Abstract
Background
For patients with acute myocardial infarction (AMI) complicated by cardiogenic shock (CS) undergoing primary percutaneous coronary intervention (PCI), the optimal timing of the initiation of intra‐aortic balloon pump (IABP) therapy remains unclear. Therefore, we performed the first meta‐analysis to compare the outcomes of IABP insertion before vs after primary PCI in this population.
Methods
Electronic databases of PubMed, EMBASE, and Cochrane Library were comprehensively searched from inception to April 1, 2019, to identify the eligible studies. The main outcomes were short‐term (in‐hospital or 30 days) and long‐term (≥ 6 months) mortality. In addition, pooled analysis of risk‐adjusted data were also performed to control for confounding factors.
Results
Seven observational studies and two sub‐analysis of randomized controlled trials involving 1348 patients were included. Compared to patients inserted IABP after PCI, patients who received IABP therapy before primary PCI had similar risks of short‐term (odds ratio [OR] 0.88, 95% CI 0.49 to 1.59) and long‐term (OR 0.99, 95% CI 0.58 to 1.68) all‐cause mortality. Moreover, a pooled analysis of risk‐adjusted data also found similar effects of the two therapies on short‐term (OR 0.65, 95% CI 0.34 to 1.25) and long‐term (OR 0.68, 95% CI 0.17 to 2.72) mortality. Besides, no significant difference was found between the two groups with respect to reinfarction, repeat revascularization, stroke, renal failure, and major bleeding.
Conclusions
The timing of the initiation of IABP therapy does not appear to impact short‐term and long‐term survival in patients with AMI complicated by CS undergoing primary PCI.
Keywords: acute myocardial infarction, cardiogenic shock, intra‐aortic balloon pump, survival, timing
1. INTRODUCTION
In patients with acute myocardial infarction (AMI), 6%‐9% can be affected by cardiogenic shock (CS) and the mortality rate is close to 50% during hospitalization.1 Despite adoption of early revascularization strategies, CS remains the leading cause of death in this population.2 Furthermore, supportive drug treatments with inotropes and vasopressors bring no benefit to patients. Cardiologists hope that mechanical circulatory support will improve clinical outcomes in this population.3, 4 The intra‐aortic balloon pump (IABP) becomes the first and most widely used device due to its ability to reduce afterload and improve coronary blood flow.4, 5, 6, 7 However, recent meta‐analyses and the landmark randomized controlled trial did not show a beneficial effect of IABP in patients with AMI complicated by CS.8, 9, 10, 11 Thus, the recommendations of IABP therapy have been reduced both in the American and European guidelines.12, 13 Nonetheless, the lack of efficacy of IABP usage might be partly influenced by the timing of initiation of IABP therapy, that is, before or after primary percutaneous coronary intervention (PCI). Nevertheless, almost all studies comparing the sequence of IABP and primary PCI are of a small scale, and current trials have shown conflicting results. Thus, we conducted this meta‐analysis to identify the optimal timing of the initiation of IABP in patients with AMI complicated by CS undergoing primary PCI.
2. METHODS
This study was performed based on the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) and meta‐analysis of observational studies in epidemiology (MOOSE) statements.14, 15
2.1. Search strategy
Two independent investigators (Lingxiao Chen and Kuo Zhou) searched the electronic databases of PubMed, EMBASE, and Cochrane Library from inception to April 1, 2019, to identify the pertinent English articles regarding the IABP inserted before vs after primary PCI for the treatment of AMI complicated by CS. The following medical subject headings and search terms were used: “acute myocardial infarction,” “cardiogenic shock,” “before primary percutaneous coronary intervention,” “after primary percutaneous coronary intervention,” and “timing.” In addition, the references of the identified articles and relevant reviews were examined to include other potentially eligible studies.
2.2. Study selection
Studies satisfying the following criteria were eligible: (a) patients who were diagnosed with CS from AMI; (b) studies that compared the strategy of IABP insertion before vs after primary PCI; and (c) studies that assessed the endpoints of interest. The selection was conducted by scanning titles and/or abstracts, and full‐text reviews were performed for further analysis. When several reports overlapped, we selected the largest and the latest one. The studies were reviewed by two independent investigators (Jinfan Tian and Yunfeng Yan) to determine whether they met the inclusion criteria. Any disagreements were resolved through discussion with a third investigator (Dongfeng Zhang).
2.3. Data extraction and quality assessment
For each eligible study, three authors (Fei Yuan, Mingduo Zhang, and Wei Wang) independently extracted the following data through a standardized form: first author, year of publication, study design, quality indicators, baseline as well as procedural characteristics, and clinical outcomes. Discrepancies were resolved by consensus. The primary endpoint was short‐term mortality (in‐hospital or 30 days). Long‐term mortality (≥ 6 months), reinfarction, stroke, repeat revascularization, acute renal failure, and major bleeding were the secondary outcomes. Deaths were classified as either cardiac or noncardiac, and classifications of other outcomes were in agreement with the included studies.
The methodological quality of the observational studies was assessed using the Newcastle Ottawa Scale.16 Studies with a Newcastle‐Ottawa score ≥ 6 (maximum, 9) were considered high quality. In addition, the quality of randomized controlled trials (RCTs) were assessed using the Cochrane risk of bias tool.17
2.4. Statistical analysis
The present study used Review Manager 5.3 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata/SE12.0 (StataCorp, College Station, Texas) for data analysis. All results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Potential heterogeneity was evaluated with the I2 statistic, and a value >50% was defined as statistical heterogeneity. For all comparisons, the DerSimonian and Lair random‐effects model was used to account for the wide range of methodological variability across the studies.
Pooled analysis of risk‐adjusted data were performed to control for confounding factors, and to test the sensitivity of the short‐term and long‐term mortality. The adjusted variables are listed in Table S1. In addition, sensitivity analysis was conducted by reanalyzing the results of studies that enrolled patients presented with ST‐segment elevation myocardial infarction (STEMI) or published in full text. In case of significant heterogeneity, sensitivity analysis was also conducted by omitting one study in each turn to test the influence of single trial. Meta‐regression analysis was carried out to assess patient characteristics with the primary endpoint, that is, male, current smoker, diabetes mellitus, hypertension, and culprit vessel of left anterior descending coronary artery. The risk of potential publication bias was assessed by the Begg's and Egger's tests.18, 19 When there was an indication of publication bias from the statistical tests, we used the trim and fill method to evaluate the influence of potentially unpublished studies on the summary estimates. All statistical tests were two‐sided and were considered to be statistically significant at P < .05.
3. RESULTS
3.1. Eligible studies
The comprehensive search yielded 1093 potentially relevant articles; after exclusion of duplicates and assessment of titles and/or abstracts, 29 articles were chosen for complete review. Finally, nine studies including 1348 patients met our inclusion criteria, published between 2005 and 201720, 21, 22, 23, 24, 25, 26, 27, 28 (Figure S1).
The main characteristics of the included studies are presented in Table 1 and quality assessment results are described in Tables S2 and S3. Seven studies were observational studies,21, 22, 23, 24, 25, 26, 27 and the remaining two were sub‐analysis of randomized controlled trials.20, 28 Five of the eligible studies only enrolled patients who presented with STEMI,22, 23, 24, 25, 27 and the remaining four included patients with non‐STEMI.20, 21, 26, 28 Two studies were abstract slides from conference proceedings. Overall, eight studies reported short‐term mortality (in‐hospital and 30‐days),20, 21, 22, 23, 24, 25, 26, 28 while four studies reported long‐term mortality (≥ 6 months).23, 25, 27, 28 In addition, five21, 22, 23, 24, 26 and three23, 27, 28 studies reported multivariable‐adjusted data of short‐term and long‐term mortality, respectively.
Table 1.
The methodology and the population characteristics of studies
| Study | No. patientsa | Period | Region | Design, center | Inclusion criteria | Exclusion criteria | Adjusted method | Follow‐up duration |
|---|---|---|---|---|---|---|---|---|
| Thiele 2005 | 9/11 | 2000‐2003 | Germany | Sub‐analysis of RCT, single | AMI with CS | Age > 75 y, mechanical complication, shock >12 h, right heart failure, sepsis, significant aortic regurgitation, severe cerebral damage, resuscitation >30 min, severe peripheral vascular disease | NA | 30 d |
| Abdel‐Wahab 2010 | 26/22 | 2005‐2008 | Germany | Retrospective registry, single | AMI with CS due to left ventricular failure | Mechanical complication, isolated right ventricular infarction, shock due to other causes, > 24 h after primary PCI | Multivariable adjusted | In‐hospital |
| Sjauw 2012 | 59/140 | 1997‐2005 | Netherlands | Prospective registry, single | STEMI with CS | NA | Propensity‐score adjusted | 30 d |
| Cheng 2013 | 87/86 | 2000‐2009 | Netherlands | Retrospective registry, single | STEMI with CS | CS developed during primary PCI or hospitalization | Multivariable adjusted | 5 y |
| Bergh 2014 | 72/67 | 2004‐2008 | Sweden | Prospective registry, single | STEMI with CS | NA | Propensity‐score adjusted |
30 d |
| Negi 2014 | 76/98 | NA | United States | Retrospective study, single | STEMI with CS | NA | NA | 1 y |
| Schwarz 2016 | 49/53 | 2005‐2010 | Germany | Retrospective registry, single | AMI with CS due to left ventricular failure | No spontaneous circulation, mechanical complication, isolated right ventricular infarction, shock due to other causes, > 24 h after primary PCI | Multivariable adjusted | In‐hospital |
| Yuan 2016 | 106/112 | 2008‐2014 | China | Prospective study, single | STEMI with CS | Incomplete data | Multivariable adjusted | 1 y |
| Fuernau 2017 | 33/242 | 2009‐2012 | Germany | Sub‐analysis of RCT, multi | AMI with CS | Resuscitation >30 min, no spontaneous circulation, coma, mechanical complication, shock >12 h, massive pulmonary embolism, severe peripheral arterial disease or aortic regurgitation, age > 90 years, shock due to other causes | Multivariable adjusted | 1 y |
Abbreviations: AMI, acute myocardial infarction; CS, cardiogenic shock; IABP, intra‐aortic balloon pump; NA, not applicable; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST‐segment elevation myocardial infarction.
Data are expressed as IABP before primary PCI/ IABP after primary PCI.
As presented in Table 2, baseline characteristics of the patients were similar between the two treatment strategies, except that dyslipidemia was more common in patients who received IABP insertion before primary PCI than the control group (48.3% vs 38.7%).
Table 2.
Baseline characteristics of the patients
| Study | Age, years | Male (%) | Current smoker (%) | Diabetes mellitus (%) | Hypertension (%) | Dyslipidemia (%) | Previous MI (%) | LVEF (%) | Systolic blood pressure, mmHg | Multivessel disease (%) | Culprit vessel of LAD (%) | IABP support time, hours |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thiele 2005 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Abdel‐Wahab 2010 | 70/71 | 88.5/72.7 | 42.3/40.9 | 50.0/45.5 | 69.2/63.6 | 57.7/54.5 | 34.6/40.9 | 23.5/23.2 | 109/105 | NA | 46.2/45.5 | 43/47 |
| Sjauw 2012 | 65.1/64.6 | 79.7/63.6 | 30.5/38.6 | 15.3/19.3 | 37.3/33.6 | 25.4/19.3 | 28.8/30.7 | NA | 109.7/104.8 | 64.4/62.1 | 55.9/61.4 | NA |
| Cheng 2013 | 65/64 | 79.3/77.9 | 24.1/29.1 | 17.2/16.3 | 29.9/27.9 | 56.3/47.7 | 29.9/18.6 | NA | 75/79 | NA | 66.7/55.8 | NA |
| Bergh 2014 | 66/66 | 69.4/76.1 | 30.6/26.9 | 29.2/14.9 | 36.1/32.8 | NA | 13.9/13.4 | 33/35 | 80/79 | 77.8/74.6 | NA | 20/24 |
| Negi 2014 | 59/61 | 63.2/74.5 | NA | NA | NA | NA | NA | NA | NA | NA | 88.2/88.8 | 56/51 |
| Schwarz 2016 | 70.6/69.7 | 73.5/66.0 | 32.7/32.1 | 51.0/41.5 | 73.5/71.7 | 61.2/52.8 | 34.7/22.6 | 24.2/25.2 | NA | NA | NA | 37/45 |
| Yuan 2016 | 63.1/65.2 | 64.2/63.4 | 44.3/47.3 | 30.2/29.1 | 36.8/38.4 | 46.2/46.4 | 10.4/14.3 | NA | 76.5/75.7 | 24.5/29.5 | 48.1/49.1 | NA |
| Fuernau 2017 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Summary | 64.6/64.9 | 71.8/69.6 | 33.8/36.7 | 28.8/24.4 | 41.9/39.2 | 48.3/38.7 | 22.6/21.9 | 28.4/29.5 | 84.9/87.9 | 50.6/48.7 | 63.0/62.4 | 38.7/41.8 |
Note: Data are expressed as IABP inserted before primary percutaneous coronary intervention/IABP inserted after primary percutaneous coronary intervention. Bold values that dyslipidemia was more common in patients who received IABP insertion before primary PCI than the control group (48.3% vs 38.7%), while other baseline characteristics of the patients were similar between the two groups.
Abbreviations: IABP, intra‐aortic balloon pump; LAD, left anterior descending coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable.
3.2. Primary endpoint
In summary, short‐term death occurred in 149 patients (36.3%) in the IABP inserted before primary PCI group compared with 264 patients (36.7%) in the IABP inserted after primary PCI group. As shown in Figure 1A, short‐term mortality was comparable between the two treatment strategies (OR 0.88, 95% CI 0.49 to 1.59, P = .67), with significant heterogeneity across studies (I2 = 76%, P = .0002). Sensitivity analysis indicated that no significant difference was found between the two groups when studies that enrolled patients with STEMI (OR 1.34, 95% CI 0.79 to 2.29, I2 = 60%) (Figure S2) or published in full text (OR 0.90, 95% CI 0.40 to 2.00, I2 = 81%) (Figure S3) were analyzed. In addition, sensitivity analysis conducted by the removal of any single trial showed that it did not essentially affect the overall pooled estimate of short‐term mortality, whereas the heterogeneity existed consistently across the studies (I2 > 50%). Moreover, a pooled analysis of risk‐adjusted data also demonstrated similar effects of the two therapies on short‐term mortality (OR 0.65, 95% CI 0.34 to 1.25, P = .19, I2 = 68%) (Figure 1B). After removing the study by Schwarz et al., the statistical heterogeneity of adjusted short‐term mortality no longer existed (OR 0.86, 95% CI 0.49 to 1.51, I2 = 47%).
Figure 1.
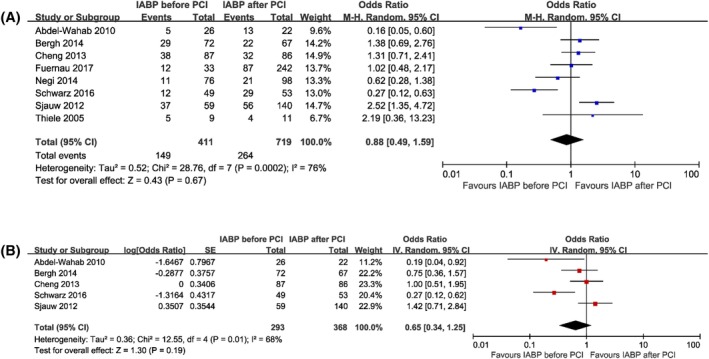
Forest plots comparing short‐term mortality for patients with acute myocardial infarction complicated by cardiogenic shock undergoing IABP insertion before or after primary percutaneous coronary intervention. A, Unadjusted short‐term mortality. B, Adjusted short‐term mortality. CI, confidence interval; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention
3.3. Secondary endpoints
In the pooled estimate, the initiation of IABP therapy before primary PCI had similar risk of long‐term mortality compared to that of inserted after primary PCI based on both unadjusted data (OR 0.99, 95% CI 0.58 to 1.68, P = .96, I2 = 57%) (Figure 2A) and risk‐adjusted data (OR 0.68, 95% CI 0.17 to 2.72, P = .59, I2 = 94%) (Figure 2B). After removing the study by Negi et al., the heterogeneity of long‐term mortality no longer existed (OR 1.19, 95% CI 0.81 to 1.75, I2 = 0%). Besides, the heterogeneity of adjusted long‐term mortality disappeared after excluding the study by Yuan et al. (OR 1.32, 95% CI 0.85 to 2.03, I2 = 0%).
Figure 2.
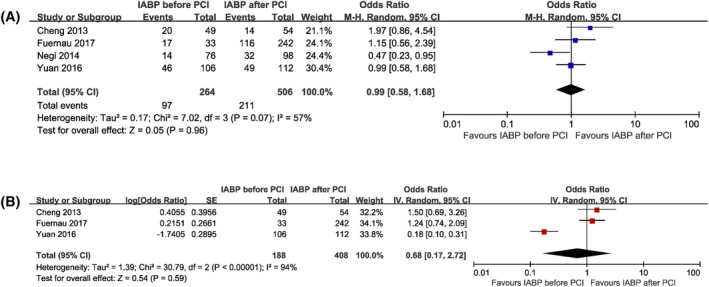
Forest plots comparing long‐term mortality for patients with acute myocardial infarction complicated by cardiogenic shock undergoing IABP insertion before or after primary percutaneous coronary intervention. A, Unadjusted short‐term mortality. B, Adjusted short‐term mortality. CI, confidence interval; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention
No significant difference was found between the two groups in terms of reinfarction (OR 1.14, 95% CI 0.60 to 2.15, P = .69, I2 = 0%), repeat revascularization (OR 0.40, 95% CI 0.09 to 1.88, P = .25, I2 = 41%), stroke (OR 0.88, 95% CI 0.35 to 2.21, P = .78, I2 = 0%), acute renal failure (OR 0.85, 95% CI 0.44 to 1.61, P = .61, I2 = 57%), and major bleeding (OR 1.02, 95% CI 0.62 to 1.68, P = .93, I2 = 2%) (Figure 3). The heterogeneity of acute renal failure no longer existed when the study by Abdel‐Wahab et al., (OR 1.07, 95% CI 0.62 to 1.84, I2 = 36%) or by Negi et al., (OR 0.66, 95% CI 0.36 to 1.20, I2 = 23%) was removed.
Figure 3.
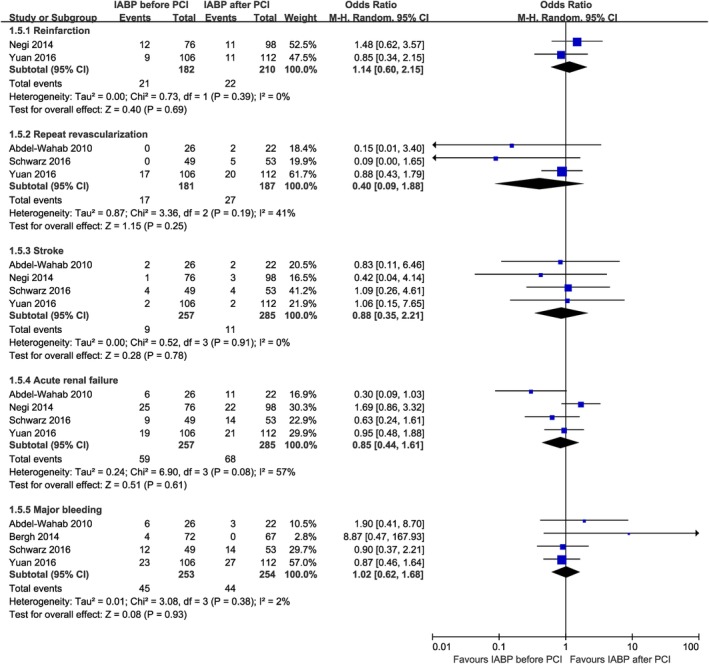
Forest plots comparing reinfarction, repeat revascularizaton, stroke, acute renal failure, and major bleeding for patients with acute myocardial infarction complicated by cardiogenic shock undergoing IABP insertion before or after primary percutaneous coronary intervention. CI, confidence interval; IABP, intra‐aortic balloon pump; PCI, percutaneous coronary intervention
3.4. Meta‐regression analysis and publication bias
Meta‐regression analysis showed significant association between patient characteristics of diabetes mellitus (regression coefficient − 0.07, 95% CI −0.21 to −0.02, P = .02) or hypertension (regression coefficient − 0.05, 95% CI −0.09 to −0.01, P = .04) and the short‐term mortality. No interaction was found between male (P = .51), current smoker (P = .42), or culprit vessel of left anterior descending coronary artery (P = .79) and the primary endpoint of short‐term mortality.
In addition, the assessment of the funnel plot was performed, and no publication bias was found for the outcomes except for major bleeding (Egger's test, P = .03; Begg's test, P = .09). One study was added with the trim and fill method, and the risk of major bleeding remained similar between the two treatment strategies (OR 1.00, 95% CI 0.53 to 1.88) (Figure S4).
4. DISCUSSION
This is the first meta‐analysis comparing the two treatment strategies of IABP inserted before and after primary PCI in patients with AMI complicated by CS. Our data suggest that the timing of initiation of IABP therapy does not have an effect on short‐term and long‐term survival in this population. Besides, the risks of reinfarction, repeat revascularization, stroke, acute renal failure, and major bleeding were similar between the two groups.
Since 1968, the IABP has been used for mechanical cardiac assistance in patients with CS.29 In theory, the deflation during systole reduces ventricular afterload and helps the ventricle push blood into the aorta, while the inflation during diastole enhances coronary artery perfusion and promotes blood flow to systemic organs.5 Based on pathophysiological considerations and benefits observed in nonrandomized studies in the pre‐PCI era, previous American Heart Association/American College of Cardiology and European Society of Cardiology guidelines gave the use of IABP a class I recommendation for the management of AMI patients with CS. Nevertheless, the results of recent meta‐analyses and the landmark intraaortic balloon pump in cardiogenic shock II (IABP‐SHOCK II) trial have cast doubt on the efficacy of IABP because IABP support does not reduce short‐term and long‐term mortality in patients with AMI complicated by CS.8, 9, 10, 11 Although the beneficial effect of IABP therapy on hemodynamic parameters has not translated to a beneficial effect on mortality in these studies, this result may be affected by multiple other factors. For example, 10%‐30% patients with CS in the non‐IABP group received emergency IABP insertion, and the frequent crossover in the randomized controlled trials definitely had an impact on the results according to the intention‐to‐treat principle.11, 30 In addition, only 13.4% patients in the IABP group inserted the balloon pump before revascularization in the IABP‐SHOCK II trial, and the timing of initiation of IABP therapy might be also of great importance in this setting.11
Over the last decade, the debate about the timing of IABP insertion has never stopped, and clinical trials have produced conflicting results. Previous experimental study with animal models of ischemia‐reperfusion demonstrated that unloading the left ventricle with IABP prior to revascularization might provide an additional infarct size reduction. 31, 32 Thereafter, a small population study with 48 patients reported that patients who underwent primary PCI assisted by IABP had a more favorable in‐hospital survival rate than those who received IABP therapy after primary PCI.21 Contrariwise, Cheng et al. (n = 173) found that IABP insertion before PCI was associated with a larger infarct size, and no difference was found between the two strategies regarding short‐term and long‐term mortality.23 Considering the small sample size of the studies and the controversial results, pooled analysis of the individual data may be informative.
The principal finding of this study is that the timing of IABP insertion that is, before or after primary PCI does not have an effect on the short‐term and long‐term mortality in patients with AMI complicated by CS. It is believed that the early initiation of IABP therapy improves myocardial perfusion and results in significant myocardial salvage than reperfusion alone.33 More importantly, hemodynamic stabilization in the setting of cardiogenic shock can prevent the relevant multi‐organ dysfunction or failure.34 One possible explanation is that the advantages of early initiation of IABP support are offset by the delay in revascularization associated with the time needed for IABP insertion.23 In patients with AMI treated with primary PCI, time to reperfusion determines the extent of reversible and irreversible myocardial injury. However, most of the included studies did not report the data of time delay or door‐to‐balloon time. The study conducted by Yuan et al. indicated an additional delay of 14 minutes in STEMI patients who received IABP therapy before primary PCI compared to those who underwent primary PCI alone (P = .04). 27 The study by Abdel‐Wahab et al. found a 35‐minute delay in patients who inserted IABP before primary PCI even without statistical difference.21 Although the delay is short, it can still cause greater damage to the microcirculation and myocardium. Therefore, this increased the incidence of major adverse cardiac events, especially in the early stage of AMI.35, 36 Notably, most of the studies eligible for this meta‐analysis are retrospective observational studies rather than RCTs, and baseline characteristics like dyslipidemia are not comparable between the two groups. Thus, any comparison in outcomes among patients with AMI complicated by CS undergoing IABP insertion before vs after primary PCI is subject to significant confounding. Moreover, the unmeasured confounders between the two mechanical circulatory support strategies might have a significant impact on the results, due to lack of detailed reporting of patient characteristics.
4.1. Limitations
Our meta‐analysis presents several limitations that merit attention. First, observational studies were mainly included in our meta‐analysis due to lack of randomized data. This introduced intense heterogeneity and potential bias. Hence, the findings of the present study should be interpreted as hypothesis‐generating only, and could not be overstated. The random‐effect model was used to account for the heterogeneity. Although sensitivity analysis with multivariable‐adjusted data was performed, the potential bias cannot be completely eliminated. Furthermore, meta‐regression analysis found that short‐term mortality might be interfered by baseline characteristics of diabetes mellitus and hypertension. Second, patients with STEMI and non‐STEMI were both enrolled in our meta‐analysis. In this case, sensitivity analysis was conducted by analyzing the results of studies that enrolled patients with STEMI exclusively, and the results were in line with the overall population. Third, data about the time needed for IABP insertion or door‐to‐balloon time were not available in most of the studies. Finally, most of the eligible studies reported in‐hospital or 30‐day mortality, and long‐term data with more than 6 months were limited.
5. CONCLUSIONS
In patients with AMI complicated by CS undergoing primary PCI, the timing of initiation of IABP therapy does not appear to impact short‐term and long‐term clinical outcomes. However, this result should be interpreted with caution based on observational data. Appropriately, powered randomized trials are warranted to investigate the relative benefit of the two strategies, that is, IABP inserted before or after primary PCI in the future.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Supporting information
Figure S1 Study selection flow diagram.
Figure S2 Separate analysis of studies that enrolled patients with ST‐segment elevation myocardial infarction.
Figure S3 Separate analysis of studies that published in full text.
Figure S4 Funnel plot for (A) detection of publication bias and (B) Trim‐and‐Fill correction for publication bias for major bleeding.
Table S1 The adjusted variables in each study.
Table S2 Quality assessment of observational studies.
Table S3 Quality assessment of randomized controlled trials.
Cui K, Lyu S, Liu H, et al. Timing of initiation of intra‐aortic balloon pump in patients with acute myocardial infarction complicated by cardiogenic shock: A meta‐analysis. Clin Cardiol. 2019;42:1126–1134. 10.1002/clc.23264
Funding information Beijing Lab for Cardiovascular Precision Medicine, Beijing, China, Grant/Award Number: PXM2019_014226_000023; Ministry of Science and Technology of the People's Republic of China, State Science and Technology Support Program, Grant/Award Number: 2011BAI11B05
REFERENCES
- 1. Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448‐454. [DOI] [PubMed] [Google Scholar]
- 2. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization and long‐term survival in cardiogenic shock complicating acute myocardial infarction. JAMA. 2006;295(21):2511‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schumann J, Henrich EC, Strobl H, et al. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev. 2018;1:CD009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah AH, Puri R, Kalra A. Management of cardiogenic shock complicating acute myocardial infarction: a review. Clin Cardiol. 2019;42(4):484‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheidt S, Wilner G, Mueller H, et al. Intra‐aortic balloon counterpulsation in cardiogenic shock. Report of a co‐operative clinical trial. N Engl J Med. 1973;288(19):979‐984. [DOI] [PubMed] [Google Scholar]
- 6. Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rios SA, Bravo CA, Weinreich M, et al. Meta‐analysis and trial sequential analysis comparing percutaneous ventricular assist devices versus intra‐aortic balloon pump during high‐risk percutaneous coronary intervention or cardiogenic shock. Am J Cardiol. 2018;122(8):1330‐1338. [DOI] [PubMed] [Google Scholar]
- 8. Ahmad Y, Sen S, Shun‐Shin MJ, et al. Intra‐aortic balloon pump therapy for acute myocardial infarction: a meta‐analysis. JAMA Intern Med. 2015;175(6):931‐939. [DOI] [PubMed] [Google Scholar]
- 9. Unverzagt S, Buerke M, de Waha A, et al. Intra‐aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;3:CD007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiele H, Zeymer U, Thelemann N, et al. Intraaortic balloon pump in cardiogenic shock complicating acute myocardial infarction: long‐term 6‐year outcome of the randomized IABP‐SHOCK II trial. Circulation. 2018;139:395‐403. 10.1161/CIRCULATIONAHA.118.038201. [DOI] [PubMed] [Google Scholar]
- 11. Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287‐1296. [DOI] [PubMed] [Google Scholar]
- 12. Neumann FJ, Sousa‐Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87‐165. [DOI] [PubMed] [Google Scholar]
- 13. O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;127(4):e362‐e425. [DOI] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25(9):603‐605. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra‐aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276‐1283. [DOI] [PubMed] [Google Scholar]
- 21. Abdel‐Wahab M, Saad M, Kynast J, et al. Comparison of hospital mortality with intra‐aortic balloon counterpulsation insertion before versus after primary percutaneous coronary intervention for cardiogenic shock complicating acute myocardial infarction. Am J Cardiol. 2010;105(7):967‐971. [DOI] [PubMed] [Google Scholar]
- 22. Sjauw KD, Engstrom AE, Vis MM, et al. Efficacy and timing of intra‐aortic counterpulsation in patients with ST‐elevation myocardial infarction complicated by cardiogenic shock. Neth Hear J. 2012;20(10):402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng JM, van Leeuwen MA, de Boer SP, et al. Impact of intra‐aortic balloon pump support initiated before versus after primary percutaneous coronary intervention in patients with cardiogenic shock from acute myocardial infarction. Int J Cardiol. 2013;168(4):3758‐3763. [DOI] [PubMed] [Google Scholar]
- 24. Bergh N, Angerås O, Albertsson P, et al. Does the timing of treatment with intra‐aortic balloon counterpulsation in cardiogenic shock due to ST‐elevation myocardial infarction affect survival? Acute Card Care. 2014;16(2):57‐62. [DOI] [PubMed] [Google Scholar]
- 25. Negi SI, Anand A, Briceno‐Gomez D, et al. Timing of intra‐aortic balloon therapy in ST elevation myocardial infarction. J Am Coll Cardiol. 2014;64(11):B21‐B22. [Google Scholar]
- 26. Schwarz B, Abdel‐Wahab M, Robinson DR, Richardt G. Predictors of mortality in patients with cardiogenic shock treated with primary percutaneous coronary intervention and intra‐aortic balloon counterpulsation. Med Klin Intensivmed Notfmed. 2016;111(8):715‐722. [DOI] [PubMed] [Google Scholar]
- 27. Yuan L, Nie SP. Efficacy of intra‐aortic balloon pump before versus after primary percutaneous coronary intervention in patients with cardiogenic Shock from ST‐elevation myocardial infarction. Chin Med J. 2016;129(12):1400‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuernau G, Ledwoch J, Eitel I, et al. Impact of timing of intra‐aortic balloon counterpulsation on mortality in cardiogenic shock: a sub‐analysis of the IABP‐shock II‐trial. J Am Coll Cardiol. 2017;69(11):1182. [Google Scholar]
- 29. Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL Jr. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA. 1968;203(2):113‐118. [PubMed] [Google Scholar]
- 30. Ohman EM, Nanas J, Stomel RJ, et al. Thrombolysis and counterpulsation to improve survival in myocardial infarction complicated by hypotension and suspected cardiogenic shock or heart failure: results of the TACTICS trial. J Thromb Thrombolysis. 2005;19(1):33‐39. [DOI] [PubMed] [Google Scholar]
- 31. Achour H, Boccalandro F, Felli P, et al. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter Cardiovasc Interv. 2005;64(2):182‐192. [DOI] [PubMed] [Google Scholar]
- 32. LeDoux JF, Tamareille S, Felli PR, Amirian J, Smalling RW. Left ventricular unloading with intra‐aortic counter pulsation prior to reperfusion reduces myocardial release of endothelin‐1 and decreases infarction size in a porcine ischemia‐reperfusion model. Catheter Cardiovasc Interv. 2008;72(4):513‐521. [DOI] [PubMed] [Google Scholar]
- 33. Smalling RW, Cassidy DB, Barrett R, Lachterman B, Felli P, Amirian J. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial‐flow, transvalvular left ventricular assist device. A comparison with intra‐aortic balloon counterpulsation and reperfusion alone in a canine infarction model. Circulation. 1992;85(3):1152‐1159. [DOI] [PubMed] [Google Scholar]
- 34. Prondzinsky R, Lemm H, Swyter M, et al. Intra‐aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152‐160. [DOI] [PubMed] [Google Scholar]
- 35. Francone M, Bucciarelli‐Ducci C, Carbone I, et al. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST‐segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54(23):2145‐2153. [DOI] [PubMed] [Google Scholar]
- 36. Prasad A, Gersh BJ, Mehran R, et al. Effect of ischemia duration and door‐to‐balloon time on myocardial perfusion in ST‐segment elevation myocardial infarction: an analysis from HORIZONS‐AMI trial (harmonizing outcomes with revascularization and stents in acute myocardial infarction). JACC Cardiovasc Interv. 2015;8(15):1966‐1974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study selection flow diagram.
Figure S2 Separate analysis of studies that enrolled patients with ST‐segment elevation myocardial infarction.
Figure S3 Separate analysis of studies that published in full text.
Figure S4 Funnel plot for (A) detection of publication bias and (B) Trim‐and‐Fill correction for publication bias for major bleeding.
Table S1 The adjusted variables in each study.
Table S2 Quality assessment of observational studies.
Table S3 Quality assessment of randomized controlled trials.


