Abstract
18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid), a prostate-specific membrane antigen–targeting radiotracer, has shown promise as a prostate cancer imaging radiotracer. We evaluated the safety, sensitivity, and impact on patient management of 18F-DCFPyL in the setting of biochemical recurrence of prostate cancer. Methods: Subjects with prostate cancer and biochemical recurrence after radical prostatectomy or curative-intent radiotherapy were included in this prospective study. The subjects underwent 18F-DCFPyL PET/CT imaging. The localization and number of lesions were recorded. The uptake characteristics of the 5 most active lesions were measured. A pre- and posttest questionnaire was sent to treating physicians to assess the impact on management. Results: One hundred thirty subjects were evaluated. 18F-DCFPyL PET/CT localized recurrent prostate cancer in 60% of cases with a prostate-specific antigen (PSA) level of ≥0.4 to <0.5, 78% with a level of ≥0.5 to <1.0, 72% with a level of ≥1.0 to <2.0, and 92% with a level of ≥2.0. Many subjects had few lesions (1 lesion in 40.8%, 2 in 8.5%, and 3 in 4.6%). The number of lesions was significantly related to PSA by ANOVA, but there was a large overlap in the PSA values for number of lesion categories. Total lesion uptake was also significantly related to PSA level. A change in treatment intent occurred in 65.5% of subjects, disease stage changed in 65.5%, and management plans changed in 87.3%. Twenty-two subjects reported mild adverse events after the scan; all resolved completely. Conclusion: 18F-DCFPyL PET/CT is safe and sensitive for the localization of biochemical recurrence of prostate cancer. This test improved decision making for referring oncologists and changed management for most subjects.
Keywords: prostate cancer, prostate specific membrane antigen, biochemical recurrence
Prostate cancer (PC) is the most prevalent cancer in men in Canada and is the cause of one third of cancer deaths in that population (1). Although biochemical recurrence after therapy can be identified with the prostate-specific antigen (PSA) test, localization of recurrence can be challenging with conventional imaging modalities that cannot match the sensitivity of this blood test (2,3). Precise localization of sites of recurrence is important, as there are options available to treat localized or oligometastatic disease (4,5).
With a new class of PET radiopharmaceuticals targeting the prostate-specific membrane antigen (PSMA), it has become feasible to detect recurrent or metastatic prostate cancer that is otherwise occult on conventional imaging modalities (6–9). 18F-DCFPyL (2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid), a radiotracer based on the glutamate-ureido-lysine motif, has the advantage of the longer 110-min half-life of 18F compared with 68Ga and of ease of regional distribution; it has been used successfully for detection of PSMA-expressing prostate cancer lesions (10–12).
In this study, we aimed to determine the proportion and characteristics of participants with biochemical recurrence who present with limited-extent disease (localized or oligometastatic) that would potentially be amenable to surgical resection or localized irradiation. We also aimed to assess the clinical impact of 18F-DCFPyL PET/CT in patient management and to evaluate the safety of this radiopharmaceutical for clinical use.
MATERIALS AND METHODS
Ethical Approval
The trial was conducted in compliance with the protocol and with good-clinical-practice guidelines as set out by Health Canada and the institutional Research Ethics Board. The study was approved by the Research Ethics Board of University of British Columbia/BC Cancer and by Health Canada. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all participants included in the study.
Selection of Subjects
Participants with any of the following criteria were enrolled: known prostate cancer with biochemical recurrence after initial curative therapy with radical prostatectomy, with a PSA level higher than 0.4 ng/mL and an additional measurement showing increase; known prostate cancer with biochemical recurrence after initial curative therapy with radiation therapy, with a PSA level higher than 2 ng/mL above the nadir after therapy; castration-resistant prostate cancer with a PSA level of at least 2.0 ng/mL with 2 consecutive rises above the nadir and castrate levels of testosterone (<1.7 nm/L); and participants with findings on other examinations (such as plain x-ray, CT, MRI, or bone scintigraphy) that are suggestive of metastatic disease but not conclusive. Participants were excluded if medically unstable, unable to lie supine for imaging, unable to provide written consent, exceeding the safe weight of the PET/CT bed (204.5 kg) or unable to fit through the PET/CT bore (70-cm diameter), or having an Eastern Cooperative Oncology Group score of more than 2. No treatment was discontinued before the 18F-DCFPyL scan.
This is an interim analysis of the first 208 participants of an investigator-initiated clinical trial (clinicaltrials.gov NCT02899312). Only participants meeting the first 2 inclusion criteria were analyzed for this paper (130/208), but all 208 are included in the safety analysis. Repeat scans in the same subjects were not included.
Study Procedures
Patient demographics were recorded, along with relevant oncologic history, laboratory values, and tumor pathology data. Referring physicians completed a questionnaire describing the intended course of treatment before the 18F-DCFPyL PET/CT scan. Participants were followed up 24 h after radiotracer administration to identify adverse events. A second questionnaire was sent to referring physicians a few weeks after the scan to assess changes in management.
18F-DCFPyL was synthesized according to a previously published method (13). The administered activity was scaled by body weight (range, 237–474 MBq), allowing a 10% variation in target activity. After a 4-h fast, participants were injected intravenously with 18F-DCFPyL. Vital signs were measured before injection, 5–15 min afterward, and after the uptake phase. The subjects could eat between the radiotracer injection and the scan. After a 120-min uptake period, patients were imaged from top of head to mid thigh on a Discovery PET/CT 600 or 690 (GE Healthcare). A CT scan for localization and attenuation correction (120 kV, automatic milliamperage selection [range, 30–200 mA], and noise index of 20) was acquired. PET data were acquired immediately after the CT data over 2–4 min/bed position, adjusted for participant girth, and reconstructed with ordered-subset expectation maximization and point-spread-function modeling.
Qualitative Image Analysis
Images were interpreted by experienced nuclear medicine physicians on an Oasis (Segami) or AW workstation (GE Healthcare). Physicians completed a qualitative interpretation case report form recording the number of positive lesions (0, 1, 2, 3, 4, 5, 6–10, or >10) and the site of recurrence (local, regional nodes, distant nodes, bone, liver, lung, or other). Regional nodes were considered pelvic, hypogastric, obturator, iliac (internal or external), or sacral; other nodal locations were considered distant. Physicians had access to all clinical data; they recorded scans as positive or negative and rated their confidence in the diagnosis for a total of 6 possible qualitative results (negative: high, moderate, or low; positive: high, moderate, or low).
Quantitative Image Analysis
Quantitative data were extracted on an AW workstation by a nuclear medicine physician, on images reconstructed without the time-of-flight option for consistency between the 2 scanners. The mean and SD of cardiac blood-pool activity in a 3-cm spheric volume of interest in the left ventricle were recorded as SUV and lean body mass SUV (SUL). Peak and SUVmax/SUL as well as total lesion uptake for the 5 most active lesions of each scan were recorded using manually corrected semiautomatic contours.
Statistical Analysis and Computations
Analysis was exploratory. Statistics were computed in R, version 3.5.1 (R Foundation for Statistical Computing). Descriptive statistics included mean, SD, or proportions, as appropriate. Vital signs were analyzed using a mixed-effects model (paired data). PSA doubling time was calculated by fitting to a linear model with logarithmic transformation. Negative doubling times due to treatment effects were excluded from calculation. Subjects were not excluded from the study on the basis of missing data; rather, for each variable or multivariate analysis, the maximum number of evaluable subjects (who had all required variables) was used and reported. Continuous distributions were compared with the Welch t test. When analyzing the effect of categoric variables against another categoric variable, the Pearson χ2 test was used with P values estimated by Monte Carlo simulation (106 repetitions). When the effect of categoric variables was assessed against a continuous variable, a linear model with ANOVA was used. SUVmax/SULmax dispersion was assessed by coefficient of variation. Statistical significance was defined as a P value less than or equal to 0.05.
RESULTS
Demographic Characteristics
One hundred thirty subjects were included in the analysis, with demographic parameters reported in Table 1 and Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). There were 94 subjects (72.3%) with biochemical recurrence after radical prostatectomy and 37 (28.5%) with biochemical recurrence after radiation therapy. Prior treatments included surgery (72.3% of cases), radiotherapy (34.6%), androgen-deprivation therapy (47.7%), or chemotherapy (0.8%), with some participants having received more than one type of therapy. Forty-five subjects received one or more types of radiotherapy: brachytherapy was administered to 27 of 45, external-beam radiotherapy to 20 of 45, intensity-modulated radiation therapy to 4 of 45, and proton therapy to 1 of 45. Overall, the subjects had a mean PSA level of 5.20 ± 6.50 ng/mL with a doubling time of 12.2 ± 11.8 mo (n = 113).
TABLE 1.
Patient Characteristics
| All included |
BR after RP only* |
BR after RT only* |
||||
| Variable | Data | n | Data | n | Data | n |
| Age (y) | 69.1 ± 6.5 | 130 | 68.4 ± 6.3 | 92 | 70.8 ± 6.9 | 35 |
| Body weight (kg) | 87.4 ± 14.4 | 130 | 86.9 ± 14.4 | 92 | 87.7 ± 13.5 | 35 |
| Height (cm) | 177.3 ± 6.8 | 130 | 176.9 ± 6.8 | 92 | 177.5 ± 6.6 | 35 |
| Injected activity (MBq) | 369.2 ± 47.2 | 130 | 367.8 ± 47.1 | 92 | 371.1 ± 46.0 | 35 |
| Uptake time (min) | 120.4 ± 1.5 | 130 | 120.5 ± 1.7 | 92 | 120.2 ± 0.6 | 35 |
| Inclusion criteria† | ||||||
| Known PC after radical prostatectomy with BR | 94 (72.3%) | 130 | 92 (100%) | 92 | 0 (0.0%) | 35 |
| Known PC after radiation therapy with BR | 37 (28.5%) | 130 | 0 (0.0%) | 92 | 35 (100%) | 35 |
| PSA at baseline (ng/mL) | 5.20 ± 6.50 | 130 | 3.03 ± 3.40 | 92 | 11.11 ± 8.94 | 35 |
| PSA doubling time (mo) | 12.2 ± 11.8 | 113 | 12.0 ± 12.3 | 78 | 12.9 ± 11.1 | 32 |
| Treatment history† | ||||||
| Surgery | 94 (72.3%) | 130 | 92 (100%) | 92 | 0 (0.0%) | 35 |
| Radiotherapy† | 45 (34.6%) | 130 | 7 (7.6%) | 92 | 35 (100%) | 35 |
| Brachytherapy | 27 (60.0%) | 45 | 0 (0.0%) | 7 | 26 (74.3%) | 35 |
| External-beam therapy | 20 (44.4%) | 45 | 5 (71.4%) | 7 | 13 (37.1%) | 35 |
| Intensity-modulated radiation therapy | 4 (8.9%) | 45 | 2 (28.6%) | 7 | 2 (5.7%) | 35 |
| Proton therapy | 1 (2.2%) | 45 | 0 (0.0%) | 7 | 1 (2.9%) | 35 |
| 223RaCl2 | 0 (0.0%) | 45 | 0 (0.0%) | 7 | 0 (0.0%) | 35 |
| Androgen-deprivation therapy | 62 (47.7%) | 130 | 39 (42.4%) | 92 | 22 (62.9%) | 35 |
| Chemotherapy | 1 (0.8%) | 130 | 1 (1.1%) | 92 | 0 (0.0%) | 35 |
Inclusion criteria.
Categories are not mutually exclusive.
PC = prostate cancer; BR= biochemical recurrence; RP = radical prostatectomy; RT = radiation therapy.
Data are mean ± SD or proportions.
Initial Tumor Characteristics
The distribution of Gleason scores was skewed toward intermediate to high grades (6: 13.2%, 3 + 4 = 7: 21.7%, 4 + 3 = 7: 28.7%, 8: 10.1%, 9: 25.6%, 10: 0.8%; n = 129). Most had an advanced pathologic T stage, with pT3 and pT4 representing 59.4% (n = 64) (Supplemental Table 2).
Clinical Assessment of PET/CT Scans
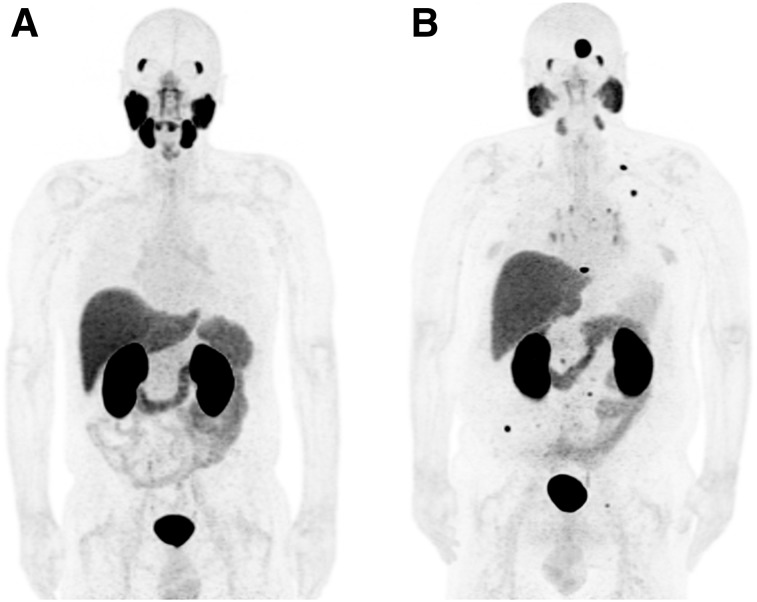
Representative 18F-DCFPyL PET/CT scans are shown in Figure 1; 84.6% were positive, with varying certainty levels (81.5% high, 13.1% moderate, and 5.4% low), showing that the readers had good confidence in their findings (Table 2; Supplemental Table 3). A high proportion of participants (53.9%) had only 3 lesions or fewer (1 lesion detected in 40.8%, 2 in 8.5%, and 3 in 4.6%).
FIGURE 1.
18F-DCFPyL PET maximum-intensity projections representative of tracer distribution. (A) Normal biodistribution (significant uptake by lacrimal glands, salivary glands, kidneys, liver, spleen, bowel, and bladder content). (B) Metastatic prostate cancer.
TABLE 2.
Qualitative Assessment of Scans
| All included |
BR after RP only* |
BR after RT only* |
||||
| Variable | Data | n | Data | n | Data | n |
| Number of lesions | 130 | 92 | 35 | |||
| 0 | 20 (15.4%) | 19 (20.7%) | 0 (0.0%) | |||
| 1 | 53 (40.8%) | 35 (38.0%) | 18 (51.4%) | |||
| 2 | 11 (8.5%) | 6 (6.5%) | 5 (14.3%) | |||
| 3 | 6 (4.6%) | 6 (6.5%) | 0 (0.0%) | |||
| 4 | 3 (2.3%) | 3 (3.3%) | 0 (0.0%) | |||
| 5 | 7 (5.4%) | 5 (5.4%) | 2 (5.7%) | |||
| 6–10 | 14 (10.8%) | 10 (10.9%) | 3 (8.6%) | |||
| >10 | 16 (12.3%) | 8 (8.7%) | 7 (20.0%) | |||
| Sites of relapse† | 130 | 92 | 35 | |||
| Local | 35 (26.9%) | 13 (14.1%) | 22 (62.9%) | |||
| Regional nodes | 57 (43.8%) | 41 (44.6%) | 14 (40.0%) | |||
| Distant nodes | 32 (24.6%) | 21 (22.8%) | 10 (28.6%) | |||
| Bone | 26 (20.0%) | 20 (21.7%) | 6 (17.1%) | |||
| Lung | 3 (2.3%) | 2 (2.2%) | 1 (2.9%) | |||
| Liver | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |||
| Other | 1 (0.8%) | 1 (1.1%) | 0 (0.0%) | |||
| Diagnosis | 130 | 92 | 35 | |||
| Positive | 110 (84.6%) | 73 (79.3%) | 35 (100%) | |||
| Negative | 20 (15.4%) | 19 (20.7%) | 0 (0.0%) | |||
| Certainty of diagnosis | 130 | 92 | 35 | |||
| High | 106 (81.5%) | 73 (79.3%) | 31 (88.6%) | |||
| Moderate | 17 (13.1%) | 14 (15.2%) | 3 (8.6%) | |||
| Low | 7 (5.4%) | 5 (5.4%) | 1 (2.9%) | |||
Inclusion criteria.
Categories are not mutually exclusive.
BR = biochemical recurrence; RP = radical prostatectomy; RT = radiation therapy.
Data are proportions.
In an ANOVA of a linear model in which PSA was analyzed with the number of lesions and Gleason score factors, the number of lesions had a significant effect (P < 0.01). However, there was substantial overlap in PSA values for differing numbers of lesions. The initial Gleason score was not significantly associated with PSA. To evaluate for a potential association between PSA value and lesion localization, Gleason score, or number of lesions, participants with disease in only 1 area were selected (n = 75). ANOVA of a linear model of PSA against lesion localization, Gleason score, and number of lesions was computed. In that subgroup, no significant association was found. Also, there was substantial overlap in PSA values when plotted against those factors (Supplemental Figs. 1–3). The Gleason score was not related to the number of lesions when evaluated by χ2, but there was lack of independence when evaluated against sites of relapse (χ2; P < 0.01).
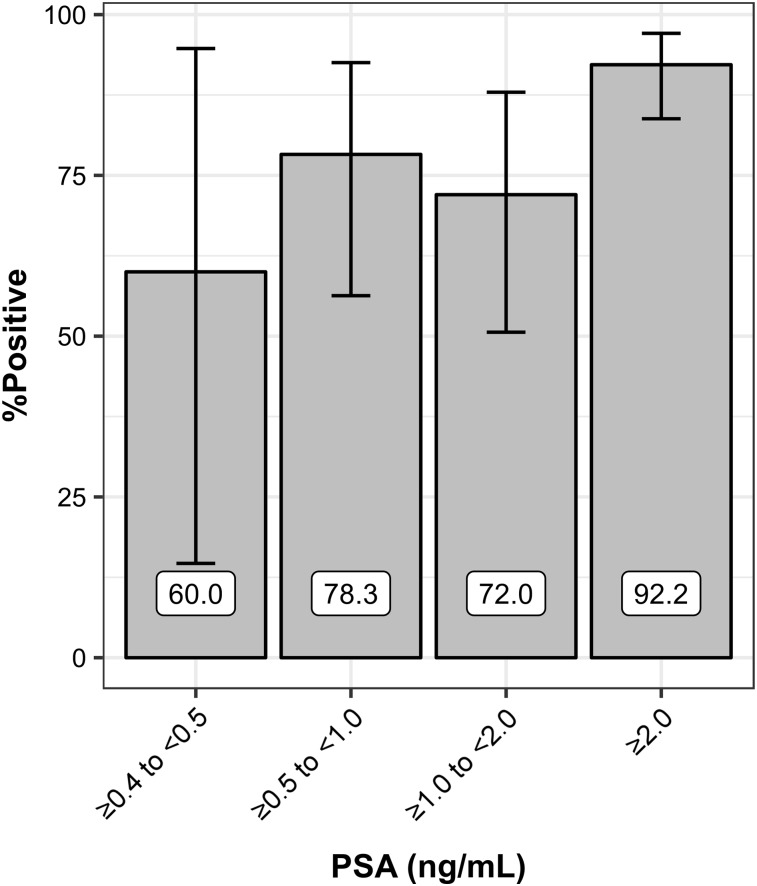
The proportion of positive scans increased with PSA level (Fig. 2; Supplemental Table 4). The PSA values for positive scans (5.80 ± 6.87 ng/mL) were significantly different (Welch t test; P < 0.001) from those for negative scans (1.86 ± 1.62 ng/mL); however, there was a large overlap in PSA values across those 2 categories.
FIGURE 2.
Proportion of positive scans based on PSA level. Error bars represent 95% confidence intervals.
Active disease was most often identified in regional nodes (43.9%), followed by prostate bed/seminal glands (26.9%), distant nodes (24.6%), bone (20.0%), lung (2.3%), and other sites (0.8%); no liver lesions were identified (Supplemental Fig. 4). Several participants had disease in more than one site. Previous treatments had an influence on lesion distribution, which differed, notably, between patients who previously had surgery with or without androgen-deprivation therapy and those who had radiotherapy with or without androgen-deprivation therapy (χ2 test; P < 0.01) (Supplemental Fig. 5). In the subset of participants treated with radiotherapy with or without androgen-deprivation therapy, there was trend toward a differing distribution of lesion localization between brachytherapy and external-beam radiotherapy (χ2 test; P = 0.051); this distribution was calculated while excluding subjects who had had multiple radiotherapy types.
Evaluation of Lesions
Background uptake was low (SUVmean, 1.22 ± 0.22) in the cardiac blood pool. The distribution of lesion uptake had an SUVmax range of 1.15–85.04 (mean, 12.43 ± 12.34). SUVpeak yielded distributions with a smaller range, 0.86–61.2 (mean, 7.60 ± 7.98). Coefficients of variation of SULmax (97%) and SULpeak (106%) were comparable to those of SUVmax (99%) and SUVpeak (105%) (Supplemental Table 5). When selecting patients who had 5 or fewer lesions (the maximum recorded on the quantitative assessment), there was a significant relationship between PSA and sum of total lesion uptake (P < 0.05) when assessed by ANOVA of a linear model that also accounted for the Gleason score (which also had a significant association with PSA in this reduced data set; P < 0.01). Lesion SUVmax and SULmax were significantly related to the initial Gleason score when evaluated by a linear model (P < 0.05).
Adverse Events
Vital signs varied at different time points: blood pressure changed from 142 ± 19/82 ± 13 to 146 ± 19/80 ± 9 mm Hg between preinjection values and immediately before the scan. Heart rate changed from 65 ± 14 to 75 ± 16 bpm, and pulse oximetry from 97.6% ± 2.1% to 97.6% ± 2.6%. Those values were statistically significant (except for pulse oximetry) but not considered clinically significant. There were no adverse events during scans. In total, 22 subjects reported mild adverse events after the scan; all resolved completely (Supplemental Table 6).
Changes in Management
Currently, referring physicians have completed postscan assessments of changes in management for 55 of 130 subjects (Table 3; Supplemental Table 7). A change in treatment intent occurred in 65.5% of subjects, with half of those being directed to palliative care and the other half to curative treatment. Disease stage changed in 65.5% of cases (97.1% of which were upstaged). Findings on 18F-DCFPyL scans prompted additional imaging in 23.6% of cases, changed plans for surgery or biopsy in 25.5%, changed plans for systemic therapy in 56.4%, and changed plans for radiotherapy in 47.3%. Physicians indicated that imaging results improved decision making in 89.1% and changed management plans in 87.3%.
TABLE 3.
Changes in Treatment Intent, Disease Stage, Investigation, Decision Making, or Management Plan
| All included |
BR after RP only* |
BR after RT only* |
||||
| Variable | Data | n | Data | n | Data | n |
| Change in treatment intent | 36 (65.5%) | 55 | 21 (56.8%) | 37 | 13 (86.7%) | 15 |
| To palliative | 18 (50.0%) | 36 | 10 (47.6%) | 21 | 6 (46.2%) | 13 |
| To curative | 18 (50.0%) | 36 | 11 (52.4%) | 21 | 7 (53.8%) | 13 |
| Change in disease stage | 36 (65.5%) | 55 | 24 (64.9%) | 37 | 10 (66.7%) | 15 |
| Upstaged | 34 (97.1%) | 35 | 23 (100%) | 23 | 9 (90.0%) | 10 |
| Downstaged | 1 (2.9%) | 35 | 0 (0.0%) | 23 | 1 (10.0%) | 10 |
| Ordering of additional diagnostic studies† | 13 (23.6%) | 55 | 6 (16.2%) | 37 | 7 (46.7%) | 15 |
| CT | 4 (30.8%) | 13 | 2 (33.3%) | 6 | 2 (28.6%) | 7 |
| MRI | 5 (38.5%) | 13 | 3 (50.0%) | 6 | 2 (28.6%) | 7 |
| Nuclear medicine | 1 (7.7%) | 13 | 1 (16.7%) | 6 | 0 (0.0%) | 7 |
| Ultrasound | 0 (0.0%) | 13 | 0 (0.0%) | 6 | 0 (0.0%) | 7 |
| Biopsy | 4 (30.8%) | 13 | 0 (0.0%) | 6 | 4 (57.1%) | 7 |
| Other‡ | 1 (7.7%) | 13 | 0 (0.0%) | 6 | 1 (14.3%) | 7 |
| Imaging results changed plans for surgery or biopsy | 14 (25.5%); NA 13 (23.6%) | 55 | 6 (16.2%); NA 10 (27.0%) | 37 | 8 (53.3%); NA 1 (6.7%) | 15 |
| Surgery or biopsy added | 9 (64.3%) | 14 | 4 (66.7%) | 6 | 5 (62.5%) | 8 |
| Surgery or biopsy cancelled | 5 (35.7%) | 14 | 2 (33.3%) | 6 | 3 (37.5%) | 8 |
| Other | 0 (0.0%) | 14 | 0 (0.0%) | 6 | 0 (0.0%) | 8 |
| Imaging results changed plans for systemic therapy | 31 (56.4%); NA 3 (5.5%) | 55 | 20 (54.1%); NA 2 (5.4%) | 37 | 9 (60.0%); NA 1 (6.7%) | 15 |
| Systemic therapy started | 23 (74.2%) | 31 | 15 (75.0%) | 20 | 6 (66.7%) | 9 |
| Systemic therapy not initiated/cancelled | 8 (25.8%) | 31 | 5 (25.0%) | 20 | 3 (33.3%) | 9 |
| Systemic therapy changed | 0 (0.0%) | 31 | 0 (0.0%) | 20 | 0 (0.0%) | 9 |
| Imaging results changed plans for radiotherapy | 26 (47.3%); NA 9 (16.4%) | 55 | 22 (59.5%); NA 6 (16.2%) | 37 | 4 (26.7%); NA 1 (6.7%) | 15 |
| Radiotherapy added | 13 (52.0%) | 25 | 11 (52.4%) | 21 | 2 (50.0%) | 4 |
| Radiotherapy cancelled | 9 (36.0%) | 25 | 8 (38.1%) | 21 | 1 (25.0%) | 4 |
| Radiotherapy prescription changed | 3 (12.0%) | 25 | 2 (9.5%) | 21 | 1 (25.0%) | 4 |
| Imaging results improved decision making | 49 (89.1%) | 55 | 33 (89.2%) | 37 | 14 (93.3%) | 15 |
| Imaging results changed subject’s management plan | 48 (87.3%) | 55 | 32 (86.5%) | 37 | 14 (93.3%) | 15 |
Inclusion criteria.
Categories are not mutually exclusive.
Repeat PET a few months after start of androgen-deprivation therapy.
BR = biochemical recurrence; RP = radical prostatectomy; RT = radiation therapy; NA = not applicable.
Data are proportions.
DISCUSSION
This study aimed to determine the sensitivity and safety of 18F-DCFPyL PET/CT for the detection of prostate cancer relapse in the context of biochemical recurrence. Since the initial publication by Rowe et al. in 2015 on 9 patients, several small studies have been published on this tracer for prostate cancer, many of them by the same groups (8,10–12,14–20). This interim analysis evaluated a large prospective cohort of subjects who participated in an investigator-initiated 18F-DCFPyL PET/CT imaging study in Vancouver, Canada.
Although the definition of oligometastatic disease in prostate cancer is still evolving, many participants had a low number of lesions that would fall under this category (53.9% had 1–3 lesions) (21–23). Although more research is needed to assess its efficacy, there is a potential for localized therapy (i.e., resection or stereotactic body radiation therapy) with minimal risk of serious adverse events (23,24). In this setting, 18F-DCFPyL PET/CT may be useful to identify disease occult on other imaging modalities that could be amenable to more aggressive treatment (25). Furthermore, in 65.4% of participants, disease was located in regional nodes or presented as local recurrence. For subjects who were treated surgically, this disease would potentially be amenable to salvage pelvic irradiation.
Although the number of lesions reported on imaging was significantly related to PSA values at baseline, there was an important overlap in PSA range between groupings based on the number of lesions. Such an overlap is to be expected, as the number of lesions is not a good indicator of tumor burden because of size variations. Conversely, there was a significant relation between total lesion uptake and PSA. However, no PSA value was predictive of oligometastatic disease in this population.
Compared with the detection rates presented by Eiber for 68Ga-PSMA HBED-CC—57.9%, 72.7%, 93%, and 96.8%, with respective PSA intervals of 0.2–0.5, 0.5–1.0, 1.0–2.0, and ≥2.0 ng/mL—our study achieved similar results, with detection rates of 60% ± 80% (exact 95% confidence interval), 78% ± 36%, 72% ± 37%, and 92% ± 14% at equivalent intervals (≥0.4 to <0.5, ≥0.5 to <1.0, ≥1.0 to <2.0, and ≥2.0, respectively) (26). The lower detection rate in the 1.0–2.0 interval for 18F-DCFPyL may be attributable to random variations and remains within the 95% confidence interval for the proportion. This is also similar to other 68Ga-PSMA studies reported in a review by Evans et al. and to detection rates reported for 18F-PSMA-1007 (61.5%, 74.5%, 90.1%, and 94.1%) (27,28). 18F-DCFPyL, in the context of the inclusion criteria of the present analysis, appears to have an overall similar sensitivity to other radiotracers.
The distribution of active disease was dependent on prior therapy. There was a greater proportion of local recurrence after radiotherapy than after surgery. This study was not designed to evaluate primary treatment modalities. Referral patterns for inclusion into the study might account for some of these differences.
Change in treatment intent occurred in 65.5% of subjects, all of whom had a change in disease stage. In comparison with 68Ga-PSMA-11, Afaq et al. reported changes in management plans in 39% of patients and Hope et al. in 59.6%. Koerber et al. reported changes in radiotherapeutic management of 56.3% in patients with PSA persistence after surgery or recurrence after definitive therapy (29–31). A systematic review by Han et al. reported a change in management in 54% (95% confidence interval, 47%–60%) (32). PSA at baseline was determined not to be a significant factor for change in management, treatment intent, or disease stage or for ordering additional diagnostic studies when assessed by logit analysis. In the metaanalysis by Han et al., the metaregression had not shown PSA to be a significant factor for change in management either, but there was a tendency toward a greater proportion of management changes in studies with greater PSA levels before PET (32).
Although a small proportion of participants reported undesirable events, all were mild and resolved completely. There was no serious adverse event. Our results indicate that 18F-DCFPyL can be considered safe for injection in humans (10,11).
As a limitation to this study, the fact that not all referring physicians (55/130) had completed the questionnaire for change in management at the time of analysis could reflect a reporting bias in favor of helpful scans.
CONCLUSION
18F-DCFPyL PET/CT imaging identified sites of recurrent prostate cancer in most subjects and was well tolerated, with no serious adverse events. A large proportion of subjects meeting the inclusion criteria for this analysis had 3 or fewer lesions identified on the scan. 18F-DCFPyL PET/CT imaging improved decision making for referring oncologists and changed management plans for most subjects.
DISCLOSURE
Financial support was received from the Canadian Institutes of Health Research (FDN-148465), BC Cancer Foundation, BC Leading Edge Endowment Fund, and Terry Fox Research Institute. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
We thank Hayley Allan and Iulia Dude, who acted as study coordinators for this trial; Tina Alden for technical coordination of the PET acquisitions; and the cyclotron/radiochemistry team (Michael Woods, Jennifer Greene Garrick, and Guillaume Langlois).
KEY POINTS
QUESTION: What is the impact of 18F-DCFPyL PET/CT on patient management in the setting of biochemical recurrence of prostate cancer?
PERTINENT FINDINGS: In this analysis of a prospective clinical trial, 18F-DCFPyl changed the management plan for 87.3% of patients and the disease stage for 65.5%, with no serious adverse events.
IMPLICATIONS FOR PATIENT CARE: 18F-DCFPyL PET/CT is safe and changed the management for most subjects.
REFERENCES
- 1.Canadian cancer statistics: a 2018 special report on cancer incidence by stage. Canadian Cancer Society website. cancer.ca/Canadian-Cancer-Statistics-2018-EN. Published June 2018. Accessed June 25, 2019.
- 2.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 3.Taneja SS. Imaging in the diagnosis and management of prostate cancer. Rev Urol. 2004;6:101–113. [PMC free article] [PubMed] [Google Scholar]
- 4.Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat Rev Urol. 2017;14:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habl G, Straube C, Schiller K, et al. Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer. 2017;17:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 7.Lenzo NP, Meyrick D, Turner JH. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics (Basel). 2018;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zacho HD, Nielsen JB, Haberkorn U, Stenholt L, Petersen LJ. 68Ga-PSMA PET/CT for the detection of bone metastases in prostate cancer: a systematic review of the published literature. Clin Physiol Funct Imaging. 2018;38:911–922. [DOI] [PubMed] [Google Scholar]
- 10.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin MA, Rowe SP, Patel HD, et al. Prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography for the preoperative staging of high risk prostate cancer: results of a prospective, phase II, single center study. J Urol. 2018;199:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giesel FL, Will L, Lawal I, et al. Intraindividual comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the prospective evaluation of patients with newly diagnosed prostate carcinoma: a pilot study. J Nucl Med. 2018;59:1076–1080. [DOI] [PubMed] [Google Scholar]
- 13.Bouvet V, Wuest M, Jans HS, et al. Automated synthesis of [18F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietlein M, Kobe C, Kuhnert G, et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietlein F, Kobe C, Neubauer S, et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017;58:947–952. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Rowe SP, Leal JP, et al. Semiquantitative parameters in PSMA-targeted PET imaging with 18F-DCFPyL: variability in normal-organ uptake. J Nucl Med. 2017;58:942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondergem M, van der Zant FM, Knol RJJ, Lazarenko SV, Pruim J, de Jong IJ. 18F-DCFPyL PET/CT in the detection of prostate cancer at 60 and 120 minutes: detection rate, image quality, activity kinetics, and biodistribution. J Nucl Med. 2017;58:1797–1804. [DOI] [PubMed] [Google Scholar]
- 18.Bauman G, Martin P, Thiessen JD, et al. [18F]-DCFPyL positron emission tomography/magnetic resonance imaging for localization of dominant intraprostatic foci: first experience. Eur Urol Focus. 2018;4:702–706. [DOI] [PubMed] [Google Scholar]
- 19.Plyku D, Mena E, Rowe SP, et al. Combined model-based and patient-specific dosimetry for 18F-DCFPyL, a PSMA-targeted PET agent. Eur J Nucl Med Mol Imaging. 2018;45:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wondergem M, van der Zant FM, Vlottes PW, Knol RJJ. Effects of fasting on 18F-DCFPyL uptake in prostate cancer lesions and tissues with known high physiologic uptake. J Nucl Med. 2018;59:1081–1084. [DOI] [PubMed] [Google Scholar]
- 21.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. [DOI] [PubMed] [Google Scholar]
- 22.Niazi T, Elakshar S, Stroian G. Local ablative stereotactic body radiotherapy for oligometastatic prostate cancer. Curr Opin Support Palliat Care. 2018;12:351–358. [DOI] [PubMed] [Google Scholar]
- 23.Koo KC, Dasgupta P. Treatment of oligometastatic hormone-sensitive prostate cancer: a comprehensive review. Yonsei Med J. 2018;59:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadvar H. Oligometastatic prostate cancer: molecular imaging and clinical management implications in the era of precision oncology. J Nucl Med. 2018;59:1338–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt-Hegemann NS, Stief C, Kim TH, et al. Outcome after PSMA PET/CT based salvage radiotherapy in patients with biochemical recurrence after radical prostatectomy: a bi-institutional retrospective analysis. J Nucl Med. 2018;60:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 27.Evans JD, Jethwa KR, Ost P, et al. Prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28–39. [DOI] [PubMed] [Google Scholar]
- 28.Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 30.Afaq A, Alahmed S, Chen SH, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. [DOI] [PubMed] [Google Scholar]
- 31.Koerber SA, Will L, Kratochwil C, et al. 68Ga-PSMA-11 PET/CT in primary and recurrent prostate carcinoma: implications for radiotherapeutic management in 121 patients. J Nucl Med. 2018;60:234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.