Abstract
Objective
The aim of the present review is to evaluate effects of intermittent hypoxia and exercise therapy in cardiometabolic parameters on adult obese people.
Database
Three well-known databases were selected: EMBASE, MEDLINE and Web of Science. Studies selection: Inclusion criteria were: (a) human healthy overweight or obese adults, (b) study randomized controlled trial, (c) original experimental study, (d) English languages and (e) therapy with intermittent hypoxia and exercise.
Design
The assessment of the methodological quality of each study was based upon the risk of bias (PEDro scale) and level of evidence (CBO Guidelines). Data extraction: five articles clearly met inclusion criteria and were reviewed to data extraction.
Results
In the hypoxia groups, weight, body mass index, waist circumference, waist–hip ratio, fat mass and lean mass improved in at least two studies in comparison with the baseline. Systolic blood pressure improved in one study. The lipid profile and the aerobic capacity were not reduced significantly.
Conclusions
Results suggest that combined hypoxia with exercise may help to improve cardiometabolic parameters in obese people.
Keywords: Exercise, Environmental physiology, Obesity, Hypoxia and women
Resumen
Objetivo
El objetivo de esta revisión es evaluar el efecto de la hipoxia intermitente y el ejercicio en parámetros cardiovasculares en adultos obesos.
Fuentes de datos
Se utilizaron 3 bases de datos para seleccionar los artículos: EMBASE, MEDLINE and Web of Science. Selección de estudios: los criterios de inclusión fueron: a) muestra compuesta por humanos adultos con sobrepeso u obesidad, b) grupos organizados aleatoriamente, c) estudios experimentales, d) escritos en inglés y e) la intervención incluya hipoxia intermitente y ejercicio.
Diseño
Para evaluar la calidad de los estudios se utilizó la escala PEDro y el nivel de evidencia con la guía CBO. Extracción de datos: 5 artículos cumplieron los criterios de inclusión y sus datos fueron extraídos y revisados.
Resultados
El peso, el índice de masa corporal, la circunferencia de la cadera, la circunferencia de la cintura, el índice cintura-cadera, la masa grasa y la masa muscular mejoraron en al menos 2 estudios comparados con la línea base en los grupos hipoxia. La presión sistólica mejoró en uno de los estudios. El perfil lipídico y la capacidad aeróbica no mejoraron significativamente.
Conclusiones
Los resultados sugieren que combinar ejercicio con hipoxia intermitente podría mejorar parámetros cardiovasculares en la población obesa.
Palabras clave: Ejercicio, Fisiología, Obesidad, Hipoxia y mujer
Introduction
The obesity epidemic continues to spread. In recent years, with the economic crisis, rates of obesity and the overweight have grown in countries like Spain, Korea, Canada and France. It is estimated that this epidemic is responsible for about 3% of total health costs. This cost will increase in coming years.1 People with excess of body weight have a higher risk of premature avoidable death.2, 3 In addition, obesity is the main factor responsible for appearance of others chronic diseases2 like diabetes type 2, ischemic heart disease or hypertension,4 causing 80%, 35% and 55% of these respectively in Europe.1
The main factors that provoke body weight excess are overeating and a low physical activity habit, which lead to a positive energy balance, and result in a body fat increase.4 The most common treatment guidelines to reduce body weight are hypocaloric diets, physical activity aerobic programs and behavioral psychotherapy.5 However, these treatments are ineffective in the long-term because after sixth months they produce a plateau in the body weight or even a recovery of lost weight.6 The low adherence to physical activity is one of the more common factors that provoke this situation.7 New treatments are necessary to improve this situation.
Although the hypoxic exposure has been more common in the field of sports performance, it may be an alternative treatment to medication with positive effects against diseases like obesity. Recent studies in this topic have led to significant differences between exposure to intermittent hypoxia (IH) and chronic hypoxia (CH) exposure. Mechanisms of physiological response to CH are different to mechanisms of physiological response to IH.8 Adipose tissue of obese subjects is poorly oxygenated.9, 10 This hypoxia in the adipose tissue is a triggering factor for inflammation-related events in obesity11: adipocyte apoptosis and macrophage infiltration, or adipokines dysfunction such as adiponectin reduction that may affect insulin sensitivity.12 Chronic hypoxia exposure may increase systemic inflammation and produce other negative effects in obese patients as increase hypercholesterolemia or blood pressure.13 But paradoxically, exposure to intermittent hypoxia may be a means to lose body mass or to improve metabolic risk factors.14 Mechanisms that explain these changes are related with disturbance of the energy balance: reduction of nutritional energy intake, a reduction of intestinal energy uptake and increased energy expenditure as a result of change of ATP metabolism.14 In this sense, intermittent hypoxia could be useful in weight homeostasis by reducing fat deposition.15
Therefore, it is logical to think that hypoxic stimulus in addition to diet and exercise can be an interesting approach to lose weight, by metabolic changes that affect to energy balance. This new strategy may be useful and practical for clinical applications in obese patients.
In spite of different studies analyze the effect of combined intermittent hypoxia with diet and exercise, to our knowledge there are no previous publications that review this issue. The aim of the present review is to evaluate effects of intermittent hypoxia and exercise therapy in cardiometabolic parameters on adult obese people.
Methods
This systematic review has been produced according to PRISMA (Preferred Reporting Items for Systematic Reviews and MetaAnalyses) guidelines.16
Data sources
Three well-known databases were selected to perform a systematic hand review: EMBASE, MEDLINE (via Ovid) and Web of Science. Experts in the field of Physiology controlled all activities. The keywords used to locate the articles were: “hypoxi*”, “exercise or training” and “obes*”. These words appeared in the title or abstract. The Boolean operators “AND” and “OR” were used. Year of publication was taken as 2005 to 2015 were delimited as filter of the search. The search was finalized on the 26th of July 2015. All references located were imported into EndNote X6.
Data extraction
Inclusion criteria were: (a) human healthy overweight or obese adults (body mass index (BMI) >27 kg/m2), (b) study randomized controlled trial, (c) original experimental study, (d) English languages articles and (e) therapy with intermittent hypoxia and exercise. Studies were only presented once as a summary at a conference, congress or seminar were excluded of this systematic review.
Study selection
The principal investigator removed duplicated articles. Two independent authors (ACC and MCC) selected the eligible articles based on title and abstract. The full text of references identified from the previous process were obtained and assessed independently by two reviewers (ACC and MCC) using the inclusion and exclusion criteria. Disagreements were resolved by consensus between two investigators after discussion. The most common reasons for the removal of papers were that the participants were not human healthy overweight or obese adults.
Assessing the Risk of Bias and determining of Level of Evidence
The assessment of the methodological quality of each study was based upon the risk of bias and level of evidence. Risk of Bias refers to internal and external validity of the studies. PEDro scale was used to measure the risk of bias,17 which is a validated scale for physical therapist interventions.18 Both reviewers of this systematic review completed an online training program to use PEDro scale before of evaluate the risk of bias in the selected articles of this study (http://www.pedro.org.au/). However, this scale does not provide evidence that the treatment is clinically useful. For this reason it is necessary to determining the level of evidence. Guidelines of the Dutch Institute for Healthcare Improvement (CBO) were used to evaluate the Level of Evidence.19
Data collection process
In the set of included studies, data extraction was completed by the principal author (ACC) using a standard table to collect information for descriptive purposes. And other author checked this process (DB). The following data were extracted: (a) author and year of publication, (b) sample characteristics: total sample size, age (mean and SD) and BMI (mean and SD), (c) design: group(s), intervention (sessions, weeks, frequency), hypoxia treatment (FiO2 and altitude meters) and exercise treatment (intensity, effort: recovery and equipment) and (d) outcome measures: instrument, outcome measure with level of evidence and reported effects (results in analyses intra group and/or between group).
Results
Literature search
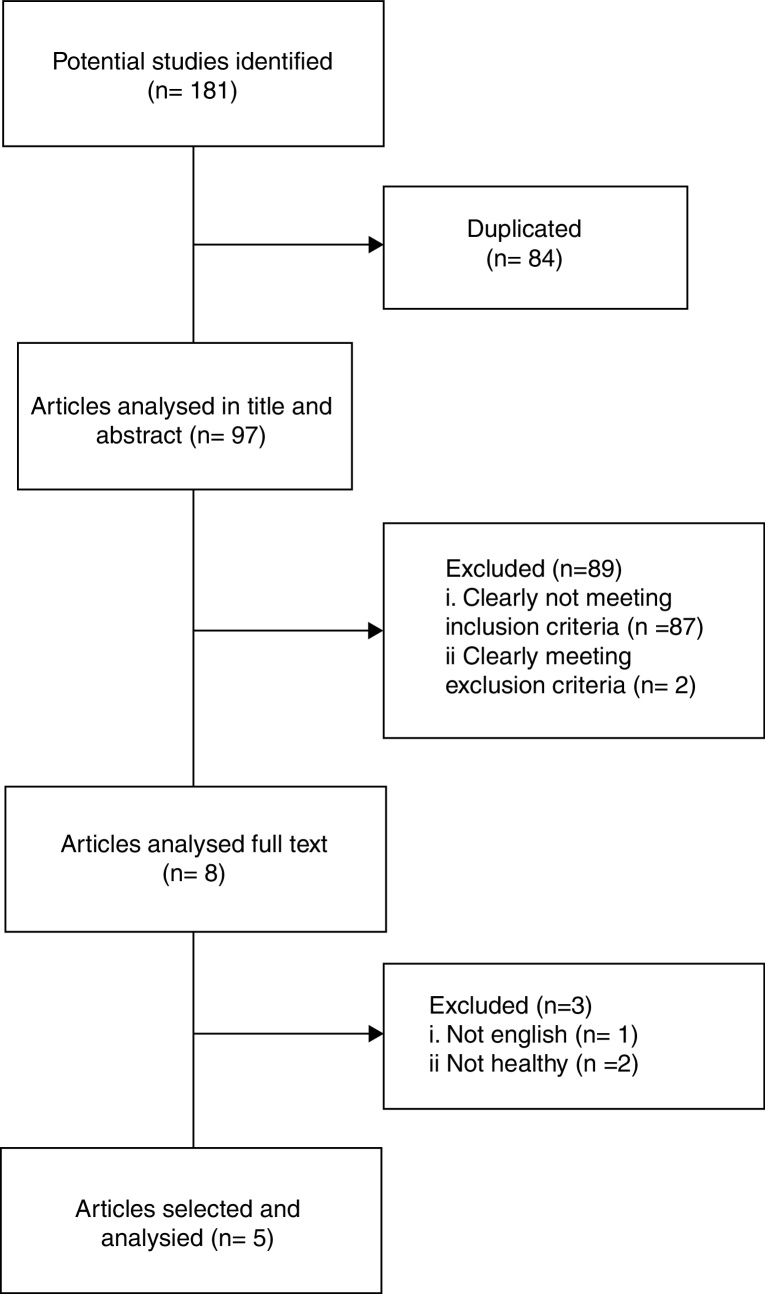
A total of 181 references were located from the three databases: 151 papers from EMBASE and MEDLINE (via Ovid) and 30 papers from Web of Science. After deleting the duplicate papers (n = 84), 97 articles of records were reviewed. Of these articles, 5 articles clearly met inclusion criteria and were reviewed to data extraction. Sample characteristics, intervention design and outcome measures of the five studies are showed in Table 1.
Table 1.
Sample characteristics, design and outcomes measures of the included studies.
| Ref | Sample size (n) | Age (mean ± SD) | Group | Design | Hypoxia treatment | Exercise treatment | Outcome (level of evidence) | Report effect |
|---|---|---|---|---|---|---|---|---|
| 22 | 32* | 52.4 ± 7.9 | Hypoxia group | 26 weeks Twice a week |
90’; FiO2 = 14.0+ 90’; FiO2 = 12.2 |
90’ (65–70% HRmax) Cycle ergometer, treadmill or cross trainer. |
Weight (2) BMI (2) Waist circumference (2) Hip circumference (3) WHR (2) Total body fat (2) Total muscle mass (2) [Glucose] (3) Hemoglobin (2) Lipid profile (2) Systolic blood pressure (2) Diastolic blood pressure (2) Peak power output (3) Peak power output per kg (3) Peak oxygen uptake (3) Peak oxygen uptake per kg (2) Peak heart rate (3) |
Δ# Δ Δ# # = = = = = = = = = # # ↑ ↑ |
| 50.3 ± 10.3 | Control group | Sea level | 90’ (65–70% HRmax) Cycle ergometer, treadmill or cross trainer. |
|||||
| 15 | 22* | 22.3 ± 1.7 | Normoxia training | 4 weeks 14 sessions per week |
Sea level | 120’ (60–70%HRmax) 40–50% 1RM; 3x(15–20): 2–3’ recovery. |
Weight (2) BMI (2) WHR (2) Total body fat (2) Total muscle mass (2) Water (3) Bone mineral content (3) Systolic blood pressure (2) Diastolic blood pressure (2) Mean blood pressure (3) Heart rate resting (2) Braquial-ankle pulse wave velocity (3) Ankle brachial index (3) |
# ↑ Δ Δ# Δ# = = = Δ = = # = = |
| 19.8 ± 2.2 | Hypoxia training | 4 weeks 14 sessions per week |
8 sessions (sea level) 3 sessions (FiO2 = 16.4–14.5%) |
120’ (60–70%HRmax) 40–50% 1RM; 3x(15–20): 2–3’ recovery. |
||||
| 23 | 32* | 29 ± 2 | Lean Group | 1 session | Hypoxia: FiO2 = 10% Normoxia: sea level. |
Resting 20% 1RM 4x(3.5’: 10’) Biceps exercise. 30% 1RM 4x(3.5’: 10’) Biceps exercise. |
Total blood pressure (3) Artery diameter (3) Breathing frequency (3) Heart rate resting (2) SpO2 (3) |
Δ # ↑MS ↑MS = ↑MS↑Ob = |
| 24 ± 2 | Obese group | Hypoxia: FiO2 = 10% Normoxia: sea level. |
Resting 20% 1RM 4× (3.5’: 10’) Biceps exercise. 30% 1RM 4x(3.5’: 10’) Biceps exercise. |
Ventilation (3) Tidal volume (3) End-tidal (3) Blood flow (3) Conductance (3) PvO2 (3) PvCO2 (3) Venous Ph (3) |
Δ # ↑MS = = ↑MS ↓Ob ↑MS ↓Ob Δ # Δ # Δ # |
|||
| 29 ± 3 | MS group | Hypoxia: FiO2 = 10% Normoxia: sea level. |
||||||
| 20 | 20* | 50.1 ± NR | Hypoxia training | 8 weeks 3 times per week |
FiO2 = 15.0% | 90’ (60% VO2max) Stepper, treadmill or bicycle ergometer. |
Weight (2) BMI (2) Hemoglobin (2) Lipid profile (2) |
↑ = = = |
| 45.5 ± NR | Sham hypoxia training | FiO2 = 20.9% | ||||||
| 21 | 45* | 42 ± 7.1 | Hypoxia group | 4 weeks 3 times per week |
FiO2 = 15.0% | 60’ (60% VO2max) Treadmill |
Weight (2) BMI (2) Waist circumference (2) Total body fat (2) Total muscle mass (2) Lipid profile (2) Peak oxygen uptake per kg (2) Time to exhaustion (3) Respiratory quotient Lactate maximal Lactate in anaerobic threshold (3) Systolic blood pressure (2) Diastolic blood pressure (2) Fasting insulin (3) HOMA index (3) |
= = Δ ↑ Δ = = = Δ = Δ = = Δ # Δ # |
| Control group | FiO2 = 20.9% |
CG: control group; EG: experimental group; NR: not reported; MS: metabolic syndrome; Ob: obese; *: n for treatment effects analysis.
=: no significant difference relative to baseline and/or the control group; ↑: statistically significant improvements in the experimental group relative to the control group; ↓: statistically significant deterioration in the experimental group relative to the control group; Δ: statistically significant improvements in the experimental group; #: statistically significant improvements in the control group.
Flowchart
Quality methodological analysis
The final score in the PEDro scale ranged from 4 to 6 points (the maximum score was 10 points). The average (SD) was 4.4 (0.89). The highest scores were obtained in creating random groups (PEDro criterion 2) and analysis of the data (PEDro criterion 10 and criterion 11) where all 5 articles satisfied these questions. The lowest scores were obtained by concealed allocation of the groups (PEDro criterion 3), blinding of the therapists (PEDro criterion 6) and intention-to-treat analysis (PEDro criterion 9).
All 5 papers had B level of evidence because all were comparative trials, but none of them were a comparative double-blind study. With regard to the level of conclusion of outcomes measure, the scores ranged from 2 to 3. The score was 2 when this variable was measured in at least 2 articles and the score was 3 when this variable only was measured in one study.
Sample characteristics and design treatment
The total sample size ranged from 2020 to 4521 for treatment effects analysis, divided in two20, 21, 22, 15 or three groups.23 The follow-up age ranged from 19.8 and 52.4 years, being the mean of 31 year (7.7). With respect to BMI all the participants of the sample were obese or overweight people (BMI >27 kg/m2).
In four papers of all, sessions of treatment performed ranged from 1221 to 56.15 A study realized only 1 session, measuring before and after of different exercises. In two of the five articles, 4 weeks of therapy were performed.21, 15 Other two performed more than 4 weeks: 8 weeks20 or 26 weeks.22 Weekly the frequency ranged from 222 to 14 sessions.15
All studies had a normoxia group that performed exercise to sea level (FiO2 = 20.9%). Lower FiO2 was of 10%, but this participants performed only one session.23 Higher partial pressure of oxygen was of 16.4%,15 corresponding with 2000 m of altitude. The FiO2 average was 13.5% (2.38). Normobaric hypoxia was simulated in all studies with hypoxia chamber, hypoxia room or with nose chip and mouthpiece.
Three of the five treatments performed aerobic exercise,20, 21, 22 one performed only strength training23 and the other study mixed training of aerobic exercise and training of strength exercise.15 Intensity of the aerobic training ranged from 60% to 70% maximum heart rate. Duration average of this exercise was 81 min (29.24). Participants performed the exercise in cycle ergometer, treadmill, cross trainer or stepper. Intensity average of the strength training was 35% 1RM, performing 3–4 bouts with dumbbells. One of the studies15 performed 15–20 repetitions with 2–3 min of recovery and the other,23 3.5 min of work and 10 min of recovery.
Key measurements and effects
The measurements with the highest Level of Conclusion (CBO Guidelines)19 were body weight, body mass index, waist circumference, waist–hip rate (WHR), total body fat, total muscle mass, hemoglobin, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, triglycerides, systolic blood pressure (SBP), diastolic blood pressure (DBP), maximal oxygen uptake (VO2max) and resting heart rate (HRres).
Medical scales were used to measure anthropometric parameters. Weight and BMI was evaluated in four studies.20, 21, 22, 15 Weight improved in three studies in compared with the baseline20, 22, 15 in the hypoxia groups. BMI improved in two articles compared with the baseline in the hypoxia group.22, 15 Besides, these parameters improved in compared with the normoxia group in one study.15 Waist circumference and WHR were evaluated in two studies. While waist circumference improved respect to baseline in both,21, 22 WHR only improved in one of them15 but, this improvements too appeared in the normoxia group. Body composition measurements were fat mass (improved in two21, 15 of three studies) and fat-free mass (improved in one21 of three papers). One study15 assessed the water and bone mineral contents but these were not improved in any case. Bioelectrical impedance system was used to evaluate the body composition.
Blood pressure was assessed in all case with a blood pressure cuff. Diastolic blood pressure was evaluated in three21, 22, 15 studies. This was not improved in any case. Systolic blood pressure too was evaluated after of three21, 22, 15 different treatments but only in one case improved.15 Heart rate was evaluated in resting and was measured in two papers. In one of them this parameters increased in the control group and not developed change in the hypoxia group.15 HRres was higher in obese adults compared with lean adults.23 Also heart rate in resting is higher in hypoxia exposure than normoxia in obese with metabolic syndrome.23
LDL, HDL, total cholesterol and triglycerides were evaluated with venous or capillary blood sample. None of these parameters developed change after of three different treatments with hypoxia and aerobic exercise.20, 21, 22 Maximal oxygen uptake was the ventilator parameters with higher score in the level of evidence because was evaluated in a study where improved compared with the normoxia group22 and in other paper where was not developed changes after of 12 sessions of aerobic training.21 An incremental exercise test was used to evaluate VO2max.
Discussion
This review expected to evaluate effects of intermittent hypoxia and exercise therapy in cardiometabolic parameters on adult obese people. In the hypoxia groups, weight, body mass index, waist circumference, waist–hip ratio, fat mass and lean mass improved in at least two studies in comparison with the baseline; and systolic blood pressure improved in one study. However, other cardiometabolic risk factor such as the lipid profile and the aerobic capacity were not reduced significantly.
Treatment's characteristics could be especially relevant in this sense. The majority of these articles performed an aerobic training program with exercise to moderate intensity. The number of sessions was very different between them, however the longest treatments were not the most effective. In fact, it has been suggested that after 3 months the adaptations to the same stimulus might be abolished and leads to a stabilization of the effects.22 In this sense, a study combines an aerobic training program with a strength training, i.e., different stimulus.15 This treatment obtained improvements in different cardiometabolic parameters despite being the highest number of treatment sessions. So, varied stimulus could be necessary in longest treatments to cause new adaptations in the organisms.
With respect to anthropometric parameters, the body weight was the variable that more improves with this therapy. Fat mass and lean mass, too, improve in some of these studies.21, 15 A definite reason why this weight loss occurs is still unknown. The mechanisms of hypoxia exposure that could explain these changes might be more energy expenditure,24 appetite suppression because of leptin levels increase25 and neuroendocrine responses to energy balance.14
Some studies reported changes on SBP following weight loss in active men.26 In this review only one study obtained improvements in this parameters after 56 sessions of training. These improvements may be attributed to that hypoxia induces the vasodilatation by increases of arteriolar diameter25 and decreased peripheral resistance in the systemic circulation.27
Our review found that hypoxic training was actually not able to reduce significantly the level of lipid parameters in obese subjects. The level of triglycerides should be reduced due to lipid oxidation. Hypoxia inducible factor is activated during exposure to hypoxia. This transcriptional factor activates the transcription of genes like PGC1α transcriptional, coactivator of lipid oxidation.28 Also, increased leptin levels lead to a reduced fat intake via food.29 However, these results did not obtain in these studies. This can be explained by several possible causes. Most likely, it seems that exercise regime could affect to the findings. In comparison with a moderate intensity, high intensity interval training burns more calories and increases post exercise fat oxidation and energy expenditure.30
Exercise intensity was too low to improve the VO2max. Higher exercise intensity seems to induce improvements in physical condition like aerobic capacity and reveal a cardioprotective effect.22 In the two papers where analyzed the aerobic capacity, subjects in hypoxic conditions improved the VO2max but none of the effects reached statistical significance.
Did not appear differences between normoxia groups and hypoxia groups in the majority of the variables (only body weight in some cases). However, it has been mentioned that if exercise is at the same relative intensity in hypoxia and normoxia, the mechanical strain is reduced under hypoxia. Similar effects obtained could be particularly important for the obese patient as who cannot attain a large workload because of orthopedic problems.21
Some limitations should be considered. Firstly, in four of the all studies the investigators did not control the food intake and physical activity. Some alterations might have occurred outside the study protocol and produce effects in the results. By other hand, size sample was not big enough to show a reduction in some variables. Time restrictions and willingness are common problems in weight loss protocols with obese people. Finally, the studies analyzed could result heterogeneous respect to the exercise protocol, hypoxic dose or outcomes measured. In this way, there are difficulties to establish consistent results.
In conclusion, the results are inconsistent, but suggest that combined intermittent hypoxia with exercise may help to improve cardiometabolic parameters in obese people. Define optimal dose of hypoxia and intensity exercise are necessary in future investigations.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This research was funding by Junta de Extremadura: GAEDAF Research Group (GR15020) and Ministerio de Educación, Cultura y Deporte (FPU15/00450).
References
- 1.WHO . WHO; Copenhagen: 2008. The challenge of obesity in the WHO European region and the strategies for response. [Google Scholar]
- 2.Barroso M., Goday A., Ramos R., Marin-Ibanez A., Guembe M.J., Rigo F. Interaction between cardiovascular risk factors and body mass index and 10-year incidence of cardiovascular disease, cancer death, and overall mortality. Prev Med. 2018;107:81–89. doi: 10.1016/j.ypmed.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Banegas J.R., Lopez-Garcia E., Gutierrez-Fisac J.L., Guallar-Castillon P., Rodriguez-Artalejo F. A simple estimate of mortality attributable to excess weight in the European Union. Eur J Clin Nutr. 2003;57:201–208. doi: 10.1038/sj.ejcn.1601538. [DOI] [PubMed] [Google Scholar]
- 4.Misra A., Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 5.Sarwer D.B., Von-Sydow G.A., Vetter M.L., Wadden T.A. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diab Obes. 2009;16:347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 6.Dansinger M.L., Gleason J.A., Griffith J.L., Selker H.P., Schaefer E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Avenell A., Grant A., McGee M., McPherson G., Campbell M., McGee M. The effects of an open design on trial participant recruitment, compliance and retention a randomized controlled trial comparison with a blinded, placebo-controlled design. Clin Trials. 2004;6:490–498. doi: 10.1191/1740774504cn053oa. [DOI] [PubMed] [Google Scholar]
- 8.Powell F.L., Garcia N. Physiological effects of intermittent hypoxia. High Alt Med Biol. 2000;1:125–136. doi: 10.1089/15270290050074279. [DOI] [PubMed] [Google Scholar]
- 9.Fleischmann E., Kurz A., Niedermayr M., Schebesta K., Kimberger O., Sessler D.I. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg. 2005;15:813–819. doi: 10.1381/0960892054222867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabon B., Nagele A., Reddy D., Eagon C., Fleshman J.W., Sessler D.I. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trayhurn P., Wood I.S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 12.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarrete-Opazo A., Mitchell G.S. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1181–R1197. doi: 10.1152/ajpregu.00208.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayser B., Verges S. Hypoxia, energy balance and obesity: from pathophysiological mechanisms to new treatment strategies. Obes Rev. 2013;14:579–592. doi: 10.1111/obr.12034. [DOI] [PubMed] [Google Scholar]
- 15.Kong Z., Zang Y., Hu Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. 2013;18:591–597. doi: 10.1007/s11325-013-0922-4. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 18.Collado-Mateo D., Adsuar J., Olivares P.R., Del Pozo-Cruz B., Parraca J.A., Del pozo-Cruz J. Effects of whole-body vibration therapy in patients with fibromyalgia: a systematic literature review. Evid-Based Complem Alternat Med. 2015 doi: 10.1155/2015/719082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbrand K., Van Croonenborg J., Wittenberg J. Guideline development. Stud Health Technol Inform. 2008;139:3–21. [PubMed] [Google Scholar]
- 20.Netzer N.C., Chytra R., Kuepper T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. 2008;12:129–134. doi: 10.1007/s11325-007-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiesner S., Haufe S., Engeli S., Mutschler H., Haas U., Luft F.C. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity (Silver Spring, MD) 2009;18:116–120. doi: 10.1038/oby.2009.193. [DOI] [PubMed] [Google Scholar]
- 22.Gatterer H., Haacke S., Burtscher M., Faulhaber M., Melmer A., Ebenbichler C. Normobaric intermittent hypoxia over 8 months does not reduce body weight and metabolic risk factors – a randomized, single blind, placebo-controlled study in normobaric hypoxia and normobaric sham hypoxia. Obes Facts. 2015;8:200–209. doi: 10.1159/000431157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limberg J.K., Evans T., Blain G., Zillner C., Proctor L., Sebranek J. Hypoxic exercise responses in lean and obese humans. Eur J Appl Physiol. 2012:24. [Google Scholar]
- 24.Lippl F.J., Neubauer S., Schipfer S., Lichter N., Tufman A., Otto B. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity (Silver Spring) 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- 25.Urdampilleta A., Gonzalez-Muniesa P., Portillo M.P., Martinez J.A. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J Physiol Biochem. 2012;68:289–304. doi: 10.1007/s13105-011-0115-1. [DOI] [PubMed] [Google Scholar]
- 26.Bailey D.M., Davies B., Baker J. Training in hypoxia: modulation of metabolic and cardiovascular risk factors in men. Med Sci Sports Exerc. 2000;32:1058–1066. doi: 10.1097/00005768-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ostadal B., Kolar F. Cardiac adaptation to chronic high-altitude hypoxia: beneficial and adverse effects. Respir Physiol Neurobiol. 2007;158:224–236. doi: 10.1016/j.resp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Zoll J., Ponsot E., Dufour S., Doutreleau S., Ventura-Clapier R., Vogt M. Exercise training in normobaric hypoxia in endurance runners III, muscular adjustments of selected gene transcripts. J Appl Physiol. 2006;100:1258–1266. doi: 10.1152/japplphysiol.00359.2005. [DOI] [PubMed] [Google Scholar]
- 29.Yingzhong Y., Droma Y., Rili G., Kubo K. Regulation of body weight by leptin, with special reference to hypoxia-induced regulation. Intern Med. 2006;45:941–946. doi: 10.2169/internalmedicine.45.1733. [DOI] [PubMed] [Google Scholar]
- 30.King J. East Tennessee State University; 2001. A comparison of the effects of interval training vs continuous training on weight loss and body composition in obese pre-menopausal women. [Google Scholar]