Abstract
Objective
The study aimed to evaluate the antioxidant property of ethanolic extract of Sida cordifolia (SAE) on alcohol-induced oxidative stress and to elucidate its mechanism of action.
Methods
Male albino rats of the Sprague–Dawley strain were grouped into four: (1) control, (2) alcohol (4 g/kg body weight), (3) SAE (50 mg/100 g body weight), and (4) alcohol (4 g/kg body weight) + SAE (50 mg/100 g body weight). Alcohol and SAE were given orally each day by gastric intubation. The duration of treatment was 90 days.
Results
The activities of toxicity markers in liver and serum increased significantly in alcohol-treated rats and to a lesser extent in the group administered SAE + alcohol. The activity of alcohol dehydrogenase and the reactive oxygen species level were increased significantly in alcohol-treated rats but attenuated in the SAE co-administered group. Oxidative stress was increased in alcohol-treated rats as evidenced by the lowered activities of antioxidant enzymes, decreased level of reduced glutathione (GSH), increased lipid peroxidation products, and decreased expression of γ-glutamyl cysteine synthase in liver. The co-administration of SAE with alcohol almost reversed these changes. The activity of glutathione-S-transferase and translocation of Nrf2 from cytosol to nucleus in the liver was increased in both the alcohol and alcohol + SAE groups, but the maximum changes were observed in the latter group.
Discussion
The SAE most likely elicits its antioxidant potential by reducing oxidative stress, enhancing the translocation of Nrf2 to nucleus and thereby regulating glutathione metabolism, leading to enhanced GSH content.
Keywords: Alcohol, Sida cordifolia, Oxidative stress, Nrf2, Glutathione, Hepatotoxicity, γ-Glutamyl cysteine synthase, Detoxification
Introduction
Liver is the major organ for the oxidation of alcohol.1 Consequently, this organ sustains the greatest damage from ethanol abuse. Ethanol metabolism increases CYP2E1 activity, which generates reactive oxygen species (ROS) and contributes to oxidative stress.2 ROS can damage or cause complete degradation of essential complex molecules in cells, including lipids, proteins, and DNA, and deplete mitochondrial antioxidants such as reduced glutathione (GSH). Induction of oxidative stress and activation of the inflammatory cascade are identified as key elements in the pathophysiology of alcoholic liver diseases.3 Chronic alcohol consumption has long been associated with progressive liver disease from steatosis to hepatic cirrhosis, and the subsequent increased risk of hepatocellular carcinoma.
Alcohol is metabolized in the liver, which results in generation of a number of potentially dangerous products such as acetaldehyde and highly reactive free radicals that contribute to alcohol-induced liver damage.4 Genes encoding a subset of drug-metabolizing enzymes have been shown to be under regulation by the antioxidant responsive element (ARE) along with a subset of antioxidant genes, such as nuclear factor erythroid-2-related factor-2 (Nrf2). It has been identified that Nrf2 is the key transcriptional factor that transmits the inducer signal to ARE.5 Under basal conditions, Nrf2 is mainly in the cytoplasm and is bound to Kelch-like ECH associated protein-1 (Keap1), which in turn facilitates the ubiquitylation and subsequent proteolysis of Nrf2 in a constitutive manner.6 In response to oxidative stress, Nrf2 dissociates from Keap1, translocates into the nucleus and elicits the antioxidant response by induction of a battery of gene products, including antioxidant and phase-II detoxification enzymes, and modulates their expression.7 These enzymes are involved in glutathione metabolism and NADPH production as well as in maintaining intracellular redox homeostasis. Thus, Nrf2 has been demonstrated to be a key transcription factor that regulates the induction of antioxidant genes.8
The popularity of herbal remedies is increasing globally and at least one quarter of patients with liver diseases use ethnobotanicals. Sida cordifolia Linn. is a small, erect, downy shrub belonging to the family malvaceae.9 Pharmacological studies have indicated that the extract of the aerial parts and roots possess strong analgesic, anti-inflammatory and hypoglycaemic activities.10 A methanolic extract of S. cordifolia exhibited significant anti-ulcerogenic properties against damage induced by aspirin and ethanol.11 The studies conducted by Silva et al.12 showed that the aqueous extract of S. cordifolia stimulates liver regeneration after 67% partial hepatectomy in rats. Studies of Shailender et al.13 evaluated an ethanolic extract of S. cordifolia for acute and sub-acute anti-inflammatory properties in albino rats and compared it with the reference drug indomethacin.
Previous studies in our department have shown that 50% ethanolic extracts of S. cordifolia have neuroprotective properties against quinolinic acid-induced oxidative stress14 and hepatoprotective properties against alcohol-induced oxidative stress.15 The objective of the present study was to evaluate the antioxidant potential of a 50% ethanolic extract of S. cordifolia on alcohol-induced oxidative stress and to elucidate its mechanism of action.
Materials and methods
Preparation of ethanolic extract of S. cordifolia root
S. cordifolia roots were collected from Trivandrum, India. The plant was authenticated by Dr Valsaladevi, Curator, Department of Botany, Kerala University. The identified and authenticated specimen was deposited in the herbarium of the Department of Botany, University of Kerala (Plant no. KU5787). Fresh plant roots (250 g) were collected, washed thoroughly, and dried in the shade. The root was then crushed and added to 500 ml of 50% ethanol. It was refluxed in a water bath for 90 minutes at 60–65°C. The whole extract was concentrated using a rotary flash evaporator. The yield of extract was 1.68%. This extract was named SAE (Sida alcoholic extract).
Male albino rats (Sprague–Dawley strain) weighing between 100 and 140 g, bred and reared in our animal house, were used for the experiment. Weight-matched animals were selected. A total of 24 rats were divided into four groups of 6 rats each.
Group I: control.
Group II: alcohol (4 g/kg body weight)/day.
Group III: SAE (50 mg extract/100 g body weight)/day).
Group IV: alcohol (4 g/kg body weight) + SAE (50 mg extract/100 g body weight)/day).
Animals were housed in polypropylene cages. Cages were kept in a room that was maintained between 28 and 32°C. The light cycle was 12 hours light and dark. Animals were handled using laboratory animal welfare guidelines.16 Rats were fed with rat feed (Ashirvad Pvt Ltd, Chandigarh, India). Food and water was given ad libitum. The dose of alcohol was selected from previous studies.12 Alcohol (1:1 diluted) and SAE were given orally by gastric intubation. Rats in the control and SAE group were given glucose solution isocaloric to the ethanol content in group II. The duration of the experiment was 90 days. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC – KU-14/2009-2010-BC-MI (22)). At the end of the experimental period the animals were sacrificed. The liver was dissected out and cleaned with ice-cold phosphate buffer saline, blot dried, and immediately transferred to ice-cold containers for various biochemical evaluations. Blood was collected in clean, dry test tubes and allowed to clot for 30 minutes at room temperature. The clear serum was removed after centrifugation at 2000 g for 10 minutes and used immediately for the assay of various parameters.
Biochemical analysis
Activity of γ-glutamyl transferase (GGT) was analysed by the method of Szasz.17 Aspartate amino transferase (AST) and alanine aminotransferase (ALT) activities were measured by the method of Reitman and Frankel.18 ROS generation in liver mitochondria was quantified using dihydro dichlorofluorescein diacetate (DCF-DA).19 Alcohol dehydrogenase activity was assayed by the method of Koivisto and Salaspuro.20 The activity of glutathione-S-transferase (GST) was determined by the method of Habig et al.21 and that of glutathione reductase (GR) by the method of David and Richard.22 The activity of glutathione peroxidase (GPx) was measured by the method of Lawrence and Burk23 as modified by Agergaard and Jensen.24 GSH was determined according to Patterson and Lazarow.25 Malondialdehyde (MDA) was estimated by the method of Ohkawa et al.26 and hydroperoxides (HP) by the procedure of Mair and Hall.27 Conjugated dienes (CD) were estimated as described by Recknagel and Ghoshal.28 Tissue protein was determined by the method of Lowry et al.29 Cytosol and nuclear fractions were isolated according to the procedure described by Cox and Emili.30 ELISA was based on the method of Engvall and Perlmann.31
Determination of intracellular ROS
Liver tissue was homogenized in sucrose buffer. A 100 μl aliquot of liver homogenate was incubated with the assay medium (20 mM Tris–HCl, 130 mM KCl, 5 mM MgCl2, 20 mM NaH2PO4, 30 mM glucose, and 5 µM DCF-DA) at 37°C for 15 minutes. H2O2 (1 µmol) was added into the mixture at the end of the assay. The formation of dichlorofluorescein (DCF) was measured at an excitation wavelength of 488 nm and emission wavelength of 510 nm for 10 minutes with a fluorescence spectrometer.
Total RNA isolation
Total RNA was isolated from the liver using TRI Reagent (Sigma Aldrich, Missouri, USA) by the method of Chomczynski and Sacchi.32
Reverse transcription-PCR
The isolated RNA was used for reverse transcriptase-polymerase chain reaction (RT-PCR) to quantify gene expression. Total RNA was reverse transcribed and PCR was performed using an Eppendorf RT-PCR kit (Eppendorf, Hamburg, Germany) with gene-specific primers. Primer sequences are given in Table 1. The PCR mixture was resolved on a 2% agarose gel containing ethidium bromide. Then the gels were subjected to densitometric scanning (Bio-Rad Gel Doc, CA, USA) to determine the OD of each and then normalized against an internal control, GAPDH (glyceraldehyde-3-phosphate dehydrogenase) using Quantity One imaging software.
Table 1.
Primer sequences used for RT-PCR analysis
| Genes | Primer sequences | Gene accession number |
|---|---|---|
| GAPDH | Forward 5′ TGA CAA CTC CCT CAA GAT TGT CA 3′ | NM 017008.4 |
| Reverse 5′ GGC ATG GAC TGT GGT CAT GA 3′ | ||
| γGCS | Forward5′CCTTCTGGCACAGCACGTTG 3′ | J 05181.1 |
| Reverse 5′TAAGACGGCATCTCGCTCCT3′ |
Statistical analysis
The results were analysed using a statistical programme SPSS/PC+, version 11.5 (SPSS Inc., Chicago, IL, USA). A one-way ANOVA was employed for comparison among the six groups. Duncan's post-hoc multiple comparison tests of significant differences among groups were employed. P < 0.05 was considered to be significant.
Results
The activities of GGT in serum, ALT and AST in liver and serum were increased significantly in alcohol-treated rats compared to control (Table 2). Their activities were reduced in the alcohol + SAE administered group and there was no change in the activities of these enzymes in the SAE alone treated group.
Table 2.
Activities of toxicity marker enzymes
| Groups | ALT (µmol pyruvate liberated/min/mg protein) | AST (µmol oxaloacetate liberated/min/mg protein | GGT (µmol p-nitroaniline liberated/min/mg protein) Serum | ||
|---|---|---|---|---|---|
| Liver | Serum | Liver | Serum | ||
| Control | 15.51 ± 1.49a | 51.68 ± 4.96a | 19.78 ± 1.90a | 185.27 ± 17.80a | 17.43 ± 1.59a |
| Alcohol | 48.81 ± 4.68b | 107.25 ± 10.29b | 78.52 ± 7.53b | 351.20 ± 33.69b | 62.40 ± 5.99b |
| SAE | 15.62 ± 1.50a | 52.66 ± 5.06a | 20.76 ± 1.99a | 187.62 ± 18.00a | 17.65 ± 1.69a |
| Alcohol + SAE | 20.29 ± 1.95c | 57.46 ± 5.38a | 27.77 ± 2.79c | 195.08 ± 18.71a | 28.76 ± 2.13c |
Values are mean ± SD. Values not sharing a common superscript differ significantly at P < 0.05.
The activity of alcohol dehydrogenase (Table 3) increased significantly in the alcohol-treated group compared with the control group. Its activity was significantly decreased in the group treated with alcohol + SAE in comparison with the control group. The ROS level in liver was increased in the alcohol-treated group compared with the control group. The level of ROS was decreased significantly in the group treated with alcohol + SAE compared with the alcohol-treated group (Table 3).
Table 3.
Activity of alcohol dehydrogenase and ROS levels in liver
| Groups | Alcohol dehydrogenase (µmol of NAD+ reduced/min/mg protein | ROS (pmol DCF/mg protein) |
|---|---|---|
| Control | 0.21 ± 0.02a | 14.3 ± 1.3a |
| Alcohol | 1.54 ± 0.14b | 37.4 ± 3.6b |
| SAE | 0.19 ± 0.02a | 12.9 ± 1.3a |
| Alcohol + SAE | 0.72 ± 0.07c | 19.5 ± 2.0c |
Values are mean ± SD. Values not sharing a common superscript differ significantly at P < 0.05.
The activities of GPx and GR, and GSH content, were significantly decreased in the liver of the alcohol-treated group compared to the control (Table 4). But co-administration of ethanol and SAE significantly increased the activities of GPx and GR and the GSH content in comparison with the alcohol-treated group. The activity of GST (Table 4) was increased in the groups treated with alcohol and alcohol + SAE. But the maximum increase was observed in the alcohol+SAE group.
Table 4.
Activities of glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST)
| Groups | GPx (µmol NADPH oxidized/min/mg protein) | GR (µmol NADPH oxidized/min/mg protein) | GST (nmol conjugate formed/min/mg protein) | GSH (mg/100 g tissue) |
|---|---|---|---|---|
| Control | 13.37 ± 1.28a | 20.75 ± 1.99a | 0.08 ± 0.007a | 518.76 ± 49.87a |
| Alcohol | 6.04 ± 0.58b | 5.16 ± 0.49b | 0.16 ± 0.019b | 392.12 ± 37.61b |
| SAE | 13.70 ± 1.34a | 21.62 ± 2.07c | 0.08 ± 0.007a | 527.37 ± 50.59a |
| Alcohol + SAE | 12.39 ± 1.13a | 18.65 ± 1.79c | 0.20 ± 0.024c | 513.89 ± 49.30a |
Values are mean ± SD. Values not sharing a common superscript differ significantly at P < 0.05.
The level of lipid peroxidation products MDA, HP, and CD (Table 5) was increased significantly in alcohol-treated rats compared with the control group but were significantly lower in the alcohol+SAE group in comparison with the alcohol group.
Table 5.
Level of lipid peroxidation products
| Groups | MDA (mmol/100 g wet tissue) | HP (mmol/100 g wet tissue) | CD (mmol/100 g wet tissue) |
|---|---|---|---|
| Control | 0.50 ± 0.05a | 8.98 ± 0.86a | 60.63 ± 5.82a |
| Alcohol | 0.99 ± 0.10b | 18.61 ± 1.79b | 146.47 ± 14.05b |
| SAE | 0.48 ± 0.04a | 8.77 ± 0.84a | 53.63 ± 5.15a |
| Alcohol+SAE | 0.57 ± 0.52c | 9.97 ± 0.96c | 73.79 ± 7.08c |
Values are mean ± SD. Values not sharing a common superscript differ significantly at P < 0.05.
The translocation of Nrf2 from cytosol to nucleus was increased in alcohol-treated rats and also in the alcohol+SAE-treated group in comparison with other groups (Fig. 1). But maximum translocation was seen in the alcohol+SAE group.
Figure 1.
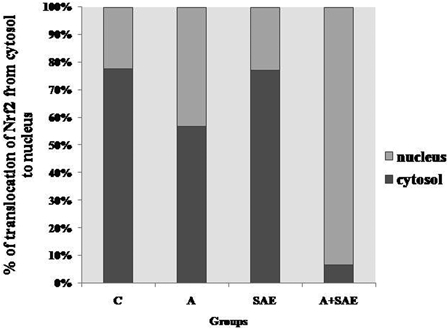
ELISA of nuclear translocation of Nrf2
The mRNA expression of γ-glutamyl cysteine synthase (γGCS) was evaluated by RT-PCR. In alcohol-treated rats, PCR products were markedly decreased compared to control rats (Fig. 2). Treatment with SAE along with alcohol increased γGCS mRNA expression in comparison with the alcohol-treated group.
Figure 2.
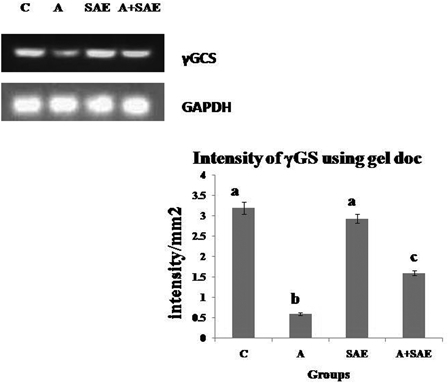
Intensity of γGCS mRNA using gel doc. The relative amount of γGCS mRNA was estimated by semi-quantitative RT-PCR. The PCR products were quantified by densitometry and standardized to their respective GAPDH controls. The mean intensity was measured and expressed as intensity/mm2. Results are expressed as average of quadriplicate experiments ± SD. Different letters indicate values statistically significant at P < 0.05. The level of γGCS mRNA was decreased significantly in alcohol-treated rats compared to control rats and there was an increase in the level in co-administered group compared to alcohol-treated group.
Discussion
Chronic alcohol feeding increased the activities of ALT, AST, and GGT in the liver and serum, indicating cellular leakage and loss of functional integrity of cell membranes in liver.33 In agreement with this we also observed increased activities of these enzymes, which are indicative of liver cell damage. Treatment of alcoholic rats with SAE decreased the activities of AST, ALT, and GGT, which indicates the hepatoprotective effect of the extract.
Alcohol is metabolized by alcohol dehydrogenase into toxic acetaldehyde, which is capable of free radical generation and cellular damage.34 Co-administration of SAE along with alcohol reduced the activity of alcohol dehydrogenase and thereby the formation of acetaldehyde. It has been well documented that alcohol increases ROS production via multiple mechanisms, including the mitochondrial electron transport chain and CYP2E1-mediated alcohol metabolism.35 Our studies are in line with these findings. Administration of SAE along with alcohol likely decreased the oxidative stress in the liver by decreasing the production of ROS.
Lipid peroxidation is accepted as being one of the principal causes of alcohol-induced liver injury. Elevated levels of lipid peroxidation products were observed in chronic alcohol-treated liver.36 The treatment with SAE significantly reduced the lipid peroxidation products MDA, HP, and CD in the liver. This is consistent with the studies of Dhalwal et al.37 who showed that the root extract of S. cordifolia exhibited superoxide scavenging activity and inhibited lipid peroxidation in rat liver homogenate.
Chronic alcohol feeding leads to a decrease in the activities of major antioxidant enzymes in liver, including superoxide dismutase and catalase.38 H2O2 is reduced to water with the help of GSH and GPx . The oxidized glutathione, in turn, is reduced by GR in the presence of NADPH. It has been reported that treatment with alcohol reduced the activities of GPx and GR in the liver.39 Our observations support this. On treatment with SAE, the changes in activities of these enzymes were reversed to near normal levels, suggesting that oxidative stress elicited by alcohol intoxication had been decreased.
GSH is the most important endogenous antioxidant, exerting a pivotal role in maintaining redox homeostasis.40 It is also an important constituent of cellular protective mechanisms in effecting detoxification of reactive metabolites in cells. The observed decrease in GSH level and other antioxidant enzyme activities in the alcohol-treated group might have been due to increased scavenging of reactive substances that were produced as a result of ethanol metabolism. Depletion of GSH is considered to be a marker of oxidative stress. γGCS is a key regulatory enzyme for the synthesis of GSH.41 Our observation of reduced expression of γGCS in rats given ethanol is supported by the report that a significant decrease in mRNA expression of γGCS was observed in the brains of alcohol-treated mice.42 Retrieval of GSH levels and expression of γGCS in SAE co-administered rats might be due to the antioxidant nature of SAE.
GST is a phase-II xenobiotic metabolizing enzyme; it catalyzes the conjugation of harmful electrophilic compounds with reduced GSH to produce less toxic or readily excreted metabolites. The elevated GST activity in the liver of ethanol-fed rats could be an adaptive response to protect tissues against ethanol-induced oxidative stress.43 These enzymes are believed to be highly regulated by Nrf2 signalling. Administration of SAE resulted in increased translocation of Nrf2 from cytosol to nucleus, coupled with reduction of oxidative stress. This might have caused enhanced activity of phase-II and antioxidant enzymes.
Nrf2 plays a key role in the adaptive response against increased oxidative stress caused by alcohol. Increases in Nrf2 protein and mRNA were observed in liver or hepatocytes of chronic alcohol-fed rats or mice.44 Our results are in line with these findings. Growing evidence supports a role of Nrf2 signalling in protecting cells from oxidative insults. A well-established mechanism that controls Nrf2 activation is that oxidative stress or Nrf2 inducers can increase Nrf2 protein stability, resulting in its accumulation in cells.45 Our study showed that SAE supplementation in an alcohol-treated group induced translocation of Nrf2. Thus, we speculate that enhanced Nrf2 expression might have activated antioxidant and phase-II enzymes by protecting the liver. This is supported by the enhanced activities of GPx, GR, and the maintained levels of GSH.
The hepatoprotective activity of the plant may be due to the presence of secondary metabolites. The phytopharmacological evaluation of ethanolic extract of S. cordifolia roots found reducing sugars, alkaloids, saponins, and steroids.46 The plant also possesses flavonol glycosides.47 Two bioactive flavones, 5,7-dihydroxy-3-isoprenyl flavones and 5-hydroxy-3-isoprenyl flavones isolated from the aerial parts of S. cordifolia, showed analgesic and anti-inflammatory activity.48 The alkaloids from S. cordifolia also show analgesic and anti-inflammatory activity.49
Conclusion
It can be concluded that SAE significantly protected the liver from alcohol-induced damage. The mechanism of action of SAE may be by reducing the metabolism of alcohol and causing decreased generation of acetaldehyde and ROS. The SAE also enhanced nuclear translocation of Nrf2, leading to activation of antioxidant and phase-II detoxification enzymes and maintaining the GSH content.
Disclaimer statements
Contributors All authors contributed equally.
Funding The authors are thankful to CSIR for the financial assistance to carry out the work efficiently.
Conflict of interest None.
Ethics approval Ethics approved.
Acknowledgement
We are thankful to the Council of Scientific and Industrial Research for the financial assistance to carry out the work.
Reference
- 1.Norberg A, Jones AW, Hahn RG, Gabrielsson JL. Role of variability in explaining ethanol pharmacokinetics: research and forensic applications. Clin Pharmacokinet 2003;42:1–31. [DOI] [PubMed] [Google Scholar]
- 2.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology 2006;43:S63–74. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 2008;47:1483–94. [DOI] [PubMed] [Google Scholar]
- 4.Maher JJ. Exploring alcohol's effects on liver function. Alcohol Health Res World 1997;21:5–12. [PMC free article] [PubMed] [Google Scholar]
- 5.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 2003;8:379–91. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 2009;48:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 2007;47:89–116. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 2005;7:385–94. [DOI] [PubMed] [Google Scholar]
- 9.Harbone JB. Phytochemical methods: a guide to modern techniques of analysis. London: Chapman and Hall Publishers; 1973. p. 4–7. [Google Scholar]
- 10.Saleem TS, Chetty CM, Ramkanth S, Rajan VS, Kumar KM, Gauthaman K. Hepatoprotective herbs – a review. Int J Res Pharm Sci 2010;1:1–5. [Google Scholar]
- 11.Philip BK, Muralidharan A, Natarajan B, Varadamurthy S, Venkataraman S. Preliminary evaluation of anti-pyretic and anti ulcerogenic activities of Sida cordifolia methanolic extract. Fitoterapia 2008;79:229–31. [DOI] [PubMed] [Google Scholar]
- 12.Silva RL, Melo GB, Melo VA, Antoniolli AR, Michellone PR, Zucoloto S,. et al. Effect of the aqueous extract of Sida cordifolia on liver regeneration after partial hepatectomy. Acta Cir Bras 2006;21:37–9. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Panchaksharimath P, Devaru S. Evaluation of anti-inflammatory activities of Sida cordifolia Linn in Albino rats. J Chem Pharm Res 2011;3:136–42. [Google Scholar]
- 14.Swathy SS, Panicker S, Nithya RS, Anuja MM, Rejitha S, Indira M. Antiperoxidative and antiinflammatory effect of Sida cordifolia Linn. on quinolinic acid induced neurotoxicity. Neurochem Res 2010;35:1361–7. [DOI] [PubMed] [Google Scholar]
- 15.Rejitha S, Prathibha P, Indira M. Amelioration of alcohol-induced hepatotoxicity by the administration of ethanolic extract of Sida cordifolia Linn. Br J Nutr 2012;108:1256–63. [DOI] [PubMed] [Google Scholar]
- 16.Hume CW. The UFAW handbook on the care and management of laboratory animals. Edinburgh/London: Churchill Livingstone; 1972. [Google Scholar]
- 17.Szasz G. A kinetic photometric method for serum gamma- glutamyl transpeptidase. Clin Chem 1969;15:124–36. [PubMed] [Google Scholar]
- 18.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 19.Driver AS, Kodavanti PR, Mundy WR. Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 2000;22:175–81. [DOI] [PubMed] [Google Scholar]
- 20.Koivisto T, Salaspuro M. Aldehyde dehydrogenase activity of the rat colon: comparison with other tissues of alimentary tract and the liver. Alcohol Clin Exp Res 1996;20:551–5. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase. The first step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 22.David M, Richard JS. Glutathione reductase. In: Bergmeyer HU, Jr (ed.). Methods of enzymatic analysis. New York: Academic Press; 1983. p. 258–65 [Google Scholar]
- 23.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976;71:952–8. [DOI] [PubMed] [Google Scholar]
- 24.Agergaard N, Jensen PT. Procedure for blood glutathione peroxidase determination in cattle and swine. Acta Vet Scand 1982;23:515–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson JW, Lazarow A. Determination of glutathione. In: Glick D, (ed.) Methods of biochemical analysis. New York: Interscience; 1983. p. 259–79. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 27.Mair RD, Hall T In: Inorganic peroxides II. Swern D, Willey C, (eds.) Vol. 2 Wiley, New York: Intersciences; 1971. p. 535–8. [Google Scholar]
- 28.Recknagel RO, Ghoshal AK. Quantitative estimation of peroxidative degeneration of rat liver microsomal and mitochondrial lipids after carbon tetrachloride poisoning. Exp Mol Pathol 1966;5:413–26. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 30.Cox B, Emili A. Tissue subcellular fractionation and protein extraction for use in mass spectroscopy-based proteomics. Nat Protoc 2006;1:1872–8. [DOI] [PubMed] [Google Scholar]
- 31.Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 1971;8:871–4. [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidiniumthiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156–9. [DOI] [PubMed] [Google Scholar]
- 33.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 2009;83:519–48. [DOI] [PubMed] [Google Scholar]
- 34.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health 2006;29:245–54. [PMC free article] [PubMed] [Google Scholar]
- 35.Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol 1978;1:163–71. [DOI] [PubMed] [Google Scholar]
- 36.Yayalaci Y, Celik I, Bati B. Hepatoprotective and antioxidant activity of linden (Tilia platyphyllos L.) infusion against ethanol-induced oxidative stress in rats. J Membr Biol 2014;247:181–8. [DOI] [PubMed] [Google Scholar]
- 37.Dhalwal K, Deshpande YS, Purohit AP. Evaluation of the antioxidant activity of Sida cordifolia. Pharma Biol 2005;43:754–61. [Google Scholar]
- 38.Krishnamoorthy G. Antioxidant efficacy of (Gaertn.) on ethanol-induced liver stress in rats. Asian J Exp Biol Sci 2012;3:22–7. [Google Scholar]
- 39.Jurczuk M, Moniuszko-Jakoniuk J, Rogalsk J. Glutathione related enzyme activity in liver and kidney of rats exposed to cadmium and ethanol. Polish J Environ Stud 2006;15:861–86. [Google Scholar]
- 40.Dickinson DA, Forman HJ. Cellular glutathione and thiol metabolism. Biochem Pharmacol 2002;64:1019–26. [DOI] [PubMed] [Google Scholar]
- 41.Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem 1975;250:1422–6. [PubMed] [Google Scholar]
- 42.Kaur J, Bansal MP. Effect of vitamin E on alcohol induced changes in oxidative stress and expression of transcription factors NFkappaB and AP-1 in mice brain cerebral hemispheres. Indian J Exp Biol 2008;46:562–7. [PubMed] [Google Scholar]
- 43.Das SK, Vasudevan DM. Effect of ethanol on liver antioxidant defense systems: a dose dependent study. Indian J Clin Biochem 2005;20:80–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong P, Cederbaum AI. Nrf2 is increased by CYP2E1 in rodent liver and HepG2 cells and protects against oxidative stress caused by CYP2E1. Hepatology 2006;43:144–53. [DOI] [PubMed] [Google Scholar]
- 45.Stewart D, Killeen E, Naquin R, Alam S, Alam J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J Biol Chem 2003;278:2396–02. [DOI] [PubMed] [Google Scholar]
- 46.Momin MA, Bellah SF, Rahman SM, Rahman AA, Murshid GM, Emran TB. Phytopharmacological evaluation of ethanol extract of Sida cordifolia L. roots. Asian Pac J Trop Biomed 2014;4:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutradhar RK, Rahman AKMM, Ahmad MU. Three new flavonol C-glycosides from Sida cordifolia Linn. J Iran Chem Soc 2007;4:175–81. [Google Scholar]
- 48.Sutradhar RK, Rahman AKMM, Ahmad MU, Bachar SC. Bioactive flavones of Sida cordifolia. Phytochem Lett 2008;1:179–82. [Google Scholar]
- 49.Sutradhar RK, Rahman AM, Ahmad M, Bachar SC, Saha A, Guha SK. Bioactive alkaloid from Sida cordifolia Linn. with analgesic and anti-inflammatory activities. Iran J Pharmacol Ther 2006;5:1–10. [Google Scholar]


