Abstract
Objective: The study was to investigate the effects of creatine (Cr) supplementation on oxidative stress markers and anaerobic performance in rats.
Methods: Sixty-four rats (Wistar) were divided into two groups: C, anaerobic exercised group (n = 32) and Cr, anaerobic exercised group supplemented with creatine (n = 32). Cr supplementation consisted of the addition of 2% Cr monohydrate to the diet. After 28 days, the rats performed acute exercise (6 × 30 seconds of vertical jumps in the water with 30 seconds rest and 50% of total body weight load attached in the back). The animals were euthanized before (pre) and at 0, 2, and 6 hours (n = 8) after acute exercise.
Results: Acute exercise induced an increase in plasma malondialdehyde (MDA) and advanced oxidation protein products (AOPP), as well as increased total lipid hydroperoxides and AOPP in gastrocnemius muscle. Cr supplementation inhibited the formation of MDA and lipid hydroperoxides in plasma. However, the antioxidant action of Cr was observed only against AOPP in gastrocnemius muscle. Cr supplementation also increased (P < 0.05) anaerobic performance compared to the C group.
Conclusion: Cr supplementation is able to inhibit the increase in plasma lipid peroxidation markers induced by high-intensity and short-duration exercise in rats; equivalent actions, however, were not observed fully in muscle tissue.
Keywords: Oxidative stress, Antioxidants, Acute exercise, Rodents
Introduction
Since Harris et al.1 demonstrated for the first time that creatine (Cr) supplementation increases intramuscular concentration of Cr and phosphocreatine (PCr), Cr monohydrate has become a popular dietary supplement among athletes and individuals practizing physical exercise. The concept of Cr supplementation improving performance is based on the fact that Cr plays an important role in energy metabolism during muscle contraction through the ATP-Cr/CP system.2 Over the past few years, the use of Cr supplementation has been extended to the medical area in view of the results obtained for the treatment of myopathies and neurodegenerative diseases.3–5 More recently, studies have shown the beneficial effects of Cr supplementation on metabolic disorders such as diabetes6 and non-alcoholic fatty liver disease.7 The recent review of Gualano et al.8 provides comprehensive information about the therapeutic effects of Cr supplementation.
Over the past decade, studies have shown that Cr exerts antioxidant activity.9 Lawler et al.10 using in vitro techniques were the first to demonstrate that Cr acts as a primary antioxidant. Subsequent studies demonstrated the protective effect of Cr against oxidative stress in cell cultures,11,12 against DNA and RNA damage,13,14 and in in vivo experiments using rats.15,16 These emerging data from in vitro methods and experiments on rodents suggest a protective antioxidant potential of Cr in different situations. However, few studies have demonstrated this protective capacity against oxidative stress induced by high-intensity exercise. Therefore, we hypothesized that supplementation with Cr would exert protective effects against oxidative stress in the plasma and muscle of rats submitted to acute high-intensity exercise. The main objective of the study was to investigate the effects of Cr supplementation on markers of oxidative stress and anaerobic performance of rats after acute anaerobic exercise.
Methods
Sixty-four male Wistar rats (120–130 g) were obtained from the Animal Care Unit at the Faculty of Medicine of Ribeirão Preto after approval by the institution's Committee on Animal Care. The animals were kept in individual cages on a 12/12 hour light/dark cycle at a mean temperature of 22°C and were divided at random into two groups: C, control group (n = 32) and Cr, creatine-supplemented group (n = 32). The animals received fresh food and water every 2 days and had free access to food during the entire experimental period. Food intake was measured daily to assess total food intake and creatine consumption. Creatine supplementation was performed by adding 2% w/w monohydrate creatine (Acros Organics, New Jersey, NY, USA) to the control AIN-93 powder diet17 for 28 days. Two percent Cr supplementation to the diet was chosen because it previously has been shown to increase plasma and total muscle Cr content.15
After 28 days of Cr supplementation the animals were submitted to an acute bout of intense exercise. Ten microliters blood samples were taken from a tail vein for lactate assay with a portable Accusport Accutrend Lactate analyzer (Boehringer Mannheim, Castle Hill, Australia). Accusport Accutrend Lactate analyzers have demonstrated reliable results when compared to standard enzymatic photofluorometry.18,19 All rat groups were euthanized by decapitation before (n = 8) and at 0 hour (n = 8), 2 hours (n = 8), and 6 hours (n = 8) after acute exercise. Blood was collected into heparinized tubes, centrifuged at 1000g for 10 minutes and plasma was separated and stored at −80°C. The gastrocnemius muscle was removed; the white portion was anatomically separated and rapidly frozen in liquid nitrogen and stored at −80°C.
Acute exercise protocol
The acute exercise protocol was performed as described by Deminice et al.20 After 1 week of water immersion (keeping the animal in 10 cm deep water for 20 minutes, 5 days/week for 1 week) the animals were adapted to the exercise protocols. The adaptation to exercise protocol consisted in adding weight to the body progressively (20% per week) using a adapted elastic backpack attached to the chest to induce the rats to jump into in a 50 cm deep cylinder tank (25 cm in diameter) for 30 seconds, 2 days/week for 2 weeks. Both water immersion and exercise adaptation was performed to reduce stress without promoting exercise training adaptations.
After 28 days of Cr supplementation all rats performed an acute bout of high-intense exercise, using the water tank described above. Rats were induced to jump for 6 minutes (30 seconds exercise interrupted by a 30 seconds rest) carrying a load of 50% body weight. In all cases, the temperature of the water was set at 31 ± 1 °C.
Biochemical analyses
Frozen muscle samples were homogenized in ice-cold 50 mM sodium phosphate, pH 7.0, and the homogenates were centrifuged at 13 000g for 10 minutes at 4°C. The supernatants were utilized for the determination of muscle malondialdehyde (MDA) and total lipid hydroperoxide as parameters of lipid peroxidation by the methods of Spirlandeli et al.21 and Costa et al.,22 respectively. Advanced oxidation protein products (AOPP) were determined by the method of Witko-Sarsat et al.23 Reduced (GSH) and oxidized glutathione (GSSG) were determined in muscle according to Rahman et al.24 Plasma and total muscle Cr were assayed by the Jaffe reaction using a method described by Deminice et al.15
Total blood hematocrit and hemoglobin were measured to correct for plasma volume shifts.25 Assays were carried out in duplicate. The coefficient of variation for each measurement was less than 5% for all assays.
Statistical analysis
Data are reported as mean ± standard error of mean. A linear mixed effects model was used to detect possible differences between groups at the time of euthanasia and possible differences in relation to time of euthanasia (pre and 0, 2, and 6 hours after exercise) in the same group. The level of significance was set at P < 0.05 in all analyses.
Results
No significant differences in final body weight, body weight gain, or food intake were observed after 4 weeks of the experiment. As expected, the Cr supplemented diet provided a significantly higher amount of Cr compared to the C diet (Table 1).
Table 1. Final weight, weight gain, diet, and creatine intake for both control (C) and creatine-supplemented group (Cr) after 4 weeks of creatine supplementation.
| C | Cr | |
|---|---|---|
| Body weight (g) | 286.2 ± 7.3 | 290.4 ± 7.5 |
| Body weight gain (g/4 weeks) | 167.3 ± 7.1 | 168.9 ± 5.8 |
| Food intake (g/day) | 20.8 ± 1.1 | 21.3 ± 1.8 |
| Creatine intake (g/day) | – | 0.42 ± 0.03 |
Values are mean ± SEM, n = 32.
The number of jumps in each of the six sets is shown in Fig. 1. Supplementation with Cr led to an increase in the number of jumps after the fourth set in the Cr group compared to the C group. These results demonstrate the capacity of Cr supplementation to improve repeated jump performance. Blood lactate concentration increased significantly (P < 0.05) immediately after the repeated jump series in both groups, without a significant difference between them. The high blood lactate concentration of 10–12 mmol/l demonstrates the high-intensity of the effort proposed in this study (Fig. 1). As expected, total muscle Cr concentration was significantly higher (P < 0.05) in the Cr group compared to C at all times of euthanasia (Fig. 1). Cr supplementation also increased plasma Cr concentration (C: pre 82.7 ± 3.5, 0 hour 112.9 ± 4.0, 2 hours 72.2 ± 4.9, and 6 hours 71.7 ± 3.0; Cr: pre 243.6 ± 9.8, 0 hour 425.4 ± 23.8; 2 hours 221.9 ± 5.8, and 6 hours 255.6 ± 10.3, µmol/l).
Figure 1.
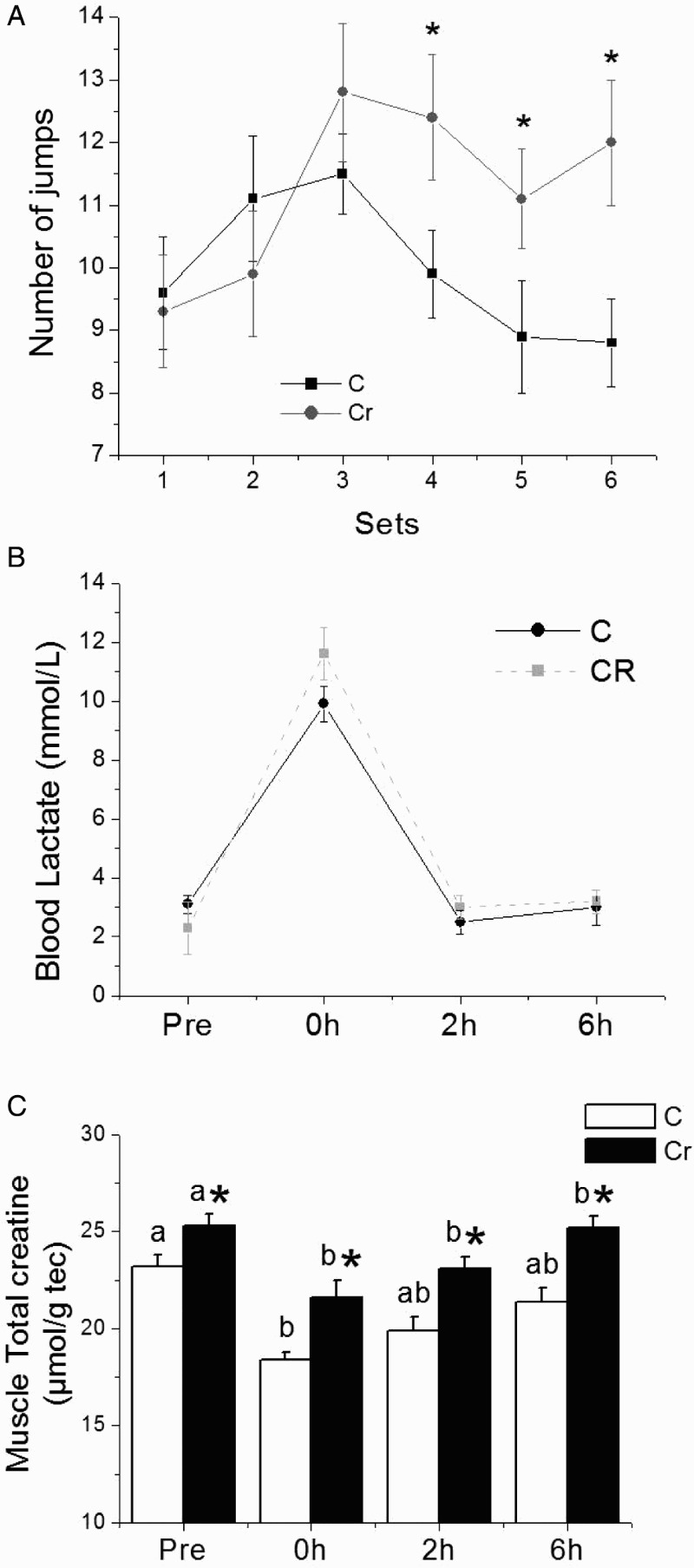
Number of jumps in each set (A); blood lactate concentration (B); and total muscle creatine determined pre and 0, 2, and 6 hours after acute exercise in the control (C) and creatine-supplemented (Cr) groups. Values are mean ± SEM, n = 8. abMeans of the same group followed by different letters were significantly different; *Significant difference in relation to the control at the same time of euthanasia (P < 0.05 by linear mixed effects model).
Figure 2 and Table 2 present plasma and white gastrocnemius muscle oxidative stress markers before and after (0, 2, and 4 hours) acute exercise for the C and Cr groups studied, respectively. Acute exercise promoted increased plasma MDA (28% at 0 hour) and AOPP (42% at 0 hour) (Fig. 2). Increased AOPP (42% at 0 hour) and lipid hydroperoxide (98% at 2 hours) were observed in gastrocnemius muscle. Decreases in GSH and GSH/GSSG ratio also were observed in gastrocnemius muscle (Table 2). Supplementation with Cr inhibited the formation of lipid peroxidation markers, MDA, and total peroxides in plasma (Fig. 2). However, an antioxidant action of Cr was observed only against AOPP in gastrocnemius muscle. Cr supplementation did not change GSH muscle levels (Table 2).
Figure 2.
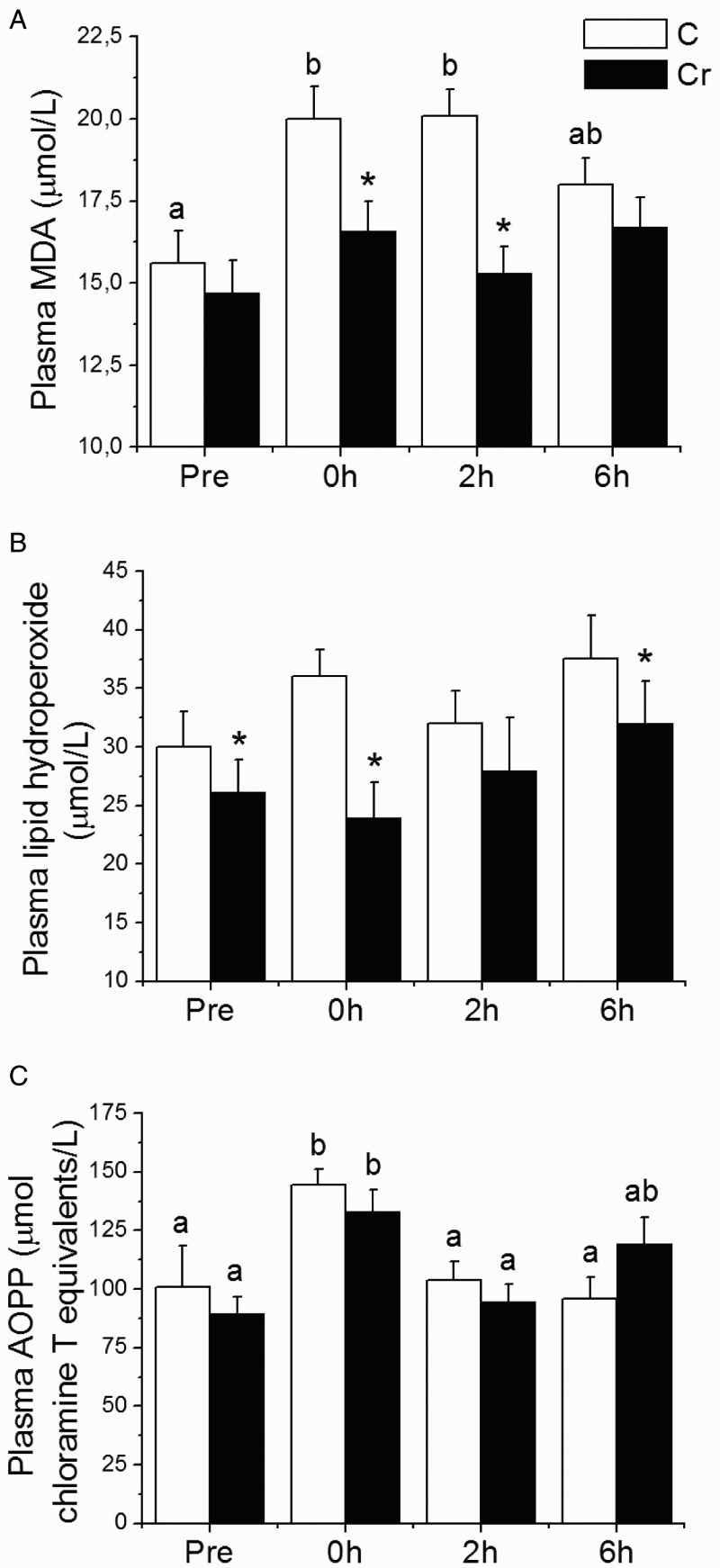
Plasma oxidative stress markers determined pre and 0, 2, and 6 hours after acute exercise in the control (C) and creatine-supplemented (Cr) groups. Values are mean ± SEM, n = 8. abMeans of the same group followed by different letters were significantly different; *Significant difference in relation to the control at the same time of euthanasia (P < 0.05 by linear mixed effects model).
Table 2. Muscle oxidative stress markers determined pre and 0, 2, and 6 hours after acute exercise in the control (C) and creatine-supplemented (Cr) groups.
| Pre | 0 hour | 2 hours | 6 hours | ||
|---|---|---|---|---|---|
| MDA (µmol/g tissue) | C | 0.16 ± 0.02 | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.15 ± 0.04 |
| Cr | 0.17 ± 0.02 | 0.14 ± 0.02 | 0.16 ± 0.03 | 0.14 ± 0.02 | |
| Lipid hydroperoxide (µmol/g tissue) | C | 23 ± 2.0a | 32.8 ± 3.4b | 49 ± 4.6c | 26.2 ± 2.8a |
| Cr | 22.9 ± 2.8a | 36.8 ± 4.5b | 44.8 ± 5.2c | 22.1 ± 3.8a | |
| AOPP (µmol/g tissue) | C | 0.62 ± 0.12a | 0.84 ± 0.14b | 0.65 ± 0.11a | 0.34 ± 0.07c |
| Cr | 0.38 ± 0.10*a | 0.63 ± 0.11*b | 0.65 ± 0.08b | 0.50 ± 0.18ab | |
| GSH (mmol/g tissue) | C | 3.2 ± 0.7a | 2.7 ± 0.4ab | 2.4 ± 0.3b | 2.2 ± 0.3b |
| Cr | 3.7 ± 0.6a | 3.2 ± 0.6ab | 3..0 ± 0.4b | 2.8 ± 0.2b | |
| GSH/GSSG | C | 7.1 ± 0.7a | 5.4 ± 1.0ab | 5.0 ± 0.4a | 4.6 ± 0.6a |
| Cr | 7.3 ± 0.8a | 5.1 ± 0.8b | 5.3 ± 0.5b | 4.7 ± 0.4b |
Values are mean ± SEM, n = 8.
abcMeans in a row followed by different letters were significantly different.
*Significant difference in relation to the control at the same time of euthanasia (P < 0.05 by linear mixed effects model).
Discussion
The main results of the present study were: (1) an acute session of repeated jumps increased plasma and muscle oxidative stress markers; (2) supplementation with Cr was able to inhibit the increases in the plasma lipid peroxidation markers MDA and lipid hydroperoxides induced by acute exercise; (3) inhibition was not observed in the muscle tissue, with the exception of the protein oxidation marker AOPP; (4) Cr supplementation improved repeated jump performance in the animals studied.
Oxidative stress is the condition that reflects an imbalance between the cellular production of pro-oxidants and the physiological capacity of the cells to remove it through the endogenous and exogenous antioxidant system.26 The generation of ROS occurs during normal cellular metabolism, but is increased under conditions of physical stress.27 Studies have indicated different mechanisms that underlie the generation of ROS induced by anaerobic exercise, such as the increased production of catecholamines, production of lactic acid and late inflammatory responses,26 elevated intracellular calcium concentrations that activate the xanthine oxidase and phospholipase pathway,28 and conditions of hypoxia and activation of phagocytic cells.28 In the present study, we chose an acute exercise protocol consisting of repeated jumps adapted to rodents to test the antioxidant capacity of Cr. This protocol was chosen since the capacity of repeated sprint exercise to promote oxidative stress is already known.26,28,29 Indeed, our data demonstrated that the acute exercise significantly increased (P < 0.05) plasma MDA at 0 hour (28%) and muscle lipid hydroperoxides at 2 hours (98%) as well as plasma at 0 hour (31%) and muscle AOPP at 0 hour (42%). Acute exercise also promoted decreased muscle GSH and GSH/GSSG ratio. This protocol is suitable for studies on Cr supplementation since it involves short-duration interval exercise (6 × 30 seconds of effort that generated 10–12 mmol/l of blood lactate concentration; Fig. 1), a type of exercise that requires significant contribution of the ATP-Cr/CP system.
Although a reasonable number of studies have demonstrated the antioxidant property of Cr using in vitro techniques10–14 and rodent studies in different situations,15,16 the present study demonstrate the protective antioxidant activity of Cr supplementation against increased lipid peroxidation induced by acute high-intensity exercise. Lawler et al.10 and Sestili et al.12 demonstrated that Cr exerts a significant direct antioxidant effect using in vitro techniques and cell cultures exposed to different oxidants, respectively. Recent studies also demonstrated the protective effect of Cr on mitochondrial DNA14 and RNA13 exposed to oxidants. Those authors concluded that the protective effect of Cr against DNA and RNA damage resulted from the capacity of Cr to directly remove free radicals.13,14 More recently, studies have demonstrated the protective effect of Cr in animal models.15,16,20 In studies conducted at our laboratory, Deminice et al.15 demonstrated the capacity of Cr to reduce homocysteine concentration and lipid peroxidation in rats. The same results were observed when hyperhomocysteinemia and lipid peroxidation were induced by acute aerobic/moderated exercise.16,20 Guimarães-Ferreira et al.30 shown reduced generation of muscle ROS in rats after short-term Cr supplementation (5 g/day for 6 days), without changes in the activity of antioxidant enzymes. According to these authors, this result indicates the capacity of Cr to directly remove ROS. Those data together indicate that Cr exerts an antioxidant effect in different situations using in vitro and in vivo methods. However, the antioxidant activity of Cr against oxidative stress induced by acute high-intensity physical exercise is still poorly understood.
Although numerous studies have demonstrated the protective antioxidant effects of Cr, the exact mechanism whereby this compound exerts these effects is still a matter of discussion.9 Lawler et al.10 reported the capacity of Cr to directly scavenge radical molecules; according to these authors, however, such capacity is lower than that of vitamin E and GSH and may be selective for specific radical molecules. Young et al.31 and Deminice and Jordao,16 demonstrated the capacity of Cr to promote up-regulation of the thiol redox system, improving GSH antioxidant capacity. As a methylated compound, Cr supplementation could suppress Cr synthesis causing a sparing effect on cysteine, a GSH precursor in the transsulfuration pathway.16 Improved hydration status, membrane stabilization and increased energetic pool of the cell are also speculated to be indirect antioxidant mechanisms.2 However, increased and/or normalized energy cell status mediated by Cr supplementation have been the most promising mechanisms among those cited.32,33 Meyer et al.32 demonstrated that increased Cr availability could normalize the PCr/Cr ratio and activate ADP recycling by mitochondrial creatine kinase, a mechanism by which it regulates oxygen uptake and reduces the generation of free radicals. Santiago et al.,33 studying the modulation of mitochondrial kinases by free radical generation, concluded that mitochondrial creatine kinase actively participates as an antioxidant system. Despite the different explanations and speculations, the exact mechanism whereby Cr exerts antioxidant activity still needs to be determined.
Although extensively studied, there is no consensus regarding the effect of antioxidant supplementation on athletic performance. Consistent data exist that antioxidant supplementation attenuates exercise-induced oxidative stress.29 However, the data showing antioxidant supplementation as the responsible factor for improvement in sports performance are inconsistent. Regarding Cr supplementation, its role in energy metabolism through the ATP-Cr/CP system is undoubtedly the most widely accepted explanation in the literature for the ergogenic activity of this substance. Indeed, recent studies have demonstrated the capacity of Cr to modulate the mitochondrial energy pool,32,33 a function that might be related to both the improvement in sprint performance and the reduction of free radicals. Therefore, further studies must consider the effects of Cr exposure on the mitochondrial ATP-Cr/CP system to understand the true contribution of the antioxidant capacity of Cr to sports performance.
In conclusion, Cr supplementation is able to inhibit the increase in plasma lipid peroxidation markers induced by high-intensity and short-duration exercise in rats; such actions, however, are not fully observed in muscle tissue. These results are original and confirm in vitro data showing that Cr exerts antioxidant activity. Furthermore, our data confirm the ergogenic activity of Cr supplementation by improving anaerobic performance in rats. These results support the practical use of Cr supplementation in high-intense repeated bout exercises. However, the relationship between the antioxidant capacity attributed to Cr and sports performance still needs to be elucidated.
Acknowledgements
The research was supported by Brazilian grants from Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil (Protocol 07/08099-5) and Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq.
Disclaimer statements
Contributors None.
Funding Supported by Brazilian funds from Fundação Araucária and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP).
Conflict of interest All authors declared that there is no potential conflict of interests regarding this article.
Ethics approval The work was approved by the Animal Research Ethics Committee of the University of Sao Paulo, Brazil.
References
- 1.Harris RC, Söderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 1992;83:367–74. [DOI] [PubMed] [Google Scholar]
- 2.Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease. Neuroscience 2002;112:243–60. [DOI] [PubMed] [Google Scholar]
- 3.Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, et al. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology 2006;67:1262–4. [DOI] [PubMed] [Google Scholar]
- 4.Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010;38:31–44. [DOI] [PubMed] [Google Scholar]
- 5.Kley RA, Tarnopolsky MA, Vorgerd M. Creatine for treating muscle disorders. Cochrane Database Syst Rev 2011;16:CD004760. [DOI] [PubMed] [Google Scholar]
- 6.Gualano B, Salles PV, Roschel H, Artioli GG, Neves M Jr, De Sá, et al. Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Med Sci Sports Exerc 2011;43:770–8. [DOI] [PubMed] [Google Scholar]
- 7.Deminice R, da Silva RP, Lamarre SG, Brown C, Furey GN, McCarter SA, et al. Creatine supplementation prevents the accumulation of fat in the livers of rats fed a high-fat diet. J Nutr 2011;141:1799–804. [DOI] [PubMed] [Google Scholar]
- 8.Gualano B, Roschel H, Lancha-Jr AH, Brightbill CE, Rawson ES. In sickness and in health: the widespread application of creatine supplementation. Amino Acids 2012;43:519–29. [DOI] [PubMed] [Google Scholar]
- 9.Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, et al. Creatine as an antioxidant. Amino Acids 2011;40:1385–96. [DOI] [PubMed] [Google Scholar]
- 10.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun 2002;290:47–52. [DOI] [PubMed] [Google Scholar]
- 11.Lenz H, Schmidt M, Welge V, Schlattner U, Wallimann T, Elsässer HP, et al. The creatine kinase system in human skin: protective effects of creatine against oxidative and UV damage in vitro and in vivo. J Invest Dermatol 2005;124:443–52. [DOI] [PubMed] [Google Scholar]
- 12.Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, et al. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 2006;40:837–49. [DOI] [PubMed] [Google Scholar]
- 13.Fimognari C, Sestili P, Lenzi M, Cantelli-Forti G, Hrelia P. Protective effect of creatine against RNA damage. Mutat Res 2009;670:59–67. [DOI] [PubMed] [Google Scholar]
- 14.Guidi C, Potenza L, Sestili P, Martinelli C, Guescini M, Stocchi L, et al. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim Biophys Acta 2008;1780:16–26. [DOI] [PubMed] [Google Scholar]
- 15.Deminice R, Portari GV, Vannucchi H, Jordao AA. Effects of creatine supplementation on homocysteine levels and lipid peroxidation in rats. Br J Nutr 2009;102:110–6. [DOI] [PubMed] [Google Scholar]
- 16.Deminice R, Jordao AA. Creatine supplementation reduces oxidative stress biomarkers after acute exercise in rats. Amino Acids 2012;43:709–15. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 18.Shimojo N, Naka K, Uenoyama H, Hamamoto K, Yoshioka K, Okuda K. Electrohemical assay system with single-use electrode strip for measuring lactate in whole blood. Clin Chem 1993;39:2312–2314. 17. [PubMed] [Google Scholar]
- 19.Roßkopf P, Lamprecht W, Liesen H. The Accusport® analyzer and its operation. In: Ramstetter E, Zieres-Nauth C, Mack M (eds.) Workshop report Accusport®. Zürich 1994-03-18. Mannheim: Boehringer; 1995. p. 33–6. [Google Scholar]
- 20.Deminice R, Vannucchi H, Simões-Ambrosio LM, Jordao AA. Creatine supplementation reduces increased homocysteine concentration induced by acute exercise in rats. Eur J Appl Physiol 2011;111:2663–70. [DOI] [PubMed] [Google Scholar]
- 21.Spirlandeli AL, Deminice R, Jordao AA. Plasma malondialdehyde as biomarker of lipid peroxidation: effects of acute exercise. Int J Sports Med 2014;35:14–8, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Costa CM, Dos Santos RCC, Lima E. A simple automated procedure for thiol measurement in human and serum samples. Braz J Pathol Med Lab 2006;42:345–350. [Google Scholar]
- 23.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 1996;49:1304–13. [DOI] [PubMed] [Google Scholar]
- 24.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 2006;1(6):3159–65. [DOI] [PubMed] [Google Scholar]
- 25.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 1974;37:247–8. [DOI] [PubMed] [Google Scholar]
- 26.Bloomer RJ, Falvo MJ, Fry AC, Schilling BK, Smith WA, Moore CA. Oxidative stress response in trained men following repeated squats or sprints. Med Sci Sports Exerc 2006;38:1436–42. [DOI] [PubMed] [Google Scholar]
- 27.Finaud J, Lac G, Filaire E. Oxidative stress: relationship with exercise and training. Sports Med 2006;36:327–58. [DOI] [PubMed] [Google Scholar]
- 28.Bloomer RJ. Effect of exercise on oxidative stress biomarkers. Adv Clin Chem 2008;46:1–50. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee AK, Mandal A, Chanda D, Chakraborti S. Oxidant, antioxidant and physical exercise. Mol Cell Biochem 2003;253:307–12. [DOI] [PubMed] [Google Scholar]
- 30.Guimarães-Ferreira L, Pinheiro CH, Gerlinger-Romero F, Vitzel KF, Nachbar RT, Curi R, et al. Short-term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. J Appl Physiol 2012;112:3913. [DOI] [PubMed] [Google Scholar]
- 31.Young JF, Larsen LB, Malmendal A, Nielsen NC, Straadt IK, Oksbjerg N, et al. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr 2010;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer LE, Machado LB, Santiago AP, da-Silva WS, De Felice FG, Galina A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 2006;281:37361–71. [DOI] [PubMed] [Google Scholar]
- 33.Santiago AP, Chaves EA, Oliveira MF, Galina A. Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 2008;90:1566–77. [DOI] [PubMed] [Google Scholar]


