Abstract
Background:
Children with tuberous sclerosis complex (TSC), caused by pathogenic variants in TSC1/TSC2, are at risk for intellectual disability. TSC2 pathogenic variants appear to increase the risk, compared with TSC1. However, the effect of TSC2 pathogenic variants on early and specific domains of development hasn’t been studied. Using an extensively phenotyped group, we aimed to characterize differences in early intellectual development between genotypes.
Methods:
The study group (n = 92) included participants with TSC enrolled in a multicenter study involving genetic testing and detailed prospective phenotyping including the Mullen Scales of Early Learning, a validated measure of cognition, language, and motor development in babies and preschool children. Mean T-scores at 24 months for each Mullen Scales of Early Learning domain were calculated for children with, versus without, a TSC2 pathogenic variant. Multivariable linear regression models were used to compare the groups, adjusting for seizures.
Results:
T-scores on every Mullen Scales of Early Learning domain were significantly worse in the TSC2 group. Below average composite scores were present in three-fourths of the TSC2 group, compared with one-fourth of those without TSC2. Having a TSC2 pathogenic variant was associated with lower composite Mullen Scales of Early Learning scores, even when corrected for seizures.
Conclusions:
In a well-characterized patient population with standardized assessment of multiple aspects of development, we found that having a TSC2 pathogenic variant was associated with significantly lower Mullen Scales of Early Learning scores at age 24 months, independent of seizures. These data suggest that a baby with a TSC2 pathogenic variant is at high risk for significant developmental delays by 24 months.
Keywords: Tuberous sclerosis complex (TSC), Genotype, Phenotype, Developmental delay, Mullen scales of early learning (MSEL), Genotype-phenotype correlation, Cognition
Introduction
Tuberous sclerosis complex (TSC), which results from pathogenic variants in TSC1 and TSC2, is a genetic disorder characterized by tumor formation throughout the body, most commonly in the skin, brain, kidneys, heart, and eyes. In addition to the tumors, patients with TSC are at risk for neurological issues including seizures, developmental delay, intellectual disability (ID), and autism.1 There is a great deal of variability in terms of TSC-related phenotypes, including neurological symptoms. For example, some individuals present early in life with infantile spasms and extreme developmental delays, whereas others may go undiagnosed until a family member is identified. Because of the variability in outcomes, it is vital to identify at-risk individuals as early as possible to develop and apply appropriate intervention and counseling strategies. As TSC genotypes are often available early in life, leveraging this information to predict phenotype could be particularly powerful. This is especially true for individuals who are at risk of developmental delay, as intervention improves outcomes.
Pathogenic variants in TSC1 and TSC2 lead to improper regulation of the mammalian target of rapamycin signaling pathway and multiple downstream effects, including uncontrolled cell growth and proliferation.2,3 A clinical diagnosis of definite TSC can be made if a person has two major features or one major feature and at least two minor features.4 Notably, a pathogenic variant in TSC1 or TSC2 cannot be found in 10% to 15% of patients who meet a clinical diagnosis of definite TSC. The cause of TSC in these patients is thought to most likely be mosaicism5,6 or an intronic variant in TSC1 or TSC27 that is undetectable by current clinical tests. The possibility of another unidentified TSC locus also remains.
Genotype-phenotype correlations have been performed for many genetic disorders to determine if genotype can help predict prognosis and assist with management.8-10 Regarding learning, an important genotype-phenotype correlation for TSC is that TSC2 pathogenic variants have been associated with increased risk of ID.11-14 TSC2 pathogenic variants are also associated with increased risk of seizures,13 which have been associated with poorer developmental outcomes.15 They have also been associated with increased tuber burden,16 which has been associated with increased risk of seizures11 and severe cerebral disease.17 Despite this, important gaps exist in our understanding of TSC genotype-phenotype associations. For example, previous genotype-phenotype studies were based on self-reported or medical history of ID, not direct assessment. Furthermore, the effect of TSC2 variants on early or specific domains of development has not been studied.
Leveraging data from a well-characterized cohort of patients with TSC who have undergone genotyping and testing with Mullen Scales of Early Learning (MSEL), we aimed to better characterize differences in early motor, language, visual reception (i.e., visual perceptual ability), and global development between different TSC genotypes. We hypothesized that patients with TSC2 pathogenic variants would be more likely to have developmental delay at 24 months than those with TSC1 pathogenic variants or those with no mutation identified (NMI).
Materials and methods
Study population
The study was performed using data from the TSC Autism Center of Excellence Research Network study (). The inclusion and exclusion criteria for the study have been previously described.18 Briefly, the study enrolled children aged three to 12 months with a clinically, or genetically, confirmed diagnosis of TSC, who were tracked for up to age 36 months.
Ninety-two children who had been genotyped for variants in TSC1 and TSC2 and who had completed the MSEL assessment at 24 months were included in the present study. The 24-month time point was chosen to reflect early cognitive, language, and motor development at a stage when the divergence of abilities would likely start to be apparent. Patients tracked the presence of seizures and seizure types with a seizure diary. Patients also underwent serial electroencephalographies. Presence of seizures was determined by the medical personnel at their study site.
Genotyping
Sixty children were genotyped during routine clinical care. For the remaining 32 children, Sanger sequencing was performed on DNA from peripheral blood as part of the TSC Autism Center of Excellence Research Network trial to identify coding variants in TSC1 and TSC2. If a pathogenic variant was not identified through Sanger sequencing, multiplex ligation-dependent probe amplification was performed to detect deletions or duplications. Variants identified were classified using American College of Medical Genetics standards and guidelines.19 A participant was classified as NMI when both Sanger sequencing and multiplex ligation-dependent probe amplification failed to identify a pathogenic, or likely pathogenic, variant. Variants of uncertain significance, likely benign, or benign were categorized as NMI. Detailed TSC disease genotypes of these patients have been reported previously.20
Mullen Scales of Early Learning
The MSEL is a validated measure of cognition, language, and motor development in babies and young children.21 There are five scales: Gross Motor, Visual Reception, Fine Motor, Receptive Language, and Expressive Language, as well as an Early Learning Composite standard score. The five scales are measured using T-scores, with a mean of 50 and an S.D. of 10, whereas the composite score has a mean of 100 and an S.D. of 15. Scores on these five scales can be classified as very low (≤30), below average (31 to 39). average (40 to 60). above average (61 to 69), and very high (70 to 80).
Statistical analysis
The genotypes were categorized as (1) TSC1 pathogenic variant, (2) TSC2 pathogenic variant, and (3) NMI. Summary statistics describing the distributions of sex, ethnicity, gestational age, seizure status, and parental ages by genotype were computed. The mean T-score at 24 months for each MSEL domain was calculated for each category (TSC1 TSC2, and NMI). Because TSC2 pathogenic variants have been associated with more severe phenotypes than TSC1 or NMI,1,22 children with, versus without, a TSC2 pathogenic variant were also compared. Additional comparisons were made excluding patients (N = 3) with TSC2 pathogenic variants known to convey a milder phenotype.23,24 Chi-squared and Fisher's exact tests were used to describe differences in categorical variables, and the Kruskal-Wallis test was used to describe differences in the distributions of continuous variables. Multivariable linear regression models were used to assess the independent effects of TSC2 pathogenic variants and seizures on MSEL scores, adjusting for maternal age. Mean scores (± standard errors) in each domain for TSC2 and non-TSC2 groups were plotted at ages nine through 36 months, with the exception of Gross Motor, which is only measured in children aged up to 33 months. For all tests, statistical significance was defined as P < 0.05. All statistical analyses were performed in R, version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sixty-three patients had a TSC2 pathogenic variant, 13 had a TSC1 pathogenic variant, and 16 had NMI. All variants identified were germline, and none appeared to be mosaic. TSC1 pathogenic variants included frameshift (50%), nonsense (42%), and splice site (8%). TSC2 pathogenic variants included frameshift (28%), nonsense (23%), missense (23%), splice site (14%), and deletion (12%). Three of the patients had TSC2 missense variants (Arg622Trp and Arg1200Trp) that had been previously associated with a milder phenotype23,24 and unless otherwise specified, were included in the TSC2 group in analyses. There were no significant differences in sex, ethnicity, gestational age, and paternal age between the three groups: TSC1, TSC2, and NMI (Table 1). As previously reported13 there was a significant difference in seizure history between the three groups, with seizures being most common in patients with TSC2 pathogenic variants. Maternal age at birth was highest in the NMI group.
TABLE 1.
Child and Parental Characteristics by Genotype
| NMI (n = 16) |
TSC1 (n = 13) |
TSC2 (n = 63) |
P Value * |
|
|---|---|---|---|---|
| Child’s sex. n (%) | 0.14 | |||
| Female | 7 (43.3) | 4 (30.8) | 37 (58.7) | |
| Male | 9 (56.2) | 9 (69.2) | 26 (41.3) | |
| Child ethnicity, n (%) | 0.73 | |||
| Hispanic | 2 (12.5) | 3 (23.1) | 14 (22.2) | |
| Non-Hispanic | 14 (87.5) | 10 (76.9) | 49 (77.8) | |
| Term Birth, n (%) | 0.06 | |||
| No | 1 (6.2) | 2 (15.4) | 1 (1.6) | |
| Yes | 15 (93.8) | 11 (84.6) | 62 (98.4) | |
| Seizures, n (%) | <0.001 | |||
| No | 5 (31.2) | 10 (76.9) | 9 (14.3) | |
| Yes | 11 (68.8) | 3 (23.1) | 54 (85.7) | |
| Maternal age at birth, mean (S.D.) years | 34.4 (4.3) | 31.8 (6.1) | 30.8 (5.1) | 0.02 |
| Paternal age at birth, mean (S.D.) years | 36.8 (5.8) | 34.5 (4.5) | 33.0 (6.8) | 0.06 |
Abbreviation:
NMI = No mutation identified.
Bold values indicate significance.
For sex, the P value is derived from chi-squared test. For other categorical variables. P values were derived from Fisher’s exact test, and for continuous variables P values were derived from the Kruskal-Wallis rank sum test. The P value is representative of the difference across the three groups.
The T-scores on every MSEL domain were significantly different among the three groups. The visual reception, fine motor, and expressive language domains were all significantly lower (P < 0.001) among those in the TSC2 group. In addition, gross motor and receptive language scales were significantly lower in the TSC2 group (P = 0.001 and P = 0.007, respectively) (Supplemental Table 1). The MSEL composite scores were also statistically different among the three groups. Table 2 demonstrates that 73% of the patients with TSC2 pathogenic variants scored below average compared with 31% of the patients with TSC1 or 23% of the patients with NMI. Notably. 57% of patients with TSC2 pathogenic variants had “very low” scores. One of the two participants in the TSC2 group who scored “very high” had a pathogenic variant previously associated with a mild phenotype (Arg1200Trp). Two of the 13 participants in the TSC2 group who scored in the average range also had genotypes previously associated with mild phenotypes (Arg622Trp and Arg1200Trp).
TABLE 2.
Twenty-Four-Month Mullen Cutoff Scores by Genotype
| NMI n (%) |
TSC1 n (%) |
TSC2 n (%) |
P Value* |
|
|---|---|---|---|---|
| Early Learning Composite T-scores | <0.001 | |||
| Very high | 0 (0.0) | 0 (0.0) | 2 (3.2) | |
| Above average | 1 (6.2) | 2 (15.4) | 2 (3.2) | |
| Average | 10 (62.5) | 8 (61.5) | 13 (20.6) | |
| Below average | 4 (25.0) | 1 (7.7) | 10 (15.9) | |
| Very low | 1 (6.2) | 2 (15.4) | 36 (57.1) |
Abbreviation:
NMI = No mutation identified.
Bold value indicate significance.
The P value was derived from Fisher’s exact test.
In comparing the TSC2 group with those without a TSC2 pathogenic variant, patients with a pathogenic variant in TSC2 scored significantly lower in all domains of the MSEL (Table 3). In multivariable linear regression models, having a TSC2 pathogenic variant, as opposed to TSC1 or NMI. was associated with five- to 10-point reduction in MSEL T-scores after adjustment for seizures and maternal age. An additional one- to three-point reduction occurred when the three participants with known mild TSC2 variants were excluded from the TSC2 group (Supplemental Table 2). Presence of seizures was correlated with reduction (9-23 points) in MSEL T-scores in all domains independent of genotype (Table 4).
TABLE 3.
Comparison of 24-Month Mullen Scales T-Scores and Mullen Composite Score Patients With and Without a TSC2 Pathogenic Variant
|
TSC2 Mean (S.D.) |
TSC1/NMI Mean (S.D.) |
P Value * |
|
|---|---|---|---|
| Gross Motor T-Score† | 36.2 (11.4) | 44.9 (9.3) | <0.001 |
| Visual Reception T-Score† | 35.7 (14.7) | 50.5 (12.0) | <0.001 |
| Fine Motor T-Score† | 33.2 (12.4) | 44.0 (12.1) | <0.001 |
| Receptive Language† T-Score | 35.5 (15.6) | 46.2 (12.4) | 0.002 |
| Expressive Language T-Score† | 35.1 (11.8) | 46.5 (10.6) | <0.001 |
| Early Learning Composite Standard Score‡ | 74.3 (23.2) | 94.5 (18.9) | <0.001 |
Abbreviations:
NMI = No mutation identified.
Bold values indicate significance.
From the Kruskal-Wallis Rank Sum Test.
T-scores have a mean of 50 and an S.D. of 10.
Early Learning Composite score has a mean of 100 and an S.D. of 15.
TABLE 4.
Multivariable Linear Regression Models for the Effect of TSC2 Genotype and Presence of Seizures on 24 Month Mullen T-Scores
| TSC2, β (95% Cl)*,† | P Value | Seizures, β (95% Cl)‡ | P Value | |
|---|---|---|---|---|
| Gross Motor | −6.20 (−11.20, −1.20) | 0.016 | −9.50 (−14.68, −4.33) | <0.001 |
| Visual Reception | −10.01 (−16.58, −3.44) | 0.003 | −13.83 (−20.63, −7.02) | <0.001 |
| Fine Motor | −5.72 (−11.47, 0.02) | 0.051 | −13.27 (−19.21, −7.32) | <0.001 |
| Receptive Language | −5.16 (−12.17, 1.84) | 0.147 | −14.05 (−21.30, −6.80) | <0.001 |
| Expressive Language | −7.23 (−12.71, −1.74) | 0.010 | −10.65 (−16.33, −4.97) | <0.001 |
| Early Learning Composite | −10.79 (−21.08, −0.49) | 0.040 | −23.25 (−33.91, −12.59) | <0.001 |
Abbreviations:
β = Unstandardized coefficient
Cl = Confidence interval
Versus TSC1 or NMI.
Adjusted for seizure status and maternal age.
Adjusted for genotype (TSC vs not) and maternal age.
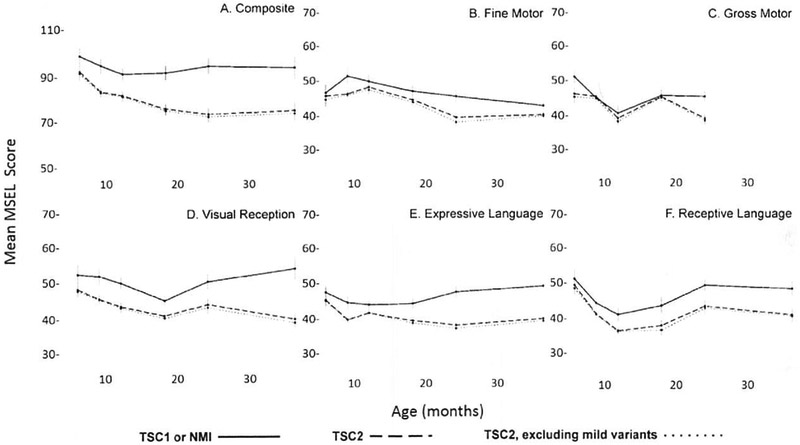
Mean trajectories for each domain and the composite score from nine through 36 months were plotted for the TSC1/NMI group, the TSC2 group, and the TSC2 group excluding children with variants known to confer a mild phenotype. Mean scores for the TSC2 group were below average (mean 50, S.D. = 10 for the individual domains; mean 100, S.D. = 15 for the composite scores) at all time points, in all categories (Fig). Mean scores for the TSC1/NMI group were consistently higher than those for the TSC2 group in all domains and the composite score. Mean scores for the TSC2 group excluding the three children with mild variants were consistently lower than those for the TSC2 group with all pathogenic variants included, although the magnitude of this difference was small.
FIGURE.
Trajectories of mean (±standard error) MSEL domain scores from nine to 36 months for participants with TSC1/NMI, TSC2, and TSC2 excluding those with pathogenic variants known to confer a mild phenotype. The composite scale (A) has a mean standard score of 100 with S.D. of 15. The other domains (B–F) have a mean T-score of 50 with S.D. of 10. Scores in the T5C2 group are consistently lower than those of TSC1/NMI group in all domains, with the lowest scores belonging to the TSC2 group, which excludes mild variants.
Discussion
Utilizing prospectively collected data from a study with standardized assessment of cognitive development by means of a validated tool, we found that patients with TSC2 pathogenic variants are significantly more likely to have developmental delay at 24 months than patients with TSC1 pathogenic variants or NMI. Although TSC2 pathogenic variants are known to be associated with a higher risk of ID in older children and adults,13,25 this study is the first to demonstrate the association of TSC2 pathogenic variants and cognitive delay in children as young as 24 months. It is also the first genotype-phenotype study of TSC to prospectively use a validated diagnostic measure of developmental status. Using this approach, we were also able to document that differences in developmental delay were global, present in every MSEL domain, along with the composite score. In addition, a greater proportion of patients with TSC2 pathogenic variants had composite scores in the lowest range (Mullen composite: standard score less than 70). Fifty-seven percent of the TSC2 group scored in the “very low” range for the composite score (compared with 15% and 6% in the TSC1 and NMI groups, respectively), indicating that the majority of people with TSC2 pathogenic variants are very delayed at 24 months. Trajectories suggest that the difference in development between patients with a TSC2 pathogenic variant, and those without, is apparent at as early as nine months and present in every domain (Fig).
Genotype-phenotype correlation studies have provided valuable prognostic information in many genetic disorders.8-10 Although they have also been helpful in TSC,26 most correlations are general, and more specific information is always desired to give families the most accurate expectations. Prognostic information is not only useful for families who are trying to envision life with TSC but also can guide management and early intervention, thus optimizing the developmental outcome of the child. For example, it is well known that early identification and control of seizures, as well as early intervention with developmental therapies, improves developmental outcomes.27 Identification of the most at-risk patients allows for closer monitoring and more rapid intervention. Owing to these recognized benefits, attempts to find correlations that may help provide prognostic information to families and physicians of patients with TSC are being undertaken across an array of specialties.18,28 Use of genotype as a predictor can be extremely advantageous as the genotype can be determined before most clinical predictors, for example, in utero.
As noted, our findings are consistent with those of other studies in demonstrating that patients with TSC2 pathogenic variants are more likely to have seizures 13,25 and that patients with seizures are more likely to have developmental delay or ID.15 It is possible that the TSC2 pathogenic variants that lead to increased risk for seizures, in turn, increase the risk for developmental delay. However, we found that the TSC2 group had significantly lower MSEL composite scores, independent of seizures. The same was observed in the gross motor, visual reception, and expressive language domains. Delays in fine motor and receptive language appeared to be similar between TSC2 and those without TSC2, when controlled for seizures. Prognostically, these correlations are important because patients with both a TSC2 pathogenic variant and seizures have two risk factors for developmental delay. Therefore they need extremely close monitoring and proactive intervention.
Our findings were also consistent with those of previous studies that determined Arg622Trp 24 and Arg1200Trp 23 TSC2 pathogenic missense variants to be associated with a milder phenotype. Although the sample size of three was too small for statistical analysis, two of the children (Arg622Trp and Arg1200Trp) had composite scores in the “average” range, whereas the third (Arg1200Trp) had a “very high” composite score. Excluding these patients from the TSC2 group in the multivariable linear regression models led to a one- to three-point reduction in mean MSEL scores in the TSC2 group (Supplemental Table 2). Trajectories (Fig) also demonstrate that excluding the TSC2 mild variants from the TSC2 group decreased the mean at all time points in all domains, along with the composite score.
Mechanistically, there are plausible explanations for why most TSC2 pathogenic variants would confer a more severe neurological phenotype than TSC1 pathogenic variants. The common consensus is that TSC1 plays a role specifically in stabilizing TSC2 and making the TSC2-GTPase-activating protein domain active, thereby inactivating Rheb-GTP, preventing Rheb-GTP from activating mammalian target of rapamycin.29 We now know that there is at least one more protein. TBCD7, found with the TSC1-TSC2 complex, so there may be additional mechanisms present to stabilize TSC2 activities.30-32 The ability of TSC2 to function in some ways independent of TSC1 would possibly explain why pathogenic variants in TSC2 are associated with more severe effects. Thousands of pathogenic variants discovered throughout the exons of TSC1 and TSC2 argues for the need to deeply dissect and characterize every functional domain of TSC1 and TSC2. In addition, the more severe phenotype of patients with TSC2 pathogenic variants could be due to a higher frequency of somatic TSC2 pathogenic variants16 given the larger size of the gene when compared with TSC1. Cortical tuber burden may also contribute as higher tuber burden has been associated with TSC2 pathogenic variants11 and severe cerebral disease,17 although effect of tuber burden on ID does not appear to be independent of seizures.33 Understanding the roles that TSC1 and TSC2 play in the cell could be helpful in identifying treatment of the nonhamartomatous symptoms of TSC, such as ID, autism, and epilepsy.
Except for a few exceptions,23,24,34-38 most reported genotype-phenotype associations in TSC are between TSC1 and TSC2, as well as in patients with NMI. Variant-specific genotype-phenotype correlations are less common due to the number of different variants in TSC, combined with smaller patient numbers per variant (http://chromium.lovd.nl/LOVD2/TSC). In our study, there were few repeats of variants in any single site, resulting in insufficient power to investigate specific pathogenic variants. Another limitation was that there were not adequate numbers to make comparisons between the TSC1 and NMI groups. Future investigations with larger sample sizes could be performed to evaluate whether there is a difference in development between the TSC1 and NMI groups. In addition, it would be useful to ascertain what percent of patients with TSC and developmental delay go on to have ID. Adjustments were made for seizure status in our study due to the well-described negative effect of seizures on development in patients with TSC.27 Tuber burden was not included as a variable because of the less clear association, but may also be useful to include in future studies.
Conclusion
In this well-characterized patient population with standardized assessment of multiple aspects of development, we found that having a TSC2 pathogenic variant was associated with significantly lower MSEL scores in all domains. It appears that the effect of TSC2 pathogenic variants on learning occurs by 24 months and is associated with a global developmental delay. Although TSC2 is associated with increased risk of seizures, which can also adversely affect development, the association between TSC2 and lower MSEL composite scores was independent of seizures. These data suggest that a baby identified to have a TSC2 pathogenic variant is at very high risk for significant global developmental delays early in development, e.g., by 24 months.
Supplementary Material
Acknowledgment
We are indebted to the generosity of the families and patients in TSC clinics across the United States who contributed their time and effort to this study. We would also like to thank the Tuberous Sclerosis Alliance for their continued support in TSC research.
Funding: This work was supported by Autism Center of Excellence Network [1U01NS082320-01], the Developmental Synaptopathies Consortium (1U54NS092090-01], and the Department of Defense [W81XWH1810537]. The Developmental Synaptopathies Consortium [U54NS092090] is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences (NCATS). Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (NINDS), Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institute of Mental Health (NIMH), and National Center for Advancing Translational Sciences (NCATS).
Footnotes
Members of the Tuberous Sclerosis Autism Center of Excellence Research Network (TACERN): Principal Investigators: Sahin, M1: Krueger, D2; Bebin, M3; Wu, JY4; Northrup, H5; Co-Investigators: Warfield S1; Peters J1; Scherrer B1; Goyal M3; Project managers: Filip-Dhima R1; Dies K1; Bruns S2; Neuropsychological assessment team: Hanson E1; Bing N2; Kent B2: O'Kelley S3; Williams ME9; Pearson D5; Data Coordinating Center at UAB: Cutter G6; TS Alliance: Roberds S7; Autism Speaks: Murray DS8: Affiliations for TACERN: 1Boston Children’s Hospital, Boston, MA, 2Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, 3University of Alabama at Birmingham, Birmingham, AL, 4University of California, Los Angeles, Los Angeles, CA, 5McGovern Medical School, University of Texas Health Science Center at Houston, Houston, TX, 6University of Alabama at Birmingham, Data Coordinating Center, Birmingham, AL, 7Tuberous Sclerosis Alliance, Silver Spring, Maryland 8Autism Speaks, New York, New York 9Keck School of Medicine of USC, University of Southern California, Children’s Hospital Los Angeles, Los Angeles, CA.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pediatrneurol.2019.03.003.
References
- 1.Northrup H, Koenig MK, Pearson DA, Au KS, Tuberous Sclerosis Complex [Updated 2018]. GeneReviews® Available at: https://www.ncbi.nlm.nih.gov/books/NBK1220/; 1999. Accessed February 2019.
- 2.Han JM, Sahin M. TSC1/TSC2 signaling in the CNS. FEBS Lett. 2011:585:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;S4:275–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013:49:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nellist M, Brouwer RW, Kockx CE, et al. Targeted Next Generation Sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Med Genet. 2015:16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin W, Kozlowski P, Taillon BE, et al. Ultra deep sequencing detects a low rate of mosaic mutations in tuberous sclerosis complex. Hum Genet. 2010:127:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyburczy ME, Dies KA, Glass J, et al. Mosaic and intronic mutations in TSC1/TSC2 explain the majority of TSC patients with no mutation identified by conventional testing. PLoS Genet. 2015:11:e1005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becerra-Muñoz VM, Gómez-Doblas JJ, Porras-Martín C, et al. The importance of genotype-phenotype correlation in the clinical management of Marfan syndrome. Orphanet J Rare Dis. 2018;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mailman MD, Heinz JW, Papp AC, et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med. 2002;4:20–26. [DOI] [PubMed] [Google Scholar]
- 10.Weese-Mayer DE, Marazita ML, Rand CM, Berry-Kravis EM Congenital central hypoventilation syndrome [Updated 2014]. GeneReviews® Available at: https://www.ncbi.nlm.nih.gov/books/NBK1427/; 2004.
- 11.Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet. 2001:68:64–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones AC, Daniells CE, Snell RG, et al. Molecular genetic and phenotypic analysis reveals differences between TSC1 and TSC2 associated familial and sporadic tuberous sclerosis. Hum Mol Genet. 1997:6:2155–2161. [DOI] [PubMed] [Google Scholar]
- 13.Kothare SV, Singh K, Chalifoux JR, et al. Severity of manifestations in tuberous sclerosis complex in relation to genotype. Epilepsia. 2014:55:1025–1029. [DOI] [PubMed] [Google Scholar]
- 14.Sancak O, Nellist M, Goedbloed M, et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype–phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet. 2005:13:731. [DOI] [PubMed] [Google Scholar]
- 15.Capal JK, Bernardino-Cuesta B, Horn PS et al. TACERN Study Group. Influence of seizures on early development in tuberous sclerosis complex. Epilepsy Behav. 2017;70:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen FE, Braams O, Vincken KL, et al. Overlapping neurologic and cognitive phenotypes in patients with TSC1 or TSC2 mutations. Neurology. 2008:70:908–915. [DOI] [PubMed] [Google Scholar]
- 17.Goodman M, Lamm SH, Engel A, Shepherd CW, Houser OW, Gomez MR. Cortical tuber count: a biomarker indicating neurologic severity of tuberous sclerosis complex. J Child Neurol. 1997:12:85–90. [DOI] [PubMed] [Google Scholar]
- 18.Capal JK, Horn PS, Murray DS, et al. TACERN Study Group. Utility of the autism observation scale for infants in early identification of autism in tuberous sclerosis complex. Pediatr Neurol. 2017:75:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards S, Aziz N, Bale S, et al. , ACMG laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015:17:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis PE, Filip-Dhima R, Sideridis G, et al. Presentation and diagnosis of tuberous sclerosis complex in infants. Pediatrics. 2017;140:e20164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullen EM. Mullen scales of early learning. MN: AGS: Circle Pines; 1995. [Google Scholar]
- 22.An KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9:88–100. [DOI] [PubMed] [Google Scholar]
- 23.Wentink M, Nellist M, Hoogeveen-Westerveld M et al. Functional characterization of the TSC2 c. 3598C> T (p. R1200W) missense mutation that co-segregates with tuberous sclerosis complex in mildly affected kindreds. Clin Genet. 2012:81:453–461. [DOI] [PubMed] [Google Scholar]
- 24.Farach LS, Gibson WT, Sparagana SP et al. TSC2 c. 1864C> T variant associated with mild cases of tuberous sclerosis complex. Am J Med Genet A. 2017;173:771–775. [DOI] [PubMed] [Google Scholar]
- 25.Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB Thiele EA Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology. 2011:76:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peron A, Au KS, Northrup H. Genetics, genomics, and genotype-phenotype correlations of TSC: Insights for clinical practice. Am J Med Genet C Semin Med Genet. 2018:178:281–290. [DOI] [PubMed] [Google Scholar]
- 27.Bombardieri R, Pinci M, Moavero R, Cerminara C, Curatolo P. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010:14:146–149. [DOI] [PubMed] [Google Scholar]
- 28.Curatolo P, Nabbout R, Lagae L, et al. Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations. Eur J Paediatric Neurol. 2018:22:738–748. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dibble CC, Elis W, Menon S, et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell. 2012:47:535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gai Z, Chu W, Deng W, et al. Structure of the TBC1D7–TSC1 complex reveals that TBC1D7 stabilizes dimerization of the TSC1 C-terminal coiled coil region. J Mol Cell Biol. 2016:8:411–425. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima A, Yoshino KI, Miyamoto T, et al. Identification of TBC7 having TBC domain as a novel binding protein to TSC1–TSC2 complex. Biochem Biophys Res Commun. 2007;361:218–223. [DOI] [PubMed] [Google Scholar]
- 33.Jansen FE, Vincken KL, Algra A, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–923. [DOI] [PubMed] [Google Scholar]
- 34.Ekong R, Nellist M, Hoogeveen-Westerveld M, et al. Variants within TSC2 exons 25 and 31 are very unlikely to cause clinically diagnosable tuberous sclerosis. Hum Mutat. 2016;37:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansen AC, Sancak O, D'Agostino MD, et al. Unusually mild tuberous sclerosis phenotype is associated with TSC2 R905Q mutation. Ann Neurol. 2006;60:528–539. [DOI] [PubMed] [Google Scholar]
- 36.Khare L, Strizheva CD, Bailey JN, et al. A novel missense mutation in the GTPase activating protein homology region of TSC2 in two large families with tuberous sclerosis complex. J Med Genet. 2001:38:347–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor SE, Kwiatkowski DJ, Roberts PS, Wollmann RL, Huttenlocher PR. A family with seizures and minor features of tuberous sclerosis and a novel TSC2 mutation. Neurology. 2003:61:409–412. [DOI] [PubMed] [Google Scholar]
- 38.van Eeghen AM, Nellist M, van Eeghen EE, Thiele EA. Central TSC2 missense mutations are associated with a reduced risk of infantile spasms. Epilepsy Res. 2013:103:83–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.