Abstract
Objectives
Exposure to sodium nitrites, a food additive, at high levels has been reported to produce reactive nitrogen and oxygen species that cause dysregulation of inflammatory responses and tissue injury. In this work, we examined the impact of dietary cod liver oil on sodium nitrite-induced inflammation in rats.
Methods
Thirty-two adult male Sprague-Dawely rats were treated with 80 mg/kg sodium nitrite in presence/absence of 5 ml/kg cod liver oil. Liver sections were stained with hematoxylin/eosin. We measured hepatic tumor necrosis factor (TNF)-α, interleukin-1 beta (IL)-1β, C-reactive protein (CRP), transforming growth factor (TGF)-β1, and caspase-3.
Results
Cod liver oil reduced sodium nitrite-induced hepatocyte damage. In addition, cod liver oil results in reduction of hepatic TNF-α, IL-1β, CRP, TGF-β1, and caspase-3 when compared with the sodium nitrite group.
Discussion
Cod liver oil ameliorates sodium nitrite-induced hepatic injury via multiple mechanisms including blocking sodium nitrite-induced elevation of inflammatory cytokines, fibrosis mediators, and apoptosis markers.
Keywords: Caspase-3, C-reactive protein (CRP), Interleukin (IL)-1β, Transforming growth factor (TGF)-β1, and Tumor necrosis factor (TNF)-α
Introduction
Humans are continuously exposed to different kinds of chemicals such as food additives. Many of these additives have been increasingly recognized as potentially hazardous for human health. One important food additive is sodium nitrite. It is well known for its role in inhibiting the growth of Clostridium botulinum spores in refrigerated meats by inhibiting iron–sulfur clusters essential to energy metabolism.1 Moreover, sodium nitrite is able to effectively delay the development of oxidative rancidity by reacting with heme proteins and metal ions and chelating free radicals, terminating the cycle of lipid oxidation that leads to rancidity.2 However, exposure to nitrites at levels above health-based risk, LD50 71 mg/kg in humans, has been reported to produce cytotoxic and weak carcinogenic effects.3,4 Reactive nitrogen species produced by exposure to nitrite have many toxic effects including hepatotoxicity, nephrotoxicity, and dysregulation of inflammatory responses and tissue injury.5,6
Recent trends in controlling and treating diseases tend to favor natural compounds, as the human diet is essential in protecting the body against the development of diseases. One of these natural products is cod liver oil, derived from the liver of genus Gadus of demersal fish in the Gadidae family. It has been used for medicinal purposes for centuries. One dose of cod liver oil supplement (5 ml) contains 500 mg vitamin A, 10 mg vitamin D, and 10 mg vitamin E as well as 1.2 g of ω-3 fatty acids.7 Cod liver oil has many favorable biological properties such as antiinflammatory, antioxidant, and antifibrosis properties.8–10
Recent understanding of the molecular events of increased levels of sodium nitrite has focused researchers' attention on oxidative stress in different body organs.8,11 Less attention has been paid to other effects like inflammation. Therefore, this study is designed to examine the impact of dietary cod liver oil on chronic exposure to oral sodium nitrite and its subsequent induced liver inflammation in rats.
Materials and methods
Animals and their treatment outlines
The animal protocol was approved by the ethical committee of the Faculty of Pharmacy, University of Mansoura (protocol code no. 2012–32). Male Sprague-Dawely rats weighing 120–140 g were used. All animals in the study were maintained under standard conditions of temperature, about 25°C, with regular 12 hours light/12 hours dark cycle and allowed free access to food and water. Rats were classified into the following groups with eight rats in each group:
Control group: Rats received the standard diet without any treatment.
Cod liver oil control group: Rats received 5 ml/kg cod liver oil (Silver Seas Co., El-Obour, Egypt) daily by oral gavage for 12 weeks.
Sodium nitrite group: Rats were given daily sodium nitrite (Sigma-Aldrich Co, St. Louis, MO, USA) at a dose of 80 mg/kg body weight dissolved in phosphate buffer solution, pH7.4, by oral gavage for 12 weeks.
Cod liver oil treated group: Rats received 5 ml/kg cod liver oil. After 60 minutes rats were treated with 80 mg/kg sodium nitrite dissolved in phosphate buffer solution daily for 12 weeks.
The doses and time course of experiments used for sodium nitrite and cod liver oil in this study were in the range of those used in other studies in the same animal species.8,12–14 In addition, the dose was determined after appropriate preliminary experiments.
Animal sacrifice and collection of samples
The animals were sacrificed by decapitation. Rat trunk blood was collected and centrifuged at 3000 rpm for 5 minutes and serum samples were separated and stored at –80° C. Rat livers were removed, cleaned with ice-cold saline, weighed, and chilled on crushed ice. A piece of the liver was homogenized in a 10-fold volume of ice-cold sodium, potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% (w/v) KCl. The homogenates were centrifuged at 600 × g at 4°C for 10 minutes. The supernatant, referred to as homogenate, was stored at –80°C till used.
Assessment of liver function tests
Serum alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma glutamyl transferase (GGT) activities were measured by standard methodologies using commercially available kits provided by Biodiagnostic Company (Giza, Egypt).
Assessment of oxidative stress
Oxidative stress was estimated in the liver homogenates through the following parameters:
Superoxide anion concentration was measured by the nitroblue tetrazolium method.15 It depends on the ability of the superoxide anion to reduce nitroblue tetrazolium to an insoluble formazan that can be measured at 560 nm.
Superoxide dismutase (SOD) activity was determined using the phenazine methosulfate method,16 in which the ability of the enzyme to inhibit the phenazine methosulfate-mediated reaction of nitrobluetetrazolium dye is measured as an indication of the activity of SOD.
ELISA determination
The levels of biochemical parameters in liver homogenate were measured by ELISA using commercially available tumor necrosis factor (TNF)-α, interleukin (IL)-1β, transforming growth factor (TGF)-β1, and C-reactive protein (CRP) ELISA kits (eBioscience Inc., San Diego, CA, USA) in accordance with the manufacturer's instructions.
Determination of caspase-3 activity assay
Caspase-3 enzyme activity assay was measured colorimetrically using commercially available kits (GenScript, Piscataway, NJ, USA) following the manufacturer's procedure.
Morphologic analysis of hepatic tissue
The liver was cut, fixed in 10% (v/v) buffered formalin and embedded in paraffin. Five micrometer thickness sections were cut and stained with Mayer's hematoxylin and eosin for examination of cell structure by light microscopy. Hepatic specimens were anonymously coded and examined in a masked manner. The morphologic changes were photographed using a digital camera-aided computer system (Nikon Digital Camera, Tokyo, Japan).
Immunohistochemistry
Immunohistochemical analyses were performed on 5-μm-thick paraffin sections cut from a paraffin block of liver. Sections were incubated with monoclonal anti-TNF-α (eBioscience Inc., San Diego, CA, USA) at 4°C. Slides were counterstained with hematoxylin and examined using a Nikon Digital Camera.
Statistical analysis
For descriptive statistics of quantitative variables, the mean ± standard error was used. Normality of the sample distribution of each continuous variable was tested with the Kolmogorov–Smirnov test. One-way analysis of variance was used to compare means between groups. If differences existed among the means, a post hoc Bonferroni correction test was used. Statistical computations were done on a personal computer using the computer software SPSS version 13 (Chicago, IL, USA). Statistical significance was predefined as P ≤ 0.05.
Results
Hepatoprotective effects of cod liver oil
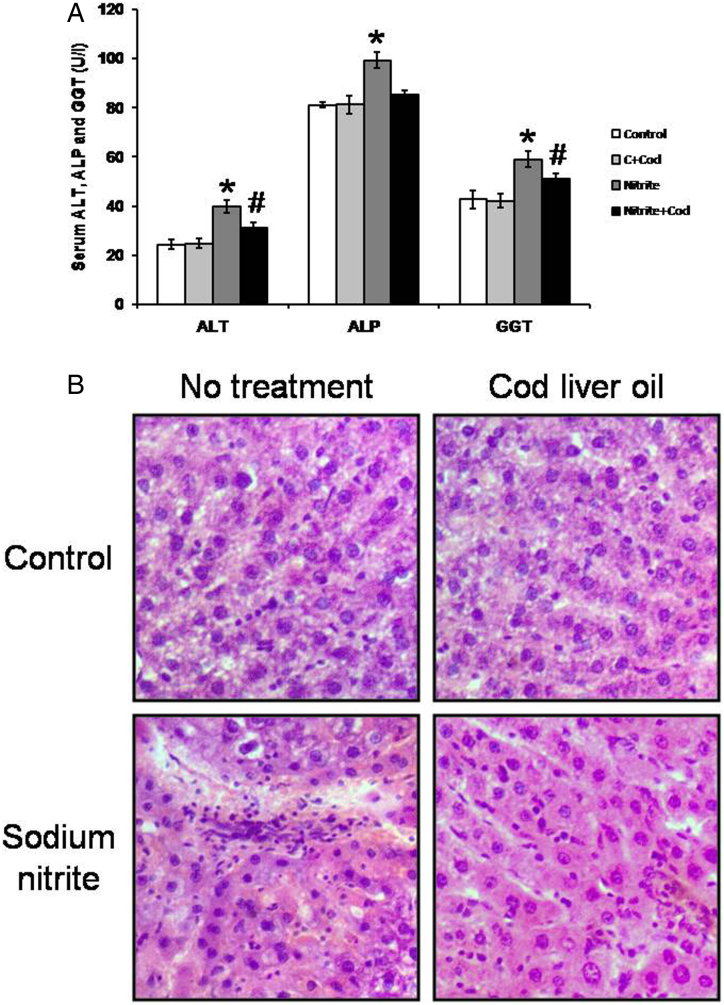
An estimation of liver enzymes in serum was conducted to evaluate the hepatoprotective effects of cod liver oil by measuring the serum activities of ALT, ALP, and GGT (Fig. 1A). Rats treated with sodium nitrite showed significant elevations in serum ALT, ALP, and GGT as compared with the control group. In parallel, liver sections stained with H&E showed fibrosis and breakdown of hepatic tissues (Fig. 1B). Treatment with cod liver oil reversed all these effects in the sodium nitrite group without affecting the control group.
Figure 1.
Effect of sodium nitrite (Nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on serum alanine aminotransferase (ALT), alkaline phosphatase (ALP) and gamma glutamyl transferase (GGT) (A) as well as liver sections stained with H/E (B). *Significant difference as compared with the rest of the groups at P < 0.05. #Significant difference as compared with the control group at P < 0.05.
Effect of cod liver oil on oxidative stress
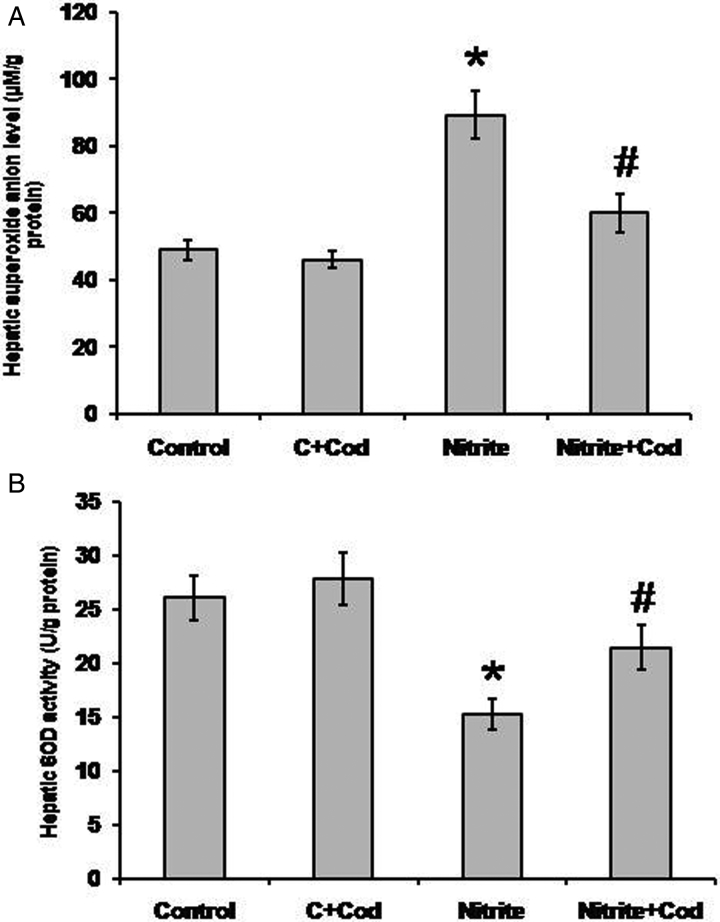
We examined the effect of sodium nitrite on oxidative stress status in hepatic homogenates and found a significant increase in the hepatic levels of superoxide anion that was associated with about 46% reduction in SOD activity as compared with the control group (P < 0.05). Treatment with cod liver oil (5 ml/kg body weight, po) daily for 12 weeks significantly blocked these effects in the sodium nitrite group but did not affect the control group (Fig. 2A and B).
Figure 2.
Effect of sodium nitrite (nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on hepatic superoxide anion concentration (A) and superoxide dismutase (SOD) activity (B). *Significant difference as compared with the rest of the groups at P < 0.05. #Significant difference as compared with the control group at P < 0.05.
Effect of cod liver oil on hepatic proinflammatory cytokines
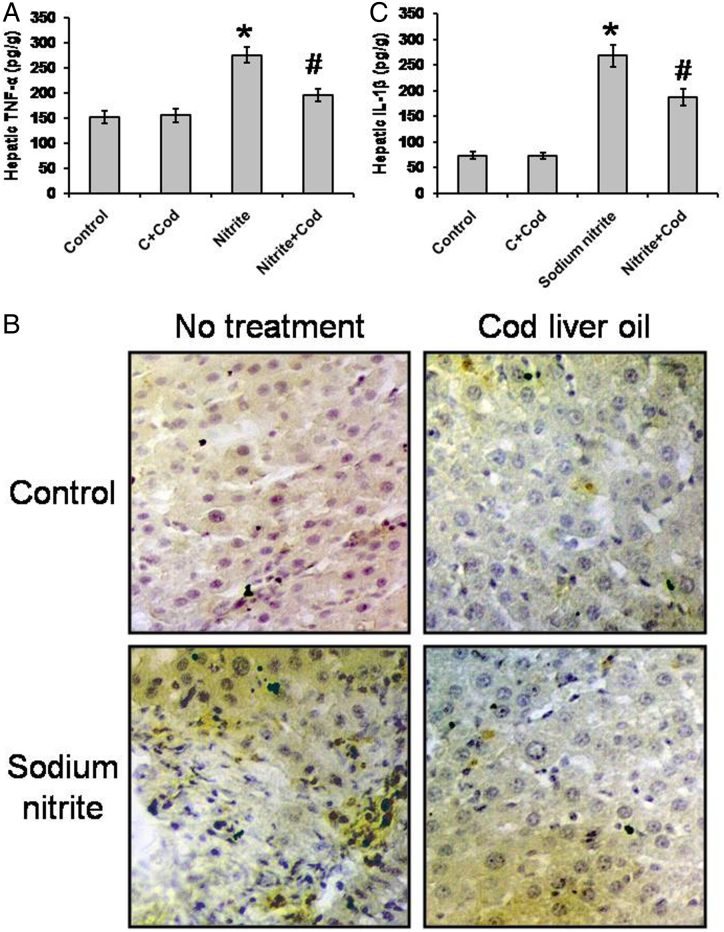
Treatment of rats with sodium nitrite was accompanied by marked cellular, molecular and biochemical changes that included liver impairment, fibrosis, mitochondrial function impairment, inflammation, and DNA degradation. Regarding proinflammatory cytokines, we found significant elevations in hepatic concentration of TNF-α (275.6 ± 15.1 pg/g) and IL-1β (267.9 ± 20.9 pg/g) in the sodium nitrite group as compared with the control group (151.13 ± 12.1 and 74.1 ± 7.3 pg/g, respectively). However, treatment of sodium nitrite-dosed rats with cod liver oil showed significant reductions in hepatic level of both TNF-α and IL-1β (196.1 ± 12.3 and 186.8 ± 16.7 pg/g, respectively) as compared with the sodium nitrite group, but still significantly higher than the control group (P < 0.05) (Fig. 3A and C). Treatment with cod liver oil did not affect the control group. In addition, rat liver sections stained with anti-TNF-α showed elevated TNF-α content in hepatocytes after sodium nitrite exposure that was reduced by treatment with cod liver oil (Fig. 3B).
Figure 3.
Effect of sodium nitrite (nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on hepatic tumor necrosis factor (TNF)-α (A) and interleukin (IL)-1β (C) as well as liver sections stained with monoclonal anti-TNF-α (B). *Significant difference as compared with the rest of the groups at P < 0.05. #Significant difference as compared with the control group at P < 0.05.
Effect of cod liver oil on hepatic acute inflammation markers
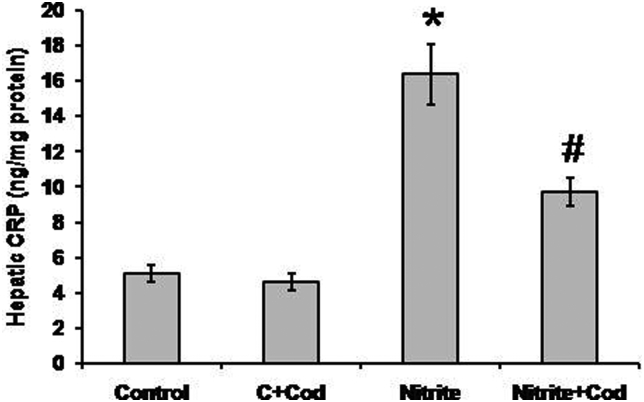
As shown in Fig. 4, we found significant elevation in hepatic concentration of CRP in the sodium nitrite group (16.4 ± 1.7 ng/mg) as compared with the control group (5.1 ± 0.43 ng/mg). Treatment of the sodium nitrite group with cod liver oil caused a significant reduction in hepatic level of CRP (9.7 ± 0.8 ng/mg) as compared with the sodium nitrite alone group. However, the hepatic level of CRP in rats treated with both sodium nitrite and cod liver oil was still significantly higher than that in the control group (P < 0.05).
Figure 4.
Effect of sodium nitrite (nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on hepatic C-reactive protein (CRP). *Significant difference as compared with the rest of the groups at P < 0.05. #Significant difference as compared with the control group at P < 0.05.
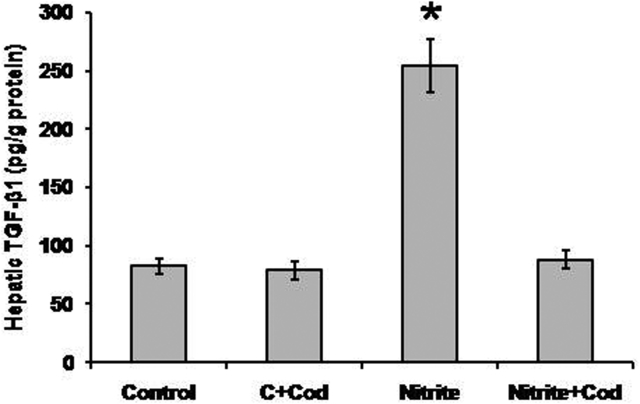
Effect of cod liver oil on hepatic fibrosis marker
As shown in Fig. 5, we found a significant increase in hepatic TGF-β1 in rats that received sodium nitrite (254.5 ± 21.3 pg/g) as compared with the control rats (82.7 ± 6.6 pg/g). Treatment of sodium nitrite-dosed rats with cod liver oil blocked the increase in hepatic TGF-β1 (88.1 ± 7.9 pg/g) as compared with rats that received sodium nitrite only. In addition, treatment of the control group with cod liver oil did not affect the hepatic level of TGF-β1.
Figure 5.
Effect of sodium nitrite (nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on hepatic transforming growth factor (TGF)-β1. *Significant difference as compared with the rest of the groups at P < 0.05.
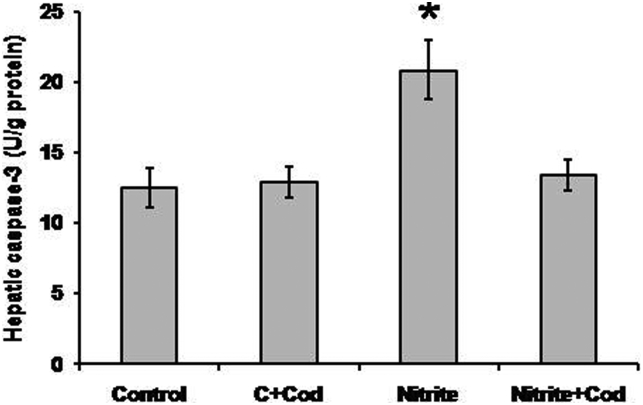
Effect of cod liver oil on hepatic apoptotic markers
Fig. 6 illustrates that sodium nitrite caused a significant increase in hepatic caspase-3 activity (20.9 ± 2.1 U/g) as compared with the control group (12.5 ± 1.1 U/g). Treatment with cod liver oil caused a significant reduction in hepatic caspase-3 activity (13.4 ± 0.9 U/g) in the sodium nitrite group but did not affect the control group.
Figure 6.
Effect of sodium nitrite (nitrite, 80 mg/kg/day) alone and its combination with cod liver oil (Cod, 5 ml/kg/day) for 12 weeks on hepatic caspase-3 activity. *Significant difference as compared with the rest of the groups at P < 0.05.
Discussion
Nitrite is an important antimicrobial additive to food products that delays the development of botulinum toxin and retards the development of rancidity during storage.17 Despite the enormous effort over the past few decades to limit or even restrict dietary nitrite consumption due to the potential to form carcinogenic N-nitrosamines, to date there are no conclusive data to indicate that dietary sources of nitrite may be unsafe. Chronic administration of sodium nitrite can result in liver impairment. Sodium nitrite caused oxidative damage to cell membrane, liver tissue damage and inhibition of oxidative stress resulting in elevating the activity of liver enzymes.8,11 Of note, we found that oral sodium nitrite resulted in a significant elevation of serum liver enzymes that was associated with elevated hepatic superoxide anion and reduced hepatic SOD activity.
We observed significant elevation in hepatic TNF-α and IL-1β in the sodium nitrite group in comparison with the control group. Similarly, Sun et al.18 found that IL-1β, IL-6, and TNF-α increased in human gastric cells after exposure to sodium nitrite. This perhaps may be explained by sodium nitrite elevation of oxidative stress and activation of proinflammatory cytokines. Many stimuli have been reported to upregulate proinflammatory cytokines, including TNF-α, through oxidative stress and activation of nuclear factor-kappa B (NF-κB).19,20 In addition, we observed that sodium nitrite caused a significant increase in hepatic CRP level, an acute-phase reactant produced by the liver in response to inflammation. CRP is synthesized by the liver in response to factors released by macrophages and adipocytes.21
The relationship between diet and health has been recognized throughout recorded history. Therefore, we tried to investigate the effect of cod liver oil on the inflammation produced by sodium nitrite in rats. We found that treatment of rats with cod liver oil significantly ameliorated liver tissue damage as well as reducing the elevated hepatic levels of TNF-α, IL-1β, and CRP in the sodium nitrite group. Cod liver oil was reported to have anti-inflammatory effects in different studies: pneumonia in mice22 and insulin resistance in humans.23 The anti-inflammatory effects of cod liver oil can be attributed to its contents of ω-3 fatty acids, eicosapentaenoic acid and docosahexaenoic acid (DHA), which have been reported to support anti-inflammatory responses in animals and humans.24–27 Several theories have been advocated to explain the ability of ω-3 fatty acids to antagonize inflammation that include competitive inhibition of conversion of arachidonate to proinflammatory lipid intermediates, generation of anti-inflammatory lipid mediators such as resolvins and protectins, and antagonism of NF-κB signaling.28–30
In addition, we found a significant elevation in hepatic TGF-β1 in sodium nitrite-treated rats. However, TGF-β1 is responsible for a series of inflammatory and fibrotic processes in liver injury.31,32 The increases in TGF-β1 were ameliorated by cod liver oil. This can be explained by ability of DHA to reduce fibrosis.33–35
Caspases are a family of cysteine proteases activated during apoptosis.36 Our results demonstrated that administration of sodium nitrite caused a significant elevation in caspase-3 activity, which was blocked by cod liver oil. However, there are no prior studies investigating the effects of cod liver oil on TNF-α, IL-β, CRP, TGF-β1, and caspase-3 in livers of sodium nitrite-treated rats with which to compare this study.
Our study concluded that cod liver oil alleviates the hepatic injury induced by sodium nitrite via multiple mechanisms including (1) blocking elevation of hepatic proinflammatory cytokines such as TNF-α and IL-1β; (2) reducing the elevation of the acute inflammation marker, CRP; (3) blocking the elevation of the hepatic fibrosis marker, TGF-β1; and (4) reducing the activation of the hepatic apoptotic enzyme, caspase-3.
Disclaimer statements
Contributors Iman O Sherif and Mohammed M Al-Gayyar contributed equally to this study.
Funding This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest None.
Ethics approval The animal protocol was approved by ethical committee in Faculty of Pharmacy, University of Mansoura (protocol code no. 2012-32).
References
- 1.Milkowski A, Garg HK, Coughlin JR, Bryan NS. Nutritional epidemiology in the context of nitric oxide biology: a risk-benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2010;22:110–9. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan GA, Jackson-Davis AL, Niebuhr SE, Xi Y, Schrader KD, Sebranek JG,. et al. Inhibition of Listeria monocytogenes using natural antimicrobials in no-nitrate-or-nitrite-added ham. J Food Prot 2012;75:1071–6. [DOI] [PubMed] [Google Scholar]
- 3.Klurfeld DM. Maternal cured meat consumption during pregnancy and risk of paediatric brain tumour in offspring: potentially harmful levels of intake. Public Health Nutr 2001;4:1303–5. [DOI] [PubMed] [Google Scholar]
- 4.Kozisek F. Influence of nitrate levels in drinking water on urological malignancies: a community-based cohort study. BJU Int 2007;99:1550–1. [DOI] [PubMed] [Google Scholar]
- 5.De Saint Blanquat G, Fritsch P, Cazottes C. Effects of dietary nitrite and nitrate on experimentally-induced inflammation in the rat. Int J Tissue React 1983;5:173–80. [PubMed] [Google Scholar]
- 6.Paik DC, Saborio DV, Oropeza R, Freeman HP. The epidemiological enigma of gastric cancer rates in the US: was grandmother's sausage the cause? Int J Epidemiol 2001;30:181–2. [DOI] [PubMed] [Google Scholar]
- 7.Brustad M, Braaten T, Lund E. Predictors for cod-liver oil supplement use-the Norwegian Women and Cancer Study. Eur J Clin Nutr 2004;58:128–316. [DOI] [PubMed] [Google Scholar]
- 8.Salama M, Abbas A, Darweish M, El-Hawwary A, Al-Gayyar M. Hepatoprotective effects of cod liver oil against sodium nitrite toxicity in rats. Pharm Biol 2013;51:1435–43. [DOI] [PubMed] [Google Scholar]
- 9.Myhrstad MC, Narverud I, Telle-Hansen VH, Karhu T, Lund DB, Herzig KH,. et al. Effect of the fat composition of a single high-fat meal on inflammatory markers in healthy young women. Br J Nutr 2011;106:1826–35. [DOI] [PubMed] [Google Scholar]
- 10.Papageorgiou N, Tousoulis D, Psaltopoulou T, Giolis A, Antoniades C, Tsiamis E,. et al. Divergent anti-inflammatory effects of different oil acute consumption on healthy individuals. Eur J Clin Nutr 2011;65:514–9. [DOI] [PubMed] [Google Scholar]
- 11.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci 2009;5:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Toxicology Program Toxicology and carcinogenesis studies of sodium nitrite (CAS NO. 7632–00–0) in F344/N rats and B6C3F1 mice (drinking water studies). Natl Toxicol Program Tech Rep Ser 2001;495:7–273. [PubMed] [Google Scholar]
- 13.Kohn MC, Melnick RL, Ye F, Portier CJ. Pharmacokinetics of sodium nitrite-induced methemoglobinemia in the rat. Drug Metab Dispos 2002;30:676–83. [DOI] [PubMed] [Google Scholar]
- 14.Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw 2013;24:114–21. [DOI] [PubMed] [Google Scholar]
- 15.Baehner RL, Boxer LA, Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood 1976;48:309–13. [PubMed] [Google Scholar]
- 16.DeChatelet LR, McCall CE, McPhail LC, Johnston RB Jr. Superoxide dismutase activity in leukocytes. J Clin Invest 1974;53:1197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binkerd EF, Kolari OE. The history and use of nitrate and nitrite in the curing of meat. Food Cosmet Toxicol 1975;13:655–61. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Aoki K, Wang W, Guo A, Misumi J. Sodium nitrite-induced cytotoxicity in cultured human gastric epithelial cells. Toxicol In Vitro 2006;20:1133–8. [DOI] [PubMed] [Google Scholar]
- 19.Elsherbiny NM, Abd El Galil KH, Gabr MM, Al-Gayyar MM, Eissa LA, El-Shishtawy MM. Reno-protective effect of NECA in diabetic nephropathy: implication of IL-18 and ICAM-1. Eur Cytokine Netw 2012;23:78–86. [DOI] [PubMed] [Google Scholar]
- 20.Al-Gayyar MM, Elsherbiny NM. Contribution of TNF-alpha to the development of retinal neurodegenerative disorders. Eur Cytokine Netw 2013;24:27–36. [DOI] [PubMed] [Google Scholar]
- 21.Manace LC, Babyatsky MW. Putting genome analysis to good use: lessons from C-reactive protein and cardiovascular disease. Cleve Clin J Med 2012;79:182–91. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Chhibber S, Mohan H. Dietary supplementation with omega-3 polyunsaturated fatty acids ameliorates acute pneumonia induced by Klebsiella pneumoniae in BALB/c mice. Can J Microbiol 2013;59:503–10. [DOI] [PubMed] [Google Scholar]
- 23.Ouellet V, Weisnagel SJ, Marois J, Bergeron J, Julien P, Gougeon R,. et al. Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J Nutr 2008;138:2386–91. [DOI] [PubMed] [Google Scholar]
- 24.Bouwens M, van de Rest O, Dellschaft N, Bromhaar MG, de Groot LC, Geleijnse JM,. et al. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr 2009;90:415–24. [DOI] [PubMed] [Google Scholar]
- 25.Hsueh HW, Zhou Z, Whelan J, Allen KG, Moustaid-Moussa N, Kim H,. et al. Stearidonic and eicosapentaenoic acids inhibit interleukin-6 expression in ob/ob mouse adipose stem cells via Toll-like receptor-2-mediated pathways. J Nutr 2011;141:1260–6. [DOI] [PubMed] [Google Scholar]
- 26.Al-Taan O, Stephenson JA, Spencer L, Pollard C, West AL, Calder PC,. et al. Changes in plasma and erythrocyte omega-6 and omega-3 fatty acids in response to intravenous supply of omega-3 fatty acids in patients with hepatic colorectal metastases. Lipids Health Dis 2013;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labonte ME, Couture P, Tremblay AJ, Hogue JC, Lemelin V, Lamarche B. Eicosapentaenoic and docosahexaenoic acid supplementation and inflammatory gene expression in the duodenum of obese patients with type 2 diabetes. Nutr J 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G,. et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 2002;196:1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W,. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue B, Yang Z, Wang X, Shi H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One 2012;7:e45990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirmaz C, Terzioglu E, Topalak O, Bayrak P, Yilmaz O, Ersoz G,. et al. Serum transforming growth factor-beta1(TGF-beta1) in patients with cirrhosis, chronic hepatitis B and chronic hepatitis C [corrected]. Eur Cytokine Netw 2004;15:112–6. [PubMed] [Google Scholar]
- 32.Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 2004;44:575–86. [DOI] [PubMed] [Google Scholar]
- 33.Baum JR, Dolmatova E, Tan A, Duffy HS. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front Physiol 2012;3:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin CR, Blanco PG, Keach JC, Petz JL, Zaman MM, Bhaskar KR,. et al. The safety and efficacy of oral docosahexaenoic acid supplementation for the treatment of primary sclerosing cholangitis – a pilot study. Aliment Pharmacol Ther 2012;35:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njoroge SW, Laposata M, Katrangi W, Seegmiller AC. DHA and EPA reverse cystic fibrosis-related FA abnormalities by suppressing FA desaturase expression and activity. J Lipid Res 2012;53:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Z, Xian M, Zhang W, McGill A, Wang PG. N-nitrosoanilines: a new class of caspase-3 inhibitors. Bioorg Med Chem 2001;9:99–106. [DOI] [PubMed] [Google Scholar]