Abstract
Introduction:
Recent evidence suggests deep brain stimulation can alter impulse control. Our objective was to prospectively evaluate the effects of subthalamic nucleus (STN) and globus pallidus internus (GPi) deep brain stimulation on impulse control disorders (ICDs) in the setting of a conservative dopamine reduction strategy.
Methods:
Patients (n=37) undergoing de novo, unilateral STN or GPi DBS lead implantation were evaluated pre-operatively and 6–12 months post-operatively for the presence of ICDs using the Questionnaire for Impulsivity in Parkinson’s disease (QUIP) and by clinical interview.
Results:
Of the patients enrolled, 23 underwent electrode implantation in the globus pallidus internus and 14 were implanted in the subthalamic nucleus. Mean time to long term follow-up was 9.7±2.4 months. Post-operative LEDD was not significantly lower than pre-operative LEDD (pre-op: 1238.53±128.47 vs. post-op: 1178.18±126.43, p=0.2972, paired t test). Mean QUIP scores were significantly lower at follow up compared to pre-operative baselne (1.51±0.45 vs. 2.51±0.58, p= 0.0447, paired t test). Patients with ICDs pre-operatively (n=14, 37.8%) had significant improvement in QUIP scores at follow-up (6.00±0.94 vs. 2.64±.98, p=.0014, paired t test). Improvement was not uniform across the cohort: 1 patient with ICD at baseline developed worsening symptoms, and 4 patients with no ICD pre-operatively developed clinically significant ICDs post-operatively.
Conclusion:
When LEDD is relatively unchanged following STN or GPi DBS for PD, ICD symptoms tend toward improvement, although worsening and emergence of new ICDs can occur. In the setting of stable LEDD, these findings suggest that the intrinsic effects of DBS may play a significant role in altering impulsive behavior.
Introduction
A large subset of Parkinson’s disease (PD) patients (~15%) suffer from impulse control disorders (ICDs) [1]. ICDs are characterized by an inability to resist an inappropriate drive which is frequently of a hedonistic nature[2]. Patients pursue certain behaviors repetitively, excessively, and compulsively to the point of interference in major areas of life functioning[3]. Common ICDs include pathological gambling, compulsive buying, hypersexuality, binge eating, or hobbyism.
Dopamine agonist (DAA) therapy is a major risk factor for the development of ICDs, and withdrawal or reduction of DAA has been shown to improve symptoms [4]. It has been argued by several expert groups that deep brain stimulation (DBS) could be used to treat ICDs, given that motor symptom control via DBS could enable therapeutic DAA reduction [5,6].
Studies comparing ICD symptoms before and after DBS have had conflicting results and suggest a more complicated picture—one in which DBS could potentially exert direct effects on impulse control. Subthalamic nucleus (STN) DBS coupled with a large reduction in dopaminergic medication has been shown to reduce pre-existing impulsive behavior [7], but onset of new ICDs and worsening of existing ICDs in the post-operative period despite reduction in dopaminergic medications have also been documented [8,9].
The results of retrospective case series and reports have revealed conflicting effects of DBS on impulse control, including improvement/resolution, worsening/new onset, and lack of change. A large prospective study of 110 patients showed that compulsive dopaminergic medication use and related ICD behaviors were reduced 1 year after bilateral STN DBS.[10] However, previous studies have been mostly retrospective [9,11–13] or cross-sectional in design and have had small cohorts [14], while the few prospective studies to date have not investigated the effects of DBS on both STN and globus pallidus internus (GPi) targets.[5,7] Finally, it remains unclear from the available evidence whether changes in ICD status in these studies were due to changes in DAA or due to changes elicited by electrical stimulation. Our center has adopted a conservative post-operative medication reduction strategy based on evidence that such a strategy minimizes adverse mood effects associated with dopamine withdrawal[15,16]. The employment of this strategy affords a unique opportunity to investigate the effects of DBS on impulsive behavior when dopamine medications are not significantly decreased in the post-operative period.
Here we present an observational, prospective evaluation of the effects of de novo unilateral DBS on impulse control in both the STN and GPi in Parkinson’s disease. Consecutive patients (GPi, n=23; STN, n=14) undergoing DBS surgery were evaluated for the presence of an ICD using the Questionnaire for Impulsivity in Parkinson’s Disease (QUIP) and using a structured clinical interview. Patients were assessed for changes between six and twelve months post-operatively.
Materials and Methods
Experimental design
Consecutive patients with Parkinson’s disease undergoing de novo unilateral STN or GPi DBS electrode placement surgery were studied. The cohort was non-randomized due to the fact that brain targets were selected by an interdisciplinary team following individual evaluations with the following departments: neurology, neurosurgery, neuropsychology, physical therapy, occupational therapy, and speech therapy. Target selection was therefore based on a multitude of factors; however, due to the lack of compelling evidence favoring one target over the other in ICD patients, pre-operative ICD status was not a factor in determining target. Laterality of the initial DBS lead implantation was determined based on a desire to improve symptoms on the most bothersome side of the body or alternatively to improve function of the dominant hand. Patients also were required to have stable anti-parkinsonian medication for at least one month prior to surgical intervention. All patients fulfilled the UK Brain Bank criteria for Parkinson’s disease. There were 37 patients enrolled in the study, with a mean age of 65.8 years (STD=8.01). The University of Florida Institutional Review Board approved the study and all patients provided informed consent before entering the study.
We recorded complete details of antiparkinsonian medication therapy for each patient. The recorded medications were those being taken at the time of pre-operative and post-operative assessment. In accordance with our center’s strategy for post-operative dopamine reduction, LEDD at post-operative assessment represented the lowest LEDD since implantation. Pre-operative assessment occurred 24 hours prior to surgery. Due to the strong association of dopaminergic agonists with the development of ICDs, we compared ICD+ and ICD− groups in the use of dopaminergic agonists (pramipexole, ropinirole, pergolide, rotigotine, apomorphine, or bromocriptine) as well as levodopa equivalent daily dosage (LEDD). Limited evidence suggests that certain non-anti-parkinsonian medications (specifically aripiprazole[17] and stimulants[18]) can influence impulsive behavior. Details of the use of these medications was obtained for all patients; however, none of the patients were prescribed these medications at any point in the study. Post-operative follow-up occurred between 6 and 12 months after implantation, mean follow-up time for all patients was 9.7±2.4 months.
Assessment of Impulse Control Disorders
Participants completed the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease Rating Scale (QUIP), a self-reported and self-completed validated measure of impulsivity in Parkinson’s disease [19]. The QUIP includes five questions for each of the four ICDs most commonly reported in Parkinson’s disease and three questions about any other activity or hobby. We utilized established thresholds to represent a positive screen for the patient-completed instrument[19]. ICD diagnosis was confirmed by a board certified neurologist according to the results of the QUIP and clinical interview and published criteria for ICDs in PD. Post-operative assessment was performed by a neurologist blinded to pre-operative QUIP score and ICD diagnosis.
Statistical analysis
For continuous variables, Shapiro-Wilk tests for normality were performed. Provided that the data were normally distributed, continuous variables were then compared using unpaired t tests for comparisons by brain target and ICD status and using paired t tests for comparisons of pre-DBS and post-DBS outcomes. For data that were not normally distributed, the Wilcoxon matched-pairs signed rank test was used for comparisons of pre-DBS and post-DBS outcomes. Statistical significance was based on an alpha of 0.05.
Results
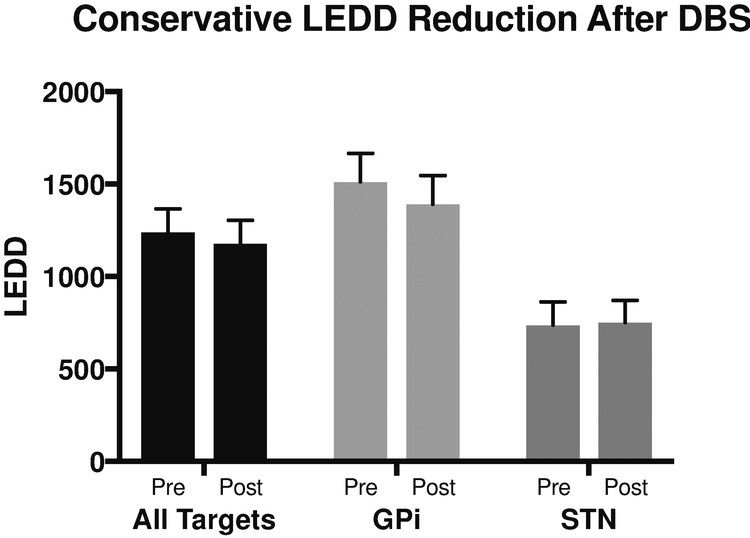
Of the 37 patients enrolled, 23 underwent electrode implantation in the globus pallidus internus and 14 were implanted in the subthalamic nucleus. Mean time to follow-up was 9.7±2.4 months. Post-operative LEDD was not significantly lower than pre-operative LEDD (pre-op: 1238.53±128.47 vs. post-op: 1178.18±126.43, p=0.2972, paired t test, Figure 1). Post-operative DAA LEDD was also not significantly lower than pre-operative DAA LEDD (74.7±126.6 vs. 59.1±107.4, p=.06, Wilcoxon matched-pairs sign rank test). As expected, patients who were ICD+ at baseline had significantly higher pre-operative LEDD compared to those who were ICD− at baseline (p= 0.0046, unpaired t test). These differences in LEDD for ICD+ and ICD− patients were maintained at the post-operative assessment (p=0 0.0347, unpaired t test). Importantly, post-operative DAA LEDD was not significantly lower than pre-operative DAA LEDD for the 14 patients who were ICD+ at baseline (pre-op: 88.2±102.4 vs. post-op: 67.5±99.3, p=.22, Wilcoxon matched-pairs signed rank test). Of these patients, only 2 underwent significant reduction in DAA LEDD post-operatively (reduction in DAA LEDD >40 mg). Of note, 5 patients were not on DAA, 4 maintained their DAA LEDD post-operatively, and 3 underwent DAA LEDD reduction <40mg. Of the 7 patients that resolved their ICDs, only 3 underwent any type of DAA reduction.
Figure 1. Conservative LEDD Reduction Post-DBS.
Pre- and post- operative LEDD by DBS target. Data are represented as mean ± standard error. *=p<.05. Abbreviations: LEDD= levodopa equivalent daily dosage, GPi=globus pallidus internus, STN=subthalamic nucleus.
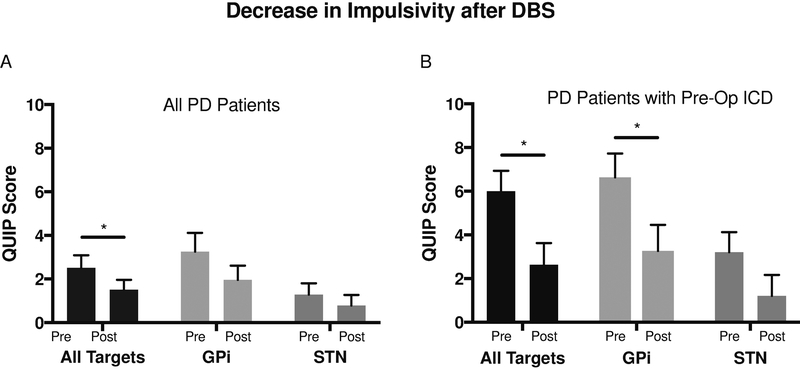
Mean QUIP scores were significantly lower at follow up compared to pre-operative baseline (1.51±0.45 vs. 2.51±0.58, p=0.0447, paired t test, Figure 2A). Patients with ICDs pre-operatively (n=14, 37.8%) had significant improvement in QUIP scores at follow-up (6.00±0.94 pre-operatively vs. 2.64±.98 at follow-up, p=.0014, paired t test, Figure 2B).
Figure 2. Decrease in Impulsivity after 6–12 months of unilateral STN or GPi DBS.
Pre- and post- operative QUIP scores by DBS target. Data are represented as mean ± standard error. *=p<.05. Abbreviations: ICD=impulse control disorder, QUIP=questionnaire for impulsivity in Parkinson’s disease, GPi=globus pallidus internus, STN=subthalamic nucleus.
Mean QUIP scores for GPi patients who were ICD+ at baseline were significantly lower post-operatively (6.64±1.09 vs. 3.27±1.18, p=0.0085, Figure 2A). A similar trend was observed for patients who underwent STN DBS, but this was not found to be statistically significant—possibly due to the smaller sample size (3.20±0.92 vs. 1.20±0.97, p=0.1419, Figure 2A).
Despite a significant decrease in overall QUIP score and subjective improvement in their symptoms, several patients (7/14, 50%) with pre-operative ICDs continued to meet diagnostic criteria for ICD. Moreover, the observed trend toward improvement was not uniform across the cohort: 1 patient worsened in symptoms and QUIP score, and 4 patients with no ICD pre-operatively developed clinically significant ICDs by the time of post-operative follow-up.
Discussion
Our data reveal that ICD symptoms can improve, worsen, emerge, or remain constant following STN and GPi DBS, even when dopamine medications are held relatively stable. There was resolution of ICDs in 50% of patients following surgery. This finding aligns with the 65% observed in a retrospective study of STN DBS[20]. In our study, 10.8% of patients that did not have ICD at baseline developed ICD post-operatively. This finding parallels the 13% rate that has been reported previously [20].
The overall pre-operative prevalence of ICDs in our cohort was 37.8%. This was considerably higher than the ICD prevalence typically reported for the Parkinson’s disease population at large (~15%) [1,14]. Higher prevalence of ICDs in the population of patients undergoing DBS has been described previously[14] and may be potentially explained by the bias of patients referred to DBS surgery tending to be younger, have higher LEDD, higher DAA LEDD, and longer disease duration—all risk factors for the development of ICD [20]. Moreover, the pre-operative prevalence of ICDs appears to be largely dependent on the impulsive behavior scale used [21], and rates from 22.5% [20] to 50%[7] have been reported. The post-operative prevalence of ICDs in our cohort was 29.7%, and this was comparable to the 25.8% post-operative prevalence found in a retrospective study of STN DBS [20].
We observed an apparent imbalance between targets with respect to both pre-operative and post-operative QUIP scores. In both cases, higher measures of impulsivity were observed in the cohort of patients undergoing GPi stimulation. This observation was unexpected due to no a priori favoring of either target based on ICD symptoms. However, it should be noted that there was a parallel imbalance in LEDD across targets. Higher LEDD is associated not only with an increased risk of impulsivity but also dyskinesias[22], and the anti-dyskinetic effects of GPi DBS have been shown to be greater compared to STN DBS[23]. As well, the severity of ICD has been associated with more severe dyskinesias[24]. Thus, a desire to suppress dyskinesias may explain the observed association between patients selected to undergo GPi DBS and greater pre-operative impulsivity scores. While the present study did not assess dyskinetic features, the above observation suggests that further exploration of a connection between ICD and dyskinesia may be of value, especially in light of the hypothesis that dyskinesias and ICD may be part of the same continuum in corticostriatal circuitry dysfunction.[25]
Our finding that changes in ICD symptoms can occur following unilateral DBS even in the setting of relatively stable dopaminergic medication is important. It corroborates pre-clinical findings suggestive of STN and GPi involvement in impulse control, and it aligns with reports that post-DBS changes in ICD symptoms do not always conform to expectations based on medication changes[14]. Pre-clinical studies in dopamine-naïve rodents revealed changes in impulsive behavior following high frequency electrical stimulation of the STN and GPi [26–29], and STN DBS in Parkinsonian humans increases measures of impulsivity independent of dopaminergic medication status.[30] It is important to emphasize that the patients in our study underwent unilateral DBS. Our findings align with previously documented changes in ICD symptoms following unilateral DBS of both the STN and GPi [8] and further establish the potential for non-motor effects of unilateral DBS, previously shown to include changes in both mood [31] and cognition[33]. However, it remains unclear how bilateral DBS might have impacted these findings. Future studies examining populations undergoing staged bilateral DBS may thus be beneficial.
The mechanisms underlying post-operative changes in impulse control are likely complex, with dopaminergic medications and electrical stimulation simultaneously affecting behavior in ways that are dependent on a multitude of factors, including the type(s) and dosage of pre- and post-surgical medication, the location of the active contact(s) of the DBS lead, and the stimulation parameters—all of which, in clinical practice, tend to be modified in the months following DBS lead implantation in an effort to optimize outcomes. Exploring the relative impact of these factors while controlling variables in a limited clinical population remains a challenge.
As this was an observational study and not a treatment study, investigators did not attempt to influence the treatment of patients. Following unilateral DBS, patients commonly do not undergo medication reduction, and it was therefore possible that patients might remain on dopamine agonists despite the presence of an ICD. The decision to reduce dopamine agonists in the setting of an ICD is often multi-factorial. Key considerations include the degree of distress caused by the ICD, the benefits of continuing medication for Parkinsonian motor symptoms, and concern for the development of dopamine agonist withdrawal syndrome (DAWS). The latter is of special concern in the present cohort, given recent data suggesting that the chief risk factors for DAWS are DAA LEDD > 150 mg, a history of DBS, and the presence of an ICD[34]. Of note, when all three risk factors are present, the risk of DAWS may exceed 90%[35]. For the 4 patients in our study who were maintained on relatively high doses of DAA (DAA LEDD >150mg) in the setting of an ICD, we suspect that these concerns motivated clinical decision making. However, this data was not tracked as a part of our protocol. Following completion of the study, the data was shared with the clinical team who pursued best clinical practice.
Our study has some limitations. The small sample size precluded sub-analysis on the impact of laterality of stimulation on ICD outcomes. The QUIP cannot capture qualitative changes in ICD symptoms that do not constitute a diagnosis conversion. Also, there was some heterogeneity in the time to follow-up. Due to the time required to complete the structured interview, the 6 to 12 month follow-up window was selected to improve completeness of data collection. The fact that our center has adopted a conservative approach to medication reduction post-DBS can be regarded as a strength of the study. The similarity in dopamine therapy in the pre- and post-operative period permitted a more controlled study of the effects of DBS, which would be skewed if both variables (medication and stimulation) had been modified. However, our study did not control for the variability inherent to DBS therapy, such as lead location and modifiable stimulation parameters. These aspects deserve further investigation, particularly in the setting of conservative dopamine reduction.
In conclusion, we show that measures of impulsivity in PD patients undergoing STN or GPi DBS therapy tend to decrease within the first year after surgery, even in the setting of relatively stable dopamine medications. However, caution should be exercised in selecting and counseling patients pre-operatively as improvement is not uniform, and some patients may worsen or develop new ICDs that did not previously exist.
Acknowledgments
PJR, SD, AG, and MSO contributed to the design of the experiment, data collection, interpretation of results, and editing of the manuscript. CH, DM, and KD contributed to interpretation of results and editing of the final manuscript.
Funding
No funding was associated with the research contained in this manuscript.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclosures
MSO serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation; he has previously received honoraria, but in the past >60 months has received no support from industry. MSO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books); he is an associate editor for New England Journal of Medicine Journal Watch Neurology. MSO has participated in CME and educational activities on movement disorders (in the last 36) months sponsored by PeerView, Prime, QuantiaMD, WebMD, MedNet, Henry Stewart, and by Vanderbilt University. The institution and not MSO receives grants from Medtronic, Abbvie, Allergan, and ANS/St. Jude, and the PI has no financial interest in these grants. MSO participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria. PJR, SD, CH, KDF, and AG have no conflicts of interest to report. No funding sources contributed to this study.
References
- [1].Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE, Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients, Arch. Neurol 67 (2010) 589–595, 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- [2].Bugalho P, Oliveira-Maia AJ, Impulse control disorders in Parkinson’s disease: crossroads between neurology, psychiatry and neuroscience, Behav. Neurol 27 (2013) 547–557, 10.3233/BEN-129019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weintraub D, David AS, Evans AH, Grant JE, Stacy M, Clinical spectrum of impulse control disorders in Parkinson’s disease, Mov. Disord. Off. J. Mov. Disord. Soc 30 (2015) 121–127, 10.1002/mds.26016. [DOI] [PubMed] [Google Scholar]
- [4].Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, Herranz Barcenas A, Vela L, Sanchez Alonso P, Mata M, Olmedilla Gonzalez N, Mahillo Fernandez I, Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study, J. Neurol. Neurosurg. Psychiatry 85 (2014) 840–844, 10.1136/jnnp-2013-306787. [DOI] [PubMed] [Google Scholar]
- [5].Amami P, Dekker I, Piacentini S, Ferré F, Romito LM, Franzini A, Foncke EMJ, Albanese A, Impulse control behaviours in patients with Parkinson’s disease after subthalamic deep brain stimulation: de novo cases and 3-year follow-up, J. Neurol. Neurosurg. Psychiatry 86 (2015) 562–564, 10.1136/jnnp-2013-307214. [DOI] [PubMed] [Google Scholar]
- [6].Okun MS, Weintraub D, Should impulse control disorders and dopamine dysregulation syndrome be indications for deep brain stimulation and intestinal levodopa? Mov. Disord. Off. J. Mov. Disord. Soc 28 (2013) 1915–1919, 10.1002/mds.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lhommée E, Klinger H, Thobois S, Schmitt E, Ardouin C, Bichon A, Kistner A, Fraix V, Xie J, Kombo MA, Chabardès S, Seigneuret E, Benabid A-L, Mertens P, Polo G, Carnicella S, Quesada J-L, Bosson J-L, Broussolle E, Pollak P, Krack P, Subthalamic stimulation in Parkinson’s disease: restoring the balance of motivated behaviours, Brain 135 (2012) 1463–1477, 10.1093/brain/aws078. [DOI] [PubMed] [Google Scholar]
- [8].Moum SJ, Price CC, Limotai N, Oyama G, Ward H, Jacobson C, Foote KD, Okun MS, Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome, PLoS One 7 (2012) e29768, 10.1371/journal.pone.0029768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim YE, Kim HJ, Kim H-J, Lee J-Y, Yun JY, Kim J-Y, Paek S-H, Jeon BS, Impulse control and related behaviors after bilateral subthalamic stimulation in patients with Parkinson’s disease, J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas 20 (2013) 964–969, 10.1016/j.jocn.2012.07.020. [DOI] [PubMed] [Google Scholar]
- [10].Eusebio A, Witjas T, Cohen J, Fluchère F, Jouve E, Régis J, Azulay J-P, Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease, J. Neurol. Neurosurg. Psychiatry 84 (2013) 868–874, 10.1136/jnnp-2012-302387. [DOI] [PubMed] [Google Scholar]
- [11].Broen M, Duits A, Visser-Vandewalle V, Temel Y, Winogrodzka A, Impulse control and related disorders in Parkinson’s disease patients treated with bilateral subthalamic nucleus stimulation: a review, Park. Relat. Disord 17 (2011) 413–417, 10.1016/j.parkreldis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [12].Demetriades P, Rickards H, Cavanna AE, Impulse control disorders following deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: clinical aspects, Park. Dis 2011 (2011), 10.4061/2011/658415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Voon V, Kubu C, Krack P, Houeto J-L, Tröster AI, Deep brain stimulation: neuropsychological and neuropsychiatric issues, Mov. Disord. Off. J. Mov. Disord. Soc 21 (Suppl 14) (2006) S305–S327, 10.1002/mds.20963. [DOI] [PubMed] [Google Scholar]
- [14].Gee L, Smith H, De La Cruz P, Campbell J, Fama C, Haller J, Ramirez-Zamora A, Durphy J, Hanspal E, Molho E, Barba A, Shin D, Pilitsis JG, The influence of bilateral subthalamic nucleus deep brain stimulation on impulsivity and prepulse inhibition in Parkinson’s disease patients, Stereotact. Funct. Neurosurg 93 (2015) 265–270, 10.1159/000381558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okun MS, Wu SS, Fayad S, Ward H, Bowers D, Rosado C, Bowen L, Jacobson C, Butson C, Foote KD, Acute and chronic mood and apathy outcomes from a randomized study of unilateral STN and GPi DBS, PLoS One 9 (2014), 10.1371/journal.pone.0114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nirenberg MJ, Dopamine agonist withdrawal syndrome and non-motor symptoms after Parkinson’s disease surgery, Brain 133 (2010), 10.1093/brain/awq165 e155–e155. [DOI] [PubMed] [Google Scholar]
- [17].Grall-Bronnec M, Sauvaget A, Perrouin F, Leboucher J, Etcheverrigaray F, Challet-Bouju G, Gaboriau L, Derkinderen P, Jolliet P, Victorri-Vigneau C, Pathological gambling associated with aripiprazole or dopamine replacement therapy: do patients share the same features? A review, J. Clin. Psychopharmacol 36 (2016) 63–70, 10.1097/JCP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].London ED, Impulsivity, stimulant abuse, and dopamine receptor signaling, Adv. Pharmacol. San. Diego Calif 76 (2016) 67–84, 10.1016/bs.apha.2016.01.002. [DOI] [PubMed] [Google Scholar]
- [19].Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, Adler CH, Potenza MN, Miyasaki J, Siderowf AD, Duda JE, Hurtig HI, Colcher A, Horn SS, Stern MB, Voon V, Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease, Mov. Disord. Off. J. Mov. Disord. Soc 24 (2009) 1461–1467, 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim YE, Kim HJ, Kim H-J, Lee J-Y, Yun JY, Kim J-Y, Paek S-H, Jeon BS, Impulse control and related behaviors after bilateral subthalamic stimulation in patients with Parkinson’s disease, J. Clin. Neurosci 20 (2013) 964–969, 10.1016/j.jocn.2012.07.020. [DOI] [PubMed] [Google Scholar]
- [21].Samuel M, Rodriguez-Oroz M, Antonini A, Brotchie JM, Ray Chaudhuri K, Brown RG, Galpern WR, Nirenberg MJ, Okun MS, Lang AE, Management of impulse control disorders in Parkinson’s disease: controversies and future approaches, Mov. Disord. Off. J. Mov. Disord. Soc 30 (2015) 150–159, 10.1002/mds.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Walker RW, Howells AR, Gray WK, The effect of levodopa dose and body weight on dyskinesia in a prevalent population of people with Parkinson’s disease, Park. Relat. Disord 17 (2011) 27–29, 10.1016/j.parkreldis.2010.10.005. [DOI] [PubMed] [Google Scholar]
- [23].Williams NR, Foote KD, Okun MS, STN vs. GPi deep brain stimulation: translating the rematch into clinical practice, Mov. Disord. Clin. Pract 1 (2014) 24–35, 10.1002/mdc3.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Voon V, Mehta AR, Hallett M, Impulse control disorders in Parkinson’s disease: recent advances, Curr. Opin. Neurol 24 (2011) 324–330, 10.1097/WCO.0b013-3283489687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Voon V, Fernagut P-O, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E, Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders, Lancet Neurol 8 (2009) 1140–1149, 10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- [26].Aleksandrova LR, Creed MC, Fletcher PJ, Lobo DSS, Hamani C, Nobrega JN, Deep brain stimulation of the subthalamic nucleus increases premature responding in a rat gambling task, Behav. Brain Res 245 (2013) 76–82, 10.1016/j.bbr.2013.02.011. [DOI] [PubMed] [Google Scholar]
- [27].Desbonnet L, Temel Y, Visser-Vandewalle V, Blokland A, Hornikx V, Steinbusch HWM, Premature responding following bilateral stimulation of the rat subthalamic nucleus is amplitude and frequency dependent, Brain Res 1008 (2004) 198–204, 10.1016/j.brainres.2004.02.032. [DOI] [PubMed] [Google Scholar]
- [28].Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C, Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1196–1200, 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Summerson SR, Aazhang B, Kemere CT, Characterizing motor and cognitive effects associated with deep brain stimulation in the GPi of hemi-Parkinsonian rats, IEEE Trans. Neural Syst. Rehabil. Eng 22 (2014) 1218–1227, 10.1109/TNSRE.2014.2330515. [DOI] [PubMed] [Google Scholar]
- [30].Frank MJ, Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making, Neural Netw. Off. J. Int. Neural Netw. Soc 19 (2006) 1120–1136, 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [31].Birchall EL, Walker HC, Cutter G, Guthrie S, Joop A, Memon RA, Watts RL, Standaert DG, Amara AW, The effect of unilateral subthalamic nucleus deep brain stimulation on depression in Parkinson’s disease, Brain Stimul 10 (2017) 651–656, 10.1016/j.brs.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Kirsch-Darrow L, Bowers D, Cognitive declines one year after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change, Clin. Neuropsychol 23 (2009) 385–405, 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yu XX, Fernandez HH, Dopamine agonist withdrawal syndrome: a comprehensive review, J. Neurol. Sci 374 (2017) 53–55, 10.1016/j.jns.2016.12.070. [DOI] [PubMed] [Google Scholar]
- [34].Garcia X, Patel S, Mohammad ME, Yu K, Sutton K, O’Donnell K, Fernandez HH, Higher doses of dopamine agonists, impulse control disorders and history of deep brain stimulation (DBS): risk factors for dopamine agonists withdrawal syndrome (DAWS)? [abstract], Mov. Disord 31 (Suppl. 2) (2016). [Google Scholar]