Abstract
Aims
Several studies and registries have demonstrated sustained reductions in blood pressure (BP) after renal denervation (RDN). The long-term safety and efficacy after RDN in real-world patients with uncontrolled hypertension, however, remains unknown. The objective of this study was to assess the long-term safety and efficacy of RDN, including its effects on renal function.
Methods and results
The Global SYMPLICITY Registry is a prospective, open-label registry conducted at 196 active sites worldwide in hypertensive patients receiving RDN treatment. Among 2237 patients enrolled and treated with the SYMPLICITY Flex catheter, 1742 were eligible for follow-up at 3 years. Baseline office and 24-h ambulatory systolic BP (SBP) were 166 ± 25 and 154 ± 18 mmHg, respectively. SBP reduction after RDN was sustained over 3 years, including decreases in both office (−16.5 ± 28.6 mmHg, P < 0.001) and 24-h ambulatory SBP (−8.0 ± 20.0 mmHg; P < 0.001). Twenty-one percent of patients had a baseline estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2. Between baseline and 3 years, renal function declined by 7.1 mL/min/1.73 m2 in patients without chronic kidney disease (CKD; eGFR ≥60 mL/min/1.73 m2; baseline eGFR 87 ± 17 mL/min/1.73 m2) and by 3.7 mL/min/1.73 m2 in patients with CKD (eGFR <60 mL/min/1.73 m2; baseline eGFR 47 ± 11 mL/min/1.73 m2). No long-term safety concerns were observed following the RDN procedure.
Conclusion
Long-term data from the Global SYMPLICITY Registry representing the largest available cohort of hypertensive patients receiving RDN in a real-world clinical setting demonstrate both the safety and efficacy of the procedure with significant and sustained office and ambulatory BP reductions out to 3 years.
Keywords: Denervation, Hypertension, Renal function, Ambulatory blood pressure monitoring, SYMPLICITY
Introduction
Activation of the sympathetic nervous system is a major mechanism in the pathogenesis of hypertension and associated comorbidities. Post-ganglionic efferent sympathetic nerve fibre activation causes increased renin release, tubular sodium reabsorption, and a reduction in renal blood flow.1 Afferent sympathetic nerve signalling arising from the kidneys are centrally integrated and result in increased sympathetic outflow to various target organs including the heart and the peripheral vasculature resulting in vasoconstriction further contributing to the rise in blood pressure (BP).2,3 Uncontrolled hypertension has been associated with progressive decline in renal function4 which has been inversely correlated to the level of muscle sympathetic nerve activity.5 The decline of renal function in hypertension varies widely depending on comorbidities and BP control6 as well as baseline estimated glomerular filtration rate (eGFR) with an average decline of 0.5 to 2.7 mL/min/1.73 m2 annually.7–9
Catheter-based renal denervation (RDN) is a treatment modality that specifically targets sympathetic overactivity commonly present in patients with uncontrolled hypertension. Indeed, RDN has been shown to reduce muscle sympathetic nerve activity,10,11 renal norepinephrine spillover,12 and BP in several patient cohorts with hypertension.13–15
The Global SYMPLICITY Registry is the largest data set of RDN-treated patients to date, which targets to include 3000 patients worldwide. The 6-month change in office and 24-h ambulatory systolic BP (SBP) for the first 1000 patients was −11.6 ± 25.3 and −6.6 ± 18.0 mmHg, respectively, for all patients (P < 0.001 for both) and −20.3 ± 22.8 and −8.9 ± 16.9 mmHg for those with severe hypertension (P < 0.001 for both).16 Small-scale trials have demonstrated sustained BP outcomes after RDN,17,18 but these trials were limited to relatively small sample sizes and not powered to evaluate long-term safety, including renal function. Furthermore, three very recent sham-controlled studies clearly demonstrated the BP lowering efficacy of RDN13–15; however, long-term data are not available. We, therefore, assessed the long-term effectiveness, safety, and effects on renal function in the Global SYMPLICITY Registry out to 3 years after RDN.
Methods
Trial design
The design and 6-month outcome data from the Global SYMPLICITY Registry (www.clinicaltrials.gov, NCT01534299) have been previously published.16,19 In brief, the prospective, open-label, single-arm, observational registry is enrolling patients with uncontrolled hypertension and/or conditions associated with sympathetic nervous system activation. The inclusion criteria are age of at least 18 years and eligibility for RDN as defined by local regulations. Patients are enrolled from a total of 196 active centres in Canada, Western Europe, Latin America, Eastern Europe, South Africa, Middle East, Asia, Australia, and New Zealand and treated with the SYMPLICITYTM renal denervation systems (Medtronic, Santa Rosa, CA, USA). The trial complies with the Declaration of Helsinki, locally appointed ethics committees approved the clinical protocol at each enrolling centre, and informed consent was obtained from all patients.
The primary objective is to assess procedural and long-term safety of RDN in a real-world setting, with recommended follow-up for 3 years. The trial procedures recommend three BP measurements at each office visit with the patient sitting quietly for at least 5 min with 1 min between each reading, and according to standard practice, 24-h ambulatory BP measurement in compliance with published guidelines.20,21 Before treatment and at each follow-up visit, investigators interviewed patients and documented any changes of prescribed antihypertensive medication class or dosage.
Definitions
Severe treatment-resistant hypertension was defined as office SBP ≥160 mmHg and 24-h ambulatory BP ≥135 mmHg, despite prescription of ≥3 antihypertensive medications, while ‘less severe hypertension’ was defined as office SBP and diastolic BP 150–180 mmHg and ≥90 mmHg, respectively, and 24-h ambulatory SBP 140–170 mmHg. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula.22 Because the MDRD formula has less precision in measuring glomerular filtration rate (GFR) at higher values, we also calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.23
Statistical analysis
Continuous variables were presented as means ± standard deviation (SD). Between-group differences in continuous variables were tested using the Wilcoxon-Mann–Whitney test. Within-group differences in continuous variables from baseline to follow-up were tested using paired t-tests. Categorical variables were presented as counts and percentages and compared between groups using the χ2 test. Changes in medication class rates between baseline and 3 years were compared with McNemar’s test. Analyses were performed on the basis of the intention-to-treat principle. Missing data were not imputed. Serial (at 6, 12, 24 and 36 months) BP and eGFR outcomes are presented in patients with matched baseline, 6-, 12-, 24-, and 36-month data available; however, long-term safety outcomes are presented in all patients in order to evaluate all safety events, and subset analyses on eGFR (i.e. the change in eGFR stratified by patients with and without diabetes mellitus) included all patients due to the limited sample size by such stratification. Kaplan–Meier time-to-event estimates were used to summarize rates of adverse events based on all available follow-up. Comparison of eGFR measurements in patients with vs. without changes in antihypertensive medications was performed to evaluate the potential effects of medication changes on renal function.
Multivariable linear regression analysis was performed to assess independent correlates of the change in office (and 24-h ambulatory) SBP at 12, 24, and 36 months. The following baseline characteristics were considered for each model: baseline SBP and diastolic BP (measured in office for the office regression model and measured using 24-h ambulatory BP monitoring for the 24-h ambulatory model), age, history of diabetes, eGFR ≥60 mL/min/1.73 m2, male gender, diabetes mellitus, body mass index, history of cardiac disease, heart failure, left ventricular hypertrophy, atrial fibrillation, current smoker, number of ablation attempts, as well as baseline number of antihypertensive medication classes, and prescription of aldosterone antagonist, alpha-1-blocker, alpha-2 agonist, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker, direct renin inhibitor, and vasodilator. For each model, only covariates with a univariate P < 0.2 were considered for the multivariable model. Multivariable predictors were then determined from these covariates using a stepwise selection method with entry/stay significance levels of 0.1/0.1.
A two-tailed P value <0.05 was considered statistically significant. Analyses were performed using SAS version 9.2 or higher (SAS Institute, Cary, NC, USA) and Institut für Herzinfarktforschung GmbH (Ludwigshafen, Germany) performed the statistical analyses. Authors had full access to the data.
Results
Baseline characteristics and procedural data
At the time of this analysis, 2237 patients had been enrolled at 196 active sites in 45 countries. Of these, 1734 patients have office BP measurements available at 6 months, 1654 at 1 year, 1258 at 2 years, and 872 at 3 years (Figure 1). Average body mass index at baseline was 31 ± 6 kg/m2, mean age was 61 ± 12 years, 20.9% had a history of CKD (eGFR <60 mL/min/1.73 m2), 38% had Type 2 diabetes mellitus, and nearly half had a history of cardiac disease (Table 1). A total of 17 patients were on haemodialysis at study entry. All patients had RDN with the mono-electrode SYMPLICITY Flex catheter (Medtronic, Santa Rosa, USA). During the RDN procedure, 13.4 ± 4.1 ablation treatments were applied in 2.1 ± 0.4 renal arteries per patient. A total of 129 ± 78 mL of contrast was used during a RDN time (the interval between RDN catheter insertion and guide catheter removal) of 49 ± 21 min.
Figure 1.
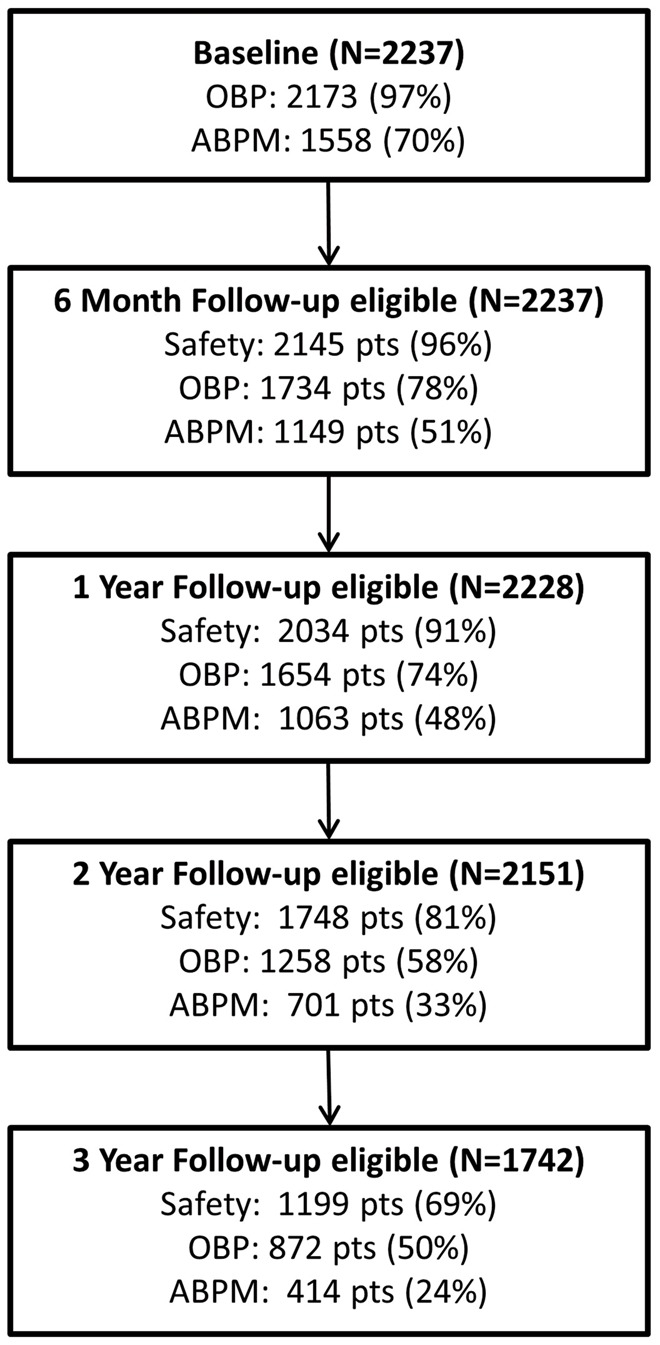
Patient disposition. ABPM, ambulatory blood pressure measurement; OBP, office blood pressure.
Table 1.
Baseline characteristics
| Characteristics | Global SYMPLICITY Registry (n = 2237) |
|---|---|
| Male (%) | 58.0 |
| Age (years) | 61 ± 12 |
| Body mass index (kg/m2) | 31 ± 6 |
| Current smoking (%) | 9.8 |
| History of cardiac disease (%) | 48.4 |
| Estimated GFR (mL/min/1.73 m2) | 76.3 ± 25.0 |
| Chronic kidney disease stage ≥3 (%) (eGFR <60 mL/min/1.73 m2) | 20.9 |
| Obstructive sleep apnoea (%) | 10.6 |
| Atrial fibrillation (%) | 12.7 |
| Diabetes Type 2 (%) | 38.0 |
| Office blood pressure (mmHg) | |
| Systolic | 166 ± 25 |
| Diastolic | 90 ± 17 |
| 24-h ambulatory blood pressure (mmHg) | |
| Systolic | 154 ± 18 |
| Diastolic | 86 ± 14 |
| True hypertension (%) | 83 |
| Masked hypertension (%) | 11 |
| White coat-hypertension (%) | 4 |
Results are presented as % or mean ± SD.
eGFR, estimated glomerular filtration rate.
Antihypertension medication
Antihypertensive medication prescription is shown in Table 2. At baseline, patients were prescribed 4.5 ± 1.4 antihypertensive medication classes, which in most patients included an angiotensin receptor blocker or ACE inhibitor, a calcium channel blocker, a diuretic, and a beta-blocker. At 3-year post-enrolment, patients were prescribed 4.4 ± 1.5 antihypertensive classes (P < 0.001 vs. baseline), reflecting a decrease in angiotensin-converting enzyme inhibitor and centrally acting sympatholytic use with a concomitant increase in aldosterone antagonist use.
Table 2.
Antihypertensive medications in patients eligible for 3-year follow-up
| Baseline (n = 1721) | 1 year (n = 1729) | 2 years (n = 1729) | 3 years (n = 1730) | P-valuea | |
|---|---|---|---|---|---|
| Antihypertensive medication classes | 4.5 ± 1.4 | 4.4 ± 1.4 | 4.4 ± 1.5 | 4.4 ± 1.5 | <0.001 |
| Beta-blockers (%) | 77.4 | 75.8 | 74.7 | 74.0 | <0.001 |
| ACE inhibitors (%) | 34.2 | 30.5 | 29.5 | 29.2 | <0.001 |
| Angiotensin receptor blockers (%) | 66.5 | 65.9 | 65.7 | 65.3 | 0.018 |
| Calcium channel blockers (%) | 77.6 | 76.4 | 76.5 | 76.2 | 0.071 |
| Diuretics (%) | 79.3 | 77.8 | 76.9 | 76.0 | <0.001 |
| Aldosterone antagonists (%) | 24.8 | 27.6 | 28.9 | 29.2 | <0.001 |
| Alpha-adrenergic blockers (%) | 35.1 | 33.1 | 32.4 | 32.4 | 0.006 |
| Direct-acting vasodilators (%) | 14.1 | 13.7 | 13.7 | 13.8 | 0.939 |
| Centrally-acting sympatholytics (%) | 38.8 | 35.6 | 35.0 | 34.3 | <0.001 |
| Direct renin inhibitors (%) | 6.2 | 4.9 | 4.7 | 4.4 | <0.001 |
Three years vs. baseline using the McNemar’s test for categorical variables and the paired t-test for number of anti-hypertensive medications.
Blood pressure
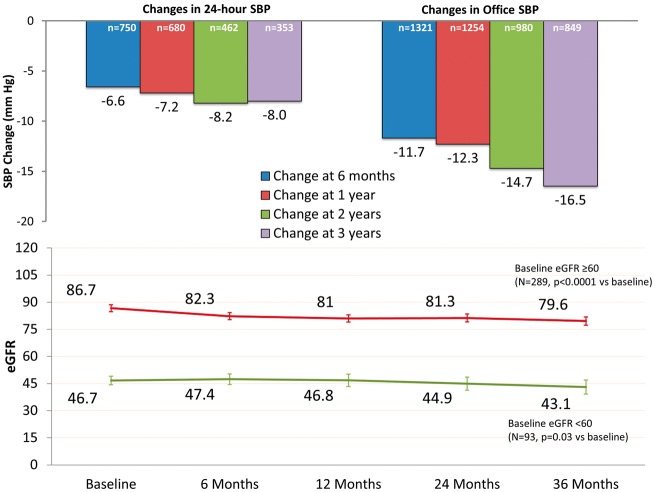
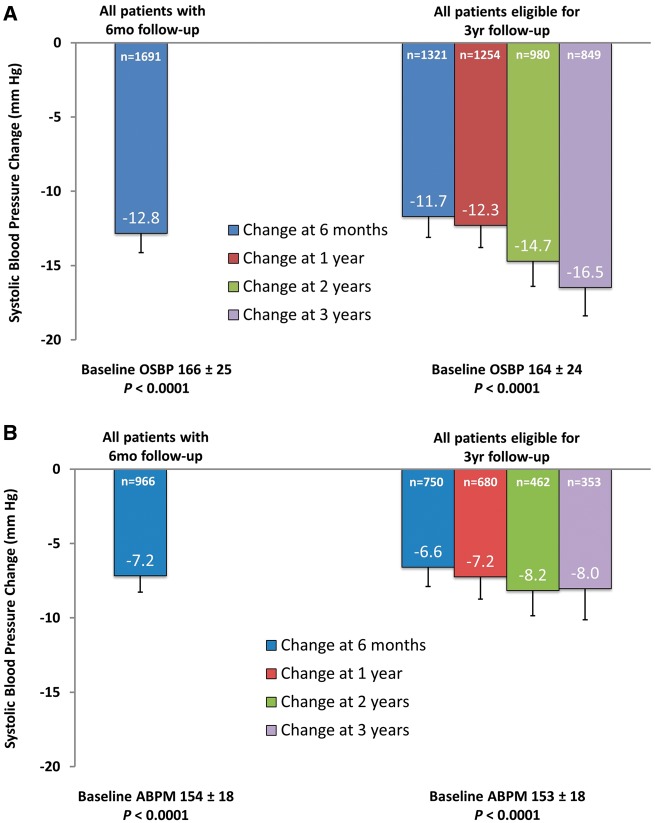
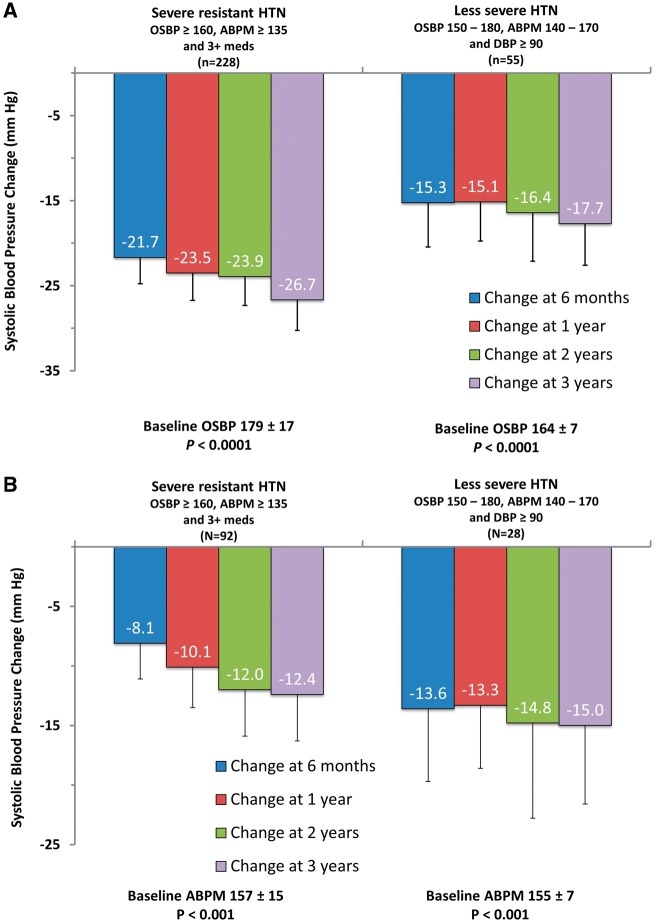
Office and 24-h ambulatory BP at baseline was 166/90 ± 25/17 and 154/86 ± 18/14 mmHg, respectively. At 6 months, office SBP decreased by −12.8 ± 26.2 mmHg (n = 1691, P < 0.0001 vs. baseline) and 24-h ambulatory SBP by −7.2 ± 17.8 mmHg (n = 966, P < 0.0001 vs. baseline); the decrease in both office and 24-h ambulatory BP was sustained at 12, 24, and 36 months (Figure 2). The 6-month change in office SBP was −21.7 ± 24.0 (n = 228, P < 0.0001) in patients with severe treatment-resistant hypertension, and −15.3 ± 19.5 (n = 55, P < 0.0001) in patients with less severe hypertension, with BP levels sustained to 3 years (Figure 3).
Figure 2.
Change in (A) office systolic blood pressure and (B) 24-h ambulatory systolic blood pressure. Error bars represent 95% confidence intervals.
Figure 3.
Change in office (A) and 24-h ambulatory (B) systolic blood pressure stratified by patients with and without severe resistant hypertension. Error bars represent 95% confidence intervals.
Baseline and procedure characteristics that correlated with a change in office and 24-h ambulatory SBP at 12, 24, and 36 months are shown in Table 3. The only baseline variable associated with a greater reduction in office (and 24 h) SBP at all three time points (12, 24, and 36 months) was higher baseline office (and 24 h) SBP. Use of alpha-adrenergic blockers and direct-acting vasodilators was associated with an increase in office SBP at 12, 24, and 36 months and current smokers were associated with an increase in 36-month 24-h SBP.
Table 3.
Multivariable predictors of baseline characteristics correlated with changes in office and ambulatory systolic blood pressure
| Covariates | Estimate (95% CI)a | P-value |
|---|---|---|
| Office SBP | ||
| 12 months | ||
| Baseline office SBP | −0.7 (−0.7 to −0.6) | <0.001 |
| Heart failure | −2.8 (−6.0 to 0.4) | 0.090 |
| Aldosterone antagonists | 3.0 (0.7–5.3) | 0.012 |
| Alpha-adrenergic blocker | 2.5 (0.3–4.6) | 0.024 |
| Direct-acting vasodilators | 3.4 (0.3–6.4) | 0.030 |
| 24 months | ||
| Baseline office SBP | −0.7 (−0.7 to −0.6) | <0.001 |
| Aldosterone antagonists | 5.0 (2.2–7.9) | <0.001 |
| Alpha-adrenergic blocker | 3.8 (1.2–6.3) | 0.004 |
| Direct-acting vasodilators | 7.0 (3.2–10.7) | <0.001 |
| 36 months | ||
| Baseline office SBP | −0.7 (−0.8 to −0.7) | <0.001 |
| Age | −0.1 (−0.2 to 0.0) | 0.074 |
| Body mass index | 0.2 (−0.0 to 0.5) | 0.094 |
| Angiotensin receptor blockers | −3.1 (−6.1 to −0.1) | 0.041 |
| Direct renin inhibitors | 10.8 (4.0–17.5) | 0.002 |
| Beta blockers | −4.0 (−7.3 to −0.7) | 0.016 |
| Alpha-adrenergic blocker | 6.2 (3.2–9.2) | <0.001 |
| Direct-acting vasodilators | 5.0 (0.5–9.6) | 0.031 |
| 24-h ambulatory SBP | ||
| 12 months | ||
| Baseline ambulatory SBP | −0.5 (−0.6 to −0.4) | <0.001 |
| Baseline ambulatory DBP | −0.1 (−0.2 to 0.0) | 0.059 |
| Centrally acting sympatholytics | 2.7 (0.7–4.7) | 0.009 |
| 24 months | ||
| Baseline ambulatory SBP | −0.6 (−0.7 to −0.5) | <0.001 |
| Baseline number of medication classes | 1.4 (0.3–2.4) | 0.009 |
| 36 months | ||
| Baseline ambulatory SBP | −0.7 (−0.8 to −0.6) | <0.001 |
| Age | −0.2 (−0.4 to −0.0) | 0.011 |
| Current smoker | 7.3 (1.1–13.5) | 0.022 |
Estimate is multivariable linear regression estimate.
CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Safety
Safety outcomes are shown in Table 4 using Kaplan–Meier estimates. At 3 years, 4.0% of patients experienced death (2.0% cardiovascular death), 3.2% stroke, and 2.6% underwent hospitalization for hypertensive crisis. Additionally, 1.6% developed end-stage renal disease, and 1.5% had an increase in serum creatinine from baseline of more than 50%. At 1 year, three patients (0.1%) were identified with newly developed renal artery stenosis. Two of these three cases, both confirmed by angiography to have 75% stenosis, were associated with a worsening of BP after an initial decline in BP following RDN; both cases were successfully treated by stenting. In the third case, a >70% stenosis in the left proximal renal artery was documented during abdominal magnetic resonance imaging; this patient was treated pharmacologically.
Table 4.
Safety results using Kaplan–Meier time-to-event analysis
| 6 months (number at riska: 2237) | 1 year (number at riska: 2112) | 2 years (number at riska: 1917) | 3 years (number at riska: 1345) | |
|---|---|---|---|---|
| Death | 0.5 (10) | 1.3 (28) | 2.8 (54) | 4.1 (59) |
| Cardiovascular events | ||||
| Cardiovascular death | 0.3 (6) | 0.8 (16) | 1.5 (28) | 2.0 (29) |
| Stroke | 0.7 (15) | 1.3 (27) | 2.1 (41) | 3.2 (47) |
| Hospitalization for new onset heart failure | 0.7 (16) | 1.1 (24) | 2.0 (38) | 3.2 (46) |
| Hospitalization for atrial fibrillation | 0.7 (15) | 1.5 (32) | 2.4 (46) | 3.0 (45) |
| Hospitalization for hypertensive crisis/hypertensive emergency | 0.8 (17) | 1.1 (24) | 1.8 (36) | 2.6 (40) |
| Myocardial infarction | 0.7 (16) | 1.1 (23) | 1.6 (31) | 2.2 (33) |
| Renal events | ||||
| New onset end-stage renal disease | 0.2 (4) | 0.4 (9) | 1.0 (19) | 1.6 (23) |
| Serum creatinine elevation >50% mg/dL | 0.4 (9) | 0.9 (19) | 1.2 (24) | 1.5 (24) |
| New artery stenosis (>70% diameter stenosis) | 0.05 (1) | 0.1 (3) | 0.2 (4) | 0.3 (4) |
| Post-procedural events | ||||
| Non-cardiovascular death | 0.1 (2) | 0.3 (7) | 1.0 (19) | 1.6 (22) |
| Renal artery reintervention | 0.2 (5) | 0.4 (8) | 0.4 (9) | 0.6 (10) |
Data are presented as Kaplan–Meier estimate % (number of events).
Number at risk at the start of each new follow-up period.
Renal function
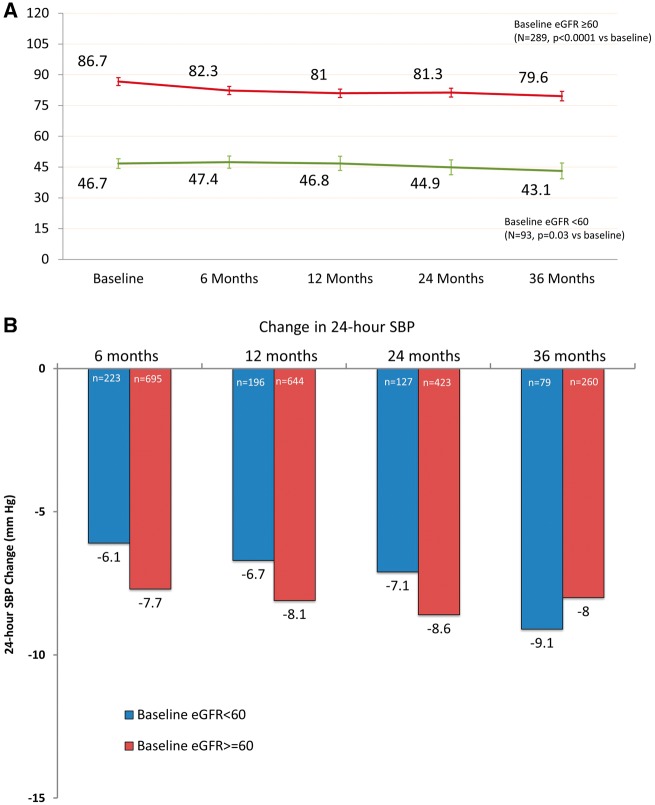
The change in eGFR following RDN is shown in Figure 4A. In patients without CKD (baseline eGFR ≥60 mL/min/1.73 m2), eGFR at baseline and 3 years was 87 ± 17 and 80 ± 20 mL/min/1.73 m2 (Δ = −7.1 ± 16.7 mL/min/1.73 m2, n = 289, P < 0.0001), respectively. For patients with CKD (baseline eGFR <60 mL/min/1.73 m2), eGFR was reduced from baseline to 3 years (47 ± 11 vs. 43 ± 19 mL/min/1.73 m2, Δ = −3.7 ± 16.2 mL/min/1.73 m2; n = 93, P = 0.03 vs. baseline). For patients with Stage 4 severe CKD at baseline (n = 37), there were two patients who progressed to Stage 5 at 6 months, four additional patients at 12 months, and two additional patients at 24 months. For patients with baseline Stage 3 moderate CKD (n = 124), there were 16 patients who progressed to Stage 4 at 6 months. There was no difference in eGFR measurements at 36 months for patients with vs. without changes in antihypertensive medication changes (70 ± 25 vs. 69 ± 25 mL/min/1.73 m2, P = 0.41).
Figure 4.
(A) Change in estimated glomerular filtration rate. Data are stratified by estimated glomerular filtration rate ≥ and <60 mL/min/1.73 m2. Error bars represent 95% confidence intervals. (B) Change in 24-h systolic blood pressure for patients with baseline estimated glomerular filtration rate ≥ and <60 mL/min/1.73 m2. There were no statistically significant differences in changes between groups.
The 6-month change in eGFR was numerically higher but did not reach statistical significance in patients with diabetes mellitus compared with those without diabetes mellitus [−4.1 ± 12.6 mL/min/1.73 m2 (n = 157) vs. −2.6 ± 13.4 mL/min/1.73 m2 (n = 224), P = 0.090] and likewise no significant difference was observed at 3 years [−7.7 ± 18.1 mL/min/1.73 m2 (n = 157) vs. −5.2 ± 15.5 mL/min/1.73 m2 (n = 224), P = 0.053].
Changes in 24-h SBP for patients with baseline eGFR <60 mL/min/1.73 m2 were not significantly different than for patients with baseline eGFR ≥60 mL/min/1.73 m2 at all measured timepoints (Figure 4B).
Discussion
In the SYMPLICITY Global Registry, the largest registry of RDN to date, SBP reduction was sustained to 3 years including decreases in both office (−16.5 mmHg) and 24-h ambulatory SBP (−8.0 mmHg). The RDN procedure showed a favourable short- and long-term safety profile. In this cohort of severe, uncontrolled hypertensive patients, renal function as assessed by eGFR declined within the range expected for hypertensive patients, with the fall in BP and these characteristics and comorbidities.24
The 6-month change in office and 24-h ambulatory SBP was largest in patients with severe resistant hypertension (−21.7 and −8.1 mmHg, respectively, P < 0.001 for both), and significant in patients with less severe hypertension (−15.3 and −13.6 mmHg, P < 0.001 for both), with results sustained to 3 years. While structural re-innervation has been demonstrated in sheep,25 findings from human studies including this report demonstrating sustained BP reduction suggest that any regrowth that may occur is unlikely to be of clinical relevance.
Although changes in prescribed antihypertensive medication regimens were allowed, patients were prescribed slightly, though not clinically meaningful, fewer antihypertensive medication classes at 36 months compared to baseline. Therefore, increases in medication cannot explain the sustained BP decrease. Although medication adherence could have improved for some patients from baseline to follow-up, potentially influencing BP reduction, a recent study suggests close to 40% of patients are not adherent to their prescribed anti-hypertensive regimen and that adherence was highly variable for each patient at different timepoints.14 In the DENERHTN trial,26 the prevalence of non-adherence to antihypertensive medications at 6 months was as high as 50% but not different between the RDN and control groups. These results are supported as well by multivariable analyses, in which higher baseline SBP was consistently associated with a greater reduction in both office and 24-h ambulatory SBP at all timepoints. Although this is the first multivariable analysis to our knowledge to examine predictors of BP change at 3 years, this finding is consistent with previous multivariable analyses of predictors of BP at 6 or 12 months after RDN.10,27,28
The sustained reductions in ambulatory SBP are also important since changes in day, night, and 24-h ambulatory BP are more closely related to cardiovascular risk than office BP measurements.29 The DENER HTN trial reported significant reductions in ambulatory SBP compared to control patients with documented resistant hypertension following a strictly controlled antihypertensive drug regimen after RDN.30 The recently published randomized, sham-controlled SPYRAL HTN-OFF MED,15 SPYRAL HTN-ON MED,14 and RADIANCE SOLO13 trials documented significant and consistent reductions in both office and ambulatory BP in patients with and without concomitant antihypertensive medication. The sustained long-term effects on BP and renal function in the Global SYMPLICITY Registry were similar to those observed in previous studies, adding relevant information about RDN in real-world patients with uncontrolled hypertension undergoing the procedure.
The Systolic Blood Pressure Intervention Trial (SPRINT) provided information about the importance of aggressive BP targets to reduce all-cause mortality in high-risk hypertensive patients.31 However, the extensive medical therapy required to achieve BP control in SPRINT was also associated with an increased risk of some adverse events, including acute kidney injury or failure.31 The present results in the context of recently published trials13–15 imply that RDN therapy may provide an important adjunctive therapy to obtain BP control with low risk of renal injury, which needs to be evaluated in future clinical trials. Indeed among 2145 subjects in the Global SYMPLICITY Registry followed for 6 months (and 1199 patients followed to 3 years), the incidence of cardiovascular death was 2.0% at 3 years and no long-term safety concerns were observed following the RDN procedure.
In total, three cases of renal artery stenosis were presented clinically at 5–6 months post-procedure and confirmed with imaging, although imaging was not requested systematically in all patients, and therefore, the true incidence cannot be scrutinized. Based on published clinical trial reports with the SYMPLICITY Flex system on 2586 patients, including this report, a total of eight new renal artery stenosis (>70% stenosis confirmed on angiography) have been reported (0.3% incidence), as well as an additional 12 cases outside of clinical studies, yielding a total of 20 cases.32 Renal artery stenosis is estimated to be present in 2–5% of hypertensive patients,33 although in a prospective study on 285 consecutive resistant hypertensive patients who underwent renal artery angiography, the incidence of renal artery stenosis was 24%.34 Thus, the incidence reported herein compares favourably with published information.
The observed eGFR decrease over 3 years was within the bounds of the expected decline in patients with severe hypertension, which ranges between 0.5 and 2.7 mL/min/1.73 m2 annually.7–9 Additionally, reduction in BP has been shown to result in a functional reduction in eGFR due to reduced perfusion pressure.35 In the DENER HTN trial, a similar 6-month change in eGFR was reported in the RDN and control groups (−4.0% vs. −6.2%, P = 0.726).30 Another study found a numerically smaller 6-month change in Cystatin C GFR in patients treated with RDN (−4.0 ± 2.8 mL/min, n = 88,) vs. a control group (−15.1 ± 11.1 mL/min, n = 12).36 In the study, renal resistive index by duplex ultrasound decreased, suggesting that RDN may improve renal perfusion through a reduction of intra-parenchymal resistance resulting in potentially renal protective effects.36 Another study showed that renal perfusion, measured using magnetic resonance imaging with arterial spin labelling, was preserved after RDN (as was eGFR) despite reduced systemic BP,37 and may help explain the preserved renal function observed after RDN. One might also speculate that the relatively small decline in eGFR in patients with CKD (baseline eGFR <60 mL/min/1.73 m2) suggests a possible renal protective effect, which is in line with clinical and preclinical data.38,39 Indeed, several studies indicate that the trajectory of the progressive decline in renal function can be altered by RDN.40,41 This finding is particularly meaningful as these patients with Stage 3 or higher CKD have a significantly higher risk for cardiovascular events, including stroke,42 and should be investigated in future clinical trials. Additionally, the decline in renal function, as measured by eGFR, is typically higher in subjects with than without diabetes.21,43,44
Limitations
As common in registries, not all patients currently enrolled in the Global SYMPLICITY Registry reached 36-month follow-up. At the time of this report, 3-year follow-up data was available for 50% of the enrolled population. It remains speculative whether patients with good or poor response to RDN decided to resign from follow-up examinations. However, when patients with complete follow-up were analysed using mixed models as a sensitivity analysis, no major differences in the results were observed. Furthermore, the Global SYMPLICITY Registry is a single-arm registry and as such does not involve control groups to compare outcomes. There is no way to rule out a Hawthorne/placebo effect, which could be caused by participation and care during the study.45 Comparison of eGFR measurements between patients with vs. without medication changes is limited since reported medication changes were not verified with medication adherence testing. The RDN procedures for this analysis were all performed with the first-generation, single-electrode SYMPLICITY Flex RDN catheter system. This device may make it more difficult to achieve a pattern of four-quadrant ablations than the current SYMPLICITY Spyral catheter technology, especially within the GSR study design that did not encourage more treatment ablations or allow for treatment in the renal artery side branches or accessories. The observed safety profile should be regarded as being device-specific; there is a need for continued long-term follow-up of patients treated with the newer SYMPLICITY Spyral catheter and revised procedural techniques.
Conclusions
The Global SYMPLICITY Registry, the largest available trial to date of outcomes after RDN in a hypertensive population, demonstrated significant BP reductions at 6 months that were sustained in the cohort that was followed to 3 years (Take home figure). No long-term safety concerns have been observed following the denervation procedure. Renal function in this cohort of severe, uncontrolled hypertensive patients as assessed by eGFR declined within the expected range (Take home figure).
Take home figure.
(A) Reduction in 24-h and office systolic blood pressure at 6, 12, 24 and 36 months. (B) Change in estimated glomerular filtration rate. Data are stratified by in estimated glomerular filtration rate ≥ and <60 mL/min/1.73 m2. Error bars represent 95% confidence intervals.
Acknowledgements
We thank Marianne Wanten, MSc, and Robin Hermans, MSc, for study management; Minglei Liu, PhD, for statistical support; Nicole Brilakis, MS, MBA, and Beth Ferri, PhD, for editorial support; and Sandeep Brar, MD and Sidney A. Cohen, MD, PhD, for study support and expert technical review (all of Medtronic).
Funding
The Global SYMPLICITY Registry is funded by Medtronic.
Conflict of interest: F.M. received speaker honoraria from Medtronic and Recor, and is supported by Deutsche Hochdruckliga, Deutsche Gesellschaft für Kardiologie, and Deutsche Forschungsgemeinschaft (SFB TRR 219). M.B. received research support and speaker fees from Medtronic and is supported by the Deutsche Forschungsgemeinschaft (SFB TRR 219). R.E.S. received speaker honorarium from Medtronic, Recor, and Ablative Solutions. K.N. is an advisor for Medtronic. L.R. is an advisor/speaker for Medtronic. M.S. is supported by career fellowships from the NHMRC, is an investigator in studies sponsored by Medtronic and Cibiem and has received research support from Boehringer Ingelheim. M.S. has received honoraria and travel support from Abbott, Boehringer Ingelheim, Servier, Novartis, and Medtronic. B.W. receives support by the National Institute for Health Research, University College London Hospitals, Biomedical Research Centre. M.F. is an employee of Medtronic. G.M. has received speaker’s fees from Medtronic.
See page 3483 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz369)
References
- 1. Böhm M, Linz D, Ukena C, Esler M, Mahfoud F.. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ Res 2014;115:400–409. [DOI] [PubMed] [Google Scholar]
- 2. Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G.. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension 1988;11:3–20. [DOI] [PubMed] [Google Scholar]
- 3. Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol 1983;245:F1–F14. [DOI] [PubMed] [Google Scholar]
- 4. Ruilope LM, Bakris GL.. Renal function and target organ damage in hypertension. Eur Heart J 2011;32:1599–1604. [DOI] [PubMed] [Google Scholar]
- 5. Grassi G, Quarti-Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G.. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension 2011;57:846–851. [DOI] [PubMed] [Google Scholar]
- 6. Bakris GL, Williams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J.. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000;36:646–661. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury EK, Langham RG, Ademi Z, Owen A, Krum H, Wing LM, Nelson MR, Reid CM; Italian Atherosclerosis, Thrombosis and Vascular Biology and Society for Invasive Cardiology–GISE Investigators. Rate of change in renal function and mortality in elderly treated hypertensive patients. Clin J Am Soc Nephrol 2015;10:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zoppini G, Targher G, Chonchol M, Ortalda V, Negri C, Stoico V, Bonora E.. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012;7:401–408. [DOI] [PubMed] [Google Scholar]
- 9. Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J.. Effect of blood pressure on early decline in kidney function among hypertensive men. Hypertension 2003;42:1144–1149. [DOI] [PubMed] [Google Scholar]
- 10. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M.. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 11. Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP.. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013;61:457–464. [DOI] [PubMed] [Google Scholar]
- 12. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD.. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 2009;361:932–934. [DOI] [PubMed] [Google Scholar]
- 13. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 14. Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 15. Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M.. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 16. Böhm M, Mahfoud F, Ukena C, Hoppe UC, Narkiewicz K, Negoita M, Ruilope L, Schlaich MP, Schmieder RE, Whitbourn R, Williams B, Zeymer U, Zirlik A, Mancia G; GSR Investigators. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension 2015;65:766–774. [DOI] [PubMed] [Google Scholar]
- 17. Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, Mahfoud F, Schlaich MP.. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J 2014;35:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD.. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 19. Böhm M, Mahfoud F, Ukena C, Bauer A, Fleck E, Hoppe UC, Kintscher U, Narkiewicz K, Negoita M, Ruilope L, Rump LC, Schlaich M, Schmieder R, Sievert H, Weil J, Williams B, Zeymer U, Mancia G.. Rationale and design of a large registry on renal denervation: the Global SYMPLICITY registry. EuroIntervention 2013;9:484–492. [DOI] [PubMed] [Google Scholar]
- 20. O'Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013;31:1731–1768. [DOI] [PubMed] [Google Scholar]
- 21. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D.. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polonia J, Azevedo A, Monte M, Silva JA, Bertoquini S.. Annual deterioration of renal function in hypertensive patients with and without diabetes. Vasc Health Risk Manag 2017;13:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Booth LC, Nishi EE, Yao ST, Ramchandra R, Lambert GW, Schlaich MP, May CN.. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015;65:393–400. [DOI] [PubMed] [Google Scholar]
- 26. Azizi M, Pereira H, Hamdidouche I, Gosse P, Monge M, Bobrie G, Delsart P, Mounier-Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Girerd X, Michel Halimi J, Zannad F, Ormezzano O, Vaisse B, Herpin D, Ribstein J, Chamontin B, Mourad JJ, Ferrari E, Plouin PF, Jullien V, Sapoval M, Chatellier G; DENERHTN Investigators. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the Renal Denervation for Hypertension (DENERHTN) Trial. Circulation 2016;134:847–857. [DOI] [PubMed] [Google Scholar]
- 27. Kandzari DE, Bhatt DL, Brar S, Devireddy CM, Esler M, Fahy M, Flack JM, Katzen BT, Lea J, Lee DP, Leon MB, Ma A, Massaro J, Mauri L, Oparil S, O'Neill WW, Patel MR, Rocha-Singh K, Sobotka PA, Svetkey L, Townsend RR, Bakris GL.. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015;36:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim BK, Böhm M, Mahfoud F, Mancia G, Park S, Hong MK, Kim HS, Park SJ, Park CG, Seung KB, Gwon HC, Choi DJ, Ahn TH, Kim CJ, Kwon HM, Esler M, Jang YS.. Renal denervation for treatment of uncontrolled hypertension in an Asian population: results from the Global SYMPLICITY Registry in South Korea (GSR Korea). J Hum Hypertens 2016;30:315–321. [DOI] [PubMed] [Google Scholar]
- 29. Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B.. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 30. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) investigators. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 31. Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT.. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Persu A, Sapoval M, Azizi M, Monge M, Danse E, Hammer F, Renkin J.. Renal artery stenosis following renal denervation: a matter of concern. J Hypertens 2014;32:2101–2105. [DOI] [PubMed] [Google Scholar]
- 33. Simon N, Franklin SS, Bleifer KH, Maxwell MH.. Clinical characteristics of renovascular hypertension. JAMA 1972;220:1209–1218. [PubMed] [Google Scholar]
- 34. Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC.. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014;113:687–690. [DOI] [PubMed] [Google Scholar]
- 35. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ; British Hypertension Society's PATHWAY Studies Group. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Böhm M.. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 2012;60:419–424. [DOI] [PubMed] [Google Scholar]
- 37. Ott C, Janka R, Schmid A, Titze S, Ditting T, Sobotka PA, Veelken R, Uder M, Schmieder RE.. Vascular and renal hemodynamic changes after renal denervation. Clin J Am Soc Nephrol 2013;8:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, Suzuki F, Nagai Y, Nakano D, Hitomi H, Kitada K, Urushihara M, Kobori H, Kohno M, Nishiyama A.. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 2012;125:1402–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlaich MP, Socratous F, Hennebry S, Eikelis N, Lambert EA, Straznicky N, Esler MD, Lambert GW.. Sympathetic activation in chronic renal failure. J Am Soc Nephrol 2009;20:933–939. [DOI] [PubMed] [Google Scholar]
- 40. Hering D, Marusic P, Duval J, Sata Y, Head GA, Denton KM, Burrows S, Walton AS, Esler MD, Schlaich MP.. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol 2017;232:93–97. [DOI] [PubMed] [Google Scholar]
- 41. Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, Veelken R, Ukena C, Uder M, Böhm M, Schmieder RE.. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens 2015;33:1261.. [DOI] [PubMed] [Google Scholar]
- 42. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY.. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 43. Hanratty R, Chonchol M, Havranek EP, Powers JD, Dickinson LM, Ho PM, Magid DJ, Steiner JF.. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol 2011;6:2605.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yokoyama H, Kanno S, Takahashi S, Yamada D, Itoh H, Saito K, Sone H, Haneda M.. Determinants of decline in glomerular filtration rate in nonproteinuric subjects with or without diabetes and hypertension. Clin J Am Soc Nephrol 2009;4:1432–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finniss DG, Kaptchuk TJ, Miller F, Benedetti F.. Biological, clinical, and ethical advances of placebo effects. Lancet 2010;375:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]