Abstract
Background
Knee MRI is increasingly used to inform clinical management. Features associated with osteoarthritis are often present in asymptomatic uninjured knees; however, the estimated prevalence varies substantially between studies. We performed a systematic review with meta-analysis to provide summary estimates of the prevalence of MRI features of osteoarthritis in asymptomatic uninjured knees.
Methods
We searched six electronic databases for studies reporting MRI osteoarthritis feature prevalence (ie, cartilage defects, meniscal tears, bone marrow lesions and osteophytes) in asymptomatic uninjured knees. Summary estimates were calculated using random-effects meta-analysis (and stratified by mean age: <40 vs ≥40 years). Meta-regression explored heterogeneity.
Results
We included 63 studies (5397 knees of 4751 adults). The overall pooled prevalence of cartilage defects was 24% (95% CI 15% to 34%) and meniscal tears was 10% (7% to 13%), with significantly higher prevalence with age: cartilage defect <40 years 11% (6%to 17%) and ≥40 years 43% (29% to 57%); meniscal tear <40 years 4% (2% to 7%) and ≥40 years 19% (13% to 26%). The overall pooled estimate of bone marrow lesions and osteophytes was 18% (12% to 24%) and 25% (14% to 38%), respectively, with prevalence of osteophytes (but not bone marrow lesions) increasing with age. Significant associations were found between prevalence estimates and MRI sequences used, physical activity, radiographic osteoarthritis and risk of bias.
Conclusions
Summary estimates of MRI osteoarthritis feature prevalence among asymptomatic uninjured knees were 4%–14% in adults aged <40 years to 19%–43% in adults ≥40 years. These imaging findings should be interpreted in the context of clinical presentations and considered in clinical decision-making.
Keywords: knee, mri, osteoarthritis, cartilage
Introduction
MRI is the most reliable non-invasive diagnostic technique to assess internal derangement of the knee joint. Increasing MRI availability has resulted in a rapid rise in its utilisation to help inform clinical management of patients with knee symptoms.1 2 Over $14 billion is spent on diagnostic imaging in the USA annually,3 yet the overall clinical benefit of the current use of knee MRI is uncertain.4 5 Findings such as meniscal tears, cartilage defects, bone marrow lesions (BMLs), osteophytes and other features suggestive of knee osteoarthritis (OA) are often interpreted as causes of pain and symptoms, triggering medical and surgical interventions.6 7 However, the relationship between MRI features of OA and knee pain is imprecise.8
In patients with knee OA, there is moderate evidence that MRI-assessed BMLs and effusion/synovitis are associated with knee pain, but conflicting or limited evidence for other MRI findings.8 Features associated with OA have also been observed on MRI in asymptomatic uninjured knees,9–11 suggesting that MRI-assessed OA features may not necessarily be the source of pain in symptomatic patients. However, estimates of the prevalence of MRI features of OA in asymptomatic uninjured knees vary across studies, from 0% to 75%.9 10 Given the large number of adults undergoing MRI to investigate the cause of knee symptoms, a reliable estimate of the prevalence of MRI features of OA in asymptomatic uninjured knees is important to inform efforts to diagnose and treat knee symptoms across the lifespan. Therefore, the aim of this systematic review and meta-analysis was to determine the prevalence of, and factors contributing to, MRI features of OA in asymptomatic uninjured knees.
Methods
Search strategy and selection criteria
This systematic review conforms to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines and is registered with PROSPERO (CRD42016053969). Study investigators searched for studies reporting the prevalence of MRI features of knee OA in asymptomatic adult knees (ie, mean age ≥18 years with no knee symptoms during any activity) with no history of injury or surgery in EMBASE, Medline, CINAHL, SPORTDiscus, Web of Science and Scopus from inception to the day of the search on 24 October 2017. The searches combined terms related to knee, asymptomatic, MRI, and pathology, without language restriction and adjusted according to individual database specifications (online appendix eMethods 1).
bjsports-2018-099257supp001.doc (1.9MB, doc)
Primary outcomes were individual MRI features assessed semiquantitatively and included in the definition of MRI-defined knee OA12: (i) cartilage defects, defined as partial-thickness or full-thickness cartilage lesions; (ii) meniscal tears, defined as high signal extending to an articular surface; (iii) BMLs, defined as areas of ill-delineated signal within trabecular bone (hypointense on T1-weighted images, hyperintense on T2-weighted fat-suppressed images); and (iv) osteophytes, defined as the presence of osteocartilagenous protrusions at articular margins. Secondary outcomes were other MRI features previously associated with knee OA (defined in detail in the online appendix eMethods 2): effusion-synovitis, subchondral cysts, ligament tears, subchondral sclerosis/attrition and infrapatellar fat pad synovitis/oedema. Two authors (AGC, HFH) independently assessed all titles and abstracts of identified reports for eligibility. Reference lists of all publications considered for inclusion were hand-searched recursively until no additional eligible publications were identified. When eligibility could not be confirmed from title and abstract, full texts were reviewed and study investigators contacted as required. If authors were able to provide data from the subset of asymptomatic participants without prior index knee injury or surgery, these were included, otherwise the article was excluded. Only full-text published articles were eligible. No publication was excluded based on language or study design. Detailed eligibility criteria are described in the online appendix eMethods 3.
Data extraction
The following information was independently extracted from the included studies by two investigators (AGC, JJS): number of participants/knees, participant characteristics (eg, age, sex, body mass index (BMI), sporting/physical activity level), MRI sequences, outcome definition (ie, specific diagnostic criteria) and reported prevalence of whole knee, as well as compartment-specific (ie, tibiofemoral and patellofemoral), abnormalities. The publication with the most participants (or most OA features assessed) was used when several publications used the same population.
Risk of bias assessment
Two reviewers (AGC, BEØ) independently assessed risk of bias using a 13-item checklist developed specifically for this review assessing quality of reporting, sample representativeness and size, comparability between respondents and non-respondents, distribution of confounders and ascertainment of MRI features of OA (online appendix eMethods 4). As per the Cochrane Handbook for Systematic Reviews recommendations, we customised specific items from the Downs and Black checklist for randomised and non-randomised studies,13 and a population-based prevalence study checklist.14 Items related to randomisation, intervention and others not relevant for the current review were excluded. Items were scored as adequate, inadequate or unable to determine. Discrepancies were resolved by discussion.
Data synthesis and analysis
Prevalence estimates of the primary outcomes at a per-knee level were calculated by pooling the study-specific estimates using random-effects proportion meta-analysis that accounted for between-study heterogeneity (Stata V.14.2 metaprop command).15 Freeman-Tukey arcsine transformation was used to normalise variance. Binomial proportion 95% CIs for individual studies were calculated around study-specific and pooled prevalences based on the score-test statistic.16 Due to the incidence of degenerative changes generally increasing substantially after 40 years of age,17 prevalence estimates of the primary outcomes were calculated separately for studies with a mean age of <40 years and for those with a mean age ≥40 years. Secondary outcomes were often inconsistently defined and thus, descriptively synthesised. Between-study heterogeneity was evaluated for each primary outcome using standard Q-tests and the I2 statistic (ie, the percentage of variability in prevalence estimates that is due to heterogeneity rather than chance, 0%=no inconsistency, 100%=maximal inconsistency).18 We further explored between-study heterogeneity by comparing results from studies grouped according to several study level characteristics (detailed in the online appendix eMethods 3) using stratified meta-analysis and meta-regression. Study level characteristics assessed were age, sex, MRI sequences employed (summarised in the online appendix eTable 1), participation in weight-bearing sports, radiographic knee OA, sample size and overall risk of bias. The prevalence estimates of primary compartment-specific outcomes (ie, tibiofemoral and patellofemoral cartilage defects, BMLs, osteophytes; medial and lateral meniscal tears) were pooled wherever reported and differences between compartments assessed with a two-proportion z-test. Publication bias of the primary outcomes secondary to small study effects was assessed using funnel plots and the Egger test when meta-analysis included ≥10 studies. We also conducted sensitivity analyses excluding studies reporting the prevalence of primary outcomes from both knees of each participant to account for potential within-person correlations. All analyses were performed using Stata V.14.2 with a significance threshold of p<0.05.
Results
Study characteristics
Forty-six cross-sectional9 11 19–62 and 17 longitudinal studies10 63–78 involving a total of 4751 individuals (5397 knees) were included in this review (figure 1, table 1). Thirty-two took place in North America, 11 in Australia, 12 in Europe, 7 in Asia and 2 in Africa. The median number of participants and knees per study was 27 (range, 4–836) and 40 (range, 4–836), respectively. The diagnostic criteria used by the studies are summarised in the online appendix eTable 1. Out of 13 possible points on the risk of bias scoring criteria, 5 studies scored 0–4 points, 26 scored 5–7 points, 25 scored 8–10 points and 7 scored 11–13 points (details in the online appendix eTable 1, efigure 1).
Figure 1.
Flow diagram for identifying studies.
Table 1.
Summary of included studies investigating the prevalence of MRI assessed knee OA features prevalence in asymptomatic uninjured populations
| Study | Cohort* | Subjects (knees), no. | Women, no. (%) | Age, years† | BMI, kg/m2† | MRI (T) | Risk of bias score |
| Alharis and Hameed,19 2012 | 80 (80) | 38 (48) | 40–60 | NR | 0.2 | 7 | |
| Antony et al,59 2016 | Childhood Determinants of Adult Health Study | 119 (119)‡ | 56 (47)§ | 35±3 (31–41)¶ | 25.7±4.3¶ | 1.5 | 11 |
| Baranyay et al,20 2007 | Melbourne Collaborative Cohort Study | 297 (297) | 186 (63) | 58±6 (40–69) | 25.2±3.8 | 1.5 | 13 |
| Beattie et al,92005 | 44 (44) | 33 (75) | 41±14 (20-68) | 25.4±4.4 | 1.0 | 7 | |
| Berry et al,29 2010 | 153 (153) | 124 (81) | 47±9 (25–60) | 32±9 | 1.5 | 6 | |
| Boden et al,30 1992 | 74 (74) | 41 (55) | 34 (16–65) | NR | 1.5 | 8 | |
| Brennan et al,63 2010 | Geelong Osteoporosis Study | 142 (142) | 142 (100) | 42±5 (30–49) | 27.3±6.3 | 1.5 | 11 |
| Brunner et al,31 1989 | Basketballers/Footballers | 5 (10)‡ | NR | NR (collegiate) | NR | 0.5/1.5 | 6 |
| Calixto et al,32 2016 | 85 (85) | 50 (59) | 50±9 | 24.0±3.4 | 3.0 | 8 | |
| Culvenor et al,44 2015 | 20 (20) | 7 (35) | 30±7 (21–44) | 22.8±1.8 | 3.0 | 7 | |
| Davies-Tuck et al,45 2008 | 20 (20) | 20 (100) | 61±6 | 25.3±4.2 | 1.5 | 7 | |
| Ding et al,46 2005 | 99 (99)‡ | 62 (63) | 45±7 (26–61) | 25.8±3.8 | 1.5 | 8 | |
| Dong et al,47 2017 | 20 (20) | 6 (30) | 35±11 | 23.5±3.0 | 1.5 | 5 | |
| Dore et al,64 2013 | Tasmanian Older Adult Cohort Study | 97 (97)‡ | 39 (40) | 65±7 (55–81) | 27.3±4.0 | 1.5 | 10 |
| Emad et al,48 2012 | 20 (40) | 12 (60) | 41±7 | 31.7±6.3 | 1.5 | 3 | |
| Fleming et al,78 2013 | 24 (24) | 5 (21) | 25±7 | 25.5±4.8 | 3.0 | 3 | |
| Foppen et al,65 2013 | 29 (55)‡ | 0 (0) | 24 (23–25)¶ | NR | 3.0 | 8 | |
| Fukuta et al,57 2002 | 115 (115) | 60 (52) | 48 (13–78) | NR | 0.5 | 7 | |
| Fukuta et al,49 2009 | 43 (43) | 34 (79) | 62 (40–79) | NR | 0.5 | 7 | |
| Guermazi et al,58 2012 | Framingham Osteoarthritis Study | 434 (434)‡ | 220 (51) | 63±8 (51–89) | 27.3±4.8 | 1.5 | 12 |
| Guymer et al,11 2007 | Victorian electoral role | 176 (176) | 176 (100) | 52±7 (40–67) | 27.1±5.5 | 1.5 | 12 |
| Hagemann et al,66 2008 | Runners | 10 (10) | 3 (30) | 37 (32–44) | NR | 1.5 | 8 |
| Jerosch et al,60 1996 | 66 (126)** | 32 (48) | 16–62** | NR | 1.0 | 8 | |
| Kaplan et al,61 2005 | Basketballers | 20 (40) | 0 (0) | 26 (21–36) | NR | 1.5 | 8 |
| Kaukinen et al,62 2016 | Oulu Knee Osteoarthritis Study | 63 (63) | 38 (60) | 55±14 | 24.8±3.2 | 3.0 | 8 |
| Kornaat and Van de Velde,672014 | Runners | 16 (32) | 3 (19) | 23±3 | 20.4±1.1 | 1.5 | 9 |
| Kornick et al,50 1990 | 54 (59)†† | 31 (48) | (20-74)†† | NR | 1.5 | 9 | |
| Krampla et al,68 2001 | Runners | 6 (6)‡ | 0 (0) | 37±8 (27–46) | NR | 1.0 | 9 |
| Kumar et al,51 2013 | 27 (42) | 9 (33) | 28±4 (20–35) | 22.7±2.1 | 3.0 | 6 | |
| Kursunoglu-Brahme et al,69 1990 | Runners | 10 (10) | 5 (50) | (20-35) | NR | 1.5 | 5 |
| Landsmeer et al,70 2016 | Prevention of Knee Osteoarthritis in Overweight Females Study | 300 (473)‡ | 300 (100) | 56±3 (50–60) | 32.2±4.3 | 1.5 | 9 |
| LaPrade et al,52 1994 | 54 (54) | 29 (54) | 29±5 (19–39) | NR | 1.0 | 5 | |
| Li et al,53 2009 | 200 (200) | 72 (36) | 31 (20–40) | NR | 1.5 | 8 | |
| Ludman et al,54 1999 | General gymnasts |
14 (26) 14 (24) |
5 (36) 4 (29) |
20 (18–23) 20 (18–22) |
NR | 1.5 | 8 |
| Major & Helms,55 2002 | Basketballers | 17 (33)‡ | 5 (29) | NR (collegiate) | NR | 1.5 | 7 |
| Marik et al,56 2016 | 9 (9) | 3 (33) | 40±18 (23-69) | 22.1±2.6 | 7 | 4 | |
| Morgenroth et al,33 2014 | 14 (14) | NR | 55±2 (35–65) | 84.6±3.2‡ ‡ | 1.5 | 5 | |
| Negendank et al,34 1990 | General contralateral meniscal tear |
18 (36) 20 (20) |
18 (56) 4 (20) |
43±16 41±12 |
67.4±14.5 79.3±14.5 |
1.0 | 9 |
| Nozaki et al,35 2004 | 57 (86) | 37 (65) | 43 (18–79) | NR | 0.3 | 4 | |
| Pan et al,71 2011 | Osteoarthritis Initiative Healthy Control Cohort | 95 (95) | 58 (61) | 55±8 (45–78) | 24.2±2.9 | 3.0 | 11 |
| Pappas et al,10 2016 | Basketballers | 24 (24) | 12 (50) | (18-22) | NR | 3.0 | 9 |
| Peers et al,41 2014 | Basketballers Swimmers |
10 (10) 10 (10) |
10 (100) 10 (100) |
20 (19–22) 20 (19–23) |
NR | 3.0 | 8 |
| Reinig et al,72 1991 | Footballers | 17 (17) | 0 (0) | (19-21) | NR | NR | 6 |
| Rennie and Finlay,42 2006 | 23 (36) | 5 (22) | 26 (15–41) | NR | 1.5 | 5 | |
| Schiphof et al,73 2014 | Rotterdam Study | 424 (836)‡ | 424 (100) | 55±4 | 26.3±4.3 | 1.5 | 10 |
| Schweitzer et al,43 1995 | 25 (50) | 7 (28) | 25 (20–46) | NR | 1.5 | 5 | |
| Shellock et al,36 1991 | Runners | 23 (23) | 15 (65) | 40 (25–55) | NR | 1.5 | 9 |
| Shellock and Mink,74 1991 | Runners | 4 (4)‡ | 2 (50)¶ | 37±4 (33–43)¶ | NR | 1.5 | 5 |
| Shellock et al,37 2003 | Triathletes | 13 (13) | 5 (38) | 48 (37–66) | NR | 1.5 | 9 |
| Souza et al,38 2013 | 19 (19) | 8 (42) | 39±10 | 23.5±3.4 | 3.0 | 6 | |
| Sowers et al,39 2011 | Michigan Study of Women’s Health Across the Nation Study | 159 (259)‡ | 159 (100) | 57±3 | 29.9±6.3 | 1.5/3.0 | 11 |
| Sritanyaratana et al,40 2014 | 20 (20) | 5 (25) | 32 (23–45) | NR | 3.0 | 3 | |
| Stahl et al,75 2008 | General runners |
12 (12) 10 (10) |
4 (33) 6 (60) |
37±11 31±5 |
75.8±12.6‡‡ 68.6±10.0‡‡ |
3.0 | 9 |
| Su et al,76 2013 | 16 (16) | 8 (50) | 33 (23–57) | 24.4 (20–29) | 3.0 | 6 | |
| Tarhan and Unlu,24 2003 | 16 (29) | 12 (75) | 28±5 (46–77) | 28.2±3.7 | 0.23 | 6 | |
| van der Heijden et al,25 2006 | 70 (70) | 41 (59) | 23±6 (14–40) | 22.3±3.0 | 3.0 | 9 | |
| Walczak et al,26 2008 | Basketballers | 14 (25)‡ | 0 (0) | 26 (20–36) | NR | 0.3/0.7/1.5 | 6 |
| Wang et al,23 2012 | 38 (38) | 18 (47) | 42±7 (30–55) | 25.2±4.1 | 1.5 | 7 | |
| Wang et al,21 2015 | 16 (16) | 4 (25) | 34±10 (18-63) | 24.5±2.3 | 3.0 | 7 | |
| Wang et al,22 2017 | 30 (30) | 11 (37) | 28±5 (18–40) | 23.4±3.3 | 1.5/3.0 | 6 | |
| Wei et al,77 2017 | Footballers | 13 (25) | 0 (0) | 20±1 (18–22) | 34.2±3.2 | 3.0 | 6 |
| Whittaker et al,27 2017 | Alberta Youth Prevention of Early Osteoarthritis Study | 73 (146) | 45 (62) | 23±3 (15–27) | 23.6±2.6 | 1.5 | 9 |
| Zanetti et al,28 2003 | Contralateral meniscal tear | 100 (100) | 41 (41) | 43 (18–73) | NR | 1.0/1.5 | 8 |
*Participants are healthy volunteers from the general population unless otherwise indicated.
†Mean ± SD (range).
‡Subset of whole cohort without previous knee injury or surgery.
§Estimated from total sample reported in original publication.
¶Values represent total sample reported in original publication.
**After excluding participant group aged <16 years.
††Number of people/knees estimated after excluding participants aged 10–20 years.
‡‡Body mass, as BMI not reported.
BMI, body mass index; NR, not reported.
Prevalence of articular cartilage defects
Forty-two studies (4322 knees from 3446 participants) reported the prevalence of cartilage defects with an overall pooled prevalence estimate of 24% (95% CI 15% to 34%; I2=97.8%). Studies with a mean age <40 years and ≥40 years had a pooled prevalence of 11% (6% to 17%) and 43% (29% to 57%), respectively, with significant evidence of between-study heterogeneity (I2=84.6% and 98.5%, respectively) (figure 2). The prevalence of cartilage defects significantly increased with age (slope=14.4% increase per 10 years; 95% CI 9.0% to 19.9%, p<0.001) (online appendix efigure 2) and a higher proportion of women (slope=4.3% increase per 10% increase in proportion of women; 95% CI 1.3% to 7.3%, p=0.006). Heterogeneity was not accounted for by other factors evaluated except: (i) risk of bias score in studies with a mean age <40 years, where a lower risk of bias resulted in a higher prevalence (p=0.03; online appendix efigure 3; and (ii) sample size in studies with a mean age ≥40 years, where a sample of ≥50 knees resulted in a significantly higher prevalence (55% (95% CI 39% to 71%)) than samples of <50 knees (15% (0% to 42%)) (p=0.014) (online appendix eTable 3).
Figure 2.
Meta-analysis of the prevalence of cartilage defects.
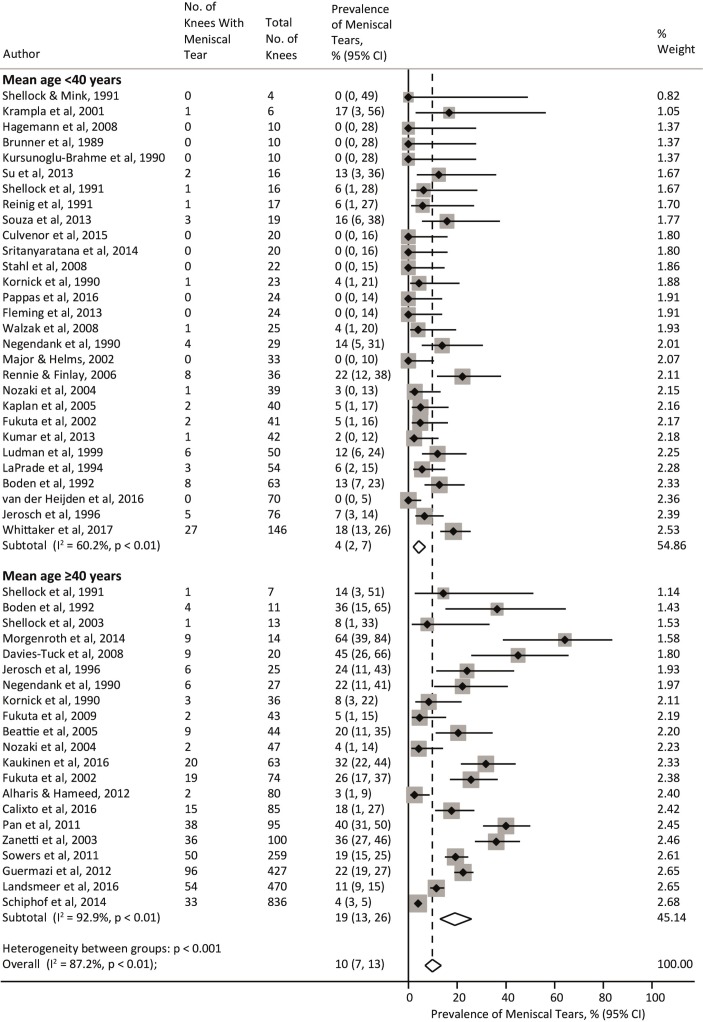
Prevalence of meniscal tears
Forty-four studies (3761 knees from 2817 participants) reported prevalence of meniscal tears with an overall pooled prevalence estimate of 10% (95% CI 7% to 13%; I2=87.2%). Studies with a mean age <40 years and ≥40 years had a pooled prevalence of 4% (2% to 7%) and 19% (13% to 26%), respectively, with significant evidence of between-study heterogeneity (I2=60.2% and 92.9%, respectively) (figure 3). The prevalence of meniscal tears significantly increased with age (slope=3.2% increase per-10 years, 95% CI 0.2% to 6.1%, p=0.036) (online appendix eFigure 2) and a higher proportion of women (slope=0.2% increase per 10% increase in proportion of females; 95% CI −1.4% to 1.8%, p=0.797). Prevalence of meniscal tears did not differ by any other study level characteristic except MRI sequences used in studies with a mean age <40 years, where use of optimal MRI sequences resulted in a significantly lower pooled prevalence (3% (0% to 7%)) than studies using suboptimal MRI sequences (7% (4% to 10%)) (p=0.034) (online appendix eTable 3).
Figure 3.
Meta-analysis of the prevalence of meniscal tears.
Prevalence of BMLs
Thirty-four studies (4089 knees from 3255 participants) reported BML prevalence with an overall pooled prevalence estimate of 18% (95%CI 12% to 24%; I2=95.6%). Studies with mean age <40 years and ≥40 years had a pooled prevalence of 14% (6% to 24%) and 21% (14% to 31%), respectively, with significant evidence of between-study heterogeneity (I2=91.2% and 96.8%, respectively) (figure 4). While BML prevalence was not associated with age (slope=4.3% increase per 10 years; 95% CI −0.4% to 9.1%, p=0.076) (online appendix eFigure 2) or percentage of women (slope=1.2% increase per 10% increase in proportion of women; 95% CI −1.5% to 3.9%, p=0.370), the large heterogeneity in those aged <40 years was partly explained by participation in weight-bearing sports. Studies of athletes playing weight-bearing sports resulted in a pooled estimate of 30% (17% to 45%) compared with general population studies of 3% (0% to 11%) (p<0.001) (online appendix eTable 3). MRI sequences employed also partly explained the heterogeneity in all studies, with a significantly higher pooled prevalence in studies using optimal sequences (<40 years p=0.027; ≥40 years p=0.002) (online appendix eTable 3). In studies with a mean age ≥40 years, a significantly higher prevalence was also observed in studies specifically excluding knees with radiographic OA (p<0.001) and in studies with a sample size ≥50 knees (p=0.029) (online appendix eTable 3).
Figure 4.
Meta-analysis of the prevalence of BMLs. BML, bone marrow lesion.
Prevalence of osteophytes
Eighteen studies (3257 knees from 2499 participants) reported osteophyte prevalence with an overall pooled prevalence estimate of 25% (95% CI 14% to 38%; I2=98.2%). Studies with a mean age <40 years and ≥40 years had a pooled prevalence of 8% (0% to 25%) and 37% (22% to 53%), respectively, with significant evidence of between-study heterogeneity (I2=94.3% and 98.6%, respectively) (figure 5). The prevalence of osteophytes significantly increased with age (slope=10.2% increase per 10 years, 95% CI 1.7% to 18.7%, p=0.021) (online appendix eFigure 2) but not with a higher proportion of women (slope=−0.1% increase per 10% increase in proportion of women; 95% CI −4.8% to 6.5%, p=0.756). Although the relatively small number of studies precluded evaluation of some study level characteristics, in studies with a mean age ≥40 years prevalence of osteophytes was significantly higher in studies that specifically excluded knees with radiographic OA (p=0.046) (online appendix eTable 3).
Figure 5.
Meta-analysis of the prevalence of osteophytes.
Compartment-specific outcomes
There were no significant differences between the prevalence of tibiofemoral and patellofemoral abnormalities (online appendix eTable 4). In studies with a mean age ≥40 years, medial meniscal tears (14% (95% CI 8% to 20%)) were significantly more common than lateral meniscal tears (5% (2% to 8%)) (p=0.009) (online appendix eTable 4).
Prevalence of secondary outcomes, sensitivity analysis and publication bias
The prevalence of secondary outcomes was generally assessed in fewer studies, with a large range of feature definitions (details in online appendix eTable 5). Prevalence of effusion/effusion-synovitis and subchondral cysts ranged from 0% to 92% (21 studies) and 0% to 24% (six studies), respectively. Prevalence of ligament tears was 0% for 16 of the 20 studies, with the remaining four studies reporting 1%–30% of mostly anterior cruciate or collateral ligament partial tears. Infrapatellar fat pad synovitis and oedema prevalence was 16%–80% (three studies) and 9%–75% (two studies), respectively. One study reported the prevalence of subchondral sclerosis/attrition, with a prevalence of 31%. Sensitivity analyses, excluding 21 studies of bilateral knees, resulted in almost identical prevalence of OA features as the full analyses (≤5% difference). Visual inspection of funnel plots stratified by age (<40 years and ≥40 years) revealed minimal asymmetry, with some evidence of small studies effect only for meniscal tears (Egger test <40 years of age p=0.027; ≥40 years of age p=0.037; online appendix efigure 4).
Discussion
This systematic review and meta-analysis of 63 studies involving 5397 knees demonstrated that OA features on MRI are common in asymptomatic uninjured knees and are generally associated with age. In young adults aged <40 years, the pooled prevalence of asymptomatic OA features ranged from 4% to 14%, with pooled prevalence estimates of 19%–43% in older adults. These findings assist both clinical providers and patients to interpret the importance of structural changes noted on MRI reports throughout the lifespan. Since more than one-third of the older population will exhibit these knee OA-related features, medical and/or surgical interventions targeting these imaging findings may not alleviate pain in patients with knee symptoms.
Clinical implications
Current management of OA-related features and atraumatic knee pain should centre on improving symptoms and functional limitations, and not be driven by imaging findings.79 80 The high rate of asymptomatic older adults (aged ≥40 years) with knee OA features on MRI helps to explain why interventions for these, such as arthroscopy, are no more efficacious in reducing symptoms than sham surgery.81 Imaging features also do not predict non-surgical treatment outcomes.79 The explosion of clinical MRI use and expenditure, by as much as 30% annually, over the past two decades1 2 has not resulted in improved treatment decisions or outcomes for people with knee pain in general practice settings.82 Alarmingly, in cases of back pain, undergoing early MRI has led to inferior outcomes.83 Future research should investigate whether explaining the normal rates of imaging features of OA to symptomatic patients presenting with imaging changes on MRI can improve outcomes and decrease the need for analgesic prescriptions, similar to that observed in the lumbar spine.84
The prevalence of MRI findings and older age
The prevalence of most knee OA-related features increased with older age, which partially explained the heterogeneity between studies. This increase of approximately 10%–15% per-decade for osteophytes and cartilage defects, and 3% per-decade for meniscal tears, suggests that these features reflect normal age-related changes. Indeed, meta-regression shows that approximately three-quarters of asymptomatic adults aged 70 years will have a cartilage lesion. A similarly high pooled prevalence of intra-articular abnormalities has also been observed in asymptomatic spines (disk/facet degeneration)85 and hips (cartilage/labral defects).86 Evidence purporting an increased risk of future radiographic OA in the presence of cartilage87 and meniscal pathology88 indicates that some of these asymptomatic OA features may not be entirely benign. As radiographic OA was already established in some knees in this review, it is possible that structural abnormalities observed were already part of the pathological OA process. However, higher rates of structural abnormalities were not evident in studies that potentially included knees with radiographic OA (ie, did not specifically exclude radiographic OA). Indeed, radiographic OA was also common in many asymptomatic knees and can also reflect normal ageing processes.89
BMLs and the association with physical activity
BMLs were the most common feature in younger adults and were not associated with age. Participation in weight-bearing sports contributed to the observed heterogeneity in BML prevalence in younger adults. The consequences of these BMLs in young athletes are not known. However, the transient nature of BMLs means that even after knee injury, when BMLs are common, most resolve without sequelae.90 While BMLs associated with established OA are an important source of knee pain, they display distinct biochemical properties from those associated with sports-related impact.91
The influence of MRI sequences acquired
The prevalence of OA features in the current review was influenced by the type of MRI sequences employed, reflecting variation in diagnostic accuracy with different MRI techniques.92 While MRI is the gold-standard imaging technique for diagnosing OA-related pathology,93 studies using non-optimal sequences to assess BMLs, such as gradient echo sequences, which are particularly prone to susceptibility artefacts,93 reported significantly lower rates. The pooled prevalence of meniscal tears in younger adults extends observations from a previous systematic review (without meta-analysis) describing the same prevalence (4%) of meniscal tears in asymptomatic, but not exclusively uninjured, athletes (mean age 20–47 years).94
Strengths and limitations
The studies included in this review used a large variety of outcome assessment tools to define MRI features. Although there were too many to assess their individual influence on prevalence rates, all methods to assess primary outcomes resulted in equivalent cut-off criteria. Thresholds to define presence of secondary outcomes were more variable and prevented meta-analysis. The detection bias associated with less experienced readers having more errors95 was reflected in risk of bias scores, with the addition of a specific item assessing reader experience. Risk of bias scores partly contributed to cartilage lesion prevalence between-study heterogeneity. In many studies, the asymptomatic uninjured controls were part of a comparator group for diseased cases; the general lack of publication bias (except for meniscal tears) confirms that prevalence rates reported were not a key determinant of publication.
Limitations of this review include the heterogeneity between studies that remained unexplained by the variables examined. Unexplained factors, such as the inherent subjective nature of grading MRIs, irrespective of experience, may contribute to OA feature prevalence. The influence of BMI was unable to be assessed as half of the studies did not report BMI. When whole knee data were not available, the highest prevalence from either compartment was analysed as the whole knee feature rate. While likely under-representing overall prevalence, this conservative approach ensured that a minimum rate was reported, as lesions in one compartment are known to increase the risk of lesions in the other compartment.96 Of the studies that reported compartment-specific abnormalities, prevalence of tibiofemoral and patellofemoral lesions were similar, while medial meniscal tears were significantly more common than lateral meniscal tears. Finally, the meta-regression analyses relied on aggregated published data, which may have underestimated the association of MRI features with older age and female sex.
Conclusion
In this systematic review, summary estimates of the prevalence of MRI features suggestive of OA among otherwise healthy asymptomatic uninjured knees ranged from 4% to 14% in young adults to 19% to 43% in older adults aged ≥40 years. These imaging findings must be interpreted in the context of clinical presentations and considered in clinical decision making.
What is already known on this subject?
Increasing availability of MRI has resulted in a rapid rise in its utilisation to help inform clinical management of patients with knee symptoms, yet the overall clinical benefit of the current use of knee MRI is uncertain.
Community-based studies have reported a high prevalence of knee osteoarthritis features detected by MRI, but these cohorts include people with knee pain and history of knee injury, a well-established risk factor for the accelerated development of knee osteoarthritis.
What are the new findings?
The prevalence of knee osteoarthritis features on MRI in otherwise healthy, asymptomatic, uninjured knees is high—up to 43% in adults aged ≥40 years.
Prevalence rates generally increase with age and are influenced by other factors such as physical activity levels and type of MRI sequences used.
Footnotes
Contributors: AGC, BEØ and KMC: designed the study and planned the analyses. AGC and HFH: completed all searches and study selection (including inclusion and exclusion of abstracts). AGC and JJS: completed all data extraction. AGC and BEØ: completed all risk of bias assessment. AG: completed all critical appraisals of magnetic resonance imaging sequences. AGC: did the meta-analyses and meta-regressions, wrote the initial draft. All authors interpreted the data, critically revised the manuscript for important intellectual content and approved the final version of the manuscript.
Funding: AGC was supported by postdoctoral funding from a European Union Seventh Framework Programme (FP7-PEOPLE-2013-ITN; 607510), and is a recipient of a National Health and Medical Research Council (NHMRC) of Australia Early Career Fellowship (Neil Hamilton Fairley Clinical Fellowship, APP1121173). HFH is supported by a NHMRC Project Grant (GNT1106852). JJS is supported by an Institutional DevelopmentAward (IDeA) from the National Institute of General Medical Sciences of theNational Institutes of Health (U54-GM104941). The funders had no role in any part of the study or in any decision about publication.
Disclaimer: These sources had no involvement in study design, interpretation of data, writing of the manuscript or the decision to submit the manuscript for publication. All other authors declare no competing interests.
Competing interests: AG is president of Boston Imaging Core Lab, LLC, and a consultant to Merck Serono, Genzyme, OrthoTrophix and TissueGene.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Solomon DH, Katz JN, Carrino JA, et al. . Trends in knee magnetic resonance imaging. Med Care 2003;41:687–92. 10.1097/01.MLR.0000062705.24024.9F [DOI] [PubMed] [Google Scholar]
- 2. Espeland A, Natvig NL, Løge I, et al. . Magnetic resonance imaging of the knee in Norway 2002-2004 (national survey): rapid increase, older patients, large geographic differences. BMC Health Serv Res 2007;7:115 10.1186/1472-6963-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iglehart JK. Health insurers and medical-imaging policy--a work in progress. N Engl J Med 2009;360:1030–7. 10.1056/NEJMhpr0808703 [DOI] [PubMed] [Google Scholar]
- 4. Odgaard F, Tuxoe J, Joergensen U, et al. . Clinical decision making in the acutely injured knee based on repeat clinical examination and MRI. Scand J Med Sci Sports 2002;12:154–62. 10.1034/j.1600-0838.2002.00246.x [DOI] [PubMed] [Google Scholar]
- 5. Brealey S, Russell I, Gilbert F. Value of knee imaging by GPs requires rigorous assessment. BMJ 2002;325:1242a–1242. 10.1136/bmj.325.7374.1242/a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergkvist D, Dahlberg LE, Neuman P, et al. . Knee arthroscopies: who gets them, what does the radiologist report, and what does the surgeon find? Acta Orthop 2016;87:12–16. 10.3109/17453674.2015.1055179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyad TF. Cartilage injuries in the adult knee: evaluation and management. Cartilage 2011;2:226–36. 10.1177/1947603510383973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yusuf E, Kortekaas MC, Watt I, et al. . Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 2011;70:60–7. 10.1136/ard.2010.131904 [DOI] [PubMed] [Google Scholar]
- 9. Beattie KA, Boulos P, Pui M, et al. . Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthritis Cartilage 2005;13:181–6. 10.1016/j.joca.2004.11.001 [DOI] [PubMed] [Google Scholar]
- 10. Pappas GP, Vogelsong MA, Staroswiecki E, et al. . Magnetic resonance imaging of asymptomatic knees in collegiate basketball players: the effect of one season of play. Clin J Sport Med 2016;26:483–9. 10.1097/JSM.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guymer E, Baranyay F, Wluka AE, et al. . A study of the prevalence and associations of subchondral bone marrow lesions in the knees of healthy, middle-aged women. Osteoarthritis Cartilage 2007;15:1437–42. 10.1016/j.joca.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 12. Hunter DJ, Arden N, Conaghan PG, et al. . Definition of osteoarthritis on MRI: results of a Delphi exercise. Osteoarthritis Cartilage 2011;19:963–9. 10.1016/j.joca.2011.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoy D, Brooks P, Woolf A, et al. . Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 15. Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods 1998;3:486–504. 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- 16. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209–12. 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 17. Litwic A, Edwards MH, Dennison EM, et al. . Epidemiology and burden of osteoarthritis. Br Med Bull 2013;105:185–99. 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, et al. . Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alharis NR, Hameed AM. Incidental meniscal findings on knee MRI in Al-Najaf city. Med J Babylon 2012;9:850–6. [Google Scholar]
- 20. Baranyay FJ, Wang Y, Wluka AE, et al. . Association of bone marrow lesions with knee structures and risk factors for bone marrow lesions in the knees of clinically healthy, community-based adults. Semin Arthritis Rheum 2007;37:112–8. 10.1016/j.semarthrit.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Chang G, Bencardino J, et al. . T1rho MRI at 3T of menisci in patients with acute anterior cruciate ligament (ACL) injury. J Magn Reson Imaging 2015;41:544–9. 10.1002/jmri.24594 [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Wang Y, Bennell KL, et al. . Cartilage morphology at 2–3 years following anterior cruciate ligament reconstruction with or without concomitant meniscal pathology. Knee Surg Sports Traumat Arthro 2017;25:426–36. 10.1007/s00167-015-3831-1 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Dempsey AR, Lloyd DG, et al. . Patellofemoral and tibiofemoral articular cartilage and subchondral bone health following arthroscopic partial medial meniscectomy. Knee Surg Sports Traumat Arthro 2012;20:970–8. 10.1007/s00167-011-1681-z [DOI] [PubMed] [Google Scholar]
- 24. Tarhan S, Unlu Z. Magnetic resonance imaging and ultrasonographic evaluation of the patients with knee osteoarthritis: a comparative study. Clin Rheumatol 2003;22:181–8. 10.1007/s10067-002-0694-x [DOI] [PubMed] [Google Scholar]
- 25. van der Heijden RA, de Kanter JL, Bierma-Zeinstra SM, et al. . Structural abnormalities on magnetic resonance imaging in patients with patellofemoral pain: a cross-sectional case-control study. Am J Sports Med 2016;44:2339–46. 10.1177/0363546516646107 [DOI] [PubMed] [Google Scholar]
- 26. Walczak BE, McCulloch PC, Kang RW, et al. . Abnormal findings on knee magnetic resonance imaging in asymptomatic NBA players. J Knee Surg 2008;21:27–33. 10.1055/s-0030-1247788 [DOI] [PubMed] [Google Scholar]
- 27. Whittaker JL, Toomey CM, Woodhouse LJ, et al. . Association between MRI-defined osteoarthritis, pain, function and strength 3-10 years following knee joint injury in youth sport. Br J Sports Med 2017. 10.1136/bjsports-2017-097576 [DOI] [PubMed] [Google Scholar]
- 28. Zanetti M, Pfirrmann CW, Schmid MR, et al. . Patients with suspected meniscal tears: prevalence of abnormalities seen on MRI of 100 symptomatic and 100 contralateral asymptomatic knees. AJR Am J Roentgenol 2003;181:635–41. 10.2214/ajr.181.3.1810635 [DOI] [PubMed] [Google Scholar]
- 29. Berry PA, Wluka AE, Davies-Tuck ML, et al. . The relationship between body composition and structural changes at the knee. Rheumatology 2010;49:2362–9. 10.1093/rheumatology/keq255 [DOI] [PubMed] [Google Scholar]
- 30. Boden SD, Davis DO, Dina TS, et al. . A prospective and blinded investigation of magnetic resonance imaging of the knee. Abnormal findings in asymptomatic subjects. Clin Orthop Relat Res 1992:177–85. [PubMed] [Google Scholar]
- 31. Brunner MC, Flower SP, Evancho AM, et al. . MRI of the athletic knee. Findings in asymptomatic professional basketball and collegiate football players. Invest Radiol 1989;24:72–5. 10.1097/00004424-198901000-00015 [DOI] [PubMed] [Google Scholar]
- 32. Calixto NE, Kumar D, Subburaj K, et al. . Zonal differences in meniscus MR relaxation times in response to in vivo static loading in knee osteoarthritis. J Orthop Res 2016;34:249–61. 10.1002/jor.23004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgenroth DC, Medverd JR, Seyedali M, et al. . The relationship between knee joint loading rate during walking and degenerative changes on magnetic resonance imaging. Clin Biomech 2014;29:664–70. 10.1016/j.clinbiomech.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Negendank WG, Fernandez-Madrid FR, Heilbrun LK, et al. . Magnetic resonance imaging of meniscal degeneration in asymptomatic knees. J Orthop Res 1990;8:311–20. 10.1002/jor.1100080302 [DOI] [PubMed] [Google Scholar]
- 35. Nozaki H, Iso Y, Suguro T, et al. . Incidence of MRI intensity changes in the knee meniscus: Comparing asymptomatic and symptomatic knees without meniscal lesion. J Med Soc Toho Uni 2004;51:156–67. [Google Scholar]
- 36. Shellock FG, Deutsch AL, Mink JH, et al. . Do asymptomatic marathon runners have an increased prevalence of meniscal abnormalities? An MR study of the knee in 23 volunteers. AJR Am J Roentgenol 1991;157:1239–41. 10.2214/ajr.157.6.1950873 [DOI] [PubMed] [Google Scholar]
- 37. Shellock FG, Hiller WD, Ainge GR, et al. . Knees of Ironman triathletes: magnetic resonance imaging assessment of older (>35 years old) competitors. J Magn Reson Imaging 2003;17:122–30. 10.1002/jmri.10234 [DOI] [PubMed] [Google Scholar]
- 38. Souza RB, Feeley BT, Zarins ZA, et al. . T1rho MRI relaxation in knee OA subjects with varying sizes of cartilage lesions. Knee 2013;20:113–9. 10.1016/j.knee.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sowers M, Karvonen-Gutierrez CA, Jacobson JA, et al. . Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J Bone Joint Surg Am 2011;93:241–51. 10.2106/JBJS.I.00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sritanyaratana N, Samsonov A, Mossahebi P, et al. . Cross-relaxation imaging of human patellar cartilage in vivo at 3.0T. Osteoarthritis Cartilage 2014;22:1568–76. 10.1016/j.joca.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peers SC, Maerz T, Baker EA, et al. . T1ρ magnetic resonance imaging for detection of early cartilage changes in knees of asymptomatic collegiate female impact and nonimpact athletes. Clin J Sport Med 2014;24:218–25. 10.1097/JSM.0000000000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rennie WJ, Finlay DB. Meniscal extrusion in young athletes: associated knee joint abnormalities. AJR Am J Roentgenol 2006;186:791–4. 10.2214/AJR.04.1181 [DOI] [PubMed] [Google Scholar]
- 43. Schweitzer ME, Tran D, Deely DM, et al. . Medial collateral ligament injuries: evaluation of multiple signs, prevalence and location of associated bone bruises, and assessment with MR imaging. Radiology 1995;194:825–9. 10.1148/radiology.194.3.7862987 [DOI] [PubMed] [Google Scholar]
- 44. Culvenor AG, Collins NJ, Guermazi A, et al. . Early knee osteoarthritis is evident one year following anterior cruciate ligament reconstruction: a magnetic resonance imaging evaluation. Arthritis Rheumatol 2015;67:946–55. 10.1002/art.39005 [DOI] [PubMed] [Google Scholar]
- 45. Davies-Tuck ML, Wluka AE, Teichtahl AJ, et al. . Association between meniscal tears and the peak external knee adduction moment and foot rotation during level walking in postmenopausal women without knee osteoarthritis: a cross-sectional study. Arthritis Res Ther 2008;10:R58 10.1186/ar2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ding C, Garnero P, Cicuttini F, et al. . Knee cartilage defects: association with early radiographic osteoarthritis, decreased cartilage volume, increased joint surface area and type II collagen breakdown. Osteoarthritis Cartilage 2005;13:198–205. 10.1016/j.joca.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 47. Dong B, Kong Y, Zhang L, et al. . Severity and distribution of cartilage damage and bone marrow edema in the patellofemoral and tibiofemoral joints in knee osteoarthritis determined by MRI. Exp Ther Med 2017;13:2079–84. 10.3892/etm.2017.4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Emad Y, Ragab Y, Gheita T, et al. . Knee enthesitis and synovitis on magnetic resonance imaging in patients with psoriasis without arthritic symptoms. J Rheumatol 2012;39:1979–86. 10.3899/jrheum.120301 [DOI] [PubMed] [Google Scholar]
- 49. Fukuta S, Kuge A, Korai F. Clinical significance of meniscal abnormalities on magnetic resonance imaging in an older population. Knee 2009;16:187–90. 10.1016/j.knee.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 50. Kornick J, Trefelner E, McCarthy S, et al. . Meniscal abnormalities in the asymptomatic population at MR imaging. Radiology 1990;177:463–5. 10.1148/radiology.177.2.2217786 [DOI] [PubMed] [Google Scholar]
- 51. Kumar D, Subburaj K, Lin W, et al. . Quadriceps and hamstrings morphology is related to walking mechanics and knee cartilage MRI relaxation times in young adults. J Orthop Sports Phys Ther 2013;43:881–90. 10.2519/jospt.2013.4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. LaPrade RF, Burnett QM, Veenstra MA, et al. . The prevalence of abnormal magnetic resonance imaging findings in asymptomatic knees. With correlation of magnetic resonance imaging to arthroscopic findings in symptomatic knees. Am J Sports Med 1994;22:739–45. 10.1177/036354659402200603 [DOI] [PubMed] [Google Scholar]
- 53. Li W, Lu Y, Ding X, et al. . MRI features and clinical relative factors of asymptoamtic adult knee cartilage lesions. Chinese J Med Imaging Technol 2009;25:2088–91. [Google Scholar]
- 54. Ludman CN, Hough DO, Cooper TG, et al. . Silent meniscal abnormalities in athletes: magnetic resonance imaging of asymptomatic competitive gymnasts. Br J Sports Med 1999;33:414–6. 10.1136/bjsm.33.6.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Major NM, Helms CA. MR imaging of the knee: findings in asymptomatic collegiate basketball players. AJR Am J Roentgenol 2002;179:641–4. 10.2214/ajr.179.3.1790641 [DOI] [PubMed] [Google Scholar]
- 56. Marik W, Nemec SF, Zbýň Štefan, et al. . Changes in cartilage and tendon composition of patients with type I diabetes mellitus. Invest Radiol 2016;51:266–72. 10.1097/RLI.0000000000000236 [DOI] [PubMed] [Google Scholar]
- 57. Fukuta S, Masaki K, Korai F. Prevalence of abnormal findings in magnetic resonance images of asymptomatic knees. J Orthop Sci 2002;7:287–91. 10.1007/s007760200049 [DOI] [PubMed] [Google Scholar]
- 58. Guermazi A, Niu J, Hayashi D, et al. . Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ 2012;345:e5339 10.1136/bmj.e5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Antony B, Venn A, Cicuttini F, et al. . Correlates of knee bone marrow lesions in younger adults. Arthritis Res Ther 2016;18:31 10.1186/s13075-016-0938-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jerosch J, Castro WH, Assheuer J. Age-related magnetic resonance imaging morphology of the menisci in asymptomatic individuals. Arch Orthop Trauma Surg 1996;115:199–202. 10.1007/BF00434553 [DOI] [PubMed] [Google Scholar]
- 61. Kaplan LD, Schurhoff MR, Selesnick H, et al. . Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy 2005;21:557–61. 10.1016/j.arthro.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 62. Kaukinen P, Podlipská J, Guermazi A, et al. . Associations between MRI-defined structural pathology and generalized and localized knee pain - the Oulu Knee Osteoarthritis study. Osteoarthritis Cartilage 2016;24:1565–76. 10.1016/j.joca.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 63. Brennan SL, Cicuttini FM, Pasco JA, et al. . Does an increase in body mass index over 10 years affect knee structure in a population-based cohort study of adult women? Arthritis Res Ther 2010;12:R139 10.1186/ar3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Doré DA, Winzenberg TM, Ding C, et al. . The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis 2013;72:1170–5. 10.1136/annrheumdis-2012-201691 [DOI] [PubMed] [Google Scholar]
- 65. Foppen W, Sluiter D, Witkamp TD, et al. . Haemophilic magnetic resonance imaging score in healthy controls playing sports. Haemophilia 2013;19:939–43. 10.1111/hae.12191 [DOI] [PubMed] [Google Scholar]
- 66. Hagemann GJ, Rijke AM, Corr PD. Do knees survive the comrades marathon? S Afr Med J 2008;98:873–6. [PubMed] [Google Scholar]
- 67. Kornaat PR, Van de Velde SK. Bone marrow edema lesions in the professional runner. Am J Sports Med 2014;42:1242–6. 10.1177/0363546514521990 [DOI] [PubMed] [Google Scholar]
- 68. Krampla W, Mayrhofer R, Malcher J, et al. . MR imaging of the knee in marathon runners before and after competition. Skeletal Radiol 2001;30:72–6. 10.1007/s002560000296 [DOI] [PubMed] [Google Scholar]
- 69. Kursunoglu-Brahme S, Schwaighofer B, Gundry C, et al. . Jogging causes acute changes in the knee joint: an MR study in normal volunteers. AJR Am J Roentgenol 1990;154:1233–5. 10.2214/ajr.154.6.2110734 [DOI] [PubMed] [Google Scholar]
- 70. Landsmeer ML, Runhaar J, van der Plas P, et al. . Reducing progression of knee OA features assessed by MRI in overweight and obese women: secondary outcomes of a preventive RCT. Osteoarthritis Cartilage 2016;24:982–90. 10.1016/j.joca.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 71. Pan J, Pialat JB, Joseph T, et al. . Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology 2011;261:507–15. 10.1148/radiol.11102234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Reinig JW, McDevitt ER, Ove PN. Progression of meniscal degenerative changes in college football players: evaluation with MR imaging. Radiology 1991;181:255–7. 10.1148/radiology.181.1.1887043 [DOI] [PubMed] [Google Scholar]
- 73. Schiphof D, van Middelkoop M, de Klerk BM, et al. . Crepitus is a first indication of patellofemoral osteoarthritis (and not of tibiofemoral osteoarthritis). Osteoarthritis Cartilage 2014;22:631–8. 10.1016/j.joca.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 74. Shellock FG, Mink JH. Knees of trained long-distance runners: MR imaging before and after competition. Radiology 1991;179:635–7. 10.1148/radiology.179.3.2027965 [DOI] [PubMed] [Google Scholar]
- 75. Stahl R, Luke A, Ma CB, et al. . Prevalence of pathologic findings in asymptomatic knees of marathon runners before and after a competition in comparison with physically active subjects-a 3.0 T magnetic resonance imaging study. Skeletal Radiol 2008;37:627–38. 10.1007/s00256-008-0491-y [DOI] [PubMed] [Google Scholar]
- 76. Su F, Hilton JF, Nardo L, et al. . Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage 2013;21:1058–67. 10.1016/j.joca.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wei W, Lambach B, Jia G, et al. . Assessing the effect of football play on knee articular cartilage using delayed gadolinium-enhanced MRI of cartilage (dGEMRIC). Magn Reson Imaging 2017;39:149–56. 10.1016/j.mri.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 78. Fleming BC, Fadale PD, Hulstyn MJ, et al. . The effect of initial graft tension after anterior cruciate ligament reconstruction: a randomized clinical trial with 36-month follow-up. Am J Sports Med 2013;41:25–34. 10.1177/0363546512464200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sakellariou G, Conaghan PG, Zhang W, et al. . EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann Rheum Dis 2017;76:1484–94. 10.1136/annrheumdis-2016-210815 [DOI] [PubMed] [Google Scholar]
- 80. Crossley KM, Callaghan MJ, van Linschoten R. Patellofemoral pain. BMJ 2015;351:h3939 10.1136/bmj.h3939 [DOI] [PubMed] [Google Scholar]
- 81. Thorlund JB, Juhl CB, Roos EM, et al. . Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ 2015;350:h2747 10.1136/bmj.h2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Brealey SD. DAMASK (Direct Access to Magnetic Resonance Imaging: Assessment for Suspect Knees) Trial Team. Influence of magnetic resonance of the knee on GPs' decisions: a randomised trial. Br J Gen Pract 2007;57:622–9. [PMC free article] [PubMed] [Google Scholar]
- 83. Webster BS, Bauer AZ, Choi Y, et al. . Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling low back pain. Spine 2013;38:1939–46. 10.1097/BRS.0b013e3182a42eb6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McCullough BJ, Johnson GR, Martin BI, et al. . Lumbar MR imaging and reporting epidemiology: do epidemiologic data in reports affect clinical management? Radiology 2012;262:941–6. 10.1148/radiol.11110618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brinjikji W, Luetmer PH, Comstock B, et al. . Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36:811–6. 10.3174/ajnr.A4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heerey JJ, Kemp JL, Mosler AB, et al. . What is the prevalence of imaging-defined intra-articular hip pathologies in people with and without pain? A systematic review and meta-analysis. Br J Sports Med 2018;52:581–93. 10.1136/bjsports-2017-098264 [DOI] [PubMed] [Google Scholar]
- 87. Cicuttini F, Ding C, Wluka A, et al. . Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum 2005;52:2033–9. 10.1002/art.21148 [DOI] [PubMed] [Google Scholar]
- 88. Englund M, Guermazi A, Roemer FW, et al. . Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 2009;60:831–9. 10.1002/art.24383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol 2013;25:108–13. 10.1097/BOR.0b013e32835a9428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boks SS, Vroegindeweij D, Koes BW, et al. . MRI follow-up of posttraumatic bone bruises of the knee in general practice. AJR Am J Roentgenol 2007;189:556–62. 10.2214/AJR.07.2276 [DOI] [PubMed] [Google Scholar]
- 91. Li X, Ma BC, Bolbos RI, et al. . Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging 2008;28:453–61. 10.1002/jmri.21437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Crema MD, Roemer FW, Marra MD, et al. . Articular cartilage in the knee: Current MR imaging techniques and applications in clinical practice and research. Radiographics 2011;31:37–61. 10.1148/rg.311105084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guermazi A, Roemer FW, Haugen IK, et al. . MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol 2013;9:236–51. 10.1038/nrrheum.2012.223 [DOI] [PubMed] [Google Scholar]
- 94. Beals CT, Magnussen RA, Graham WC, et al. . The prevalence of meniscal pathology in asymptomatic athletes. Sports Med 2016;46:1517–24. 10.1007/s40279-016-0540-y [DOI] [PubMed] [Google Scholar]
- 95. Krampla W, Roesel M, Svoboda K, et al. . MRI of the knee: how do field strength and radiologist’s experience influence diagnostic accuracy and interobserver correlation in assessing chondral and meniscal lesions and the integrity of the anterior cruciate ligament? Eur Radiol 2009;19:1519–28. 10.1007/s00330-009-1298-5 [DOI] [PubMed] [Google Scholar]
- 96. Stefanik JJ, Niu J, Gross KD, et al. . Using magnetic resonance imaging to determine the compartmental prevalence of knee joint structural damage. Osteoarthritis Cartilage 2013;21:695–9. 10.1016/j.joca.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2018-099257supp001.doc (1.9MB, doc)