising results, although mild side effects on bone marrow and kidneys have been reported (3–6). Fractionation of the treatment to allow recovery of bone marrow, and use of 177Lu instead of 90Y, have resulted in fewer side effects on normal tissues (6,7). Coinfusion with positively charged amino acids, such as lysine and arginine, reduces renal uptake of the somatostatin analog, and coadministration of these amino acids is now routinely used in treatment with 177Lu-octreotate or 90Y-octreotate (8). However, despite these efforts to minimize damage to normal tissue, radionuclide therapy with somatostatin analogs is still limited by the assumed risk of kidney toxicity.
A strategy to further reduce the risk of toxicity after therapy includes the use of radioprotectors (9). α1-microglobulin (A1M) is a small plasma protein (26 kDa) with the ability to protect normal tissues from oxidative stress by binding and neutralizing free radicals and by reducing oxidants and oxidative lesions (10,11). A1M has also been shown to inhibit the propagation of cell death to bystander cells—that is, cells not exposed directly to radiation but residing near irradiated cells (12). A recombinant form of human A1M (rA1M) has been proposed as a kidney protector during 177Lu-octreotate treatment of NET (13). rA1M, with its antioxidation properties and its similar biodistribution and pharmacokinetics to that of the 111In-labeled somatostatin analog octreotide (14), is an interesting candidate for renal protection during treatment with 177Lu-octreotate. Inhibition of renal damage by coadministration with rA1M has recently been studied in non–tumor-bearing mice up to 6 mo after injection of 150 MBq of 177Lu-octreotate (15). The results indicate that rA1M could potentially protect kidney tissue from radiation-induced damage, allowing for reduced toxicity or dose escalation. However, adding a radiation-protecting agent, such as an antioxidant, to radionuclide therapy may result in protective effects not only on normal tissue but also on tumor tissue. To the best of our knowledge, this is the first study investigating the potential radioprotective effect of rA1M on tumor tissue.
The aim of this work was therefore to investigate whether coadministration of 177Lu-octreotate with rA1M impacts, first, the biokinetics of 177Lu, with particular interest on the tumor uptake, and second, the treatment effect of 177Lu-octreotate on tumor tissue.
MATERIALS AND METHODS
Animal Models
Animal experiments were performed on NET-bearing female 4-wk-old BALB/c nude mice (Janvier and Charles River). The animals were kept under a standard laboratory day and night cycle and were given water and food ad libitum. All animal procedures were approved by the Ethics Committee for Animal Research in Gothenburg (approval 107-2015). GOT2 (human medullary thyroid carcinoma) and GOT1 (human small intestine NETs) tumors were transplanted subcutaneously between the shoulders, as described earlier (16,17). At the start of the study, the mean tumor volume was 0.5 cm3 (SEM, 0.1 cm3); the animals receiving the GOT2 were 13–32 wk old, and the animals receiving the GOT1 tumors were 12–27 wk old.
Radiopharmaceutical
177Lu-octreotate was obtained from the Nuclear Research and Consultancy Group (IDB Holland), and radiolabeling was conducted according to the manufacturer’s instructions. The amount of peptide-bound 177Lu in the injection solution was higher than 97%, as shown using instant thin-layer chromatography (Whatman Chromatography paper [3 mm] [GE Healthcare] and 0.1 M sodium citrate [Labservice AB]). After preparation of the 177Lu-octreotate, the stock solution was diluted with saline solution to the desired activity concentration. Syringes containing 177Lu-octreotate (0.1 mL) were prepared from the stock solution. The activity of the syringes were measured before and after injection by a well-type ionization chamber (CRC-15R; Capintec) to determine the amount of injected activity in each animal.
rA1M
Human rA1M (RMC-035; 5.9 mg/mL) was obtained from A1M Pharma AB and was diluted with a solution containing sterile endotoxin-free 10 mM sodium phosphate (pH 7.4), 0.15 M NaCl, and 12 mM histidine (A1M Pharma AB), to an rA1M concentration of 1.1 mg/mL. The mice were weighed, and syringes containing a 5 mg/kg dose of rA1M were prepared for each mouse.
Biodistribution in GOT2-Bearing Mice
The biodistribution of 177Lu-octreotate was studied in female BALB/c mice bearing GOT2 tumors. In total, 32 mice were intravenously injected with 5 MBq (SD, 8%) of 177Lu-octreotate in the tail vein at 3 pm (±1 h). Half of the mice also received an intravenous 5 mg/kg injection of rA1M directly after the 177Lu-octreotate injection. The animals (n = 4/group) were killed by cardiac puncture under anesthesia with sodium pentobarbital (APL) at 1, 24, 72, or 168 h after administration. Samples of blood, lungs, liver, spleen, kidneys, tumor, femur (including bone marrow), adrenal gland, and pancreas were collected and weighed directly after excision. The 177Lu activity in the samples was measured using a γ-counter equipped with a 7.6-cm (3-in) NaI(Tl) detector (2480 Wizard2; Wallac). The 177Lu activity concentration in the tissue samples, , was calculated as percentage injected activity per gram:
where is the activity in the sample at the time of death, corrected for radioactive decay to time of administration (t = 0), is the injected activity at time t = 0 and is the mass of the sample. For bone, the 177Lu activity concentration was calculated together with bone marrow.
Therapy Study in GOT1-Bearing Mice
The effect on tumor volume of rA1M alone or rA1M in combination with 177Lu-octreotate treatment was studied in female BALB/c mice bearing GOT1 tumors. The 40 mice were divided into 4 groups (n = 10/group). One group received 177Lu-octreotate (30 MBq), one group received rA1M (5 mg/kg), one group received both, and one group served as untreated controls. The injected 177Lu activity level was chosen to give a limited therapeutic effect (noncurative) to enable detection of differences in tumor volume among the groups (18).
The mean tumor volume in the groups at the time of injection (day 0) was approximately 0.5 cm3: 0.51 cm3 (SEM, 0.09 cm3) in the A1M group, 0.50 cm3 (SEM, 0.08 cm3) in the 177Lu-octreotate group, 0.47 cm3 (SEM, 0.07 cm3) in the coadministration group, and 0.51 cm3 (SEM, 0.08 cm3) in the control group.
The tumor response was followed over time by measurement once or twice a week with digital slide calipers. The volume was estimated assuming an elliptic shape:
where is the longest diameter and and are the 2 perpendicular diameters. Tumor response was studied as the tumor volume relative to that at treatment, or as the area under the curve (AUC) for each individual tumor using the trapezoidal rule.
The animals were killed by cardiac puncture under anesthesia with sodium pentobarbital (APL) when the tumor size exceeded 10% of the body weight or the general condition of the mouse was reduced. The mice in the rA1M group were killed and tumor samples collected on day 37 or 44, at the latest. All remaining mice were killed 70 d after the treatment.
Statistical Analysis
In the biodistribution study, 2-way ANOVA was used to determine statistically significant differences between groups. Statistical significance was considered present for probabilities higher than 95% (P < 0.05).
In the therapy study, the difference between groups was determined by performing Kruskal–Wallis 1-way ANOVA with pairwise comparison, using IBM SPSS Statistics, version 25, on the AUC calculated up to the time point when the first mouse was killed (day 21). Statistical significance was considered present for probabilities higher than 95%.
RESULTS
Biodistribution Study
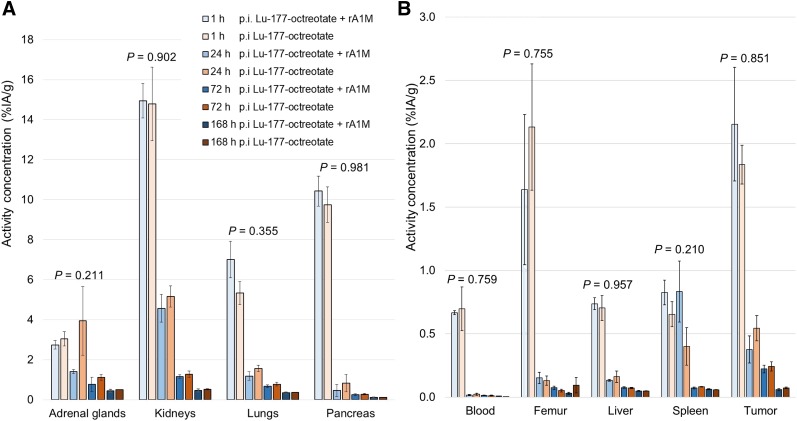
The concentration of 177Lu in the investigated organs and tissues at different time points is shown in Figure 1. In almost all organs and tissues, the maximal activity concentration was reached within the first hour after injection for both groups. Thereafter, the 177Lu concentration decreased rapidly. The highest 177Lu activity concentration was observed in the kidneys at 1 h after the injection for both the 177Lu-octreotate group and the coadministration group: 15% (SEM, 2) and 15% (SEM, 1) injected activity per gram, respectively. High concentrations were also obtained for pancreas, adrenals, and GOT2 tumors, all with known expression of somatostatin receptor type 2. The results from the 2-way ANOVA showed no statistically significant differences in 177Lu activity concentration with time for any organ or tissue between the 2 groups (Fig. 1).
FIGURE 1.
Mean 177Lu activity concentration in adrenal glands, kidney, lungs, and pancreas (A) and in blood, femur, liver, spleen, and GOT2 tumors (B) at 1, 24, 72, and 168 h after injection (p.i.) with 177Lu-octreotate (5 MBq) or 177Lu-octreotate (5 MBq) and rA1M (5 mg/kg) (n = 4/group). Error bars show SEM, and P values show results from 2-way ANOVA of interaction of 2 groups over time. Note difference in y-axis scales.
Therapy Study
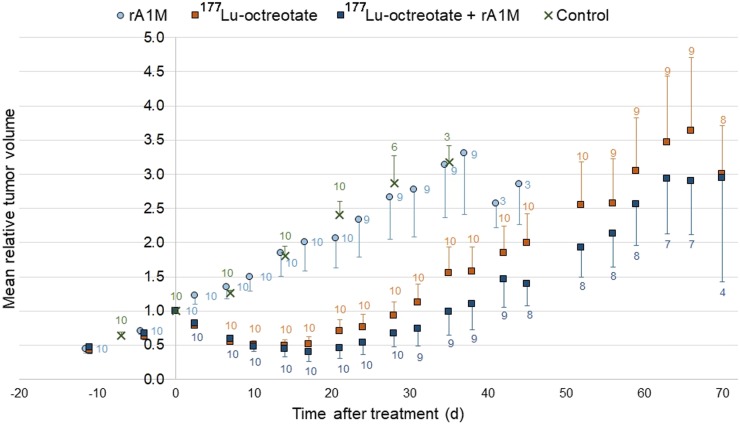
The mean tumor volume of GOT1 tumors in the rA1M group and the untreated control group did not decrease over time (Fig. 2). For the 177Lu-octreotate group and the coadministration group, a therapeutic response was observed. The mean tumor volume decreased during the first 14 d, followed by tumor regrowth (Fig. 2).
FIGURE 2.
Mean relative volume of GOT1 tumors in BALB/c nude mice vs. time after injection (or study start) for control mice and mice injected with rA1M (5 mg/kg), 177Lu-octreotate (30 MBq), or both rA1M (5 mg/kg) and 177Lu-octreotate (30 MBq). Data labels represent number of animals, and vertical error bars indicate SEM.
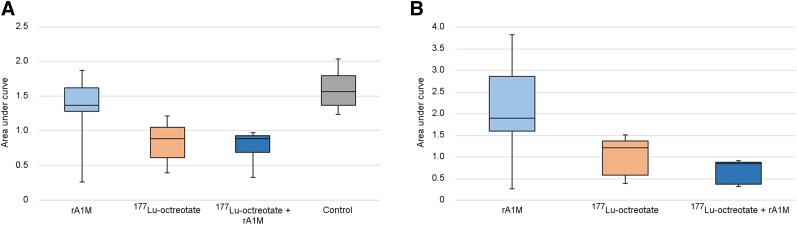
The AUC for each individual tumor from a plot of relative tumor volume versus time after treatment was calculated with a normalized time-axis (Fig. 3); a tumor with no change in volume during the integrated time would have an AUC of 1, and a nonresponding tumor with an increase in tumor volume would have a value greater than 1. The 1-way ANOVA showed that the mean AUCs (calculated from day 0 to day 21) for the rA1M group (1.4; SEM, 0.2) and the control group (1.6; SEM, 0.1) were significantly higher than those for the 177Lu-octreotate group (0.84; SEM, 0.09) and the coadministration group (0.79; SEM, 0.07). AUC did not significantly differ between the 177Lu-octreotate group and the coadministration group or between the control group and the rA1M group.
FIGURE 3.
Response of GOT1 tumors in BALB/c nude mice injected with rA1M (5 mg/kg), 177Lu-octreotate (30 MBq), or 177Lu-octreotate (30 MBq) and rA1M (5 mg/kg) or untreated control, determined as AUC, from individual data behind Figure 2. (A) AUC calculated from day 0 to day 21, when all mice remained in study. (B) AUC calculated from day 0 to day 37 for mice still followed on day 37. AUC of 1 corresponds to no net change in tumor volume during period. Box plots show interquartile range (Q1–Q3) with median value, and whiskers show range from minimum to maximum.
DISCUSSION
Higher amounts of 177Lu-octreotate can be administered to patients with NET if the risk of side effects on normal tissues can be reduced. rA1M is a new, promising candidate for renal protection in radiation therapy. However, when radiation-protecting agents are used, it is essential that the radiobiologic effect on tumor tissue is not reduced. In this study, we investigated whether coadministration of rA1M and 177Lu-octreotate in NET-bearing mice affected the biokinetics of 177Lu and the change in tumor volume, compared with corresponding data after injection of 177Lu-octreotate alone.
The result showed no statistically significant difference in biodistribution of 177Lu-octreotate over time in GOT2-bearing mice with or without coadministration of rA1M. Of special interest is that there was no reduction of uptake in tumor tissue and no increased uptake in kidneys and bone marrow (femur), which are the 2 main organs at risk in 177Lu-octreotate treatment. Thus, coadministration with rA1M will not influence the absorbed dose delivered to tumor or normal tissues from 177Lu-octreotate exposure. Furthermore, no statistically significant difference in the therapeutic effect of 177Lu-octreotate was observed in GOT1-bearing mice with or without coadministration of rA1M. No statistically significant difference in tumor growth was observed between GOT1-bearing mice injected with rA1M and controls.
Both animal tumor models used in this study, GOT1 and GOT2, are relevant for this type of study, as the basis for future clinical trials. They are human NETs and show a close resemblance to the clinical situation, with a slow tumor growth rate and preserved neuroendocrine features. In the biodistribution study, the impact of change in tumor volume over time was reduced using GOT2,which has a lower uptake of and a lower therapeutic response to 177Lu-octreotate than GOT1. GOT1 expresses all subtypes of somatostatin receptors and especially high amounts of somatostatin receptor subtype 2 (17) and was chosen for the therapy study. Since initial tumor size may influence the uptake of 177Lu-octreotate, as we have observed in another model (19), efforts were made to minimize the variation in size of the tumors on day 0 both within and between the groups. The fact that more than one GOT1 transplantation donor was used in this study may also contribute to the difference in biologic response between mice. However, no relationship between transplantation donor and uptake of 177Lu-octreotate or therapeutic response was seen.
The findings in the biodistribution study are well in line with published results from the GOT2 animal model (20), but with a few exceptions: in the present study, the 177Lu concentration was slightly higher in adrenal glands and spleen and lower in pancreas 24 h after injection. It is known that parameters such as the amount of activity and the sex may affect the result (21,22), and the low administered amount of 177Lu-octreotate in the present study was chosen with consideration of previous studies to avoid, for example, receptor saturation (18,21). In this study, the mice were purchased from 2 different suppliers: Charles River in Germany and Janvier in France. The relatively large variations observed for spleen and adrenal glands may be due to differences in mice obtained from different suppliers.
In the therapy study, the response of GOT1 tumors in the mice receiving 177Lu-octreotate with or without rA1M can be divided into 2 phases: a reduction phase during which the tumor volume is smaller than the volume at treatment, and a regrowth phase during which the tumor has regrown and increased above the volume at treatment (Fig. 2). A similar response of GOT1 tumors to 177Lu-octreotate and coadministration treatments was observed, especially during the reduction phase. There was a tendency toward delayed regrowth in the coadministration group compared with the 177Lu-octreotate group, but the difference was not statistically significant. As expected, a differential response was observed between the rA1M group and the groups receiving 177Lu-octreotate (alone or together with rA1M), with the exception of one tumor that had an unexpectedly strong therapeutic response. The mean tumor size in the rA1M group increased over time, resembling the behavior of the untreated tumors in the control group.
The results from the therapy study are consistent with a previous study of response in GOT1 after treatment with 30 MBq of 177Lu-octreotate (18). The therapeutic response was almost immediate and lasted for about 38 d for the coadministration group and 31 d for the 177Lu-octreotate group. The therapeutic effect of 177Lu-octreotate was quite variable between individuals: the mean maximal decrease in tumor volume was about 50% (Fig. 2), but for some tumors the response was much stronger. Although the amount of 177Lu-octreotate was deliberately chosen to induce a moderate remission, some tumors had a period when they were not visible to the unaided eye. A few of these tumors never entered the regrowth phase before the end of the study. When these mice were killed, the skin where the tumors had been located was removed, and no signs of the tumors were visible. This was also the case for one tumor in the rA1M group that had a therapeutic response. The fact that this tumor was slightly smaller than the mean volume in this group at the start of the study, at 0.18 cm3 versus a mean of 0.54 cm3 (SEM, 0.09 cm3), may have contributed to the observed effect.
It was recently shown that coadministration with rA1M could inhibit renal damage in non–tumor-bearing mice up to 6 mo after injection of 150 MBq of 177Lu-octreotate (15). In the present study, we found that coadministration with rA1M did not prevent 177Lu-octreotate from inducing remission of tumors in vivo. Why does rA1M protect the kidneys, but not the tumors, from 177Lu-octreotate–induced damage? A plausible explanation may be that, like 177Lu-octreotate, rA1M is colocalized to the renal cortex (14), whereas A1M did not to influence the radiobiologic effects on NETs. The mechanisms behind these findings should be explored further.
CONCLUSION
No difference in the biodistribution of 177Lu in NET-bearing mice was found after coadministration of rA1M and 177Lu-octreotate, compared with administration of 177Lu-octreotate alone. rA1M had no negative effects on the therapeutic response of NET after exposure to 177Lu-octreotate. Together with results from previous studies demonstrating reduced radiobiologic effects on the kidneys when 177Lu-octreotate is combined with rA1M, we conclude that coadministration of rA1M could be a potential possibility for improving 177Lu-octreotate therapy by reducing the side effects to the kidneys.
DISCLOSURE
This study was supported by the Swedish Research Council (grants 21073 and 00696), the Swedish Cancer Society (grant 3427), BioCARE (a National Strategic Research Program at the University of Gothenburg), the Swedish state under an agreement between the Swedish government and the county councils (the ALF-agreement; ALFGBG-725031), the King Gustav V Jubilee Clinic Cancer Research Foundation, Sahlgrenska University Hospital Research Funds, the Wilhelm and Martina Lundgren Research Foundation, the Assar Gabrielsson Cancer Research Foundation, and the Herbert & Karin Jacobsson Foundation. Magnus Gram and Bo Åkerström are cofounders and shareholders of A1M Pharma AB, which holds patents related to A1M use and production. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the staff of the Central Radiopharmacy of Sahlgrenska University Hospital for preparing the 177Lu-octreotate, especially Annika Bergentall, MSc, Hana Hameed Bakr, Petra Bergström, PhLic, and Ylva Surac, MSc. Thanks also are given to A1M Pharma AB (Lund, Sweden) for offering scientific input and providing the rA1M.
KEY POINTS
QUESTION: Does the potential kidney protector rA1M affect the therapeutic response of 177Lu-octreotate?
PERTINENT FINDINGS: No differences in biodistribution of 177Lu-octreotate were observed between mice with human neuroendocrine tumors with or without coadministration with rA1M. Therapeutic response of 177Lu-octreotate was also not affected by coadministration with rA1M.
IMPLICATIONS FOR PATIENT CARE: Coadministration of rA1M could allow for a better kidney protection during 177Lu-octreotate therapy, while keeping the tumor treatment efficacy. This would increase the therapeutic window, and thus allow for higher administered activity and enhanced cure rate.
REFERENCES
- 1.ICRP Publication 107: nuclear decay data for dosimetric calculations. Ann ICRP. 2008;38(3). [DOI] [PubMed] [Google Scholar]
- 2.Uusijärvi H, Bernhardt P, Ericsson T, Forssell-Aronsson E. Dosimetric characterization of radionuclides for systemic tumor therapy: influence of particle range, photon emission, and subcellular distribution. Med Phys. 2006;33:3260–3269. [DOI] [PubMed] [Google Scholar]
- 3.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26:2124–2130. [DOI] [PubMed] [Google Scholar]
- 4.Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med. 2002;43:610–616. [PubMed] [Google Scholar]
- 5.Kwekkeboom DJ, Kam BL, van Essen M, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer. 2010;17:R53–R73. [DOI] [PubMed] [Google Scholar]
- 6.Vegt E, de Jong M, Wetzels JF, et al. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51:1049–1058. [DOI] [PubMed] [Google Scholar]
- 7.Valkema R, Pauwels SA, Kvols LK, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with 90Y-DOTA0,Tyr3-octreotide and 177Lu-DOTA0, Tyr3-octreotate. J Nucl Med. 2005;46(suppl 1):83S–91S. [PubMed] [Google Scholar]
- 8.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forssell-Aronsson E, Spetz J, Ahlman H. Radionuclide therapy via SSTR: future aspects from experimental animal studies. Neuroendocrinology. 2013;97:86–98. [DOI] [PubMed] [Google Scholar]
- 10.Akerström B, Maghzal GJ, Winterbourn CC, Kettle AJ. The lipocalin alpha1-microglobulin has radical scavenging activity. J Biol Chem. 2007;282:31493–31503. [DOI] [PubMed] [Google Scholar]
- 11.Olsson MG, Olofsson T, Tapper H, Akerstrom B. The lipocalin alpha1-microglobulin protects erythroid K562 cells against oxidative damage induced by heme and reactive oxygen species. Free Radic Res. 2008;42:725–736. [DOI] [PubMed] [Google Scholar]
- 12.Olsson MG, Nilsson EJ, Rutardottir S, Paczesny J, Pallon J, Akerstrom B. Bystander cell death and stress response is inhibited by the radical scavenger α1-microglobulin in irradiated cell cultures. Radiat Res. 2010;174:590–600. [DOI] [PubMed] [Google Scholar]
- 13.Ahlstedt J, Tran TA, Strand SE, Gram M, Akerstrom B. Human anti-oxidation protein A1M: a potential kidney protection agent in peptide receptor radionuclide therapy. Int J Mol Sci. 2015;16:30309–30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlstedt J, Tran TA, Strand F, et al. Biodistribution and pharmacokinetics of recombinant α1-microglobulin and its potential use in radioprotection of kidneys. Am J Nucl Med Mol Imaging. 2015;5:333–347. [PMC free article] [PubMed] [Google Scholar]
- 15.Kristiansson A, Ahlstedt J, Holmqvist B, et al. Protection of kidney function with human antioxidation protein α1-microglobulin in a mouse 177Lu-DOTATATE radiation therapy model. Antioxid Redox Signal. 2019;30:1746–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johanson V, Ahlman H, Bernhardt P, et al. A transplantable human medullary thyroid carcinoma as a model for RET tyrosine kinase-driven tumorigenesis. Endocr Relat Cancer. 2007;14:433–444. [DOI] [PubMed] [Google Scholar]
- 17.Kölby L, Bernhardt P, Ahlman H, et al. A transplantable human carcinoid as model for somatostatin receptor-mediated and amine transporter-mediated radionuclide uptake. Am J Pathol. 2001;158:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalmo J, Spetz J, Montelius M, et al. Priming increases the anti-tumor effect and therapeutic window of 177Lu-octreotate in nude mice bearing human small intestine neuroendocrine tumor GOT1. EJNMMI Res. 2017;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt A, Bernhardt P, Nilsson O, et al. Biodistribution and dosimetry of 177Lu-labeled [DOTA0,Tyr3]octreotate in male nude mice with human small cell lung cancer. Cancer Biother Radiopharm. 2003;18:593–599. [DOI] [PubMed] [Google Scholar]
- 20.Dalmo J, Rudqvist N, Spetz J, et al. Biodistribution of 177Lu-octreotate and 111In-minigastrin in female nude mice transplanted with human medullary thyroid carcinoma GOT2. Oncol Rep. 2012;27:174–181. [DOI] [PubMed] [Google Scholar]
- 21.Schüler E, Osterlund A, Forssell-Aronsson E. The amount of injected 177Lu-octreotate strongly influences biodistribution and dosimetry in C57BL/6N mice. Acta Oncol. 2016;55:68–76. [DOI] [PubMed] [Google Scholar]
- 22.Melis M, Krenning EP, Bernard BF, de Visser M, Rolleman E, de Jong M. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: species and gender differences. Nucl Med Biol. 2007;34:633–641. [DOI] [PubMed] [Google Scholar]