Abstract
Astrocytes are the major glial subtype in the brain and mediate numerous functions ranging from metabolic support to gliotransmitter release through signaling mechanisms controlled by Ca2+. Despite intense interest, the Ca2+ influx pathways in astrocytes remain obscure, hindering mechanistic insights into how Ca2+ signaling is coupled to downstream astrocyte-mediated effector functions. Here, we identified store-operated Ca2+ release-activated Ca2+ (CRAC) channels encoded by Orail and STIM1 as a major route of Ca2+ entry for driving sustained and oscillatory Ca2+ signals in astrocytes after stimulation of metabotropic purinergic and protease-activated receptors. Using synaptopHluorin as an optical reporter, we showed that the opening of astrocyte CRAC channels stimulated vesicular exocytosis to mediate the release of gliotransmitters, including ATP. Furthermore, slice electrophysiological recordings showed that activation of astrocytes by protease-activated receptors stimulated interneurons in the CA1 hippocampus to increase inhibitory postsynaptic currents on CA1 pyramidal cells. These results reveal a central role for CRAC channels as regulators of astrocyte Ca2+ signaling, gliotransmitter release, and astrocyte- mediated tonic inhibition of CA1 pyramidal neurons.
INTRODUCTION
Astrocytes are the primary glial cell type in the brain and regulate many aspects of brain function including neurogenesis and synaptogenesis, regulation of blood flow and metabolic support, ion homeostasis, and clearance of neurotransmitters (1–4). Growing evidence indicates that astrocytes also play a direct role in modulating neuronal activity and synaptic functions through the release of neuroactive molecules (4, 5). Termed “gliotransmitters,” these small molecules such as glutamate, adenosine triphosphate (ATP), adenosine, and D-serine (5, 6) can directly modulate brain functions such as plasticity (7, 8), synchronization of neuronal spiking (9), and even behavior (10). As electrically nonexcitable cells, astrocytes exhibit a form of excitability based on intracellular Ca2+ elevations. Neurotransmitters including glutamate and ATP bind to high-affinity G protein-coupled receptors (GPCRs) on astrocytes to evoke inositol 1,4,5-trisphosphate (IP3)–mediated release of Ca2+ from endoplasmic reticulum (ER) Ca2+ stores (5, 11) and evoke astrocyte Ca2+ signaling behaviors ranging from sustained signals to oscillations (12,13). Because Ca2+ mediates numerous effector functions including ion homeostasis, metabolism, gene expression, cell growth, and secretion (14), identification of the mechanisms by which Ca2+ enters the cell to evoke astrocyte Ca2+ signaling is of great interest.
Yet, the available literature shows gaps in our understanding of the “Ca2+ excitability” of astrocytes. What pathways are involved in generating astrocyte Ca2+ elevations? What are the dynamic properties of these Ca2+ signals? How are they coupled to secretion of gliotransmitters and other downstream effector functions? Progress in resolving these questions has been slow, largely because the sources of Ca2+ mobilization and Ca2+ entry in astrocytes remain poorly understood (12, 13, 15–17). There is evidence for the involvement of Ca2+ release from intracellular stores (4, 12,15,18,19). However, because release of Ca2+ from intracellular stores is typically coupled to activation of store-operated Ca2+ entry (SOCE) (20) and previous studies were not designed to discriminate between SOCE and store release, the possibility that SOCE could contribute to astrocyte Ca2+ signaling has not been seriously considered in past work.
In metazoans, a primary mechanism of mobilizing [Ca2+]i (intracellular calcium concentration) elevations is SOCE. SOCE is activated by the engagement of cell surface receptors that activate phospholipase C through G proteins or tyrosine kinase cascades to cleave phosphatidylinositol 4,5-bisphosphate and produce IP3 (20). The ensuing depletion of intracellular Ca2+ stores activates the ER Ca2+ sensors stromal interaction molecule 1 (STIM1) and STIM2, which translocate to the junctional ER to interact with and activate store-operated Ca2+ release-activated Ca2+ (CRAC) channels formed by the Orail, Orai2, and Orai3 proteins (20). Key hallmarks of CRAC channels include store-dependent activation, voltage independence, exquisite Ca2+ selectivity, and a low unitary conductance, making them ideally suited for generating oscillatory and long-lasting [Ca2+]i signals needed for transcriptional, enzymatic, and secretory effector cascades (20). Orai1 and STIM1 are expressed in glioblastoma cell lines (21), cortical astrocytes (22), spinal astrocytes (23), and white matter glia of the optic nerve (24) where they mediate SOCE in response to thapsigargin (TG)–mediated depletion of ER Ca2+ stores. However, the contribution of CRAC channels for regulating the Ca2+ excitability of astrocytes, particularly in response to physiological agonists, and their role in gliotransmitter release are unknown.
Here, we investigated the role of CRAC channels for astrocyte Ca2+ excitability and gliotransmitter release using genetic knockouts of Orail and STIM1 and wide-field, two-photon, and total internal reflection fluorescence (TIRF) microscopy. Our results showed that CRAC channels formed by Orai1 and STIM1 are a major route of Ca2+ entry in hippocampal astrocytes and are essential for generating Ca2+ signals in the cell body and astrocytic processes in situ after metabotropic receptor stimulation. Moreover, we showed that stimulation of CRAC channels directly triggered slow vesicular release of gliotransmitters, including ATP. Using slice electrophysiology, we showed that activation of CRAC channels in astrocytes increased the activity of GABAergic interneurons in the CA1 hippocampus, resulting in enhanced GABAergic transmission to CA1 pyramidal neurons. Together, these results identify CRAC channels as a major route of Ca2+ entry in astrocytes and a principal mechanism for evoking gliotransmitter release for modulating neuronal circuits.
RESULTS
Hippocampal astrocytes exhibit SOCE mediated by Orai1 and STIM1
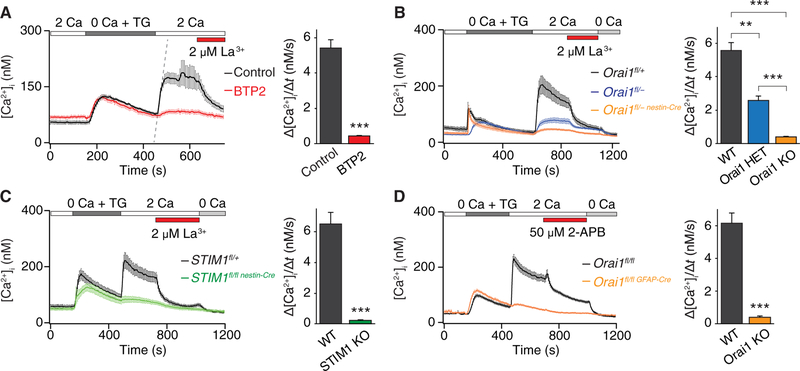
Astrocytes display intracellular Ca2+ fluctuations implicated in many downstream effector functions including release of gliotransmitters (25–27). However, the contributions of specific plasma membrane Ca2+ entry pathways that mediate these functions are poorly understood. To address a potential role for CRAC channels in generating cytosolic Ca2+ elevations and gliotransmitter release, we began our studies in hippocampal astrocytes by measuring cytosolic Ca2+ transients after depletion of ER Ca2+ stores with the SERCA inhibitor TG. TG was administered in a Ca2+-free Ringer’s solution, and SOCE was quantified using the classical method of measuring the rate and extent of Ca2+ influx after readdition of extracellular Ca2+. These experiments revealed that mouse hippocampal astrocytes exhibited robust SOCE with the typical pharmacological hallmarks of CRAC channels (28), including blockade by low concentrations of the trivalent ion La3+ (Fig. 1A), inhibition by the channel inhibitor bistrifluoromethyl pyrazole 2 (BTP2) (Fig. 1A), and potentiation at low doses and inhibition at high doses by the compound 2-aminoethyldiphenyl borate (2-APB) (fig. S1, A and B). These results indicate that storeoperated CRAC channels are functionally expressed in mouse brain astrocytes and mediate robust SOCE.
Fig. 1. Hippocampal astrocytes exhibit SOCE mediated by Orai1 and STIM1.
(A) Depletion of ER Ca2+ stores with TG (1 μM) in a Ca2+-free Ringer’s solution evoked store release and subsequent SOCE when extracellular Ca2+ (2 mM) was added back. SOCE was blocked by 2 μM LaCl3 and after preincubation with BTP2 (1 μM for 2 hours). The right graph shows summary of the rate of Ca2+ influx in control and BTP2-treated cells. Summary data are means ± SEM of n = 35 to 36 cells for each group from three independent experiments. Ca2+ influx rates were calculated by measuring the initial slope of Ca2+ entry over 18 s after readdition of 2 mM Ca2+ solution (as shown by the dotted line for the control condition). (B) SOCE is abolished in cultured hippocampal astrocytes from mice with brain-specific Orai1 KO. Ca2+ influx rates were attenuated after readdition of external Ca2+. The right graph shows the summary of the rate of Ca2+ influx in WT (Orai1fl/+ and Orai1fl/fl), Orai1 HET (Orai1fl/+ nest,n-Cre and Orai1fl/−), and Orai1 KO (Orai1fl/fl nestin-Cre and Orai1fl/−nestin-Cre) cells. Summary data are means ± SEM of n = 39 to 56 cells for each group from four to six independent experiments. (C) SOCE was abolished in cultured astrocytes from STIM1 KO mice (STIM1fl/fl nestin-Cre). Summary data (right graph) are means ± SEM, n = 25 to 30 cells for each group from three to four independent experiments. (D) SOCE was abolished in cultured astrocytes from astrocyte-specific Orai1 KO mice (Orai1fl/fl GFAP-Cre). Summary data are means ± SEM of n = 34 to 40 cells for each group from three independent experiments. **P < 0.01, ***P < 0.001 by analysis of variance (ANOVA) followed by Tukey test for comparison of multiple groups (B) or by unpaired t test for comparison of two groups (A, C, and D).
The best-described CRAC channels are formed by Orai1 and activated by the ER Ca2+ sensor STIM1. Immunostaining revealed that Orai1 is expressed in the glial fibrillary acidic protein (GFAP)-positive hippocampal astrocytes from wild-type (WT) but not from Orai1 knockout (KO) cultures (fig. S1C). Moreover, expression of a dominantnegative pore mutant of Orai1, E106A, suppressed SOCE, suggesting the involvement of Orai proteins in this pathway (fig. S1D). To directly determine the contribution of Orai1 and STIM1 for mediating SOCE in astrocytes, we examined Ca2+ signals in brain-specific KO mice of these proteins, which were generated by crossing Orai1fl/fl (or STIM1fl/fl) mice with mice expressing Cre-recombinase driven by the nestin promoter (29). Because nestin is expressed in neural stem/progenitor cells early in brain development, the progeny of this cross (Orai1fl/fl nestin-Cre or STIM1fl/fl nestin-Cre) should lack Orai1 or STIM1 in both neurons and glia in the brain (30, 31). Consistent with this expectation, examination of TG-mediated Ca2+ entry in primary astrocytes obtained from Orai1 or STIM1 KO mice indicated that SOCE was abrogated upon loss of Orai1 or STIM1 (Fig. 1, B and C) with no change in the resting baseline [Ca2+] levels or the amount of Ca2+ released from ER stores (fig. S1, E and F). The decrease in SOCE showed a gene dosage effect with the homozygous Orai1 KO cells, showing significantly greater impairment in Ca2+ influx compared to Orai1 heterozygous (HET) cells (Fig. 1B). SOCE was observed in astrocytes derived from the hippocampus or the cortex, and La3+ sensitivity and dependence on Orai1 did not differ between astrocytes from these two regions (fig. S1, G and H).
SOCE was also lost in astrocytes derived from the astrocyte-selective Orai1fl/fl GFAP-Cre mice (Fig. 1D), in which Orai1 was deleted by crossing Orai1fl/fl mice with mGFAP-Cre transgenic mice. Polymerase chain reaction (PCR) measurements in these mice indicated that Orai1 was deleted in astrocytes from Orai1fl/fl GFAP-Cre mice but preserved in neurons (fig. S1I), providing a second, more astrocyte-selective genetic line for probing the role of Orai1 in astrocytes. In addition, astrocytes grown using the “AWESAM” protocol that yields stellate astrocytes with complex morphology, long processes, and a more in vivo-like transcriptome than traditional astrocyte cultures (32, 33) also exhibited robust SOCE, which was lost in Orai1 KO astrocytes (fig. S1, J and K). Collectively, these results indicate that Orai1 and STIM1 are essential for mediating SOCE in mouse astrocytes.
SOCE in astrocytes is activated by metabotropic purinergic and protease-activated receptors
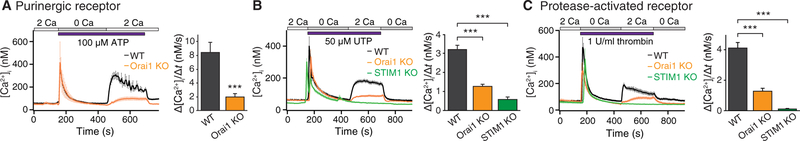
To investigate the physiological activators of SOCE in astrocytes, we surveyed several agonists of G protein-coupled purinergic, glutamatergic, cholinergic, and protease-activated receptors (PARs) that can result in depletion of ER Ca2+ stores. We administered these agonists in Ca2+-free solution to release intracellular Ca2+ stores and examined SOCE after readdition of extracellular Ca2+. The purinergic agonist ATP, the P2Y-specific (P2Y2 and P2Y4) receptor agonist uridine triphosphate (UTP), and the PAR agonist thrombin robustly activated SOCE in astrocytes (Fig. 2, A to C). Purinergic receptors, including metabotropic P2Y receptors, are implicated in the Ca2+ excitability of astrocytes and the reciprocal communication between neurons and glia (34). Likewise, PARs, which are abundantly expressed in astrocytes (35–37), are linked to many astrocyte functions including gliotransmitter release (38), production of proinflammatory mediators (39), and astrogliosis (40).
Fig. 2. Stimulation of purinergic and PAR GPCRs activates SOCE in hippocampal astrocytes.
(A) Cultured hippocampal astrocytes were treated with ATP (100 μM) in a Ca2+-free Ringer’s solution to deplete internal stores. Readdition of 2 mM extracellular Ca2+ elicited SOCE that was significantly decreased in Orail KO (Orai1fl/fl nestln-Cre and Orai1fl/fl GFAP-Cre) cells, as measured by the rate of Ca2+ influx. Summary data are means ± SEM of n = 22 to 26 cells for each group from three to four independent experiments. (B) Stimulation of P2Y receptors with UTP (50 μM) activated store release in Ca2+-free solution and subsequent sustained SOCE in 2 mM Ca2+ solution. SOCE was significantly attenuated in Orail KO (Orai1fl/fl GFAP-Cre) and STIM1 KO (STIM1fl/fl nestin-Cre) astrocytes. Summary data are means ± SEM of n = 25 to 57 cells for each group cells from three to six independent experiments. (C) Stimulation of PARs with thrombin (1 U/ml) activated store release in Ca2+-free solution followed by SOCE in 2 mM Ca2+ solution. SOCE was significantly attenuated in Orail KO (Orai1fl/fl GFAP-Cre) and STIM1 KO (STIM1fl/fl nestin-Cre) astrocytes. Summary data are means ± SEM of n = 24 to 53 cells for each group from three to six independent experiments. ***P < 0.001 by ANOVA followed by Tukey test for comparison of multiple groups (B and C) or by unpaired t test for comparison of two groups (A).
These experiments indicated that application of ATP or UTP in a Ca2+-free solution produced a transient Ca2+ rise followed by a sustained Ca2+ elevation when extracellular Ca2+ was reapplied. The rate and extent of Ca2+ influx after readdition of extracellular Ca2+ was attenuated in astrocytes from Orai1 KO and STIM1 KO mice (Fig. 2, A and B). In a complementary test, application of ATP or UTP in the presence of extracellular Ca2+ resulted in a biphasic Ca2+ signal, the sustained phase of which was lost in Orai1 KO and STIM1 KO astrocytes (fig. S2, A and B). Similarly, application of thrombin elicited Ca2+ signals consistent with SOCE, which were diminished in Orai1 KO and STIM1 KO astrocytes (Fig. 2C and fig. S2, C and D). These results indicate that CRAC channels contribute to the Ca2+ signals mediated by purinergic receptors and PARs. In contrast to astrocytes, neurons failed to show elevations in [Ca2+]i after administration of thrombin (fig. S2E), consistent with previous studies showing that PAR-linked Ca2+ signaling is active primarily in hippocampal CA1 astrocytes but not in neurons (36, 37). Together, these findings suggest that Orai1 channels are essential for mediating intracellular Ca2+ rises downstream of many types of metabotropic stimuli in astrocytes.
Activation of Orai1 channels stimulates vesicular exocytosis
The finding that Orai1 channels are critical for receptor-mediated mobilization of Ca2+ influx into astrocytes led us to next ask whether Orai1-mediated Ca2+ entry evoked the release of gliotransmitters. We addressed this question using several different methods: monitoring vesicular exocytosis with the genetically encoded fluorescent reporter, synaptopHluorin (spH), and the styryl dye, FM1–43, and directly measuring specific gliotransmitters released into the external media.
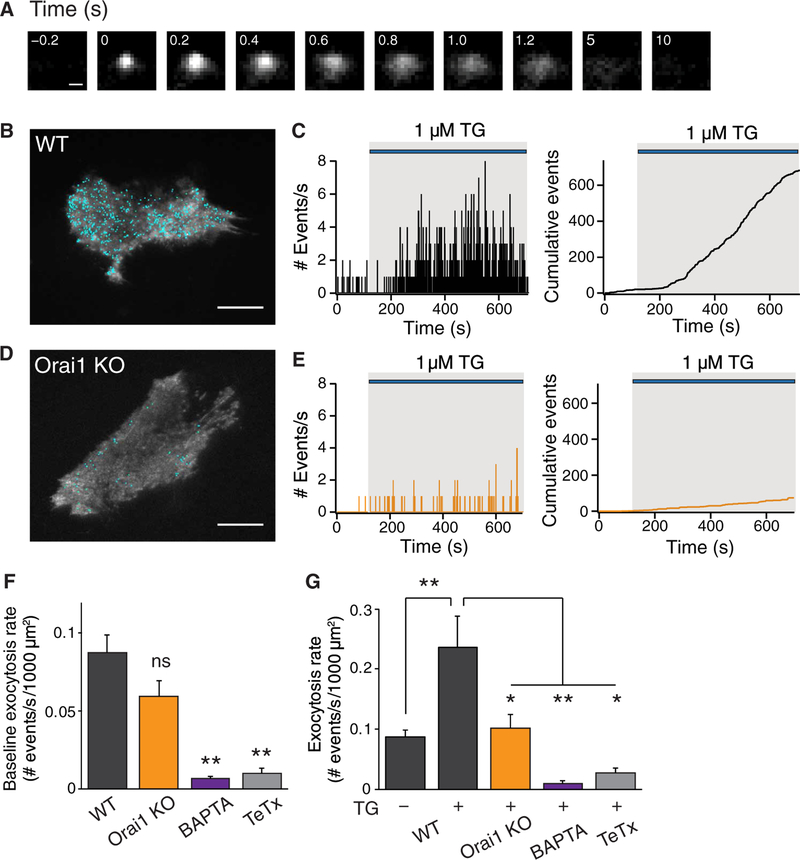
spH is a fusion protein made up of the transmembrane synaptic vesicle protein VAMP2 (synaptobrevin 2) and a pH-sensitive green fluorescent protein called pHluorin fused to its C terminus inside the vesicle lumen (41). The highly acidic intraluminal environment of vesicles (pH ~5.5) causes pHluorin fluorescence [pKa ~ 7.1 (where Ka is the acid dissociation constant)] to be quenched. However, upon vesicular fusion, the intraluminal pH equilibrates with the neutral pH of the extracellular medium (pH ~7.4) resulting in rapid dequenching and ~20-fold increase in spH fluorescence. Fluorescence is quenched once again after endocytosis and reacidification. We transiently transfected spH into hippocampal astrocytes and monitored spH fluorescence 24 to 48 hours later using TIRF microscopy, which enabled us to detect distinct exocytotic events on the footprint of spH-expressing astrocytes (Fig. 3A and movies S1 and S2). At rest, the exocytotic events occurred with an average frequency of 0.09 ± 0.01/s or 5 ± 1 fusions/min per 1000 μm2 of astrocyte footprint. The fluorescence of the events decayed with heterogeneous kinetics with an average decay half-width of ~1.7 s in resting cells (fig. S3, A to C). These kinetic features are comparable to the properties of spontaneous spH fusion events previously described and could, as previously suggested, reflect a mix of both kiss-and-run and full fusions (25,26).
Fig. 3. Orai1 channels stimulate vesicular exocytosis after store depletion.
(A) Fluorescence changes during a single vesicle fusion event monitored with spH. Images were captured every 200 ms, and the time of appearance of the fusion event was set to 0. Scale bar, 1 mm. (B) Location of spH events (shown in blue dots) are mapped onto the footprint of a TG-stimulated WT (Orai1fl/+) astrocyte. Scale bar, 20 μm. (C) Histogram of the number of spH fusion events measured each second. The right plot shows the integral of these events over the time course of the experiment. Stimulation with TG evoked an increase in the rate of exocytosis. (D) Location of the spH events (blue dots) mapped onto the footprint of a TG-stimulated Orail KO (Orai1fl/fl GFAP-Cre) astrocyte. Scale bar, 20 μm. (E) Histogram of the number of spH fusion events measured each second. The right plot shows the integral of these events over the time course of the experiment. (F) Summary of the average exocytosis rate during a 2-min unstimulated baseline for each of the indicated conditions. (G) Summary of the average exocytosis rate for each of the indicated conditions. The average exocytosis rate during TG (1 μM) treatment was calculated from the maximum slope of the cumulative events plot over a 200-s window. TG-evoked spH exocytosis was significantly suppressed in Orail KO cells, by preincubation with BAPTA-AM (acetoxy methyl ester) (5 μM) or by coexpressing the light chain of tetanus toxin (TeTx) in astrocytes. WT, n = 21 cells; Orail KO, n = 17 cells; BAPTA, n = 7 cells; TeTx, n = 5 cells. Bar graphs show means ± SEM. *P < 0.05, **P < 0.01 by ANOVA followed by Tukey test. ns, not significant.
Stimulating WT cells with TG to activate CRAC channels enhanced the rate and extent of exocytosis (Fig. 3, B and C). TG also caused the appearance of large-amplitude exocytotic events with similar half-widths as seen at baseline (fig. S3A). The average rate of exocytosis increased about threefold to 14 ± 3 fusions/min per 1000 μm2 of astrocyte footprint, reaching its peak value 267 ± 30 s after TG administration. In contrast to astrocytes from WT mice, exocytosis evoked by TG was significantly impaired in astrocytes from Orail KO mice (Fig. 3, D and E). Basal exocytosis was not significantly altered in the Orail KO mice (Fig. 3F). Rather, the primary defect appeared to be in the extent of the increase in secretion rate after cell stimulation, which was significantly smaller in Orail KO astrocytes in response to TG stimulation (Fig. 3G).
To verify that the spH events we observed were truly vesicle fusions, we examined the effects of coexpressing the light chain of tetanus toxin (TeTx), a protease toxin that cleaves VAMP2 and prevents SNARE (soluble N-ethylmaleimide–sensitive factor attachment protein receptor)–mediated exocytosis (42). spH exocytosis was abrogated with TeTx both at baseline (Fig. 3F) and in response to TG (Fig. 3G), indicating that spH-monitored vesicular fusion events in astrocytes are driven by SNARE-mediated exocytosis. Furthermore, chelating intracellular Ca2+ with BAPTA [1,2-bis(2-aminophenoxy)ethane- N,N,N′,N′-tetraacetic acid] nearly completely abolished spH fusion events at baseline (Fig. 3F) and after TG stimulation (Fig. 3G), consistent with the role of [Ca+]i elevations in inducing vesicular exocytosis.
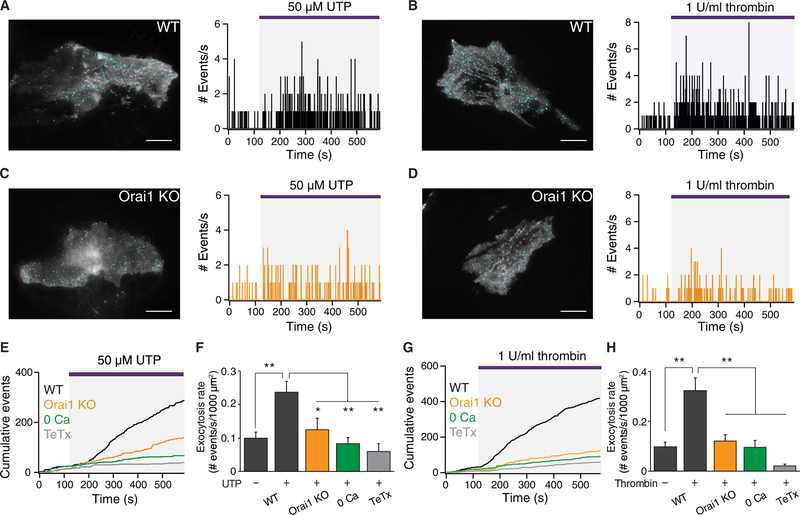
Next, we stimulated astrocytes with the GPCR agonists UTP and thrombin (Fig. 4, A and B). UTP and thrombin increased the rate of exocytosis to 14 ± 2 and 19 ± 3 fusions/min per 1000 μm2 of astrocyte footprint, respectively. UTP and thrombin evoked exocytosis considerably faster than TG, with the maximal rates peaking at 148 ± 22 s and 158 ± 32 s after agonist application, respectively, suggesting that receptor-mediated Ca2+ entry was more effective at triggering exocytosis than Ca2+ entry triggered by passive depletion of ER stores by TG. In addition to increasing the rate of exocytosis, UTP and thrombin also resulted in the appearance of large-amplitude exocytotic events, which could reflect compound or multivesicular release (fig. S3, B and C). As seen for TG, deletion of Orail decreased the rate of exocytosis in response to cell stimulation by UTP and thrombin (Fig. 4, C and D). Thrombin stimulation also evoked increased spH exocytotic events in WT astrocytes with stellate morphology grown using the AWESAM protocol, but not in similarly grown Orail KO astrocytes (fig. S4, A to D). Together, these results indicate that Orail channels are essential for store depletion–evoked and receptor-stimulated vesicular exocytosis in hippocampal astrocytes.
Fig. 4. Exocytosis evoked by UTP and thrombin is abrogated in Orai1 KO astrocytes.
(A) Location of spH events (blue dots) mapped onto the footprint of a WT (Orai1fl/+) astrocyte stimulated with 50 μM UTP (left image). Histogram of the number of spH fusion events measured each second (right plot). UTP was administered after a 2-min baseline. (B) Left: Location of spH events (blue dots) mapped onto the footprint of a WT (Orai1fl/+) astrocyte stimulated with thrombin (1 U/ml). Right: Histogram of the number of spH fusion events measured each second. Thrombin was administered after a 2-min baseline. (C and D) Location of spH events mapped onto the footprint of an Orai1 KO (Orai1fl/fl GFAP-Cre) astrocyte stimulated with 50 μM UTP (C) or thrombin (1 U/ml) (D). Histogram of the number of spH fusion events measured each second (right plot). (E) Cumulative event plots for WT, Orai1 KO, Ca2+-free, and TeTx-expressing astrocytes stimulated by UTP. (F) The average rate of UTP-evoked exocytosis per 1000 μm2 was significantly suppressed in Orai1 KO cells, in Ca2+-free solution, or by TeTx. WT, n = 19 cells; KO, n = 12 cells; Ca2+-free, n = 10 cells; TeTx, n = 7 cells. (G) Cumulative event plots for WT, Orai1 KO, Ca2+-free, and TeTx-expressing astrocytes stimulated by thrombin. (H) Average rate of thrombin-evoked exocytosis per 1000 μm2 in the indicated conditions. WT, n = 18 cells; KO, n = 17 cells; Ca2+-free, n = 7 cells; TeTx, n = 9 cells. Scale bars, 20 μm. Bar graphs show means ± SEM. *P < 0.05, **P < 0.01 by ANOVA followed by Tukey test.
Administration of UTP or thrombin in a Ca2+-free Ringer’s solution did not substantively induce exocytosis (Fig. 4, E to H), despite mobilization of [Ca2+]i (Fig. 2, B and C), indicating that store release by itself does not drive vesicular exocytosis. Together, these data show that Ca2+-dependent vesicular exocytosis evoked by GPCR stimulation requires Ca2+ entry through Orail channels across the plasma membrane.
Vesicular exocytosis is mediated by local Ca2+ signals around CRAC channels
The finding that store release alone in the absence of SOCE was less efficient at evoking gliotransmitter release led us to next consider whether vesicle fusion events were coupled to local spatial microdomains around CRAC channels rather than global Ca2+ rises. To explore this idea, we studied the differential effects of fast and slow Ca2+ chelators, BAPTA and EGTA, on vesicle fusion. Chelators reduce the lateral spread of the Ca2+ microdomain to an extent determined by the chelator’s on-rate. EGTA and BAPTA have similar affinities for Ca2+, but the Ca2+ binding on-rate of EGTA is 150-fold slower. Therefore, EGTA suppresses global [Ca2+] elevations but produces a local region of relatively unbuffered [Ca2+] around the pore. In contrast, BAPTA has a faster Ca2+ binding on-rate, causing Ca2+ to rapidly drop to resting levels within tens of nanometers of the Ca2+ channel, suppressing both local and global Ca2+ elevations. The differential effects of EGTA and BAPTA on Ca2+ buffering have been used to map the local coupling of Ca2+ channels to BK channels (43), the neurotransmitter release machinery (44, 45), and gene transcription (29,46,47). We found that both EGTA and BAPTA strongly suppressed the TG- and thrombin-mediated global rises in [Ca2+]i resulting from activation of CRAC channels as measured by Fura-2 (fig. S5A). However, whereas BAPTA nearly completely suppressed both spontaneous and UTP-evoked vesicle fusion events, EGTA was less effective (fig. S5, B and C). Similar results were seen in thrombin-stimulated cells (fig. S5, D and E). These results suggest that vesicular fusion events in response to CRAC channel activation are stimulated primarily by local submembrane Ca2+ elevations rather than global Ca2+ rises.
Loss of Orai1 impairs exocytosis as monitored by FM1–43
In a second method to monitor exocytosis, we used the dye FM1–43, which reversibly binds to synaptic membranes and can be incorporated into synaptic vesicles and has been used to monitor vesicular exocyto- sis in many cell types, particularly neurons (48, 49). FM1–43 is reported to preferentially label lysosomal-like vesicles (27), raising the possibility that this method may provide a readout of lysosomal secretion. We found that activation of SOCE by store depletion stimulated exocytosis of FM1–43-labeled vesicles in cultured hippocampal astrocytes (fig. S6A). FM1–43 destaining was markedly attenuated by deleting Orai1 and suppressed after preincubation with the CRAC channel inhibitor BTP2 (fig. S6, B and C). These results are consistent with the previous findings using spH and further support a crucial role for Orai1 in mediating gliotransmitter exocytosis from astrocytes.
Activation of CRAC channels evokes ATP release from hippocampal astrocytes
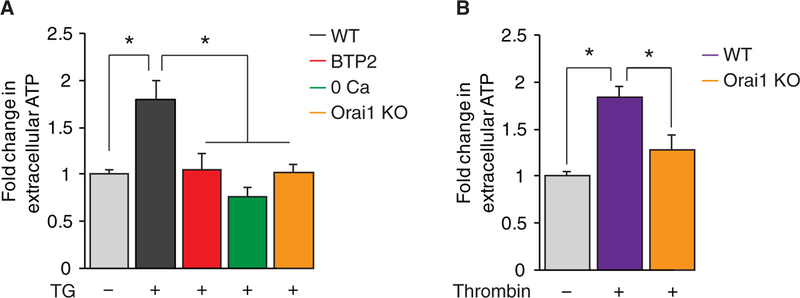
Although pHluorins and FM1–43 provide a convenient way to monitor regulated exocytosis, these tools do not reveal the identity of the gliotransmitters being released. ATP is a prominent glio- transmitter released from astrocytes that regulates the physiology of neurons and astrocytes (50–54). To determine whether CRAC channels are involved in the release of this key gliotransmitter, we measured ATP in the supernatant of cultured astrocytes. These measurements revealed that CRAC channel activation in response to depletion of ER Ca2+ stores by TG (Fig. 5A) or thrombin (Fig. 5B) significantly enhanced ATP secretion. ATP secretion was impaired in Ca2+-free solution (Fig. 5A), suggesting that store release by itself was insufficient to stimulate secretion. Deletion of Orai1 attenuated both TG- and thrombin-evoked ATP secretion (Fig. 5, A and B). Likewise, blockade of CRAC channel-mediated Ca2+ influx with BTP2 also abrogated TG-induced ATP release from astrocytes (Fig. 5A). Together, these results provide evidence that Orai1-mediated CRAC channels are essential for the secretion of ATP from astrocytes.
Fig. 5. Agonist-evoked ATP secretion is abrogated in Orai1 KO astrocytes.
(A) SOCE stimulates ATP secretion from cultured astrocytes. ATP levels were measured using a luciferin-luciferase luminescence assay from the supernatant of multiwell plates after 10 min of stimulation. TG-mediated ATP secretion depended on external Ca2+ and was suppressed in Orai1 KO astrocytes and WT astrocytes after preincubation with CRAC channel inhibitor BTP2 (1 μM for 2 hours). n = 9 to 23 wells for each group from three to five independent cultures. (B) Thrombin stimulated ATP secretion from cultured WT astrocytes but not from Orai1 KO astrocytes. n = 10 to 16 wells for each group from three to four independent cultures. Bar graphs show means ± SEM. *P < 0.05 by ANOVA followed by Tukey test.
Orai1 channels generate GPCR-mediated Ca2+ fluctuations in astrocyte processes in situ
The results presented above indicated that CRAC channels function as a major route of receptor-mediated Ca2+ entry in primary cultured astrocytes. Does this phenomenon also occur in astrocytes in their normal environment in situ? Astrocytes in vivo display complex morphology, with numerous intricate branches and fine tertiary processes that propagate intracellular Ca2+ fluctuations and waves (19, 55). As in primary cultured astrocytes, Ca2+ fluctuations in astrocytes in brain slices are also mediated largely through Ca2+ influx across the plasma membrane (19, 55), but the identity of the channels responsible for Ca2+ entry is unclear. To visualize Ca2+ signals in astrocyte fine processes in their native environment and examine the role of Orai1 channels for these Ca2+ signals, we used the genetically encoded fast Ca2+ indicator GCaMP6f to image Ca2+ fluctuations using two-photon laser scanning microscopy (2PLSM) in acutely prepared slices from WT and conditional Orai1 KO mice. GCaMP6f was selectively expressed in hippocampal CA1 astrocytes by injecting the dorsolateral hippocampus in adult mice (P35-P57) with an AAV5 viral expression vector carrying the GfaABC1D astrocyte-specific promoter (19). We imaged Ca2+ activity using 2PLSM in single GCaMP6f-expressing astrocytes in transverse hippocampal slices from WT (Orai1fl/fl) and Orai1 KO (Orai1fl/fl GFAP-Cre) mice (Fig. 6A). We focused our studies on astrocytes in the CA1 stratum radiatum layer because these astrocytes have been implicated in many functions including uptake and release of transmitters and modulation of hippocampal neural circuits by ATP-mediated stimulation of interneuron activity (56–58).
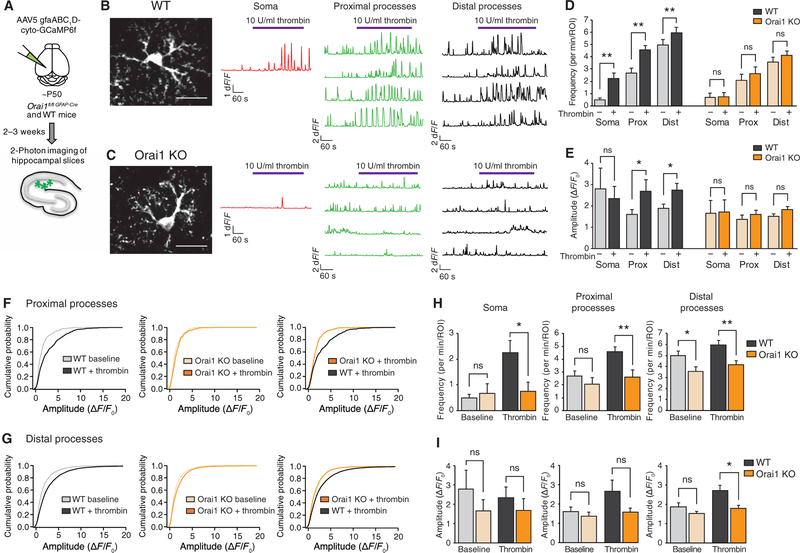
Fig. 6. Orai1 channels generate GPCR-mediated Ca2+fluctuations in astrocyte processes in situ.
(A) Illustration of the experimental approach. GCaMP6f was expressed in astrocytes of the hippocampal CA1 using stereotaxic injections of AAV5 virus with an astrocyte-specific gfaAB1D promoter. After 2 to 3 weeks, to allow for expression, Ca2+ fluctuations in astrocytes expressing GCaMP6 were imaged using 2PLSM. (B and C) Images of GCaMP6f-expressing WT (Orai1fl/fl) (B) or Orai1 KO (Orai1fl/fl GFAP-Cre) (C) astrocytes. Each image is the maximum intensity projection of the time series (540 s). Scale bar, 20 μm. Traces on the right show representative Ca2+ fluctuations measured in individual ROIs from the soma, proximal processes, and distal processes. Thrombin (10 U/ml) was used to activate Gq protein-coupled PARs on astrocytes and evoke Ca2+ signaling. Movies of the Ca2+ signals in these examples are shown in movies S3 and S4. (D and E) Summary of the Ca2+ oscillation frequency (D) and amplitude (E) at baseline and after administration of thrombin in WT (Orai1fl/fl, black bars) and Orai1 KO (Orailfl/fl GFAP-Cre, orange bars) astrocytes. (WT, n = 11 cells from five mice; Orai1 KO, n = 8 cells from four mice). Statistical analysis was done using paired t test. Prox, proximal processes; Dist, distal processes. (F and G) Cumulative probability plots of the amplitudes of each Ca2+ oscillation in each region of interest (ROI) measured in the proximal (F) and distal (G) processes. (H and I) Comparison of WT and Orai1 KO Ca2+ oscillations, at baseline and after thrombin application. Loss of Orai1 significantly reduced the frequency (H) and amplitude (I) of the Ca2+ fluctuations in the proximal and distal processes. Statistical analysis was done using unpaired t test. Bar graphs show means ± SEM. *P < 0.05, **P < 0.01.
Examination of GCaMP6f-expressing astrocytes using 2PLSM revealed an intricate pattern of astrocyte arborization expected of astrocytes in situ. In contrast to cultured astrocytes, astrocytes in situ showed robust on-going spontaneous activity seen as transient rises in Ca2+ in their soma and in the processes (movie S3). To compare the behavior of WT and Orai1 KO astrocytes, we mapped Ca2+ fluctuations in regions of interest (ROIs) in three anatomically defined compartments: the soma, the primary proximal processes coming off the cell body, and the distal tertiary processes (Fig. 6, B and C, and fig. S7A). These measurements showed that WT astrocytes showed spontaneous Ca2+ oscillations, which occurred at a relatively low frequency of 0.5 ± 0.14/min in the soma but at considerably higher frequencies in the proximal and distal processes (2.7 ± 0.4/min and 5.0 ± 0.4/min, respectively) (Fig. 6D). We observed Ca2+ signals with a traveling wave-like quality, as well as more compartmentalized, local Ca2+ signals in the processes (movies S3 and S4), similar to the observations by Srinivasan et al. (19). Consistent with previous observations (19, 55), spontaneous Ca2+ activity declined in the absence of extracellular Ca2+, indicating that it critically depends on Ca2+ entry across the plasma membrane (fig. S7B).
To examine how these Ca2+ signals are altered by GPCR stimulation, we used the PAR agonist thrombin, which evokes robust Orail-dependent Ca2+ signals in astrocytes as described above (Fig. 2C and fig. S2, C and D). Application of thrombin increased Ca2+ activity in all three astrocyte compartments—soma, proximal processes, and distal processes—with the frequency of somatic Ca2+ oscillations in WT astrocytes increasing nearly fivefold (from 0.5 ± 0.14 to 2.3 ± 0.5/min) (Fig. 6D). Thrombin also significantly increased both the amplitude and frequency of Ca2+ fluctuations in the proximal and distal processes (Fig. 6, D and E), with the amplitude of Ca2+ fluctuations in the proximal processes increasing by 65% and those in the distal processes increasing by 45%. Analysis of the amplitude distributions revealed that the increase in the overall amplitude of the thrombin-evoked Ca2+ spikes occurred primarily because of emergence of large-amplitude oscillations in the proximal and distal processes (Fig. 6, F and G, and fig. S7, C and D).
Examination of the behavior of Orail KO astrocytes revealed that, at baseline, the frequency and amplitude of Ca2+ oscillations in the cell body and proximal processes were similar to those seen in WT astrocytes (Fig. 6H). The only notable difference from WT astrocytes was a modest decline (~30%) in the frequency of baseline Ca2+ oscillations in the distal processes (Fig. 6H). However, the amplitude of these signals was comparable to those seen in WT astrocytes (Fig. 6I). Thus, these results suggest that, in unstimulated astrocytes, CRAC channels make only minor contributions to spontaneous baseline Ca2+ signals.
In response to thrombin, however, Orail KO astrocytes displayed several major differences from WT astrocytes. First, in contrast to the marked enhancement of Ca2+ fluctuations seen in WT astrocytes, thrombin failed to boost the frequency of Ca2+ fluctuations in Orail KO astrocytes (Fig. 6D). In all three compartments (cell body, proximal processes, and distal processes), the rate of Ca2+ fluctuations remained unchanged after thrombin administration. As a result, the frequency of Ca2+ fluctuations after thrombin stimulation was markedly lower in Orail KO astrocytes compared to stimulated WT astrocytes (Fig. 6H). Second, unlike WT astrocytes, Orail KO astrocytes also did not show an increase in the amplitude of Ca2+ fluctuations in response to thrombin administration (Fig. 6E). The amplitude of Ca2+ fluctuations after thrombin stimulation was also lower in Orail KO astrocytes compared to stimulated WT astrocytes in the distal processes (Fig. 6I). A direct comparison of distribution of the amplitudes of Ca2+ spikes further revealed that the proximal and distal processes of Orail KO astrocytes exhibited a significant lack of larger amplitude events seen in WT astrocytes in response to thrombin stimulation (Fig. 6, F and G, and fig. S7C). Together, these results indicate that loss of Orail substantially attenuates the frequency and amplitude of Ca2+ fluctuations both in the cell body and processes of astrocytes in situ after GPCR stimulation.
Astrocyte Orai1 channels regulate GABAergic transmission to CA1 pyramidal neurons
The above findings indicating that Orail channels function as a major route of Ca2+ entry in astrocytes to stimulate gliotransmitter release led us to next examine the role of this pathway for modulating the activity of neighboring hippocampal neurons. To investigate this question, we used hippocampal CAl slice electrophysiology to monitor the effects of astrocyte-mediated release of gliotransmitters on hippocampal neurons. Thrombin-mediated stimulation of PARs expressed in astrocytes enhances the activity of GABAergic interneurons in the stratum radiatum region, likely through astrocytic release of ATP and stimulation of P2Y receptors on interneurons (57). The ensuing increase in electrical activity of interneurons is then detected as a slow burst of spontaneous inhibitory postsynaptic currents (sIPSCs) on CAl pyramidal neurons (57, 58). We examined the role of astrocyte Orail channels for this effect by recording IPSCs in CAl pyramidal neurons using patch-clamp electrophysiology in hippocampal slices.
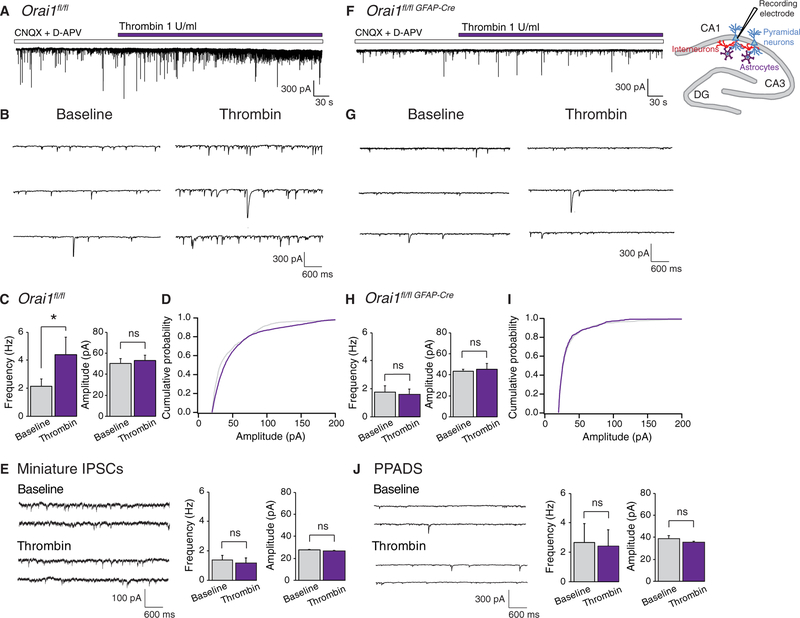
Consistent with previous reports (57, 58), we found that administration of thrombin to hippocampal slices evoked an increase in sIPSCs on CA1 pyramidal neurons that developed gradually over tens of seconds (Fig. 7, A and B). The frequency of sIPSCs increased twofold after thrombin administration, without an obvious change in the average amplitude of the events (Fig. 7, C and D). This result indicates that stimulation of astrocyte Ca2+ signaling by thrombin increases the activity of GABAergic interneurons in the CA1 hippocampus. Moreover, the thrombinmediated IPSC burst required action potential activity as measurements of the quantal miniature ISPC (mIPSC) responses in the presence of tetrodotoxin (TTX) showed no change in the mIPSC frequency or amplitude (Fig. 7E).
Fig. 7. Astrocyte Orai1 channels regulate GABAergic input to CA1 pyramidal cells.
(A) Administration of thrombin evokes a burst of spontaneous IPSCs on Orail (WT) CA1 pyramidal neurons. Patch-clamp slice recordings were performed from CA1 pyramidal neurons held at −70 mV. (B) sIPSC traces from the experiment in (A) shown on an expanded timescale. (C) Summary of sIPSC frequency and amplitude in CA1 neurons from WT slices before and after application of thrombin. Thrombin evokes an increase in sIPSC frequency with no change in overall amplitude in WT slices (*P = 0.02 by paired t test, n = 8 cells). (D) Amplitude distribution of the sIPSC events in WT slices. (E) Thrombin does not alter the frequency or amplitude of mIPSCs in WT slices. mIPSCs were isolated in the presence of 1 μM TTX (n = 8 cells). (F) The thrombin- induced sIPSC response in CA1 neurons is abolished in Orai1fl/fl GFAP-Cre slices. (G) sIPSC traces from the experiment in (F) shown on an expanded timescale. (H) Summary of sIPSC frequency and amplitude in Orai1fl/fl GFAP-Cre slices before and after application of thrombin (n = 6 cells). (I) Amplitude distribution of the sIPSC events in Orai1 KO slices. (J) The broad-spectrum ATP receptor inhibitor PPADS (30 μM) abolishes the thrombin-mediated increase in frequency of sIPSCs in WT slices (n = 4 cells). Bar graphs show means ± SEM. *P < 0.05 by paired t test.
In conditional Orai1 KO (Orai1fl/fl GFAP-Cre) mice, we observed that the frequency and amplitude of sIPSCs at baseline were unchanged compared to WT astrocytes (Fig. 7, F to I). This result suggests that, in the absence of exogenous agonists, the inhibitory tone on CA1 pyramidal neurons was not altered by loss of astrocytic Orai1 channels. However, the thrombin-mediated enhancement of sIPSC response seen in WT mice was abolished in the Orai1fl/fl GFAP-Cre mice (Fig. 7, F to H). As a consequence, steady-state IPSC activity after thrombin administration was markedly lower in astrocytes from Orai1fl/fl GFAP-Cre mice compared to WT mice (Fig. 7, C and H). Further, administration of the broad-spectrum ATP receptor antagonist PPADS abolished the thrombin-mediated IPSC burst on CA1 pyramidal cells in WT slices (Fig. 7J), a finding consistent with previous reports indicating that astrocytic release of ATP modulates the activity of neighboring interneurons (52, 57, 58). Because PARs mediate Ca2+ signaling primarily in astrocytes but not in CA1 neurons (36, 37), these results suggest that thrombin-evoked activation of CA1 astrocytes results in the release of gliotransmitters to stimulate the excitability of nearby CA1 interneurons, which in turn increases GABAergic transmission to CA1 pyramidal neurons. Collectively, these results indicate that Orai1 channels in astrocytes are critical for astrocyte-mediated stimulation of stratum radiatum interneurons and astrocyte-mediated increase in tonic inhibition of CA1 pyramidal neurons.
DISCUSSION
Despite evidence demonstrating that the Ca2+ excitability of astrocytes is important for many functions including the release of gliotransmitters (6, 50, 52, 59–63), the identity of the specific pathways that generate astrocyte Ca2+ signals, and how these are linked to effector functions remains poorly understood. In this study, we found that CRAC channels formed by Orai1 and STIM1 are a major route of Ca2+ influx in hippocampal astrocytes and that their activation generates sustained Ca2+ signals and oscillations in response to stimulation of various GPCRs. Moreover, we showed that the CRAC channelmediated Ca2+ signals triggered gliotransmitter exocytosis and secretion of the gliotransmitter ATP. The release of ATP from astrocytes and the ensuing activation of purinergic receptors stimulated hippocampal interneurons to increase GABAergic transmission on CA1 pyramidal cells. These results reveal a function of CRAC channels as important regulators of astrocyte gliotransmission and identify astrocytic Orail channels as a new target for modulating neuronal activity.
We demonstrated that cortical and hippocampal astrocytes exhibited SOCE with the pharmacological and biophysical hallmarks of CRAC channels, including blockade by low concentrations of La3+, modulation by 2-APB, and inhibition by the CRAC channel inhibitor BTP2. SOCE in astrocytes was abrogated by conditional KO of Orail and STIM1, consistent with previous observations (22, 24, 64). Our data do not directly rule out a role for the CRAC channel molecules Orai2, Orai3, and STIM2. However, TG-mediated SOCE was >95% abolished by deletion of Orail and STIM1, indicating that Orail and STIM1 are essential for SOCE in hippocampal astrocytes. Likewise, loss of Orail abrogated sustained and oscillatory Ca2+ signals evoked by the GPCR agonists ATP, UTP, and thrombin without detectably affecting Ca2+ release from intracellular stores. These results indicate that CRAC channels function as a major route of Ca2+ entry in hippocampal astrocytes after stimulation of GPCRs.
Astrocyte morphology in intact tissue is complex, with numerous fine networks of processes extending from the soma. Unlike astrocytes in culture, measurements of astrocyte Ca2+ signals in brain slices using GCaMP6f revealed substantial spontaneous activity especially in the proximal and distal processes. These Ca2+ fluctuations were reduced in Ca2+-free extracellular solutions, indicating that they were mediated in large part by transmembrane Ca2+ fluxes, in agreement with conclusions from past reports (19, 55, 65). However, loss of Orail elicited only a modest decrease in Ca2+ signaling at rest, indicating that Orail does not substantially account for the fluctuations seen in unstimulated cells. By contrast, stimulation of astrocytes in tissue slices evoked an increase in Ca2+ fluctuations that were dependent on the presence of Orail channels. Specifically, the thrombin-mediated increase in the frequency of Ca2+ fluctuations in the cell body and processes was essentially eliminated in the absence of Orail channels (Fig. 6, D and H). Loss of Orail also attenuated the amplitude of the thrombin-mediated Ca2+ rises in the distal processes. Because astrocytes express a large repertoire of GPCRs for hormones and neurotransmitters (66) whose activation is linked to many downstream effects including gliotransmitter release, cytokine production, and proliferation (4, 23, 38, 57), the finding that Orail channels play an essential role in triggering astrocytic Ca2+ elevations in response to GPCR activation suggests that CRAC channels could serve as a key Ca2+ entry pathway for driving these and other downstream effector functions.
Consistent with this possibility, measurements of vesicular exocytosis using spH and FM1–43 showed that knocking out Orail impaired GPCR-evoked gliotransmitter release (Figs. 3 to 5). Specifically, spH measurements showed that the increase in vesicular exocytosis triggered by TG, UTP, or thrombin was essentially eliminated in Orail KO astrocytes. Likewise, FMl-43 measurements similarly showed that loss of Orail markedly attenuated the extent of FMl-43 destaining. Last, direct measurements of ATP showed that the release of this key gliotransmitter was impaired in Orail KO astrocytes. These results indicate that CRAC channels are essential for evoked vesicular exocytosis after stimulation of purinergic receptors and PARs in astrocytes.
The loss of Orail did not impair Ca2+ store release in response to stimulation of P2Y receptors and PARs (Fig. 2) yet evoked vesicular exocytosis, and ATP secretion was markedly impaired (Figs. 4 and 5). The inability of IP3R-mediated store release in the absence of Ca2+ influx to evoke substantive vesicular exocytosis points to an essential requirement for Ca2+ entry across the plasma membrane to evoke exocytosis from astrocytes. This conclusion challenges prevailing viewpoints that have attributed GPCR-mediated Ca2+ elevations and gliotransmitter release primarily to Ca2+ release from stores (12,17, 67, 68). However, these previous studies of IP3R-medidated Ca2+ transients did not directly probe whether GPCR-evoked Ca2+ signals in fact are the exclusive result of Ca2+ release from stores or also include Ca2+ entry across the plasma membrane through store-operated channels. On the basis of results presented here, we favor the interpretation that store release alone is insufficient and that Ca2+ entry through SOCE is essential for vesicular exocytosis. The ability of the slow Ca2+ chelator EGTA to block global Ca2+ signals but not vesicular exocytosis as effectively as the fast Ca2+ chelator BAPTA is also consistent with this notion, reaffirming the importance of local Ca2+ signals close to sites of Orail channels for initiating exocytosis.
A notable feature of astrocytic vesicular release is its slow and sustained nature, which differs from the considerably faster and precisely timed nature of exocytosis in neurons (69). We observed that the increase in the rate of exocytosis developed slowly, especially after TG stimulation, and was typically maintained for minutes after agonist administration (Figs. 3 and 4). These observations are consistent with previous findings showing delays in exocytosis of several seconds after cell stimulation (26, 27, 53, 70). We believe that the slow kinetics of astrocytic exocytosis likely reflects the slow kinetics of CRAC channel activation, which requires accumulation of STIM1 and trapping of Orail at the ER-plasma membrane junctions (20). The slow and distributed nature of astrocyte gliotransmitter release suggests that, rather than affecting individual fast synaptic events, astrocytes play a role in modulating the homeostatic state or global activity of neuronal circuits by targeting extrasynaptic low-affinity receptors (15).
To examine the implications of Orail-mediated gliotransmitter release on neural network activity, we used slice electrophysiology to monitor astrocyte-mediated modulation of interneuron activity. As shown in previous reports (56, 57), stimulation of astrocytes by the PAR agonist thrombin increased IPSC activity on CAl pyramidal neurons on slow timescales of tens of seconds lasting several minutes (Fig. 7). The thrombinmediated increase in tonic inhibition on CAl pyramidal neurons was lost in slices from Orai1f/f GFMP-Cre mice, indicating that astrocyte Orail channels have a vital role in driving the interneuron-mediated inhibition of CAl neurons. Moreover, the ATP receptor antagonist PPADS abolished the thrombin-mediated IPSC burst in slices from WT mice, consistent with a model in which astrocyte stimulation enhances interneuron activity through Orail-dependent release of ATP. These findings agree with previous observations showing that activation of astrocytes increases extracellular ATP levels, which can modulate neuronal (and astrocytic) function in myriad ways including increased inhibition in the neocortex (52, 56) and increased interneuron activity in the hippocampus CAl (56, 57). Together, these results indicate that, by driving astrocyte Ca2+ signaling and gliotransmitter release, astrocyte CRAC channels are likely to have a critical role in modulating hippocampal excitability under physiological conditions and may influence neuronal network activity in pathological conditions such as epilepsy and stroke.
MATERIALS AND METHODS
Transgenic mice
C57BL/6 mice were cared for in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals. Animals were group-housed in a sterile ventilated facility, under standard housing conditions (l2:l2-hour light/dark cycle with lights on at 7:00 a.m. and temperatures of 20° to 22°C with ad libitum access to water and food), and maintained with in-house breeding colonies. Male and female mice were used in approximately equal numbers. All research protocols were approved by the Northwestern University Institutional Animal Care and Use Committee.
Tissue-specific deletion of Orail in the brain was accomplished as previously described (29). Briefly, Orai1fl/fl mice (provided by Amgen Inc.) and Orai1fl/+ were crossed with nestin-Cre mice (00377l from the Jackson Laboratory) to generate Orai1fl/fl nestin-Cre (brain-specific KO) and Orai1fl/+ nestin-Cre (brain-specific heterozygote). In addition to conditional deletion of Orail in the nervous system, the Orai1fl/fl × nestin-Cre cross also produced germline transmission in some instances, resulting in Orai1fl/− and Orai1fl/− nestin-Cre genotypes, which were used in some cases for Ca2+ imaging experiments of Orail HET and KO cultures, as indicated in the figure legends. To delete Orail selectively in astrocytes, Orai1fl/fl mice were crossed with mGFAP-Cre mice (0l2887 from the Jackson Laboratory) to yield Orai1fl/fl GFAP-Cre mice. In this line, Cre recombinase is controlled by a mouse GFAP regulatory sequence targeted to postnatal astrocytes. STIMl KO mice were prepared by crossing STIM1fl/fl (courtesy of S. Feske, New York University) with nestin-Cre mice to obtain STM1fl/fl nestin-Cre mice.
Primary cultures
Primary astrocytes were isolated from neonatal (P0 to P3) mice by standard techniques for astrocytes (32, 71) with minor modifications. Hippocampi or cortices were dissected, and meninges were removed under a dissection microscope in 4°C dissection medium [l0 mM Hepes in Hanks’ balanced salt solution (HBSS)]. The tissue was minced and trypsinized (0.25% trypsin; Invitrogen) for l0 min in a 37°C water bath. Tissue was washed twice with HBSS and dissociated gently by trituration in culture media consisting of Dulbecco’s modified Eagle’s medium with l0% fetal bovine serum and l% penicillin-streptomycin solution. Dissociated cells were filtered through a 70-μm strainer to collect cell suspension and cultured in 25-mm2 tissue culture flasks with l0 ml of medium. Half of the medium was exchanged every 3 to 4 days, and microglia were removed by forcefully shaking by hand for l0 to l5 s before each medium change. After cells reached near confluence (l2 to l4 days in vitro), medium was removed from the cells and exchanged with preheated trypsin-EDTA (0.05%). After 5 min in the incubator, culture medium was added to inactivate the trypsin, and cells were collected and centrifuged for 5 min. The supernatant was removed, and the cell pellet was resuspended in culture medium. Cells were plated on poly-L-lysine-coated glass-bottom dishes (MatTek, l4-mm diameter, l0,000 to l5,000 cells per coverslip), 24-well plates (l5,000 to 20,000 cells per well), or six-well plates (~l00,000 cells per well). Plated astrocytes were maintained in the incubator and used in experiments after 2 days and within l to 2 weeks of plating. Half of the medium was exchanged with fresh medium every 4 days.
Stellate-like astrocytes were cultured using methods adapted from the recently developed AWESAM protocol (32, 33) with minor modifications. Astrocytes were cultured in flasks as described above. On days 7 to 10 in vitro, cells were trypsinized as described above and resuspended in NB+ medium containing Neurobasal (Invitrogen) with heparin-binding epidermal growth factor (HBEGF) (5 ng/ml), 2% B-27 supplement (Invitrogen), 2 mM L-glutamine, and 1% penicillinstreptomycin solution. Cells were plated on poly-L-lysine–coated glass-bottom dishes (5000 to 10,000 cells per coverslip) and maintained in the incubator for at least 1 week before use in experiments.
Primary neuronal hippocampal cultures were prepared from neonatal (P0 to P1) mice (72). Hippocampi were dissected as described above. Once the tissue was trypsinized and washed with HBSS, it was dissociated gently by trituration in neuronal media consisting of Neurobasal supplemented with 2% B-27, 2 mM L-glutamine, and 1% penicillin-streptomycin. Cells were plated on poly-D-lysine- coated glass-bottom dishes (~20,000 cells per coverslip) or six-well plates (~200,000 cells per well) and maintained in the incubator for 2 to 3 weeks. Half of the neuronal medium was exchanged with fresh medium once a week.
DNA purification
Cultured hippocampal astrocytes or neurons were detached from six-well plates using a cell scraper. Detached cells and media were transferred to a 1.5-ml microcentrifuge tube and centrifuged for 5 min at 300g. Supernatant was removed, and the cell pellet was re-suspended in phosphate-buffered saline (PBS). Total DNA was purified using the DNeasy kit (Qiagen), according to the manufacturer’s instructions for cultured cells. Orai1 gene deletion was checked by PCR using the following primers: 5′-GGGACAAAACACTAACCTGT-CAT-3′, 5′-GGAGTAGAATTCAGTGGGAGAGT-3′, and 5′-TAT-GGTAAGGCTGGGAGACAC-3′. The expected sizes of the PCR products were as follows: WT, ~131 base pairs (bp); floxed, ~257 bp; and KO ~207 bp.
Viral microinjections
Postnatal days 35 to 57 Orai1fl/fl GFAP-Cre and WT littermate mice were deeply anesthetized with isoflurane and head-fixed on a stereotaxic frame. Ophthalmic ointment was applied to protect the eyes during surgery. Thermal support was provided using a feedback-controlled heating pad (Warner). Mice were given preoperative analgesic coverage (buprenorphine, 0.3 mg/kg, subcutaneously (sc)]. Small craniotomies were performed directly over hippocampus in the left hemisphere. The stereotaxic coordinates were as follows: 2.0 mm posterior to bregma, 1.5 mm lateral to midline, and 1.6 mm ventral to the pial surface. Injection pipettes were fabricated from glass capillary micropipettes (Wiretrol II, Drummond Scientific Company) by pulling (PP-830, Narishige) to a fine tip and beveling (Micro Grinder EG-400, Narishige) to a sharp edge. Pipettes were backfilled with mineral oil and then loaded with viral vector by tip filling. Pipettes were advanced slowly to their targets where the viral vector carrying a construct coding for the Cre-independent GCaMP6f (AAV5 gfapABC1D-cyto-GCaMP6f; Addgene plasmid no. 52925 at a titer of 1.4 × 1013 viral genomes/ml) was injected at a volume of 0.5 ml over 5 min. Syringes were left in place for 10 min before retraction to allow for virus diffusion. Animals received postoperative analgesic coverage (meloxicam, 1.5 mg/kg, sc, once every 24 hours for 2 days).
Solutions and chemicals
The standard Ringer’s solution used for wide-field Ca2+ imaging studies contained the following: 155 mM NaCl, 4.5 mM KCl, 10 mM D-glucose, 5 mM Hepes, 1 mM MgCl2, and 2 mM CaCl2. The Ca2+-free Ringer’s solution contained 3 mM MgCl2, 1 mM EGTA (Sigma-Aldrich), and no added CaCl2. pH was adjusted to 7.4 with 1 N NaOH. Stock solutions of TG, 2-APB, and YM-58483 (BTP2; Calbiochem) were dissolved in dimethyl sulfoxide and used at the indicated concentrations. ATP (Sigma-Aldrich), UTP (Sigma-Aldrich), and L-glutamic acid (Sigma-Aldrich) were all dissolved in water and used at the indicated concentrations. Thrombin was dissolved in 0.1% albumin and used at the indicated concentrations. EGTA-AM and BAPTA-AM were obtained from Invitrogen and loaded into astrocytes by incubating the cells for 35 to 45 min with 5 μM AM buffer at 37°C.
For GCaMP6f imaging and electrophysiological recordings of slices, the external artificial cerebrospinal fluid (aCSF) solution contained the following: 125 mM NaCl, 2.4 mM KCl, 1.2 mM Na2PO4, 25 mM NaHCO3, 25 mM glucose, 2 mM CaCl2, and 1 mM MgCl2, equilibrated with 95% O2 and 5% CO2. Ca2+-free aCSF solution contained the following: 125 mM NaCl, 2.4 mM KCl, 1.2 mM Na2PO4, 25 mM NaHCO3, 25 mM glucose, and 3 mM MgCl2, with 1 mM EGTA, equilibrated with 95% O2 and 5% CO2. The internal solution for electrophysiological recordings contained the following: 95 mM CsF, 25 mM CsCl, 10 mM Hepes, 10 mM EGTA, 2 mM Mg-ATP, 0.3 mM Na3-guanosine triphosphate, 10 mM QX-314, 5 mM tetraethylammonium chloride (TEA-Cl), and 5 mM 4-aminopyridine (4-AP) (pH 7.3 with CsOH).
Wide-field Ca2+ imaging
Astrocytes grown on glass-bottom dishes were loaded with Fura-2 by incubating cells in 2 mM Fura-2–AM (Invitrogen) in growth medium for 35 min at 37°C. Fura-2–containing medium was washed off, and cells were incubated for an additional 5 to 10 min before imaging. All experiments were performed at room temperature. Single-cell [Ca2+]i measurements were performed as described previously (29). Image acquisition and analysis were performed using SlideBook (Denver, CO). Dishes were mounted on the stage of an Olympus IX71 inverted microscope, and images were acquired every 6 s at excitation wavelengths of 340 and 380 nm and an emission wavelength of 510 nm. For data analysis, ROIs were drawn around single cells, background was subtracted, and F340/F380 ratios were calculated for each time point. A rise in the ratio ofemission when excited at 340 nm over the ratio when excited at 380 nm indicated a rise in [Ca2+];.
[Ca2+]i was estimated from F340/F380 ratio using the standard equation: , where R is the F340/F380 fluorescence ratio and values of Rmin and Rmax were determined from an in vitro calibration of Fura-2 pentapotassium salt. β was determined from the Fmin/Fmax ratio at 380 nm and Kd is the apparent dissociation constant of Fura-2 binding to Ca2+ (132 nM). The values of these parameters were as follows: Rmin = 0.36, Rmax = 19.37,. For each cell, the rate of SOCE (Δ[Ca2+]i/Δt) was calculated from the slope of a line fitted to three points (18 s) after the readdition of 2 mM Ca2+ Ringer’s solution. Baseline [Ca2+]i was calculated by averaging [Ca2+] values over a 2-min baseline for each experiment. Store release was calculated by measuring the area under the curve during TG application in Ca2+-free solution.
Plasmids and transfection
Cultured astrocytes were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The E106A Orai1-YFP (yellow fluorescent protein) plasmid has been described previously (73). spH was a gift from E. Kavalali (University of Texas Southwestern). The TeTx light chain plasmid was purchased from Addgene (pGEMTEZ-TeTxLC, plasmid no. 32640). Experiments were performed 24 to 48 hours after transfection.
spH imaging
Astrocytes were transfected with 200 ng of spH plasmid per coverslip. After 24 to 48 hours, astrocyte growth medium was washed away and replaced with Ringer’s solution. Dishes were mounted on the stage of Nikon X1 Spinning Disk Confocal equipped with TIRF illumination. spH fusion events were visualized on an Andor iXon3 EMCCD (electron multiplying charge-coupled device) camera using a 60× objective and 488-nm laser filter set with the laser light brought to the angle for internal reflection. Images were acquired every 200 ms. Spontaneous events were imaged for a 2-min baseline before the solution was changed by pipette perfusion.
To determine the number, rate, and location of the vesicle fusion events, time-lapse images were analyzed with a MATLAB program developed in-house. Time-lapse images (in the form of 16-bit.tiff files) were imported into MATLAB as three-dimensional (3D) matrices of one intensity value per pixel. The total cell area was calculated in the first frame by using the MATLAB edge detection function and multiplying the number of pixels within the edge of the cell by the area in a pixel. Background was removed by averaging the mean background intensity and subtracting it from all pixels in each frame. To reduce potential confounding factors of cell movement during solution exchange, the frames during and immediately after cell movement (generally a total ~15 s) were not analyzed. To detect events, each pixel was analyzed throughout the time-lapse imaging run to detect local intensity peaks. These local intensity peaks were then analyzed to see whether they qualified as exocytotic events using criteria described by Schmoranzer et al. (74). Specifically, to count as an event, the intensity of the detected peak had to exceed a threshold determined by the mean intensity plus three SDs of that pixel in frames 50 to 5 time points before the peak (the four time points immediately preceding the peak were excluded from this calculation to account for the rise time of the signal). To account for the spatial spread of the fluorophore molecules, event spread was analyzed using two criteria: (i) The average of the four pixels immediately surrounding the peak had increased intensity compared with baseline in the after 0.6 s, and (ii) the central pixel intensity decreased from its peak value after 0.6 s. If local intensity peaks were found to be exocytotic events according to these criteria, then their coordnates in the matrix and times were stored so that their locations on the cell footprint and event times could be recovered.
FM1–43 imaging
Cultured astrocytes were incubated at room temperature in HBSS (with 1.2 mM Ca2+) containing 8 mM FM1–43 lipophilic styryl dye (Invitrogen). After a 10-min loading period, the cells were washed three times with Ca2+-free HBSS for 5 min before imaging. Fluorescence images were obtained at an excitation wavelength of 480 nm and an emission wavelength of 620 nm. Images were acquired every 15 s for 10 to 15 min, background-subtracted, and normalized to the value at t = 0.
ATP measurements
ATP released from astrocytes plated on 24-well plates was measured from the cell supernatant with the ENLITEN ATP luciferin-luciferase assay (Promega). Cells were washed twice with growth medium. After 30-min incubation at 37°C, medium was removed from cells and replaced with conditioned HBSS (with or without 1.2 mM Ca2+). The ectonucleotidase inhibitor ARL67156 (200 mM) was applied to each well. The HBSS solution was collected after 10 min, and 50 μl was mixed with 50 ml of luciferin/luciferase reagent. Bioluminescence was detected with a luminometer (Berthold Detection Systems). The intensity of the emitted light is proportional to ATP concentration. Data are expressed as a fold change in ATP concentration compared to unstimulated condition performed on the same day.
Immunostaining
Astrocytes plated on coverslips were fixed with 4% paraformaldehyde in PBS for 15 min at 4°C. Cells were washed with PBS, permeabi- lized by blocking in 4% bovine serum albumin/0.1% Triton X-100 in PBS for 1 hour. Cells were incubated with primary antibodies at 1:800 for GFAP (Sigma-Aldrich) and 1:200 for Orai1 (Alomone) overnight, followed by secondary antibody tagged to Alexa Fluor 488 or Alexa Fluor 594 at 1:500 for 1 hour. Nuclei were labeled with 4′,6-diamidino-2-phenylindole.
Slice preparation
Hippocampal slices were cut from the brains of 3- to 5-week-old mice for electrophysiology and 14 to 22 days after viral injections for GCamP6f imaging. Animals were deeply anesthetized with isoflurane, and the brains were removed quickly. Horizontal slices, either 250 μm (for GCamP6f imaging) or 300 μm (for slice electrophysiology), were cut using a tissue slicer (Compresstome model VF-200–0Z, Precisionary Instruments) in ice-cold sucrose aCSF. For the electro- physiological slice experiments, the composition of this solution was as follows: 85 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 25 mM NaHCO3, 25 mM glucose, 75 mM sucrose, 0.5 mM CaCl2, and 4 mM MgCl2, saturated with 95% O2 and 5% CO2. For GCamP6f imaging, slices were cut in a solution containing the following: 110 mM choline chloride, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 25 mM D-glucose, 11.6 mM ascorbic acid, 3.1 mM pyruvic acid, 7 mM MgCl2, and 0.5 mM CaCl2, saturated with 95% O2 and 5% CO2. Slices were then quickly transferred into a recovery chamber with aCSF solution maintained at 30°C and containing the following: 125 mM NaCl, 2.4 mM KCl, 1.2 mM Na2PO4, 25 mM NaHCO3, 25 glucose, 1 mM CaCl2, and 2 mM MgCl2, saturated with 95% O2 and 5% CO2. After 30 min in the recovery chamber, slices were subsequently transferred into a storage chamber with aCSF containing 2 mM CaCl2, where they were stored for 0.5 to 6 hours until they were used for calcium imaging using 2PLSM or electrophysiology.
GCamP6f imaging using 2-photon microscopy
GCamP6f signals were imaged in astrocytes expressing GCamP6f in brain slices using a Nikon A1R-MP+ multiphoton microscope with a Chameleon Vision titanium sapphire laser and 25× Nikon objective lens. The data were collected using Nikon Elements software. Astrocytes were selected from the CA1 stratum radiatum region and were typically located ~50 to 120 mm below the surface. The hippocampus (DG, CA1, and CA3) was identified using autofluorescence visible at 750-nm excitation at low magnification. Once the stratum radiatum region was identified, excitation was switched to 950 nm to visualize single GCaMp6f-expressing cells at high magnification. Images were acquired at 1 frame/s. Cells were excited at 950 nm at a laser power of 10%. Imaging was performed at room temperature. During the experiment, slices were continuously perfused with aCSF solution, which was switched for aCSF with thrombin (10 U/ml) or Ca2+-free aCSF at the indicated times.
Slice electrophysiology
Patch-clamp recordings were performed using an Axopatch 200B amplifier interfaced to an ITC-18 input-output board and an iMac G5 computer. Currents were filtered at 2 kHz with a four-pole Bessel filter and sampled at 5 kHz. Stimulation, data acquisition, and analysis were performed using in-house routines developed on the Igor Pro platform. Recordings were performed from CA1 pyramidal neurons, and the holding potential was −70 mV. CA1 pyramidal cells were identified by their location in the pyramidal layer. IPSCs were isolated by the inclusion of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 mM) and D-(−)-2-amino-5-phosphonopentanoic acid (D-APV) (50 μM) in the extracellular solution to block glutamate receptors. mIPSCs were recorded in the presence of TTX (1 μM).
Data analysis
All data are expressed as means ± SEM. For datasets with two groups, statistical analysis was performed with two-tailed t test to compare between control and test conditions. For datasets with greater than two groups, one-way ANOVA followed by Tukey post hoc test was used to compare groups. Statistical analysis was performed with a confidence level of 95%, and results with P < 0.05 were considered statistically significant.
The average rate of spH-labeled exocytosis was calculated by measuring the slope of a line fit to the cumulative events plot for each cell. The baseline rate was measured by fitting a line to the first 120 s of the experiment. For measurements of agonist-evoked exocytosis, the cursors were set at least 120 s after agonist application and spanned 200 s. For the Ca2+-free exocytotic rate analysis, the cursors for the fit were set immediately after agonist application to obtain the store-release contribution to the release rate. No difference was seen in the exocytosis rates between Orai1fl/fl nestin-Cre and Orai1fl/l GFAP-Cre astrocytes, and therefore, the results from these cells were pooled into an “Orai1 KO” group.
spH event kinetics were measured with a line scan [12-pixel length (3.24 mm)] through the center of individual events. The fluorescence values were calculated as ΔF/F0, where F0 was the average of the baseline fluorescence for 5 s (25 time points) before the event. The half-width of each event was calculated using the Peak Analyzer function of OriginLab as the width of the curve at half of its maximum peak value.
The frequency and amplitude of Ca2+ fluctuations in GCaMP6f-expressing astrocytes in slices were analyzed using ImageJ and OriginLab. ROIs were drawn manually on the 2D maximum intensity projection of the time series (~540 s) in each experiment around the soma, the primary proximal processes coming off the cell body, and the distal tertiary processes (fig. S7A). The numbers and average sizes of the ROIs drawn around the soma, proximal processes, and distal processes were not statistically significantly different between WT and Orai1fl>fl GFAP-Cre cells. Background was measured in a region of the imaging field not containing any GCamP6f expression. The background-subtracted mean intensity of each ROI was measured at each time point using the ImageJ Multi Measure plugin, and F0 was calculated by averaging the first 20 time points for each ROI. The intensity of the GCamP6f fluctuations at each time point was calculated as ΔF/F0 for each ROI, and frequency and amplitude of the fluctuations were measured using Peak Analyzer function of OriginLab. A moving baseline with asymmetric least squares smoothing was used, and peaks were determined from the local maxima that exceeded a threshold of 10% over the local baseline. The frequency and amplitude for all the peaks in each ROI was averaged and calculated for each compartment (soma, proximal processes, and distal processes) in each cell. Analysis was done over a 3-min duration immediately before and at least 90 s after the administration of thrombin. Cells were excluded from analysis if they had noticeable z-drift or x-y drift during the experiment.
IPSCs were analyzed using MiniAnalysis software (Synaptosoft Inc.). Events were analyzed over a 5-min duration immediately before and 3 min after the administration of thrombin. The threshold for detection of events was set at 20 pA.
Supplementary Material
Fig. S1. Astrocytes exhibit SOCE mediated by Orai1 and STIM1.
Fig. S2. Stimulation of purinergic and PAR GPCRs activates SOCE in hippocampal astrocytes.
Fig. S3. Kinetics of individual spH fusion events in WT astrocytes.
Fig. S4. spH-monitored vesicular exocytosis in AWESAM stellate astrocyte cultures.
Fig. S5. Local Ca2+ signals contribute to CRAC channel–mediated vesicular exocytosis.
Fig. S6. Exocytosis as monitored by FM1–43 is impaired in Orai1 KO astrocytes.
Fig. S7. Ca2+ fluctuations in astrocyte processes in situ.
Movie S1. spH-monitored exocytotic events in a WT astrocyte.
Movie S2. spH-monitored exocytotic events in an Orai1 KO (Orai1fl/fl GFAP-Cre) astrocyte.
Movie S3. GCaMP6f signals monitored by 2PLSM in an astrocyte from a WT hippocampal slice.
Movie S4. GCaMP6f signals in an astrocyte from an Orai1 KO (Orai1fl/fl GFAP-Cre) hippocampal slice.
Acknowledgments:
We thank members of the laboratory for helpful discussions. We thank C. Maguire and M. Raineri Tapies for technical support with animal care, E. Kavalali (University of Texas Southwestern) for the gift of spH plasmids, and J. Rappaport for discussions on analysis of the spH fusion events. TIRF and multiphoton imaging work were performed at the Northwestern University Center for Advanced Microscopy supported by NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
Funding: This work was supported by NIH grants NS057499 and R01 GM114210 to M.P. A.B.T. was supported by NIH predoctoral fellowship F30 NS090817, the Julius B. Kahn fellowship, and the Medical Scientist Training Program.
Footnotes
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Kimelberg HK, Nedergaard M, Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7, 338–353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuchero JB, Barres BA, Glia in mammalian development and disease. Development 142, 3805–3809 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verkhratsky A, Nedergaard M, Hertz L, Why are astrocytes important? Neurochem. Res. 40, 389–401 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Verkhratsky A, Nedergaard M, Physiology of Astroglia. Physiol. Rev. 98, 239–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perea G, Araque A, GLIA modulates synaptic transmission. Brain Res. Rev. 63, 93–102 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Araque A, Astrocytes process synaptic information. Neuron Glia Biol. 4, 3–10 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Henneberger C, Papouin T, Oliet SHR, Rusakov DA, Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, Takano H, Moss SJ, McCarthy K, Haydon PG, Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Ishikawa T, Abe R, Nakayama R, Asada A, Matsuki N, Ikegaya Y, Astrocyte calcium signalling orchestrates neuronal synchronization in organotypic hippocampal slices. J. Physiol. 592, 2771–2783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X, Li L-P, Wang Q, Wu Q, Hu H-H, Zhang M, Fang Y-Y, Zhang J, Li S-J, Xiong W-C, Yan H-C, Gao Y-B, Liu J-H, Li X-W, Sun L-R, Zeng Y-N, Zhu X-H, Gao T-M, Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Araque A, Carmignoto G, Haydon PG, Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol. 63, 795–813 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Volterra A, Liaudet N, Savtchouk I, Astrocyte Ca2+ signalling: An unexpected complexity. Nat. Rev. Neurosci. 15, 327–335 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Parpura V, Store-operated calcium entry in neuroglia. Neurosci. Bull. 30, 125–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapham DE, Calcium signaling. Cell 131, 1047–1058 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD, What is the role of astrocyte calcium in neurophysiology? Neuron 59, 932–946 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton NB, Attwell D, Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 11, 227–238 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Parpura V, Grubisic V, Verkhratsky A, Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta 1813, 984–991 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Kanemaru K, Sekiya H, Xu M, Satoh K, Kitajima N, Yoshida K, Okubo Y, Sasaki T, Moritoh S, Hasuwa H, Mimura M, Horikawa K, Matsui K, Nagai T, Iino M, Tanaka KF, In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca2+ indicator. Cell Rep. 8, 311–318 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS, Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18, 708–717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakriya M, Lewis RS, Store-Operated Calcium Channels. Physiol. Rev. 95, 1383–1436 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo Y-H, Matrougui K, Mongin AA, Trebak M, STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 465, 1249–1260 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon J, An H, Sa M, Won J, Shin JI, Lee CJ, Orai1 and Orai3 in combination with stim1 mediate the majority of store-operated calcium entry in astrocytes. Exp. Neurobiol. 26, 42–54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Xia J, Munoz FM, Manners MT, Pan R, Meucci O, Dai Y, Hu H, STIMs and Orai1 regulate cytokine production in spinal astrocytes. J. Neuroinflammation 13, 126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papanikolaou M, Lewis A, Butt AM, Store-operated calcium entry is essential for glial calcium signalling in CNS white matter. Brain Struct. Funct. 222, 2993–3005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowser DN, Khakh BS, Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. Proc. Natl. Acad. Sci. U.S.A. 104, 4212–4217 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malarkey EB, Parpura V, Temporal characteristics of vesicular fusion in astrocytes: Examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J. Physiol. 589, 4271–4300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T, Sun L, Xiong Y, Shang S, Guo N, Teng S, Wang Y, Liu B, Wang C, Wang L, Zheng L, Zhang CX, Han W, Zhou Z, Calcium triggers exocytosis from two types of organelles in a single astrocyte. J. Neurosci. 31, 10593–10601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jairaman A, Prakriya M, Molecular pharmacology of store-operated CRAC channels. Channels 7, 402–414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Somasundaram A, Shum AK, McBride HJ, Kessler JA, Feske S, Miller RJ, Prakriya M, Store-operated CRAC channels regulate gene expression and proliferation in neural progenitor cells. J. Neurosci. 34, 9107–9123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H, Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science 294, 2186–2189 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Bates B, Rios M, Trumpp A, Chen C, Fan G, Bishop JM, Jaenisch R, Neurotrophin-3 is required for proper cerebellar development. Nat. Neurosci. 2, 115–117 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Wolfes AC, Ahmed S, Awasthi A, Stahlberg MA, Rajput A, Magruder DS, Bonn S, Dean C, A novel method for culturing stellate astrocytes reveals spatially distinct Ca2+ signaling and vesicle recycling in astrocytic processes. J. Gen. Physiol. 149, 149–170 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfes AC, Dean C, Culturing in vivo-like murine astrocytes using the fast, simple, and inexpensive AWESAM protocol. J. Vis. Exp., e56092 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fields RD, Burnstock G, Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Ubl JJ, Reiser G, Four subtypes of protease-activated receptors, co-expressed in rat astrocytes, evoke different physiological signaling. Glia 37, 53–63 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF, Protease-activated receptor-1 in human brain: Localization and functional expression in astrocytes. Exp. Neurol. 188, 94–103 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF, Astrocytic control of synaptic NMDA receptors. J. Physiol. 581, 1057–1081 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS, Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 28, 6659–6663 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sofroniew MV, Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20, 160–172 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon R, Traynelis SF, Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. J. Neurosci. 25, 4319–4329 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrone J, Li Z, Murthy VN, Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat. Protoc. 1, 2970–2978 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Jahn R, Hanson PI, Otto H, Ahnert-Hilger G, Botulinum and tetanus neurotoxins: Emerging tools for the study of membrane fusion. Cold Spring Harb. Symp. Quant. Biol. 60, 329–335 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Prakriya M, Lingle CJ, Activation of BK channels in rat chromaffin cells requires summation of Ca(2+) influx from multiple Ca(2+) channels. J. Neurophysiol. 84, 1123–1135 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Adler EM, Augustine GJ, Duffy SN, Charlton MP, Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J. Neurosci. 11, 1496–1507 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rozov A, Burnashev N, Sakmann B, Neher E, Transmitter release modulation by intracellular Ca2+ buffers in facilitating and depressing nerve terminals of pyramidal cells in layer 2/3 of the rat neocortex indicates a target cell-specific difference in presynaptic calcium dynamics. J. Physiol. 531, 807–826 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deisseroth K, Bito H, Tsien RW, Signaling from synapse to nucleus: Postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron 16, 89–101 (1996). [DOI] [PubMed] [Google Scholar]
- 47.Kar P, Nelson C, Parekh AB, Selective activation of the transcription factor NFAT1 by calcium microdomains near Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 286, 14795–14803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Tsien RW, Properties of synaptic transmission at single hippocampal synaptic boutons. Nature 375, 404–408 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Betz W, Bewick G, Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255, 200–203 (1992). [DOI] [PubMed] [Google Scholar]
- 50.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S, Astrocytes control breathing through pH-dependent release of ATP. Science 329, 571–575 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SB, ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y, Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLOS Biol. 12, e1001747 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R, Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 282, 28749–28758 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu X.-s., Duan S, Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9, 945–953 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Rungta RL, Bernier L-P, Dissing-Olesen L, Groten CJ, LeDue JM, Ko R, Drissler S, MacVicar BA, Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64, 2093–2103 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Tan Z, Liu Y, Xi W, Lou H-F, Zhu L, Guo Z, Mei L, Duan S , Glia-derived ATP inversely regulates excitability of pyramidal and CCK-positive neurons. Nat. Commun. 8, 13772 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowser DN, Khakh BS, ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 24, 8606–8620 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maggio N, Cavaliere C, Papa M, Blatt I, Chapman J, Segal M, Thrombin regulation of synaptic transmission: Implications for seizure onset. Neurobiol. Dis. 50, 171–178 (2013). [DOI] [PubMed] [Google Scholar]
- 59.Malarkey EB, Ni Y, Parpura V, Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56, 821–835 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS, TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 33, 10143–10153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS, TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15, 70–80 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malarkey EB, Parpura V, Mechanisms of glutamate release from astrocytes. Neurochem. Int. 52, 142–154 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perea G, Navarrete M, Araque A, Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 32, 421–431 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Moreno C, Sampieri A, Vivas O, Peña-Segura C, Vaca L, STIM1 and Orai1 mediate thrombin-induced Ca2+ influx in rat cortical astrocytes. Cell Calcium 52, 457–467 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Haustein MD, Kracun S, Lu X-H, Shih T, Jackson-Weaver O, Tong X, Xu J, Yang XW, O’Dell TJ, Marvin JS, Ellisman MH, Bushong EA, Looger LL, Khakh BS, Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Porter JT, McCarthy KD, Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 51, 439–455 (1997). [DOI] [PubMed] [Google Scholar]
- 67.Fiacco TA, Agulhon C, Taves SR, Petravicz J, Casper KB, Dong X, Chen J, McCarthy KD, Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron 54, 611–626 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Fiacco TA, McCarthy KD, Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci. 38, 3–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sudhof TC, Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80, 675–690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pryazhnikov E, Khiroug L, Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56, 38–49 (2008). [DOI] [PubMed] [Google Scholar]
- 71.McCarthy KD, de Vellis J, Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Beaudoin GMJ III, Lee S-H, Singh D, Yuan Y, Ng Y-G, Reichardt LF, Arikkath J, Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 7, 1741–1754 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M, STIM1-Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 586, 5383–5401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmoranzer J, Goulian M, Axelrod D, Simon SM, Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J. Cell Biol. 149, 23–32 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prakriya M, Lewis RS, Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J. Physiol. 536, 3–19 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG, Orail is an essential pore subunit of the CRAC channel. Naxture 443, 230–233 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Astrocytes exhibit SOCE mediated by Orai1 and STIM1.
Fig. S2. Stimulation of purinergic and PAR GPCRs activates SOCE in hippocampal astrocytes.
Fig. S3. Kinetics of individual spH fusion events in WT astrocytes.
Fig. S4. spH-monitored vesicular exocytosis in AWESAM stellate astrocyte cultures.
Fig. S5. Local Ca2+ signals contribute to CRAC channel–mediated vesicular exocytosis.
Fig. S6. Exocytosis as monitored by FM1–43 is impaired in Orai1 KO astrocytes.
Fig. S7. Ca2+ fluctuations in astrocyte processes in situ.
Movie S1. spH-monitored exocytotic events in a WT astrocyte.
Movie S2. spH-monitored exocytotic events in an Orai1 KO (Orai1fl/fl GFAP-Cre) astrocyte.
Movie S3. GCaMP6f signals monitored by 2PLSM in an astrocyte from a WT hippocampal slice.
Movie S4. GCaMP6f signals in an astrocyte from an Orai1 KO (Orai1fl/fl GFAP-Cre) hippocampal slice.