Abstract
PURPOSE
Although the clinical use of multiparametric prostate magnetic resonance imaging (mpMRI) is increasing, the adherence to parameters for mpMRI, which had been described in the Prostate Imaging-Reporting and Data System version 2 (PI-RADS v2) for an optimum image acquisition is unknown. In this paper, we aimed to determine the compliance with the minimum acceptable technical parameters for prostate mpMRI defined by PI-RADS v2 in tertiary care centers in Turkey.
METHODS
We sent a survey to all radiology departments of tertiary referral hospitals in Turkey (n=120) to evaluate their adherence to PI-RADS v2 technical specifications. Statistical analysis was performed using chi-square, Fisher exact, ANOVA, and the Student t tests. The cutoff values for image acquisition times were also determined with receiver operating characteristics (ROC) analysis. P values <0.05 were considered statistically significant.
RESULTS
One hundred and eleven clinics responded to our survey (response rate, 92.5%). Prostate MRI was reported to be performed in 61 centers, of which 26 (42.6%) used 3 T (Tesla) scanner while 35 (57.4%) used 1.5 T. The adherence to slice thickness, in-plane phase and frequency resolutions on T2-weighted imaging were 68.9%, 41%, and 9.8%, respectively. The adherence to the same parameters on diffusion-weighted imaging (DWI) were higher compared with T2-weighted imaging (85.2%, 62.3%, and 78.7%, respectively). In comparative analysis, the adherence to slice thickness, field of view (FOV) and in-plane phase resolution on T2-weighted imaging were higher for 3 T compared with 1.5 T scanners (P = 0.004, P = 0.041, and P = 0.001, respectively). T2-weighted imaging acquisition time was significantly longer for the centers that adhered to FOV (P = 0.034) and in-plane T2-weighted imaging phase resolution (P = 0.028). The DWI scan time was significantly longer when they adhered to DWI-FOV (P = 0.014) and b value ≥1400 s/mm2 (P = 0.008). The calculated cutoff of scan times were 220 s in T2-weighted imaging and 312 s in DWI to ensure the compliance with voxel sizes and b value criteria.
CONCLUSION
The tertiary referral centers in Turkey did not meet majority of the technical specifications of PI-RADS v2 during prostate MRI acquisition. Awareness to the minimum acceptable technical parameters of mpMRI should be increased to potentially improve the quality of prostate cancer imaging.
The Prostate Imaging-Reporting and Data System (PI-RADS) was first published in 2012. The minimum acceptable technical parameters for multiparametric prostate magnetic resonance imaging (mpMRI) had been described in this document elaborately (1). In 2015, the PI-RADS guidelines were revised and version 2 (v2) was released. The technical specifications have been updated and acquisition recommendations for axial T2-weighted imaging, diffusion-weighted imaging (DWI), and dynamic contrast enhanced (DCE) imaging have been detailed in that edited version (2). Use of prostate MRI in prostate cancer has substantially increased (3), and prostate MRI is recognized as one of the biomarkers such as blood tests (e.g., serum prostate specific antigen [PSA], 4K test) and tissue based genomic classifiers (e.g., Oncotype DX, Decipher) (4). The biggest concern about prostate MRI as a potential biomarker amongst others is its inhomogeneous quality regarding image acquisition and interpretation (5, 6). Currently, only one study has evaluated the adherence of imaging centers to the technical specifications of PI-RADS v2 (7).
It has become a common practice to use MRI in healthcare all over the world in the last decade. Interestingly, based on 2015 data of the Organisation for Economic Cooperation and Development (OECD), Turkey is the country where the highest number of MRI scans (n=144 per 1000 individuals per year) were performed whereas corresponding numbers were 36 and 118 for the European Union (EU) and the United States (US), respectively. However, the number of MRI scanners per million was 10.2 in Turkey, whereas corresponding numbers were 13.7 and 39 for the EU and the US, respectively (8). While use of MRI has become more popular in clinical care in Turkey in the last decade, the share of prostate MRI within this workload is still unknown. On the other hand, localized prostate cancer care has already started to implement prostate MRI and few centers reported use of MRI and its guidance in targeted biopsies and surgery in Turkey (9–11). In this study, we aimed to determine the compliance with the minimum acceptable technical parameters for prostate mpMRI defined by PI-RADS v2 in tertiary care reference centers in Turkey.
Methods
This retrospective study was approved by the ethics committee (approval number: 31829978-050.01.04-E.1800012835). We reached the radiology departments (n=120) of all third level referral hospitals in Turkey, including state university hospitals, training and research hospitals of the Ministry of Health, and private university hospitals by phone or mail in February 2018. We asked them to complete a survey form on mpMRI parameters, if prostate mpMRI was being performed in their departments (Table 1). The responses were compared with PI-RADS v2 minimum acceptable technical parameters. Turkey’s 2017 population data were received from the official website of the Turkish Statistical Institute (TSI) (12).
Table 1.
The questionnaire sent to all tertiary referral centers in Turkey
| Questions |
|---|
| Magnet strength? |
| Brand of MRI scanner? |
| Are you using endorectal coil? |
| How many channels does your pelvic-surface coil have? |
| Do you obtain coronal T2? |
| Do you obtain sagittal T2? |
| Do you obtain precontrast axial T1? |
| Do you obtain postcontrast T1? |
| Do you obtain at least one sequence encompassing aortic bifurcation? |
| For axial T2, slice thickness? |
| For axial T2, gap between slices? |
| For axial T2, FOV (two plane)? |
| For axial T2, matrix (phase x frequency)? |
| For axial T2, NEX? |
| For axial T2, total acquisition time? |
| For DWI, TR? |
| For DWI, TE? |
| For DWI, slice thickness? |
| For DWI, gap between slices? |
| For DWI, FOV (two plane)? |
| For DWI, matrix (phase x frequency)? |
| For DWI, maximum b value (acquisition)? |
| For DWI, NEX for maximum b value? |
| For DWI, total acquisition time? |
| For DCE, temporal resolution? |
| For DCE, TR? |
| For DCE, TE? |
| For DCE, slice thickness? |
| For DCE, gap between slices? |
| For DCE, FOV (two plane)? |
| For DCE, matrix (phase x frequency)? |
| For DCE, total acquisition time? |
MRI, magnetic resonance imaging; FOV, field of view; NEX, number of excitations; DWI, diffusion-weighted imaging; TR, repetition time; TE, echo time; DCE, dynamic contrast enhancement.
Statistical analysis
The comparison of field strength and institute types for the compliance with the parameters was done by chi-square and Fisher exact test. The acquisition times of the institutes were compared with each other by the ANOVA test (with Bonferroni correction). The data showed a normal distribution and Student t test was used to compare acquisition time with the adherence to technical specifications. The cutoff image acquisition times to reach compliance with PI-RADS v2 specifications were calculated with receiver operating characteristic (ROC) analysis. Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS version 20.0; IBM Corp.). P values <0.05 were considered statistically significant (alpha error level <5%).
Results
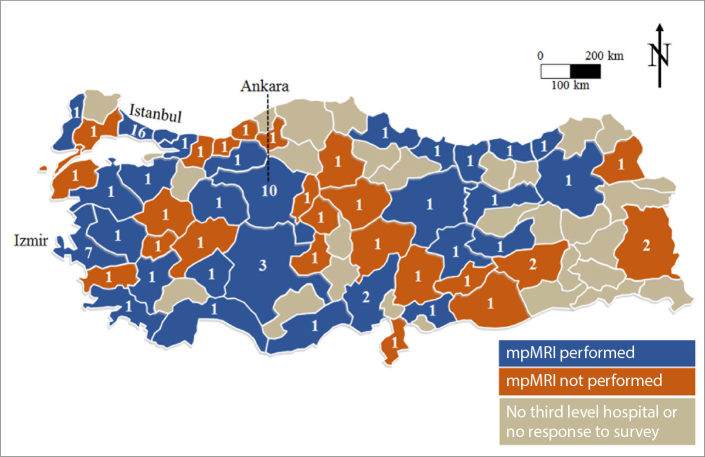
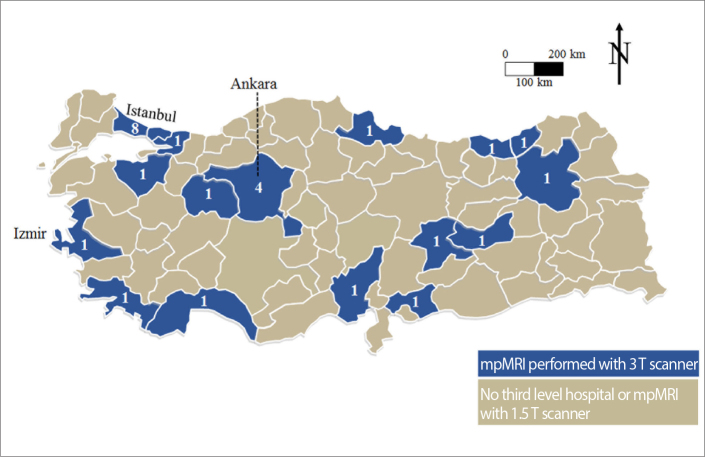
One hundred and eleven of 120 clinics responded to our survey (response rate, 92.5%). Sixty-one of 111 (55%) centers reported to perform MRI of the prostate (59 centers multiparametric, 2 centers biparametric) (Fig. 1). 3 Tesla (T) scanner was used in 26 (42.6%), while 1.5 T scanner was used in 35 (57.4%) clinics (Fig. 2). The vendors of MRI devices were Siemens in 35, Philips in 17 and General Electric in 9 clinics.
Figure 1.
Map shows the status and number of tertiary referral centers performing MRI of the prostate in Turkey.
Figure 2.
Number of tertiary referral centers performing MRI of the prostate with 3 Tesla scanners in Turkey.
Thirty-three clinics (54.1%) were in the three largest cities of Turkey (Fig. 1). MpMRI of the prostate was documented to be performed only in 13 of 59 cities with <1 million population according to 2017 data of TSI. Of the 61 centers performing prostate MRI, 36 were state universities, 14 were private universities, and 11 were training and research hospitals of the Ministry of Health.
The adherence to the minimum acceptable technical parameters of PI-RADS v2 is presented in Table 2. The compliance with the parameters was as follows for T2-weighted imaging: slice thickness, 68.9%; inter-slice gap, 32.8%; field-of-view (FOV), 75.4%; in-plane frequency resolution, 9.8%; in-plane phase resolution, 41%. The adherence to slice thickness, inter-slice gap, in-plane frequency, and phase resolution were higher in DWI compared with T2-weighted imaging and were 85.2%, 50.8%, 78.7%, and 62.3%, respectively. The compliance with FOV in DWI was 37.7% and there were 2 centers using narrower FOV than 160 mm, which is suggested as the lower limit in PI-RADS v2 (2). These 2 centers were considered to be incompatible with the PI-RADS v2 technical specifications. The adherence to highest b value ≥1400s/mm2 was 57.4%. The adherence to slice thickness, inter-slice gap, in-plane frequency, and phase resolution in DCE were 39%, 50.8%, 93.2%, and 89.9%, respectively. The adherence to temporal resolution ≤10 seconds (s) in DCE MRI was 55.9%. The mean acquisition times of axial T2-weighted imaging, DWI, and DCE imaging were 233, 274, and 247 s, respectively.
Table 2.
Adherence to the technical parameters of PI-RADS v2 in Turkey
| Questions | PI-RADS v2 recommendation | Mean | Min–max | Adherence, n (%) | |
|---|---|---|---|---|---|
| Pelvic coil, how many channels? | ≥16 channel | 16.8 | 2–64 | 52 (85.2) | |
| Coronal T2 | Should be obtained | 55 (90.2) | |||
| Sagittal T2 | Should be obtained | 58 (95.1) | |||
| Precontrast axial T1 | Should be obtained | 59 (96.7) | |||
| Postcontrast T1 | Should be obtained | 53 (86.9) | |||
| One sequence covering aortic bifurcation | Should be obtained | 42 (68.9) | |||
|
| |||||
| Axial T2: | Slice thickness (mm) | ≤3 | 3.25 | 2.5–4.5 | 42 (68.9) |
| Gap | 0 | 0.42 | 0–2.5 | 20 (32.8) | |
| Field of view (mm) | 120–200 | 205 | 140–320 | 46 (75.4) | |
| Frequency voxel size (mm) | ≤0.4 | 0.67 | 0.31–1.05 | 6 (9.8) | |
| Phase voxel size (mm) | ≤0.7 | 0.81 | 0,31–1.34 | 25 (41) | |
| NEX | 2.5 | 1–5 | |||
| Acquisition time (s) | 233 | 76–490 | |||
|
| |||||
| Diffusion: | Slice thickness (mm) | ≤4 | 3.67 | 3–6 | 52 (85.2) |
| Gap | 0 | 0.38 | 0–1.5 | 31 (50.8) | |
| Field of view (mm) | 160–220 | 255 | 140 – 461 | 23 (37.7) | |
| Frequency voxel size (mm) | ≤2.5 | 2.02 | 0.7–3.24 | 48 (78.7) | |
| Phase voxel size (mm) | ≤2.5 | 2.32 | 0.89–3.96 | 38 (62.3) | |
| TR (ms) | ≥3000 | 4876 | 400–8300 | 54 (88.5) | |
| TE (ms) | ≤90 | 80 | 55–116 | 47 (77) | |
| Maximum b value (s/mm2) | ≥1400 | 1302 | 600–2400 | 35 (57.4) | |
| NEX of maximum b value | 6.9 | 1–20 | |||
| Acquisition time (s) | 274 | 54–639 | |||
|
| |||||
| Dynamic*: | Temporal resolution (s) | ≤10, preferred <7 | 14.5 | 3.4–61 | 33 (55.9), 10 (16.9) |
| Slice thickness (mm) | ≤3 | 3.34 | 0.9–4.8 | 23 (39) | |
| Gap | 0 | 0.4 | 0–3 | 30 (50.8) | |
| Frequency voxel size (mm) | ≤2 | 1.38 | 0.58–3.54 | 55 (93.2) | |
| Phase voxel size (mm) | ≤2 | 1.64 | 0.66–3.85 | 53 (89.9) | |
| TR (ms) | ≤100 | 30 | 2.7–500 | 56 (94.9) | |
| TE (ms) | ≤5 | 3.18 | 0.8–50 | 56 (94.9) | |
| Acquisition time (s) | ≥120 | 247 | 63–551 | 53 (89.9) | |
PI-RADS v2, Prostate Imaging-Reporting and Data System; min, minimum; max, maximum; NEX, number of excitations; TR, repetition time; TE, echo time.
Results of 59 clinics.
In comparative analysis, the adherence to slice thickness, FOV, and in-plane phase resolution at T2-weighted imaging were significantly higher for the centers using 3 T scanners (P = 0.004, P = 0.041, and P = 0.001, respectively). No significant difference was found regarding compliance with the other parameters between 1.5 T and 3 T scanners (Table 3).
Table 3.
Comparison of tertiary referral centers using 1.5 T and 3 T devices regarding compliance with the parameters of PI-RADS v2
| Parameters/adherence | 1.5 T (n=35), n (%) | 3 T (n=26), n (%) | P |
|---|---|---|---|
| T2 slice thickness | 19 (54.3) | 23 (88.5) | 0.004 |
| T2 gap | 13 (37.1) | 7 (26.9) | 0.40 |
| T2 FOV | 23 (65.7) | 23 (88.5) | 0.041 |
| T2 voxel (frequency) | 3 (8.6) | 3 (11.5) | 0.70 |
| T2 voxel (phase) | 8 (22.9) | 17 (65.4) | 0.001 |
| DWI TR | 31 (88.5) | 23 (88.5) | 0.99 |
| DWI TE | 30 (85.7) | 17 (65.4) | 0.062 |
| DWI slice thickness | 29 (82.9) | 23 (88.5) | 0.54 |
| DWI gap | 20 (57.1) | 11 (42.3) | 0.25 |
| DWI FOV | 12 (34.3) | 11 (42.3) | 0.52 |
| DWI voxel (frequency) | 30 (85.7) | 18 (69.2) | 0.12 |
| DWI voxel (phase) | 23 (65.7) | 15 (57.7) | 0.52 |
| DWI maximum b value | 17 (48.6) | 18 (69.2) | 0.11 |
| *DCE temporal resolution (7 s) | 7 (21.2) | 3 (11.5) | 0.49 |
| *DCE temporal resolution (10 s) | 20 (64.5) | 13 (50) | 0.42 |
| *DCE TR | 31 (93.9) | 25 (96.2) | 1.00 |
| *DCE TE | 31 (93.9) | 25 (96.2) | 1.00 |
| *DCE slice thickness | 12 (36.4) | 11 (42.3) | 0.64 |
| *DCE gap | 15 (45.4) | 15 (57.7) | 0.35 |
| *DCE voxel (frequency) | 31 (93.9) | 24 (92.3) | 1.00 |
| *DCE voxel (phase) | 28 (84.8) | 25 (96.2) | 0.22 |
| *DCE duration | 29 (82.9) | 24 (92.3) | 0.69 |
T, Tesla; FOV, field of view; DWI, diffusion-weighted imaging; TR, repetition time; TE, echo time; DCE, dynamic contrast enhancement.
DCE results of 59 clinics (1.5 T n=33, 3 T n=26).
There was no significant difference between instutition types regarding compliance with any of the parameters. Mean durations of three major sequences were shorter in the private universities, but it was not statistically significant (Table 4).
Table 4.
Image acquisition times of tertiary referral centers in Turkey
| Institution type | Mean (s) | SE (s) | 95% CI (s) | Min–max (s) | P |
|---|---|---|---|---|---|
| T2 State university (n=35) | 243 | 17 | 210–275 | 91–490 | 0.45 |
| T2 Training hospital (n=10) | 237 | 28 | 182–292 | 150–480 | |
| T2 Private university (n=14) | 206 | 18 | 170–241 | 76–330 | |
|
| |||||
| DWI State university (n=35) | 282 | 22 | 239–325 | 54–639 | 0.61 |
| DWI Training hospital (n=10) | 283 | 30 | 223–342 | 134–499 | |
| DWI Private university (n=14) | 246 | 26 | 195–297 | 62–366 | |
|
| |||||
| *DCE State university (n=35) | 248 | 18 | 212–284 | 65–551 | 0.94 |
| *DCE Training hospital (n=10) | 254 | 21 | 213–296 | 145–344 | |
| *DCE Private university (n=14) | 240 | 33 | 176–304 | 63–439 | |
SE, standard error; CI, confidence interval; DWI, diffusion weighted imaging; DCE, dynamic contrast enhancement.
DCE results of 59 clinics.
In comparison of acquisition times with the adherence to the minimum acceptable technical specifications, T2-weighted imaging acquisition time was significantly longer for the centers which adhered to FOV and in-plane phase resolution for T2-weighted imaging (P = 0.034 and P = 0.028, respectively) (Table 5). DWI acquisition time was also significantly longer when they adhered to FOV for DWI and b value ≥1400s/mm2 (P = 0.014 and P = 0.008, respectively) (Table 6). The compliance with temporal resolution ≤10 s was also related with echo time (TE) and repetition time (TR) in DCE imaging (P = 0.011 for both).
Table 5.
The comparative analysis of T2-weighted imaging acquisition time with the parameters
| T2 parameters | T2 acquisition time | ||
|---|---|---|---|
|
| |||
| Mean (s) | Standard error | P | |
| Slice thickness ≤3 mm (n=19) | 238 | 15 | 0.54 |
| Slice thickness >3 mm (n=42) | 222 | 15 | |
|
| |||
| Gap = 0 mm (n=20) | 254 | 16 | 0.22 |
| Gap >0 mm (n=41) | 223 | 16 | |
|
| |||
| FOV ≤200 mm (n=46) | 247 | 14 | 0.034 |
| FOV >200 mm (n=15) | 189 | 17 | |
|
| |||
| Frequency voxel size ≤0.4 mm (n=6) | 267 | 32 | 0.31 |
| Frequency voxel size >0.4 mm (n=55) | 229 | 13 | |
|
| |||
| Phase voxel size ≤0.7 mm (n=25) | 264 | 17 | 0.028 |
| Phase voxel size >0.7 mm (n=36) | 212 | 15 | |
FOV, field of view.
Table 6.
The comparative analysis of DWI acquisition time with the parameters
| DWI parameters | DWI acquisition time | ||
|---|---|---|---|
|
| |||
| Mean (s) | Standard error | P | |
| TR ≥3000 ms (n=54) | 274 | 17 | 0.95 |
| TR <3000 ms (n=7) | 276 | 25 | |
|
| |||
| TE ≤90 ms (n=47) | 284 | 16 | 0.21 |
| TE >90 ms (n=14) | 239 | 21 | |
|
| |||
| Slice thickness ≤3 mm (n=52) | 282 | 16 | 0.20 |
| Slice thickness >3 mm (n=9) | 227 | 46 | |
|
| |||
| Gap = 0 mm (n=31) | 301 | 21 | 0.075 |
| Gap >0 mm (n=30) | 247 | 21 | |
|
| |||
| 160 mm ≤ FOV ≤220 mm (n=23) | 321 | 28 | 0.014 |
| FOV <160 mm or FOV >220 mm (n=38) | 245 | 16 | |
|
| |||
| Frequency voxel size ≤0.4 mm (n=48) | 280 | 17 | 0.43 |
| Frequency voxel size >0.4 mm (n=13) | 251 | 33 | |
|
| |||
| Phase voxel size ≤0.7 mm (n=38) | 289 | 20 | 0.21 |
| Phase voxel size >0.7 mm (n=23) | 250 | 22 | |
|
| |||
| Maximum b value ≥1400 s/mm2 (n=35) | 308 | 17 | 0.008 |
| Maximum b value <1400 s/mm2 (n=26) | 228 | 25 | |
DWI, diffusion-weighted imaging; TR, repetition time; TE, echo time; FOV, field of view.
In ROC analysis, the optimal cutoff value of T2 acquisition time was found as 220 s for adherence to voxel sizes (for phase: sensitivity 76%, specificity 69.4%, and area under the curve [AUC] 0.707; for frequency: sensitivity 83.3%, specificity 54.5%, and AUC 0.665). The cutoff value of DWI duration was calculated as 221 s (sensitivity 85.7%, specificity 65.4%, and AUC 0.74) for compliance with b value ≥1400s/mm2. When the cutoff time was taken as 312 s, sensitivity and specificity were found as 40% and 76.9%, respectively. The mean sequence duration of the centers which did not adhere to b value ≥1400s/mm2 was 228 s, while it was 308 s for the centers meeting the b value criteria. The cutoff was calculated as 1.87 milliseconds (ms) for TE (sensitivity 100%, specificity 61.5%, and AUC 0.72) and 7.06 ms for TR (sensitivity 100%, specificity 15.4%, and AUC 0.553) to be able to comply with temporal resolution ≤10 s in DCE imaging.
Discussion
In this study, we found that the technical specifications of prostate mpMRI performed in tertiary referral centers in Turkey did not meet majority of the recommendations of PI-RADS v2. The lowest compliance was 9.8% in T2-weighted imaging frequency voxel size, while the highest one was 94.9% in TE and TR in DCE MRI. There was only one center meeting all technical specifications of PI-RADS v2. The minimum acceptable technical requirements was defined by expert consensus in PI-RADS v2 guidelines. There was no study focused on relation between image quality and technical specifications prior to release of PI-RADS v2. A previous study of Esses et al. (7) reported that the lowest compliance was 16.8% in T2-weighted imaging in-plane frequency dimension and the adherence to TE and TR were 100% in DCE MRI. In our study, adherence to the majority of the criteria was lower compared with that study except for some parameters such as temporal resolution, slice thickness, and in-plane phase dimension in DCE MRI.
The PI-RADS v2 recommended to perform prostate mpMRI at 3 T scanners and suggested to use endorectal coil (ERC), especially when acquired at 1.5 T (2). While prostate MRI was more often performed with 1.5 T (59%) in Turkey, ERC was reported to be used only in 2 centers (one 3 T, one 1.5 T). In Esses et al. (7), 1.5 T scanners were used in 30.8% (23.1% without ERC, 7.7% with ERC) of 107 participant centers in the US.
The centers using 3 T more often adhered to slice thickness, FOV and in-plane dimension (phase) in T2-weighted imaging in our study. In the study of Esses et al. (7), the compliance with in-plane (phase) dimension of T2-weighted imaging, gap in DWI and in-plane (frequency) dimension in DCE MRI was also significantly higher with 3 T devices. Higher field strength scanners provide higher signal-to-noise ratio and this factor might have enabled higher number of matrix or narrower FOV.
In PI-RADS v2, the recommendations mainly focused on high spatial resolution, but there was no proposal for contrast resolution of any sequence. In our study, mean number of excitations (NEX) was 2.5 for T2-weighted imaging, whereas PI-RADS v2 did not make any suggestions. In the future versions of PI-RADS, NEX≥2 may be added for higher contrast resolution in T2-weighted imaging. The scan time is directly proportional to NEX, so careful checking of acquisition times may be important, and this can potentially enhance contrast resolution. In this context, use of 3 T scanners could be encouraged.
The PI-RADS v2 had no recommendation for acquisition times of T2-weighted imaging and DWI. In our study, 220 s was calculated as a cutoff value for T2-weighted imaging duration in order to comply with voxel dimensions. Also, the compliance with b value ≥1400 s/mm2 was significantly higher for the centers acquiring DWI in 312 s or longer. These cutoffs may increase the adherence to voxel sizes in T2-weighted imaging and b value ≥1400 s/mm2 in DWI.
Temporal resolution should be at least ≤10 s and is preferred to be <7 s for DCE MRI in PI-RADS v2 (2). The compliance with the 10 s criterion was 55.9%, while it was 16.9% for 7 s criterion in our survey. The adherence to 7 s criterion was found as 9.6% in the study of Esses et al. (7). Previous clinical studies suggested that a temporal resolution faster than 10 s does not provide any additional benefit in the diagnosis of the prostate cancer (13, 14).
Temporal resolution is directly related to the number of matrix (phase) and TR (15). The adherence to TE and TR were relatively high (94.9%) in our study. Mean durations of the centers were 3.18 and 30 ms for TE and TR, respectively. The average durations of TE and TR were 1.62 and 4.44 ms for the centers with temporal resolution ≤10 s. The adherence to temporal resolution ≤10 s was higher when TE and TR was below 1.87 and 7.06 ms, respectively. Esses et al. (7) found mean TE and TR as 1.7 and 4.4 ms, respectively. The respective TE, TR, and temporal resolution values of previous studies on MRI of the prostate were as follows: 2.3 ms, 3.7 ms, 5.6 s; 1.89 ms, 4.1 ms, 2.3 s; and 1.96 ms, 5.5 ms, 3 s (16–18). The PI-RADS v2 recommendations of TE <5 ms and TR <100 ms seem to be too long. In subsequent versions, shortening the TE and TR may be considered to improve the adherence to temporal resolution in DCE MRI.
The most important limitation of our study was that the data was based on the questionnaires and not on the actual DICOM data. It was assumed that all centers responded to our survey correctly. Another limitation was the inclusion of only tertiary referral centers, excluding private practice where mpMRI of the prostate is also being performed. Although the direct impact of adherence to PIRADS technical standards on image quality is still unknown, our results can potentially assist others on which technical specifications among minimum acceptable technical parameters in PI-RADS guidelines should be further investigated to obtain good quality prostate MRI. Although the results may not be generalized to the entire clinical practice in Turkey, this is a relatively powerful survey with a high response rate of 92.5%. Larger scale studies evaluating the adherence to PI-RADS specifications in the entire world is needed, considering increasing popularity of the prostate MRI.
In conclusion, the adherence to voxel dimensions in T2-weighted imaging, b value ≥1400 s/mm2 in DWI and temporal resolution <7 s in DCE were low in Turkey. The adherence to slice thickness, FOV, and in-plane dimension (phase) in T2-weighted imaging was higher when 3 T (vs. 1.5 T) scanners were used. Inclusion of recommendations regarding acquisition times and contrast resolution in the future versions of PI-RADS can potentially enhance the compliance with the technical specifications. Awareness to the minimum acceptable technical parameters of mpMRI can potentially improve the quality of prostate cancer imaging and future research is needed to explore impact of adherence to PI-RADS technical specifications on the resultant prostate MRI quality.
Main points.
The adherence to MRI acquisition parameters of PI-RADS v2 is low in Turkey.
Some recommendations of PI-RADS v2 for technical specifications may need to be revised.
The image acquisition duration of T2-weighted imaging longer than 220 s can enhance the compliance with voxel size, which can potentially improve image quality.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Jelle O, Barentsz JO, Richenberg J, et al. ESUR prostate MR guidelines. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PI-RADS Prostate Imaging Reporting and Data System Version 2. American College of Radiology; 2015. [Google Scholar]
- 3.Rosenkrantz AB, Hemingway J, Hughes DR, Duszak R, Jr, Allen B, Jr, Weinreb JC. Evolving use of prebiopsy prostate magnetic resonance imaging in the medicare population. J Urol. 2018;200:89–94. doi: 10.1016/j.juro.2018.01.071. [DOI] [PubMed] [Google Scholar]
- 4.VanderWeele DJ, Turkbey B, Sowalsky AG. Precısıon management of localized prostate cancer. Expert Rev Precis Med Drug Dev. 2016;1:505–515. doi: 10.1080/23808993.2016.1267562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenkrantz AB, Verma S, Choyke P, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol. 2016;196:1613–1618. doi: 10.1016/j.juro.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RT, Spilseth B, Froemming AT. How and why a generation of radiologists must be trained to accurately interpret prostate mpMRI. Abdom Radiol (NY) 2016;41:803–804. doi: 10.1007/s00261-016-0745-4. [DOI] [PubMed] [Google Scholar]
- 7.Esses SJ, Taneja SS, Rosenkrantz AB. Imaging facilities’ adherence to PI-RADS v2 minimum technical standards for the performance of prostate MRI. Acad Radiol. 2018;25:188–195. doi: 10.1016/j.acra.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 8.https://data.oecd.org/healthcare/magnetic-resonance-imaging-mri-exams.htm https://doi.org/10.1787/health_glance-2017-en
- 9.Acar O, Esen T, Çolakoğlu B, et al. Multiparametric MRI guidance in first-time prostate biopsies: what is the real benefit? Diagn Interv Radiol. 2015;21:271–276. doi: 10.5152/dir.2015.46014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavukçu HH, Aytaç Ö, Balcı NC, Kulaksızoğlu H, Atuğ F. The efficacy and utilisation of preoperative multiparametric magnetic resonance imaging in robot-assisted radical prostatectomy: does it change the surgical dissection plan? Turk J Urol. 2017;43:470–475. doi: 10.5152/tud.2017.35589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okcelik S, Soydan H, Ates F, et al. Evaluation of PCA3 and multiparametric MRI’s: collective benefits before deciding initial prostate biopsy for patients with PSA level between 3–10ng/mL. Int Braz J Urol. 2016;42:449–455. doi: 10.1590/S1677-5538.IBJU.2015.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.http://www.turkstat.gov.tr/Start.do
- 13.Othman AE, Falkner F, Weiss J, et al. Effect of temporal resolution on diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging of the prostate. Invest Radiol. 2016;51:290–296. doi: 10.1097/RLI.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 14.Ream JM, Doshi AM, Dunst D, et al. Dynamic contrast-enhanced MRI of the prostate: an intraindividual assessment of the effect of temporal resolution on qualitative detection and quantitative analysis of histopathologically proven prostate cancer. J Magn Reson Imaging. 2017;45:1464–1475. doi: 10.1002/jmri.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RB, McVeigh ER. High temporal resolution phase contrast MRI with multiecho acquisitions. Magn Reson Med. 2002;47:499–512. doi: 10.1002/mrm.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: ınterobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology. 2015;277:741–750. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI-RADS version 2 decision rules: ımpact on prostate cancer detection. Radiology. 2017;283:119–129. doi: 10.1148/radiol.2016161124. [DOI] [PubMed] [Google Scholar]
- 18.Kuhl CK, Bruhn R, Krämer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology. 2017;285:493–505. doi: 10.1148/radiol.2017170129. [DOI] [PubMed] [Google Scholar]