Abstract
PURPOSE
We aimed to evaluate the feasibility, accuracy, and complications of computed tomography (CT)-guided percutaneous transthoracic needle biopsy (PTNB) of cavitary lesions.
METHODS
Consecutive PTNB procedures in an academic institution over a 4-year period were reviewed, 53 of which were performed on patients with cavitary lesions. The demographic data of patients, lesion characteristics, biopsy technique and complications, initial pathologic results, and final diagnosis were reviewed. A final diagnosis was established through surgical correlation, microbiology or clinico-radiologic follow-up for at least 18 months after biopsy.
RESULTS
The overall accuracy of PTNB was 81%. In 33 patients (62%) the cavitary lesion was found to be malignant (23 lung cancers and 10 metastases). The sensitivity and specificity for malignancy was 91% and 100%, respectively. In 20 patients (38%) a benign etiology was established (16 infections and 4 noninfectious etiologies), with PTNB demonstrating a sensitivity of 81% and specificity of 100% for infection. Wall thickness at the biopsy site, lesion in lower lobe, and malignancy were significant independent risk factors for diagnostic success. Minor complications occurred in 28% of cases: 13 pneumothoraces (5 requiring chest tube), 1 small hemothorax, and 1 mild hemoptysis. A nonsignificant higher chest tube insertion rate was seen in cavities with a thinner wall.
CONCLUSION
PTNB of cavitary lesions provides high accuracy, sensitivity, and specificity for both malignancy and infection and has an acceptable complication rate. Wall thickness at the biopsy site, lesion in lower lobe, and malignancy were significant independent risk factors for diagnostic success. Samples for microbiology should be obtained in all patients, especially in the absence of on-site cytology, due to the high prevalence of infection in cavitary lesions.
A cavity is defined as “a gas-filled space within pulmonary consolidation, a mass, or a nodule” (1). Cavities occur in malignant tumors and benign diseases (Fig. 1). Malignant causes of cavitation occur from internal desquamation of tumor cells with liquefaction in primary lung cancer and pulmonary metastases (2). Benign causes of cavitation may be due to necrosis, including caseous and ischemic necrosis, cystic dilatation of airways within consolidation, or cyst-forming infections. Although certain computed tomography (CT) findings, such as a nodular or irregular inner contour, a spiculated or lobulated margin, or enhancing mural nodule, are more common in malignant nodules (3, 4), imaging features between benign and malignant cavitary nodules show considerable overlap.
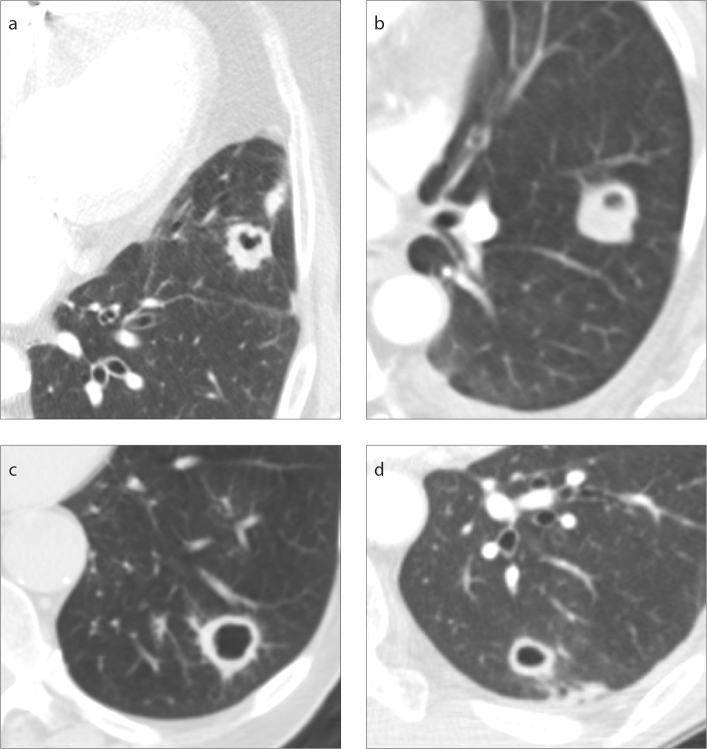
Figure 1. a–d.
Examples of cavitary lesions which were diagnosed on percutaneous transthoracic needle biopsy (PTBN) as (a) bladder cancer metastasis, (b) aspergillus infection, (c) non-small cell lung cancer, and (d) mycobacterium avium-intracellulare (MAI) infection.
CT-guided percutaneous transthoracic needle biopsy (PTNB) is a recognized method for establishing the diagnosis of pulmonary lesions, as it has high diagnostic accuracy ranging from 82%–97% and a low complication rate (5–7). Most previous studies refer to biopsy of solid pulmonary lesions. The safety and diagnostic accuracy of PTNB for cavitary lesions have been reported in only a few studies (8, 9). Zhuang et al. (8) showed that the diagnostic accuracy of CT-guided PTNB for malignant cavitary disease was 96.3% but did not specifically address accuracy for benign causes of cavitary disease such as infection. Yeow et al. (9) demonstrated that in the setting of benign pathologic results, the overall diagnostic accuracy rates with percutaneous needle biopsy for focal lung lesions decreased from 95% to 86%, and they found a 94% diagnostic accuracy rate for cavitary lesions, but they did not specifically look at how benignity affected the accuracy of diagnosing cavitary lesions. The aim of this retrospective analysis was to evaluate the feasibility, diagnostic accuracy, and complications of PTNB of cavitary pulmonary lesions, both malignant and benign.
Methods
This study was designed as a retrospective single-center study and was performed in accordance with the Health Insurance Portability and Accountability Act (HIPAA). Due to the retrospective nature of the study protocol, the institutional ethical review board waived the need for informed consent. This study was designed and conducted according to recently published Standards for Reporting of Diagnostic Accuracy (STARD) (10).
Medical record review
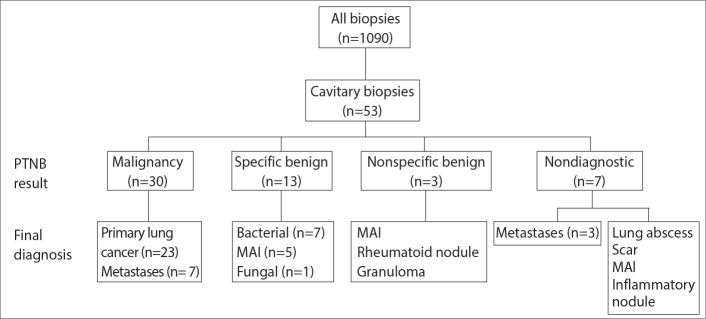
A search of the radiology database was performed to identify patients who had undergone CT-guided PTNB over a 4-year period from July 2009 through June 2013. Two investigators identified cavitary lesions undergoing PTNB on chest CT images by consensus, and these subjects were enrolled in this study (Fig. 2).
Figure 2.
A flow chart of the subjects included in this study.
One investigator retrospectively reviewed the medical record, including patient notes, imaging, and surgical, pathologic, and other laboratory reports. Abstracted data included patient demographics, and smoking history. With regard to the lung biopsy, largest diameter on axial images, cavitary wall thickness at the biopsy site, and location of the cavity were recorded as was the presence of emphysema, whether fine-needle aspiration alone or with core biopsy was performed, length of the needle path, histopathologic results of the biopsy specimen, and complications. Postbiopsy records were reviewed and abstracted data included follow-up surgery and pathology, diagnostic imaging, and treatment prescribed.
Biopsy procedure
All biopsies were performed by one of five experienced thoracic radiologists (with 3–30 years of experience) according to a standard protocol under moderate sedation (11). All procedures were performed under CT guidance using a 16 MDCT scanner (Lightspeed, General Electric) with slice thickness 2.5–5 mm, kV 100–140, and mA 100–260. Patients were positioned either prone or supine, depending on the location of the lesion. Biopsies were planned to avoid crossing large vessels, fissures, or bullae. Sampling of the maximal wall thickness of the lesion was preferable, when accessible.
A 19G thin walled co-axial needle (Chiba-Ultrathin, Cook) was advanced to a position within the wall of the cavity (Fig. 3). Additional intra-cavitary fluid was aspirated at the discretion of the operators. Once the needle was in the wall of the lesion, the inner stylet was removed and a 22G aspiration needle (Chiba, Cook) was advanced through the lumen of the introducer-needle. Then, tissue samples were aspirated. Drops of saline were placed in the well of the introducer needle during needle exchanges to form a water seal to prevent air from entering the needle, and to reduce the risk of air embolism.
Figure 3.
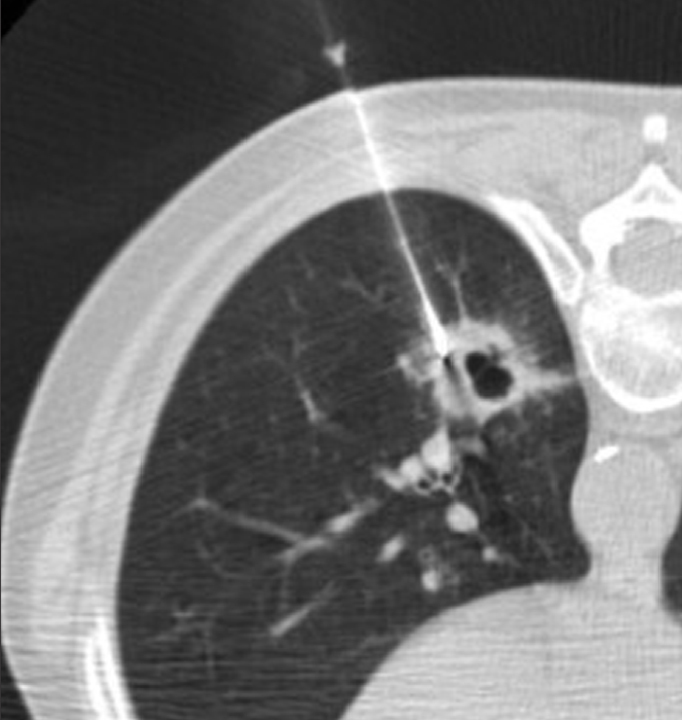
A 61-year-old patient with squamous cell carcinoma of the lung in left lower lobe diagnosed at PTNB and confirmed by surgical lobectomy. CT shows biopsy needle targeting the maximal wall thickness and avoiding vessels.
Aspirated specimens were submitted on glass slides to the on-site cytotechnologist in attendance for rapid interpretation, regarding the adequacy of each specimen for diagnosis. If infection was being considered or if the rapid interpretation was not diagnostic for malignancy, further aspirates were obtained and submitted for microbiology including Gram stain, fungal stain, AFB smear, and aerobic and anaerobic culture. In cases where the rapid interpretation was not diagnostic for malignancy, core biopsy specimens were obtained using a 20G spring-loaded biopsy needle (Quick-core disposable biopsy needle; Cook or Temno, Allegiance Healthcare Corp.) with a 1 or 2 cm needle throw if deemed safe by the operator. Core biopsy specimens were submitted in formalin for pathologic examination. In all 53 cases fine needle aspirations (FNA) for cytology were obtained, with the addition of core needle biopsies in 18 cases. Samples for microbiology were obtained in 30 cases. In 6 cases, intracavitary fluid was also aspirated.
Immediately after the specimens were obtained, but before the removal of the coaxial introducer needle, a limited CT scan was performed to rule out the presence of pneumothorax and hemorrhage. If pneumothorax was present, air was aspirated at the time of removal of the biopsy needle. After removal of the coaxial introducer needle, the patient was immediately rolled on to a stretcher in a biopsy-site down position to reduce the rate and size of a pneumothorax (12). Chest radiographs were obtained at 1 and 3 hours after the procedure, according to department protocol.
Complications were categorized in accordance with the Society of Interventional Radiology clinical practice guidelines (13). The presence of a pneumothorax, hemoptysis, air embolism and procedure-related mortality, as well as the need for intervention to control complications such as chest tube insertion, were recorded.
Statistical analysis
The histopathologic reports of needle biopsy were divided into the following categories: malignant or suspicious for malignancy, specific benign, nonspecific benign, and nondiagnostic. Malignant or suspicious for malignancy included cases in which a specific malignancy was diagnosed or suspicion for malignancy was present. Specific benign included cases in which a benign neoplasm such as hamartoma or specific infection was diagnosed. A biopsy sample was regarded as nonspecific benign if pathologic findings such as fibrosis, inflammation without identification of specific microorganisms, and necrosis were reported but no specific organisms or disease could be identified. If specimens contained normal respiratory elements, including lung tissue, respiratory epithelial cells, histiocytes, or blood that did not represent a mass or lesion, this was considered as a nondiagnostic category. The final diagnosis was established through surgical correlation, microbiology or clinico-radiologic follow-up for at least 18 months after biopsy.
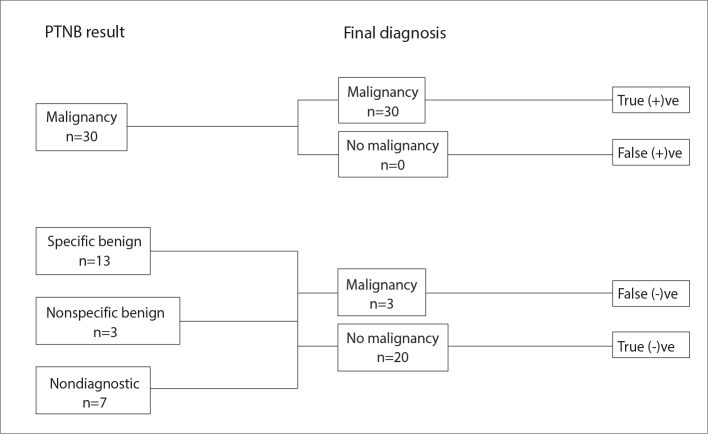
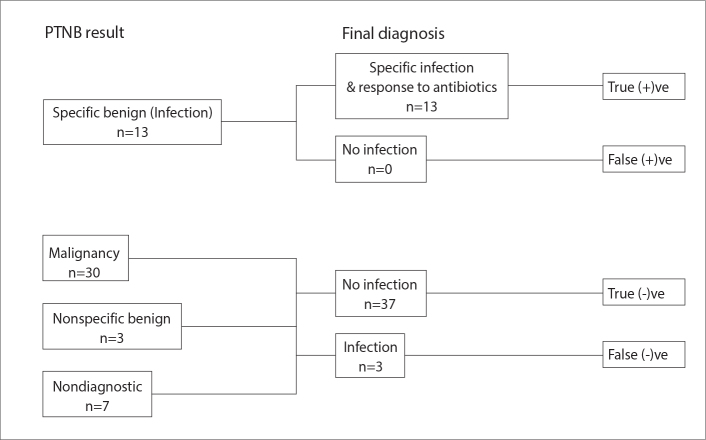
The overall accuracy for a specific diagnosis for malignancy, infection or a benign etiology was determined. The sensitivity and specificity for the diagnosis of malignancy and for the diagnosis of infection was calculated as outlined (Figs. 4, 5). Diagnostic success was defined as a PTNB result which was concordant with the final diagnosis (i.e., a malignant PTNB result with final diagnosis of same malignancy and a specific benign PTNB result with the same specific benign final diagnosis). Any nonspecific benign PTNB result or nondiagnostic specimen, or discordance of the PTNB result and final diagnosis was considered a diagnostic failure.
Figure 4.
A flowchart demonstrating how the sensitivity and specificity for the diagnosis of malignancy was calculated including diagnostic results with respect to malignancy.
Figure 5.
A flowchart demonstrating how the sensitivity and specificity for the diagnosis of infection was calculated including diagnostic results with respect to infection.
Demographic data, lesion and procedure characteristics were evaluated using the nonparametric Mann-Whitney U test for numeric values and Fisher exact test for categoric values. Multivariable logistic regression, using variables yielding a P < 0.1 in the univariable logistic regression analysis, was performed to identify independent risk factors for diagnostic success. The Hosmer-Lemeshow goodness-of-fit test was used to assess model performance. Odds ratios (ORs) with 95% CIs were calculated. A P value less than 0.05 was considered statistically significant. Statistical analyses were performed using statistical software, Stata for Mac (StataCorp, version 15.1).
Results
A total of 1090 percutaneous CT-guided lung biopsies were performed over a 4-year period, 53 (5%) of which were performed on cavities (22 men, 31 women; mean age, 65.4 years; age range, 29–89 years) (Fig 2). Twelve patients were nonsmokers, 28 were ex-smokers and 13 were current smokers. In the majority of cases (64%, 34/53), the biopsied cavity was a solitary lesion. In 19 cases there were multiple lesions, and the decision regarding which lesion to biopsy was based on size in 15 cases (with the largest lesion biopsied) and on the safest route in 4 cases. The mean maximal lesion diameter was 33±18 mm (range, 8–82 mm) and mean maximal cavity wall thickness was 12±8 mm (range, 2–63 mm). Characteristics of the patients and the lesions are summarized in Table 1.
Table 1.
Patient demographics and lesion characteristics
| Characteristic | No |
|---|---|
| Age (years)a | 65±14 |
|
| |
| Sex | |
| Male | 22 |
| Female | 31 |
|
| |
| Smoking history | |
| Nonsmoker | 12 |
| Smoker | 13 |
| Ex-smoker | 28 |
|
| |
| Underlying malignancy | |
| No | 30 |
| Lung cancer | 6 |
| Other malignancy | 17 |
| Head and neck cancer | 6 |
| Transitional cell cancer | 3 |
| Breast cancer | 2 |
| Hematologic | 1 |
| Endometrial | 1 |
| Rectal | 1 |
| Pancreatic | 1 |
| Prostate | 1 |
|
| |
| Multiplicity | |
| Single lesion | 34 |
| Multiple lesions | 19 |
|
| |
| Lesion size (mm)a | 33±18 |
|
| |
| Wall thickness (mm)a | 12±8 |
Data are mean±standard deviation.
Of the 53 cavities that underwent PTNB, the biopsy was positive for malignancy in 30 cases, including 23 primary lung cancers (adenocarcinoma in 16, squamous cell carcinoma in 6, and poorly differentiated carcinoma in 1), and 7 metastases (head and neck cancer in 3, transitional cell carcinoma in 2, and 1 each of colon and endometrial cancer). A specific benign finding was identified in 13 cases, all being infection, including 7 bacterial infections, 5 mycobacterium avium-intracellulare infections (MAI), and 1 fungal infection. Three cavities were diagnosed as nonspecific benign, but confirmed as MAI and granulomatous nodule (unidentified cause) in each case by surgery, and as a rheumatoid nodule in 1 case by clinical and CT follow-up. Nondiagnostic PTNB occurred in 7 cases: 3 cases were confirmed by surgical pathology as a metastasis, bacterial lung abscess, and an old postinflammatory scar. One case was confirmed as MAI by bronchoscopy. The final diagnosis of the remaining 3 nondiagnostic biopsy samples were made by clinical and imaging follow-up: 2 cases were metastases (as the nodule increased in size and there were multiple nodules), and 1 case resolved at follow-up and was likely to have been inflammatory in etiology. Table 2 summarizes the results obtained from PTNB and the final diagnosis.
Table 2.
Results of diagnosis obtained from PTNB and the final diagnosis (n=53)
| Lesion | PTNB, n | Final diagnosis, n |
|---|---|---|
| Malignant lesions | ||
| Lung cancer | 23 | 23 |
| Metastases | 7 | 10 |
|
| ||
| Benign lesions | ||
| Bacterial infection | 7 | 8 |
| MAI infection | 5 | 7 |
| Fungal infection | 1 | 1 |
| Granuloma | 0 | 1 |
| Rheumatoid nodule | 0 | 1 |
| Nonspecific benign (scar/inflammation/fibrosis) | 3 | 2 |
|
| ||
| Nondiagnostic | 7 | 0 |
PTNB, percutaneous transthoracic needle biopsy; MAI, mycobacterium avium-intracellulare infection.
The overall accuracy for a specific diagnosis for malignancy, infection or a specific noninfectious benign etiology was 81%. A diagnosis of malignancy was made in 33 patients (62%), of which 23 were primary lung cancers and 10 were metastases. PTNB demonstrated a sensitivity of 91% and specificity of 100% for malignancy. PTNB correctly established the diagnosis of lung cancer in all cases. In the three cases of metastasis, which were not diagnosed by biopsy, only FNA was obtained.
In 20 patients a benign etiology was established (38%), including 16 infections and 4 noninfectious benign etiologies. PTNB demonstrated an 81% sensitivity and 100% specificity for a diagnosis of infection. In 13 cases of infection (81%) a specific organism was identified by PTNB. Of the 13 patients with diagnosis of infection by PTNB, 9 did not have any antibiotic treatment prior to the PTNB and the other 4 had antibiotic treatment only. Of the 3 patients without a specific microbiologic diagnosis by PTNB but who subsequently went on to have a specific infectious diagnosis made either surgically or bronchoscopically, 2 did not receive antibiotic treatment prior to PTNB and 1 patient did.
Regarding the four noninfectious benign entities (granuloma, scar, rheumatoid nodule, and inflammatory nodule which resolved on follow-up imaging), PTNB yielded a nonspecific benign result in 2 cases and a nondiagnostic result in 2 cases. In two of these four cases only an FNA was obtained.
Core needle biopsy (CNB) was performed in 18 cases: FNA and CNB confirmed a final diagnosis of malignancy in 10, infection in 6 and other benign causes in 2 (rheumatoid nodule, and granuloma). In the 10 cases of malignancy in which a CNB was obtained, FNA and CNB made the diagnosis in 9 cases and FNA only made the diagnosis in 1 case in which the CNB only identified necrosis. CNB also did not add to the diagnostic accuracy of FNA and microbiology in either the infectious or noninfectious benign cases.
In the 6 patients with a known history of lung cancer, the final diagnosis was lung cancer in 3 cases, and MAI, lung abscess, and a rheumatoid nodule (one case of each). In the 17 cases with an extra-pulmonic malignancy, the final diagnosis was metastasis from the known malignancy in 10 cases, a new primary lung cancer in 4 cases, a lung abscess in 2 cases, and aspergillosis in one case.
There were 10 cases of diagnostic failure, including 3 cases that demonstrated nonspecific benign histology and 7 cases of a nondiagnostic sample. Benign lesions were significantly associated with diagnostic failure (P = 0.03). A trend towards a higher diagnostic failure rate was seen in cavities with a thinner wall at the biopsy site (P = 0.086). Although 70% of the cases of diagnostic failure were in nodules with a wall less than 10 mm, there were 23 nodules with a wall less than 10 mm which were a diagnostic success. Table 3 details the demographic data, lesion characteristics and biopsy technique of diagnostic success and failure groups. These variables in addition to diagnostic success were included in the initial multivariable logistic model. The model fit was acceptable as indicated by the Hosmer-Lemeshow test (P = 0.8). After model refinement, wall thickness at the biopsy site (OR, 1.21; 95% CI, 1.02–1.44; P = 0.01), lesion in lower lobe (OR, 17.39; 95% CI, 0.84–64.86; P = 0.048), and malignancy (OR, 14.71; 95% CI, 1.56–139.11; P = 0.007) remained significant independent risk factors for diagnostic success.
Table 3.
Patient demographics and lesion characteristics in success and failure groups
| Variable | Diagnostic success (n=43) | Diagnostic failure (n=10) |
|---|---|---|
| Age (years)a | 69 (30–89) | 66 (33–77) |
|
| ||
| Sex, n | ||
| Male | 19 | 3 |
| Female | 24 | 7 |
|
| ||
| Lesion size (mm)a | 34 (8–82) | 24 (9–69) |
|
| ||
| Wall thickness (biopsy site, mm)a | 11 (3–51) | 7 (3–22) |
|
| ||
| Location, n | ||
| Upper & middle lobe | 20 | 8 |
| Lower lobe | 23 | 2 |
|
| ||
| Final diagnosis, n | ||
| Benign | 13 | 7 |
| Malignant | 30 | 3 |
|
| ||
| Needle path, n | ||
| <3 cm | 10 | 2 |
| ≥3 cm | 33 | 8 |
|
| ||
| Biopsy needle category, n | ||
| FNA | 28 | 7 |
| Core | 15 | 3 |
FNA, fine needle aspiration.
Data are median (min–max).
There were no procedure related deaths or major complications. Minor complications occurred in 15 patients (28%). Postbiopsy pneumothorax occurred in 13 patients (24.5%), 5 patients (9.4%) requiring chest tube. One patient had mild hemoptysis and one patient had a small hemothorax but neither required intervention. A nonsignificant higher chest tube rate occurred after biopsy of cavities with a thinner wall but no statistical difference was found between patients requiring and not requiring chest tube in relation to patient age, sex, lesion characteristics, presence of emphysema, length of the needle path, and final diagnosis (Table 4). Asymptomatic perilesional hemorrhage was noted by CT at time of biopsy in 17 patients (32%). There was no statistical difference in the rate of all complications or in the rate of major complications (pneumothorax requiring chest tube, hemothorax or hemoptysis) between FNA alone and FNA combined with core biopsy (P = 0.27 and P = 0.65, respectively).
Table 4.
Risk factors for chest tube insertion
| Variable | No chest tube (n=48) | Chest tube insertion (n=5) | P |
|---|---|---|---|
| Age (years)a | 65±14 | 69±7 | 0.600 |
|
| |||
| Sex, n | |||
| Male | 19 | 3 | 0.638 |
| Female | 29 | 2 | |
|
| |||
| Lesion size (mm)a | 34±19 | 25±16 | 0.308 |
|
| |||
| Wall thickness (biopsy site, mm)a | 14±12 | 8±6 | 0.227 |
|
| |||
| Emphysema, n | |||
| Present | 31 | 4 | 0.651 |
| Absent | 17 | 1 | |
|
| |||
| Location, n | |||
| Upper & middle lobe | 25 | 3 | |
| Lower lobe | 23 | 2 | 1 |
|
| |||
| Final diagnosis, n | |||
| Benign | 20 | 0 | 0.144 |
| Malignant | 28 | 5 | |
|
| |||
| Needle path, n | |||
| <3 cm | 12 | 0 | 0.577 |
| ≥3 cm | 36 | 5 | |
|
| |||
| Biopsy needle category, n | |||
| FNA | 31 | 4 | 0.651 |
| Core | 17 | 1 | |
FNA, fine-needle aspiration.
Data are mean ± standard deviation.
Discussion
In this retrospective analysis of PTNB of cavitary pulmonary lesions, we evaluated the diagnostic accuracy, complication rates, and risk factors for diagnostic failure and development of pneumothorax requiring chest tube insertion. The overall accuracy for a specific diagnosis was 81%. The sensitivity and specificity for malignancy were 91% and 100%, respectively; and for infection were 81% and 100%, respectively. Independent factors for diagnostic success were wall thickness at the biopsy site, lesion location in the lower lobe, and malignancy. We found benignity was a significant independent risk factor for diagnostic failure, which agrees with the findings in prior studies (9, 14). Prior studies have also revealed improved diagnostic success with a wall thickness of >5 mm which is also in keeping with our findings of improved diagnostic success with increased wall thickness at the biopsy site (8). Our sensitivity and specificity for diagnosing malignancy is similar to the findings of Zhang et al. (8) which determined overall sensitivity and specificity of PTNB for diagnosing malignancy of 96% and 98%, respectively (8). While other papers have demonstrated that in the setting of benign pathologic results the overall diagnostic accuracy rates with percutaneous needle biopsy for focal lung lesions decreases, this study is unique in that it specifically addressed the sensitivity and specificity of PTNB for diagnosing infection in cavitary lesions, which is often an important diagnostic consideration in such lesions (9).
The causes of solitary cavitary lesions detected by plain radiography have been reported as malignancy in 37.7%–55.4% and benign in 44.6%–62.3% (15, 16). In this study, we found 62% of cases were malignant and 38% were benign. In 10 of the 23 patients with a known underlying malignancy (43%), the final diagnosis of the cavitary nodule biopsied was not related to the known primary cancer and was a secondary malignancy in 4 cases, an infection in 5 cases, and a rheumatoid nodule in one case. This underscores the importance of performing biopsies in these cases. We recommend microbiology evaluation on all cases without malignant cells identified on rapid on-site evaluation. In the absence of on-site evaluation, a fine needle aspirate should be sent to microbiology on all cavitary lesions.
A prior study on pulmonary cavitary biopsy reported a higher percentage of nondiagnostic samples when cavity maximal wall thickness was less than 5 mm (8). We also experienced a higher diagnostic failure rate in cavities with a thinner wall at the biopsy site. Therefore, we suggest targeting the thickest wall of a cavitary nodule where technically feasible and safe.
PTNB of cavities was associated with an overall complication rate of 28% (15 of 53), with pneumothorax being the most common (13 of 15). Five patients (9.4%) required chest tube insertion. This incidence of pneumothorax is within previously reported ranges for pneumothorax rate (range, 17%–36.8%) and chest tube insertion (range, 1%–14.2%) after PTNB of pulmonary nodules (5–7, 17–19). The development of perilesional hemorrhage was also evaluated and occurred in 32% (17/52) of patients. This is within the rate of hemorrhage observed after PTNB in recent literature (26.8%–41.1%) (20, 21). In the absence of hemoptysis, perilesional hemorrhage was not considered a major complication as there have been studies suggesting that a degree of pulmonary hemorrhage may even be a protective advantage during PTNB in the prevention of pneumothorax development (21).
In this study, no significant difference was found in the rate of pneumothorax requiring chest tube insertion with respect to patient age, sex, presence of emphysema, and location of lung lesions, in agreement with earlier reports (18, 19). In addition, lesion size and length of the needle path were not associated with an increased risk of chest tube insertion. Although a higher chest tube rate was seen in cavities with a thinner wall, this was not statistically significant. A previous study on PTNB cavitary lesions also reported that no significant risk factors for pneumothorax development were present in relation to wall thickness (8).
This study found mild hemoptysis in one case (1.9%), which is within the reported incidence range (0.2%–8.4%) (5–7, 19, 20). We did not experience any cases of air embolism. Air embolism is a rare but potentially fatal complication, with a reported incidence of 0.06% in a large series (22). The considered risk factors of air embolism were coughing during the procedure, positive-pressure ventilation, needle tip placed within the pulmonary vein, and procedure performed in a patient with vasculitis (23). Cystic or cavitary lesions are also reported as possible risk factors for air embolism in several published reports (24).
This study has several limitations. The retrospective nature of the study may have introduced a selection bias. Our analysis for risks associated with chest tube insertion may have been underpowered to detect significant differences due to the overall low number of chest tubes inserted. Larger studies would be useful in the future to uncover other potentially significant differences. Finally, our results are based on the experience at a single academic medical center with procedures performed by one of five experienced thoracic radiologists and may not be widely applicable to centers which are not as experienced in CT-guided PTNB or where on-site cytopathology is not available.
In conclusion, CT-guided percutaneous biopsy of cavitary pulmonary lesions is a safe procedure which provides high sensitivity for specific diagnoses, both malignant and infectious. Wall thickness at the biopsy site, lesion location in the lower lobe, and malignancy were significant independent risk factors for diagnostic success. Samples for microbiology should be obtained in all patients due to the high prevalence of infection in cavitary lesions.
Main points.
CT-guided percutaneous transthoracic needle biopsy of cavitary nodules is safe, with a high sensitivity and specificity for malignancy and infection.
When feasible, the thickest wall of the cavitary nodule should be biopsied.
Samples should be sent for microbiology evaluation in all cases without malignant cells identified on rapid on-site evaluation, or when rapid-onset evaluation is not available.
Wall thickness at the biopsy site, lesion in lower lobe, and malignancy were significant independent risk factors for diagnostic success.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 2.Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21:3053–3033. doi: 10.1128/CMR.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Honda O, Tsubamoto M, Inoue A, et al. Pulmonary cavitary nodules on computed tomography: differentiation of malignancy and benignancy. J Comput Assist Tomogr. 2007;31:943–949. doi: 10.1097/RCT.0b013e3180415e20. [DOI] [PubMed] [Google Scholar]
- 4.Park Y, Kim TS, Yi CA, Cho EY, Kim H, Choi YS. Pulmonary cavitary mass containing a mural nodule: differential diagnosis between intracavitary aspergilloma and cavitating lung cancer on contrast-enhanced computed tomography. Clin Radiol. 2007;62:227–232. doi: 10.1016/j.crad.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. AJR Am J Roentgenol. 2000;175:239–243. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 6.Poulou LS, Tsagouli P, Ziakas PD, Politi D, Trigidou R, Thanos L. Computed tomography-guided needle aspiration and biopsy of pulmonary lesions: a single-center experience in 1000 patients. Acta Radiol. 2013;54:640–645. doi: 10.1177/0284185113481595. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita J, Masago K, Kato R, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol. 2015;204:29–34. doi: 10.2214/AJR.14.13151. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang YP, Wang HY, Zhang J, Feng Y, Zhang L. Diagnostic accuracy and safety of CT-guided fine needle aspiration biopsy in cavitary pulmonary lesions. Eur J Radiol. 2013;82:182–186. doi: 10.1016/j.ejrad.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Yeow KM, Tsay PK, Cheung YC, Lui KW, Pan KT, Chou AS. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol. 2003;14:581–588. doi: 10.1097/01.RVI.0000071087.76348.C7. [DOI] [PubMed] [Google Scholar]
- 10.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. Radiology. 2015;277:826–832. doi: 10.1148/radiol.2015151516. [DOI] [PubMed] [Google Scholar]
- 11.Wu CC, Maher MM, Shepard JA. CT-guided percutaneous needle biopsy of the chest: preprocedural evaluation and technique. AJR Am J Roentgenol. 2011;196:W511–514. doi: 10.2214/AJR.10.4657. [DOI] [PubMed] [Google Scholar]
- 12.O’Neill AC, McCarthy C, Ridge CA, et al. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology. 2012;262:314–319. doi: 10.1148/radiol.11103506. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Wallace MJ, Cardella JF, et al. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21:969–975. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Montaudon M, Latrabe V, Pariente A, Corneloup O, Begueret H, Laurent F. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol. 2004;14:1234–1240. doi: 10.1007/s00330-004-2250-3. [DOI] [PubMed] [Google Scholar]
- 15.Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135:1269–1271. doi: 10.2214/ajr.135.6.1269. [DOI] [PubMed] [Google Scholar]
- 16.Woodring JH, Fried AM. Significance of wall thickness in solitary cavities of the lung: a follow-up study. AJR Am J Roentgenol. 1983;140:473–474. doi: 10.2214/ajr.140.3.473. [DOI] [PubMed] [Google Scholar]
- 17.Saji H, Nakamura H, Tsuchida T, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: the angle of the needle trajectory is a novel predictor. Chest. 2002;121:1521–1526. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- 18.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol. 1999;172:1049–1053. doi: 10.2214/ajr.172.4.10587145. [DOI] [PubMed] [Google Scholar]
- 19.Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–754. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 20.Tai R, Dunne RM, Trotman-Dickenson B, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. 2015 doi: 10.1148/radiol.2015150381. 150381. [DOI] [PubMed] [Google Scholar]
- 21.De Fillippo M, Sabe L, Silva M. CT-guided biopsy of pulmonary nodules: is pulmonary hemorrhage a complication or an advantage. Diagn Interv Radiol. 2014;20:421–425. doi: 10.5152/dir.2014.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomiyama N, Yasuhara Y, Nakajima Y, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Hiraki T, Fujiwara H, Sakurai J, et al. Nonfatal systemic air embolism complicating percutaneous CT-guided transthoracic needle biopsy: four cases from a single institution. Chest. 2007;132:684–690. doi: 10.1378/chest.06-3030. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi S, Endoh H, Honda T, Komura N, Satoh K. Cerebral air embolism complicating percutaneous thin-needle biopsy of the lung: complete neurological recovery after hyperbaric oxygen therapy. J Anesth. 2001;15:233–236. doi: 10.1007/s005400170008. [DOI] [PubMed] [Google Scholar]