Abstract
Objectives: Exposure to 2,5-hexanedione (2,5-HD) is well known to be associated with reproductive dysfunctions in both humans and animals. However, the role of oxidative stress in 2,5-HD-induced toxicity in testes and sperm has not yet been studied.
Methodology: The present study investigated the influence of 2,5-HD on antioxidant systems in the testes and epididymal sperm of rats following exposure to 0, 0.25, 0.5, and 1% 2,5-HD in drinking water for 21 consecutive days.
Results: Administration of 0.5% 2,5-HD significantly (P < 0.05) decreased epididymis weight, whereas 1% 2,5-HD-treated rats showed significantly decreased body weight, testis, and epididymis weights compared with the control group. Exposure to 2,5-HD caused a significant dose-dependent increase in the activities of superoxide dismutase, catalase, and glutathione peroxidase in both testes and sperm compared with the control group. Moreover, 2,5-HD-exposed rats showed significant decrease in glutathione-S-transferase activity and glutathione level with concomitant significant elevation in the levels of hydrogen peroxide and malondialdehyde in both testes and sperm. Testicular and epididymal atrophy with significant, dose-dependent, decrease in epididymal sperm number, sperm motility, and viability were observed in 2,5-HD-treated rats.
Conclusion: 2,5-HD exposure impaired testicular function and sperm characteristics by disruption of the antioxidant systems and consequently, increased oxidative stress in the treated rats.
Keywords: 2,5-Hexanedione; Antioxidant systems; Oxidative stress; Testes; Sperm; Rats
Introduction
The most substantial exposures of humans to 2,5-hexanedione (2,5-HD), an active metabolite of the common industrial solvent, n-hexane occur in occupational settings where its air concentrations exceeded 500 ppm (v/v).1 The low-level environmental exposure to 2,5-HD is ubiquitous because n-hexane is a component of petroleum.2 Nigeria is the second leading gas flaring country in the world at the end of 2011. Nigerians’ acute exposure to n-hexane could occur via inhalation of vapors or emissions from refined petroleum products at the work place, whereas the most widespread form of low-level exposure to n-hexane for the general population could be through consumption of contaminated food or groundwater from spill sites, refineries, underground storage tanks at gas stations, and waste sites.3 The American Conference of Governmental Industrial Hygienists recommended that free urinary 2,5-HD be measured in biological monitoring of individuals exposed to hexane instead of total 2,5-HD and proposed a reference value of 0.4 mg/l for free 2, 5-HD (ACGIH, 2001).4 Indeed, findings from shoe factory workers confirmed that free 2,5-HD measurement is a better indicator for evaluating risk of exposure to n-hexane.5,6
2,5-HD is a testicular toxicant that targets the Sertoli cell, the supportive cell within seminiferous tubules. 2,5-HD promotes rapid microtubule assembly, induces tubulin cross-linking, alters microtubule-dependent transport in these cells, which consequently disturbs the germ cell niche by impeding seminiferous tubule fluid secretion.2 The disruption of the Sertoli cell microenvironment has been reported to cause germ cell apoptosis and ultimately testicular atrophy.7–10 Although 2,5-HD is well known to cause Sertoli cell dysfunction by interfering with Sertoli cell microtubule assembly kinetics,2,10 there is still a paucity of information on the involvement of free radicals in the 2,5-HD-induced pathogenesis in the testes and sperm.
Recently, exposure to 2,5-HD was demonstrated to induce oxidative stress, resulting in hepatorenal damage in male rats.11 Similarly, administration of 2,5-HD to female rats caused significant oxidative damage to the erythrocytes, ovarian and uterine tissues with marked disruption of hormonal balance.12,13 Reactive oxygen species (ROS) are essential mediators of normal sperm function because of their involvement in the induction and development of sperm hyperactivation, capacitation, and acrosome reaction.14 However, excessive production of ROS above normal levels due to exposure to xenobiotics causes oxidative stress which is well known to be detrimental to the normal testicular function. Oxidative stress induces apoptosis in the testes, impairs sperm movement to fertilize the oocyte, and damages sperm DNA to produce a viable pregnancy.15,16 To counteract the damaging effect of ROS, the testes and sperm cells are endowed with extensive antioxidant defense mechanisms, including the antioxidant enzymes (e.g. superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST)) and non-enzymatic antioxidants (e.g. glutathione, vitamin C, and vitamin E).
Hitherto, the involvement of oxidative stress in 2,5-HD-induced testicular toxicity has not been reported. The objective of the present study was therefore to explore whether oxidative stress plays any role or not in the toxic effects previously observed in reproductive system of animals exposed to 2,5-HD. To the best of our knowledge, this is the first study to assess the antioxidant status in the testes and sperm following exposure of rats to 2,5-HD at environmentally relevant concentrations.
Materials and methods
Chemicals
2,5-HD (98.99%), epinephrine, glutathione (GSH), thiobarbituric acid, hydrogen peroxide (H2O2), 5,5′-dithio-bis-2-nitrobenzoic acid and 1-chloro-2, 4-dinitrobenzene were purchased from Sigma Chemical Co. (St Louis, MO, USA). All other reagents were of analytical grade and obtained from the British Drug Houses (Poole, Dorset, UK).
Animal model and experimental design
Thirty-two healthy and sexually matured male Wistar rats (190 ± 6 g) obtained from the Department of Biochemistry, University of Ibadan, Ibadan, Nigeria, were used for this study. They were housed in plastic cages placed in a well-ventilated rat house, provided rat pellets and water ad libitum and subjected to natural photoperiod of 12-hour light : 12-hour dark. All the animals received humane care according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science and published by the National Institute of Health.17 The experimental etiquettes were carried out after approval by the University of Ibadan Ethical Committee. The ethical regulations have been followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments.18 The rats were randomly divided into four groups of eight rats per group. Group I animals received normal drinking (tap) water alone and served as a control. Animals in groups II, III, and IV were exposed to 0.25, 0.5, and 1% 2,5-HD, respectively, in drinking water for 21 consecutive days according to established procedure.19 It has been reported that the toxic effects observed in workers chronically exposed to n-hexane were similar to those found in rats chronically treated with 2,5-HD in drinking water.20 Twenty-four hours after the last treatment, all the rats were sacrificed by cervical dislocation under light ether anesthesia. The testes and epididymis were quickly removed and weighed. The body weights of all rats were recorded twice: at the beginning of the study and just before they were sacrificed.
Sperm progressive motility assay
The motility of the sperm from the control and 2,5-HD-treated rats was evaluated according to the method of Zemjanis.21 Briefly, epididymal sperm was obtained by cutting the cauda epididymis with surgical blades and released onto a sterile clean glass slide. The sperm was subsequently diluted with 2.9% sodium citrate dehydrate solution which had been pre-warmed to 37°C, mixed thoroughly and covered with a 24 × 24 mm coverslip. At least 10 microscopic fields were observed under a phase contrast microscope at ×200 magnification to evaluate the sperm motility. Sperm motility was calculated by scoring the number of all progressive sperm, followed by the non-progressive and then the immotile sperm in the same field. The data were expressed as percentage of sperm progressive motility.
Epididymal sperm concentration
The epididymal sperm number (ESN) from the control and 2,5-HD-treated rats was obtained by the method described in the WHO manual.22 Briefly, the sperm was obtained by mincing the caudal epididymis in normal saline and filtering through a nylon mesh. An aliquot of 5 µl of the sperm was mixed with 95 µl of diluent (0.35% formalin containing 5% NaHCO3 and 0.25% trypan blue). Subsequently, 10 µl of the diluted sperm was transferred to the hemocytometer, allowed to sediment by standing for 5 minutes in a humid chamber to prevent drying before they were counted using the improved Neubauer (Deep 1/10 m; LABART, Munich, Germany) chamber with a light microscope at ×400.
Sperm viability and morphological abnormalities
A portion of the sperm suspension placed on a glass slide was smeared out with another slide and stained with a reagent containing 0.2 g eosin and 0.6 g fast green dissolved in distilled water and ethanol in a ratio of two to one (2:1) for morphological examination, whereas sperm viability was determined by staining with 1% eosin and 5% nigrosine in 3% sodium citrate dehydrate solution according to Wells and Awa.23 A total of 400 sperm cells from each rat were used for morphological examination.
Preparation of testes and epididymal sperm for biochemical assays
The sperm from the control and 2,5-HD-treated rats was collected according to Adedara and Farombi.24 Sperm was collected as quickly as possible after a rat was sacrificed. Each right caudal epididymis was placed in cold phosphate buffered saline solution and cut with surgical blades into pieces. The solution was pipetted several times to obtain the sperm suspension and then filtered through a nylon mesh. The sperm suspensions were subsequently homogenized at 4°C with a glass Teflon homogenizer for 10 seconds and centrifuged at 2000×g for 10 minutes to obtain the supernatant, which was used for biochemical assays. The right testes were homogenized in four volumes of phosphate buffer (pH 7.4) and the resulting homogenate was centrifuged at 10 000×g for 15 minutes at 4°C and the supernatant was thereafter used for the biochemical estimations.
SOD activity was determined by the method described by Misra and Fridovich.25 CAT activity was determined using H2O2 as a substrate according to the method of Clairborne.26 GST was assayed by the method of Habig et al.27 Reduced GSH was determined at 412 nm using the method described by Jollow et al.28 GPX activity was determined according to the method of Rotruck et al.29 Protein concentration was determined by the method of Lowry et al.30 Hydrogen peroxide generation was assessed by the method of Wolff.31 Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Farombi et al.32 and expressed as µmol MDA/mg protein.
Histopathology
The testes and epididymides were carefully removed and fixed with Bouin's solution. After dehydration procedures, the samples were embedded in paraffin. The tissues were thereafter cut to produce 4–5 µm sections by a microtome and the slides were subsequently stained with hematoxylin and eosin (H&E). All slides were coded before examination with a light microscope and photographed using a digital camera by investigators who were blinded to control and 2,5-HD-treated groups.
Statistical analysis
All data were tested and confirmed to be normally distributed and homoscedastic with the Levene test and residual distribution. Further statistical analyses were carried out using one-way analysis of variance to compare the experimental groups followed by the Dunnett post hoc test to identify significantly different groups (SPSS for Windows, version 17). Values of P < 0.05 were considered significant.
Results
Effect of 2,5-HD on fluid intake, body, and organ weights of rats
The fluid intake, body weight gain, and relative organ weights of the rats are presented in Table 1. There was a significant (P < 0.05) reduction in the daily fluid intake by 2,5-HD-exposed rats when compared with the control. The rats that were treated with 1% 2,5-HD showed a significant reduction in body weight gain when compared with the control. 2,5-HD at 0.25% concentration did not produce any effect on the body, testes, or epididymis weights of the treated rats. Administration of 0.5% 2,5-HD caused a significant decrease in epididymis weight only, whereas 1% 2,5-HD-treated rats showed marked decreased in body, testes, and epididymis weight compared with the control.
Table 1. Fluid intake, body weight, absolute, and relative organ weights in rats exposed to 2,5-HD for 21 consecutive days.
| Control | 0.25% 2,5-HD | 0.5% 2,5-HD | 1% 2,5-HD | |
|---|---|---|---|---|
| Fluid intake/day/rat (ml) | 25.32 ± 2.02 | 24.68 ± 2.08 | 18.79 ± 1.62a | 15.11 ± 0.97a |
| Final body weight | 247.01 ± 7.84 | 238.51 ± 7.33 | 232.86 ± 6.51 | 198.02 ± 8.25a |
| Testes (g) | 1.99 ± 0.04 | 1.95 ± 0.59 | 1.93 ± 0.60 | 1.32 ± 0.31a |
| Testes (g/100 g bw) | 0.81 ± 0.02 | 0.81 ± 0.05 | 0.83 ± 0.06 | 0.67 ± 0.01a |
| Epididymis (g) | 0.23 ± 0.04 | 0.21 ± 0.04 | 0.18 ± 0.03a | 0.14 ± 0.06a |
| Epididymis (g/100 g bw) | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.03a | 0.07 ± 0.02a |
Data are expressed as mean ± SD for eight rats per group.
aP < 0.05 vs. Control.
2,5-HD-induced oxidative stress in testes and sperm of rats
The activities of antioxidant enzymes, namely SOD, CAT, GST, and GPx, in the testes and sperm following the administration of 2,5-HD in rats after 21 days are presented in Fig. 1 . Administration of 2,5-HD resulted in a significant (P < 0.05) dose-dependent increase in the activities of SOD, CAT, and GPx, whereas it markedly decreased GST activity in both testes and sperm when compared with the control group. Furthermore, the rats exposed to 2,5-HD showed a significant decrease in the GSH level with concomitant significant elevation in the levels of H2O2 and MDA in testes and sperm of the treated rats when compared with control (Fig. 2). Testicular GSH level decreased by 23, 32, and 40%, whereas sperm GSH level decreased by 28, 43, and 55% in rats treated with 0.25, 0.5, and 1% 2,5-HD, respectively, when compared with the control. Moreover, while the level of testicular H2O2 was increased by 71, 83, and 115%, sperm H2O2 level was increased by 88, 123, and 203% in rats treated with 0.25, 0.5,% and 1% 2,5-HD, respectively, when compared with the control. Similarly, testicular MDA level was elevated by 71, 89, and 111% while sperm MDA level was elevated by 47, 64, and 101% in rats treated with 0.25, 0.5, and 1% 2,5-HD, respectively, when compared with the control.
Figure 1.
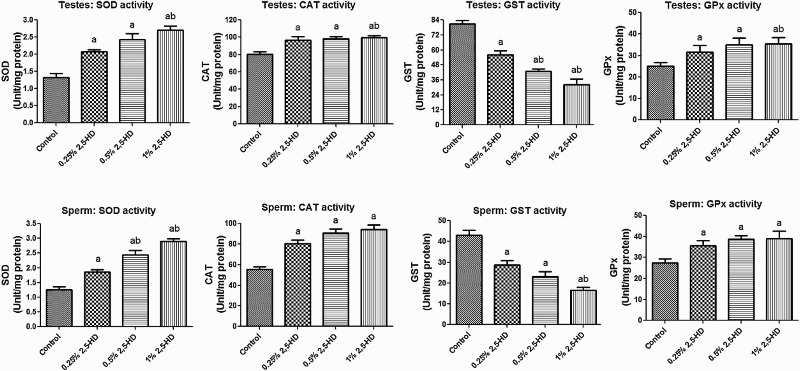
SOD, CAT, GST, and GPx activities in testes and sperm following 21 consecutive days of 2,5-HD treatment in rats. Each bar represents mean ± SD of eight rats. aP < 0.05 vs. Control; bP < 0.05 vs. 0.25% 2,5-HD.
Figure 2.
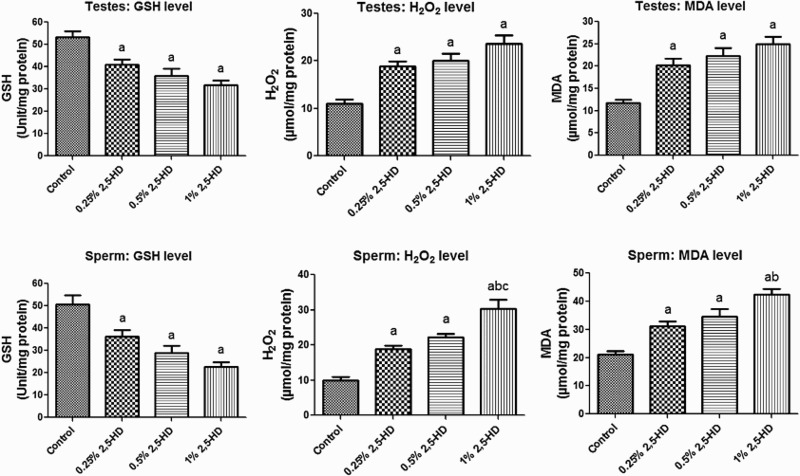
GSH, H2O2 production, and lipid peroxidation levels in testes and sperm following 21 consecutive days of 2,5-HD treatment in rats. Each bar represents mean ± SD of eight rats. aP < 0.05 vs. Control; bP < 0.05 vs. 0.25% 2,5-HD, cP < 0.05 vs. 0.5% 2,5-HD.
Sperm functional characteristics in rats exposed to 2,5-HD
The effects of 2,5-HD on sperm characteristics after the exposure period are presented in Figs. 3–4. The results indicate a significant (P < 0.05) dose-dependent decrease in ESN, sperm motility, and viability in rats exposed to 2,5-HD when compared with the control. However, a dose-dependent increase in the sperm abnormalities, consisting majorly tailless heads, curved mid-pieces, and bent mid-pieces were frequently observed in 2,5-HD-treated rats. Following exposure of rats to 0.25, 0.5, and 1% 2,5-HD, sperm motility decreased by 24, 30, and 38%, ESN reduced by 35, 45, and 60%, whereas sperm viability decreased by 19, 28, and 55%, respectively, when compared with the control. Conversely, total sperm abnormality increased by 68, 90, and 107% in rats exposed to 0.25, 0.5, and 1% 2,5-HD, respectively, when compared with the control.
Figure 3.
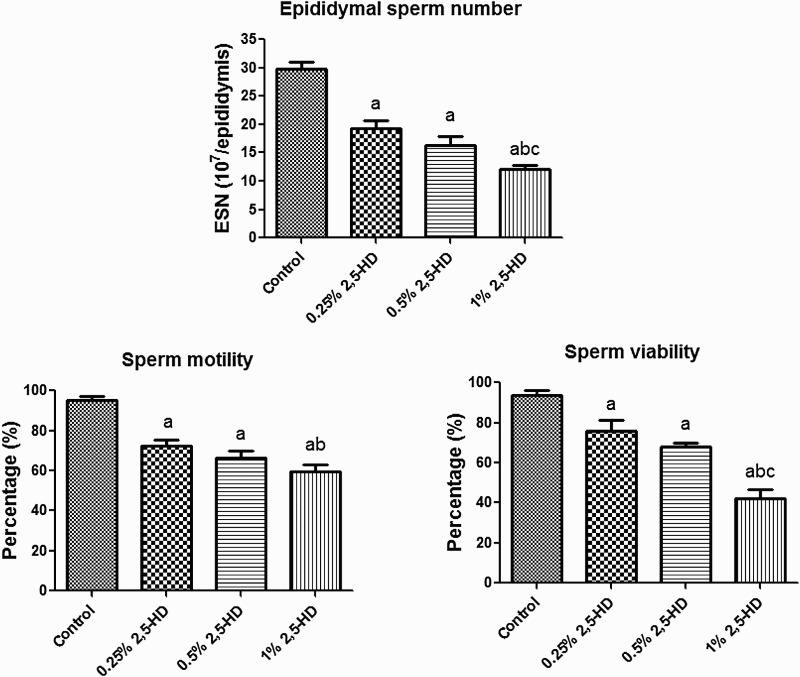
Epididymal sperm number, motility, and viability in experimental rats following 21 consecutive days of 2,5-HD treatment in rats. Each bar represents mean ± SD of eight rats. aP < 0.05 vs. Control; bP < 0.05 vs. 0.25% 2,5-HD, cP < 0.05 vs. 0.5% 2,5-HD.
Figure 4.
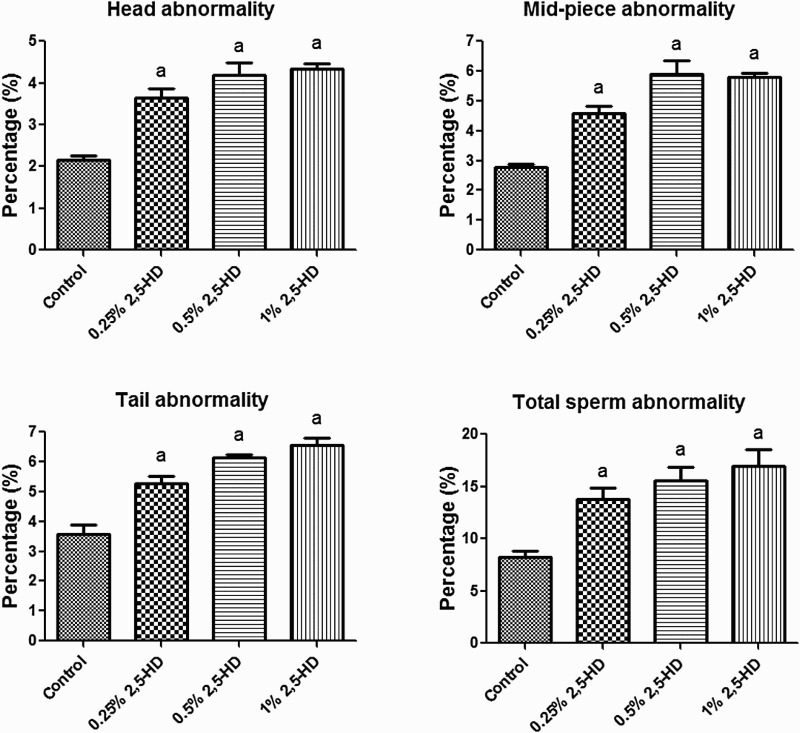
Specific (i.e. head, mid-piece, and tail) and total sperm morphological abnormalities in experimental rats following 21 consecutive days of 2,5-HD treatment in rats. Each bar represents mean ± SD of eight rats. aP < 0.05 vs. Control.
Histopathology of testes and epididymis of 2,5-HD-treated rats
The representative photomicrographs of the control group and 2,5-HD-treated testes and epididymis are shown in Figs. 5 and 6, respectively. The microscopic examination showed normal testicular morphology in control and 0.25% 2,5-HD-treated rats. The treatment-related lesions observed in 0.5 and 1% 2,5-HD-treated rats includes vacuolization and marked degeneration of the seminiferous tubules. Light microscopy revealed that the epididymis of control and 0.25% 2,5-HD-treated rats has normal architecture. However, progressive degeneration of epididymis characterized with severe erosion of the epididymal lining and reduced epithelia layer integrity was observed in 0.5 and 1% 2,5-HD-exposed rats.
Figure 5.
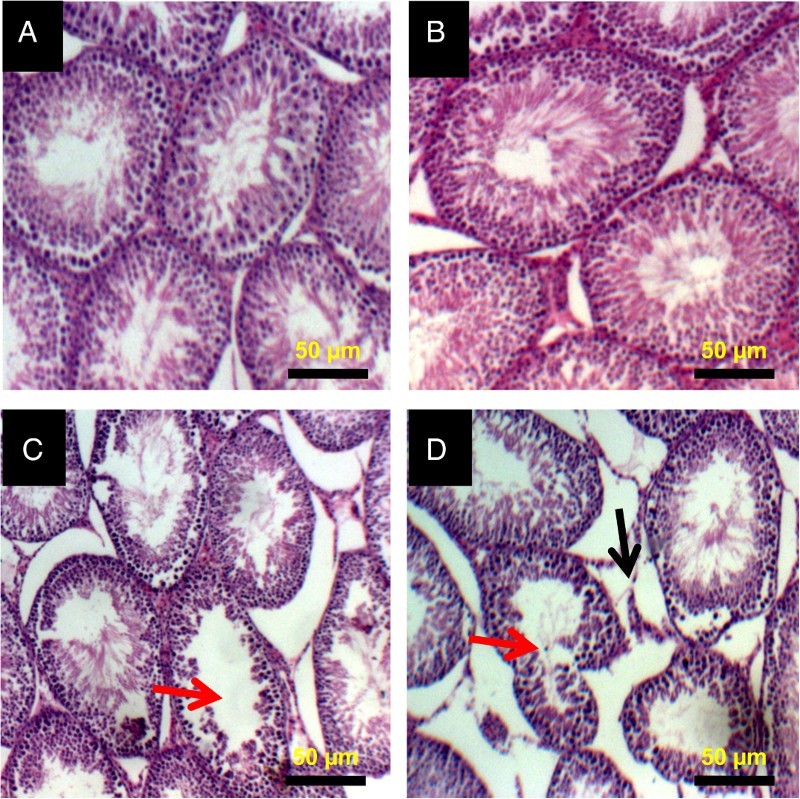
Histopathology of testes from control and 2,5-HD-treated rats. Representative photomicrographs of testes from control (A) and 0.25% 2,5-HD (B) showed normal morphology. Treatment-related lesions such as vacuolization (red arrow) and marked degeneration of the seminiferous tubules (black arrow) were observed in the testes of 0.5% 2,5-HD (C) and 1% 2,5-HD-treated rats (D).
Figure 6.
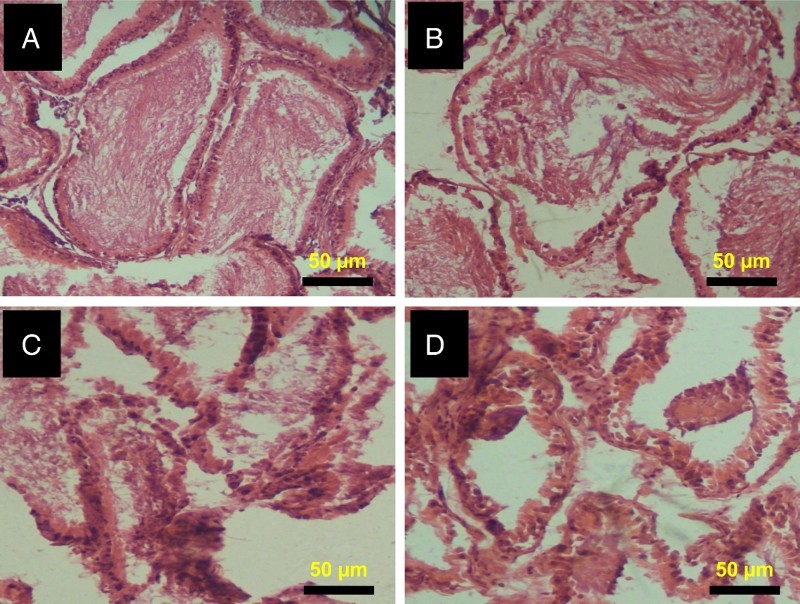
Histopathology of epididymis from control and 2,5-HD-treated rats. Representative photomicrographs of epididymis from control (A) and 0.25% 2,5-HD (B) showed normal morphology. Epididymis from 0.5% 2,5-HD (C) and 1% 2,5-HD (D) groups showed progressive degeneration of epididymis characterized with severe erosion of the epididymal lining and reduced epithelia layer integrity.
Discussion
The present investigation clearly demonstrated that the general metabolic condition of rats treated with 1% 2,5-HD was not within normal range evidenced in the treatment-related decrease in the fluid intake, average body, testes, and epididymis weights. The significant decrease in body weight in animals exposed to 1% 2,5-HD could be due to organ failure and metabolic dysfunction previously reported.11 Exposure of rats to 2,5-HD reportedly did not affect food and fluid intake, whereas the detrimental effects on the liver and kidney in the treated rats were strongly associated with biochemical alterations such as significant elevation in serum aminotransferases, alkaline phosphatase, albumin, bilirubin, urea, creatinine, electrolytes, metabolic disorders, oxidative stress, and histological damage.11 Testicular weight is a valuable index of male reproductive toxicity. The reduction in the testes weight indicates the degenerative capacity of the 2,5-HD which could be attributed to inhibition of seminiferous tubule fluid secretion and the loss of germ cells.33–35 The normal spermatogenesis and sperm function are protected from oxidative stress by the antioxidant defense system comprising of SOD, CAT, GPx, GST, and GSH. The basic biochemistry of antioxidant enzymes involves the rapid dismutation of superoxide anion to H2O2 by SOD thereby preventing the former from participating in Haber–Weiss reaction to produce the highly pernicious hydroxyl radicals.3,34 Moreover, the cell is further protected from the oxidizing action of H2O2 by its subsequent conversion to water and oxygen by CAT or GPx.
In the present study, elevated levels of H2O2 and MDA in testes and sperm of rats exposed to 2,5-HD indicate oxidative injury possibly due to inability of the antioxidant enzymes to scavenge the oxidants generated in the testicular and epididymis milieu. MDA is a stable end product of lipid peroxidation which is well known to be an indirect indicator of increased intracellular ROS generation. The testicular germ cell and sperm are particularly vulnerable to lipid peroxidation due to the high polyunsaturated fatty acids content in their plasma membranes.16,36 The dose-dependent increase in the activities of SOD, CAT, and GPx in rats administered with 2,5-HD suggests the adaptive response of the testicular and sperm cells to oxidative stress. Glutathione is an important intracellular redox buffer that functions as a non-enzymic antioxidant by direct interaction of its thiol group with ROS. Glutathione serves as a co-substrate for GST in biochemical conjugation of xenobiotics and it helps in the maintenance of endogenous vitamin E and C levels.37,38 The dose-dependent decline in GST activity and GSH level in both testes and sperm could prevent efficient detoxification of peroxide-containing metabolites generated during oxidative stress in the 2,5-HD-treated rats.
Normal epididymal structure and its internal microenvironment are essential for sperm maturation and maintenance of sperm viability and motility. The decreased sperm endpoints including epididymal sperm count, progressive motility, and viability with elevated sperm abnormalities reflect spermatotoxic effects of 2,5-HD possibly due to the testicular and epididymal atrophy caused by ROS. The elevated levels of ROS often generated during exposure to environmental contaminants are well known to suppress the function of the testis namely spermatogenesis and steroidogenesis.16,39 Decreased motility, impaired fertilization, and oxidative DNA damage are the three inter-related mechanisms that account for oxidative stress-mediated male infertility.40 Thus, the toxic effects seen on the male reproductive system in the present study are attributable to its primary effect on testicular and epididymal antioxidant defense systems. However, its secondary effect due to organ failure and metabolic dysfunction previously reported,11 may be a contributory factor in the 2,5-HD-induced reproductive toxicity.
Moreover, the histopathological examination of the testes and epididymis sections corroborated that the disruption of antioxidant status resulted in severe oxidative damage in the 2,5-HD-treated rats. Administration of 2,5-HD caused severe treatment-related lesions characterized by vacuolization and degeneration of the seminiferous tubules, whereas severe erosion of the epididymal lining and reduced epithelia layer integrity was observed in the treated rats. Our findings are in agreement with previous studies where 3 weeks of 1% 2,5-HD exposure through drinking water caused mild changes such as vacuolization in testes of exposed rats.41,42 Recently, much attention has been focused on the protective effects of antioxidants and naturally occurring phytochemicals against testicular oxidative damage resulting from exposure to environmental compounds.24 The protective effects of several antioxidants are believed to be due to their ROS scavenging activity prior to induction of oxidative damage to cellular macromolecules. Indeed, the induction of oxidative stress in the reproductive tissues of 2,5-HD-treated rats reported in the present study may provide credence to the previous study on the chemoprotective effects of resveratrol, a potent antioxidant, on the testicular toxicity induced by 2,5 HD in rats.33
In conclusion, the data from the present investigation showed for the first time that exposure to 2,5-HD impairs sperm characteristics and alters testicular and epididymal architectures through induction of oxidative stress, in rats treated with toxic amount of 2,5-HD. Thus, the results of the present study may have translational toxic implications to male population in Nigeria where the quantity of gas being flared is still substantial and the lack of adherence to the use of gloves and respirators by factory workers is common. Further investigation on the uptake and levels of 2,5-HD in testes, influence of 2,5-HD on testicular steroidogenesis, hypothalamic–pituitary–testicular axis and pituitary–thyroid axis functions is warranted.
Acknowledgments
This work was supported in part by the Multidisciplinary Research Grants under the Staff Training and Research Capacity Building Programme of the John D. and Catherine T. Mac-Arthur Foundation Grant (USA) endowment from the University of Ibadan, Nigeria, awarded to Professor E.O. Farombi. The technical assistance of Messrs Omoko Ejiro of the Department of Veterinary Surgery and Reproduction, University of Ibadan, is highly appreciated. The University Writing Center, Georgia Southern University, Statesboro GA 30460, USA is gratefully appreciated for editing the scientific English expression of the manuscript.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest The authors declare that there are no conflicts of interests.
Ethics approval The experimental etiquettes were carried out after approval by the University of Ibadan Ethical Committee.
References
- 1.Smith PA. Toxicology of organic solvents. In: Luttrell William E., Jederberg Warren W., Still Kenneth R., (eds.) Toxicology principles for the industrial hygienist. Virginia: AIHA; 2008. pp. 187–207. [Google Scholar]
- 2.Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, Kwon EJ, Patel SR, Rasoulpour RJ, Schoenfeld HA, Thompson S. 2,5-hexanedione-induced testicular injury. Ann Review Pharmacol Toxicol 2003;43:125–47. doi: 10.1146/annurev.pharmtox.43.100901.135930 [DOI] [PubMed] [Google Scholar]
- 3.Adedara IA, Ebokaiwe AP, Farombi EO. Tissues distribution of heavy metals and erythrocytes antioxidant status in rats exposed to Nigerian bonny light crude oil. Toxicol Ind Health 2013;29:162–8. doi: 10.1177/0748233711427049 [DOI] [PubMed] [Google Scholar]
- 4.American Conference of Governmental Industrial Hygienists (ACGIH) TLVs and BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. American Conference of Governmental Industrial Hygienists, Cincinnati, OH, 2001, pp. 1–185. [Google Scholar]
- 5.Prieto MJ, Marhuenda D, Roel J, Cardona A. Free and total 2,5-hexanedione in biological monitoring of workers exposed to n-hexane in the shoe industry. Toxicol Lett 2003;145:249–60. doi: 10.1016/S0378-4274(03)00302-3 [DOI] [PubMed] [Google Scholar]
- 6.Neghab M, Soleimani E, Rajaeefard A. Assessment of occupational exposure to n-hexane: a study in shoe making workshops. Res J Environ Toxicol 2011;5:293–300. doi: 10.3923/rjet.2011.293.300 [DOI] [Google Scholar]
- 7.Boekelheide K, Johnson KJ, Richburg JH. Sertoli cell toxicants. In: Griswold MD, Skinner MK, (eds.) Sertoli cell biology. San Diego, CA: Elsevier Inc; 2005. pp. 345–82. [Google Scholar]
- 8.Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol 2007;35:719–27. doi: 10.1080/01926230701481931 [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki H, Sandrof MA, Boekelheide K . Suppression of radiation-induced testicular germ cell apoptosis by 2,5-hexanedione pretreatment. I. Histopathological analysis reveals stage dependence of attenuated apoptosis. Toxicol Sci 2010;117:449–56. doi: 10.1093/toxsci/kfq203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catlin NR, Huse SM, Boekelheide K. The Stage-specific testicular germ cell apoptotic response to low-dose X-irradiation and 2,5-hexanedione combined exposure. I: validation of the laser capture microdissection method for qRT-PCR array application. Toxicol Pathol 2014;42:1221–8. doi: 10.1177/0192623314526319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adedara IA, Abolaji AO, Odion BE, Okwudi IJ, Omoloja AA, Farombi EO. Impairment of hepatic and renal functions by 2,5-hexanedione is accompanied by oxidative stress in rats. J Toxicol 2014;2014:239240. doi: 10.1155/2014/239240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farombi EO, Abolaji AO, Adedara IA, Soladogun A, Salau V, Oguaka M. Oxidative stress and changes in antioxidative defence system in erythrocytes of female rats exposed to 2, 5-hexanedione. Arch Bas App Med 2014;2:29–33. [Google Scholar]
- 13.Abolaji AO, Adedara IA, Soladogun A, Salau V, Oguaka M, Farombi EO. Exposure to 2,5-hexanedione is accompanied by ovarian and uterine oxidative stress and disruption of endocrine balance in rats. Drug Chem Toxicol 2015;38:400–07. doi: 10.3109/01480545.2014.974265 [DOI] [PubMed] [Google Scholar]
- 14.Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev 1995;7:659–68. doi: 10.1071/RD9950659 [DOI] [PubMed] [Google Scholar]
- 15.Gil-Guzman E, Ollero M, Lopez MC, Sharma RK, Alvarez JG, Thomas AJ Jr, Agarwal A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Human Reprod 2001;16:1922–30. doi: 10.1093/humrep/16.9.1922 [DOI] [PubMed] [Google Scholar]
- 16.Adedara IA, Vaithinathan S, Jubendradass R, Mathur PP, Farombi EO. Kolaviron prevents carbendazim-induced steroidogenic dysfunction and apoptosis in testes of rats. Environ Toxicol Pharmacol 2013;35:444–53. doi: 10.1016/j.etap.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 17.National Research Council Guide for the care and use of laboratory animals. Washington: National Academies Press; 2011. [Google Scholar]
- 18.Public Health Service (PHS) Public Health Service Policy on Humane Care and Use of Laboratory Animals. US Department of Health and Human Services, Washington, DC. [PL 99–158. Health Research Extension Act, 1985]; 1996. [Google Scholar]
- 19.Bryant BH, Yamasaki H, Sandrof MA, Boekelheide K. Spermatid head retention as a marker of 2,5-hexanedione-induced testicular toxicity in the rat. Toxicol Pathol 2008;36:552–9. doi: 10.1177/0192623308317426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto-Castelló MJ, Hernández-Viadel ML, Cardona A, Marhuenda D, Felipo V. Activation of soluble guanylate cyclase by nitric oxide is increased in lymphocytes from both rats chronically exposed to 2,5-hexanedione and workers chronically exposed to n-hexane. Toxicology 2007;229:73–8. doi: 10.1016/j.tox.2006.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Zemjanis R. Collection and evaluation of semen. In: Zemjanis R, (ed.) Diagnostic and therapeutic technique in animal reproduction, 2nd edn Baltimore, MA, USA: William and Wilkins Company, Waverly Press, Inc; 1970. pp. 139–53. [Google Scholar]
- 22.World Health Organization Laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th edn New York: Cambridge University Press; 1999. 76:4–33. [Google Scholar]
- 23.Wells ME, Awa OA. New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci 1970;53:227. doi: 10.3168/jds.S0022-0302(70)86184-7 [DOI] [PubMed] [Google Scholar]
- 24.Adedara IA, Farombi EO. Chemoprotection of ethylene glycol monoethyl ether-induced reproductive toxicity in male rats by kolaviron, isolated biflavonoid from Garcinia kola seed. Hum Exp Toxicol 2012;31:506–17. doi: 10.1177/0960327111424301 [DOI] [PubMed] [Google Scholar]
- 25.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170–5. [PubMed] [Google Scholar]
- 26.Clairborne A. Catalase activity. In: Greewald AR, (ed.) Handbook of methods for oxygen radical research. Boca Raton, FL: CRC Press; 1995. pp. 237–42. [Google Scholar]
- 27.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 28.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974;11:151–69. doi: 10.1159/000136485 [DOI] [PubMed] [Google Scholar]
- 29.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–90. doi: 10.1126/science.179.4073.588 [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 31.Wolff SP. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 1994;233:182–9. doi: 10.1016/S0076-6879(94)33021-2 [DOI] [Google Scholar]
- 32.Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 2000;38:535–41. doi: 10.1016/S0278-6915(00)00039-9 [DOI] [PubMed] [Google Scholar]
- 33.Jiang YG, Peng T, Luo Y, Li MC, Lin YH. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2,5-hexanedione. Chin Med J (Engl) 2008;121:1204–9. [PubMed] [Google Scholar]
- 34.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. In: Cheng CY, (ed.) Molecular mechanisms in spermatogenesis. New York: Landes Bioscience and Springer Science + Business Media; 2008. pp. 154–71. [Google Scholar]
- 35.Pacheco SE, Anderson LM, Sandrof MA, Vantangoli MM, Hall SJ, Boekelheide K. Sperm mRNA transcripts are indicators of sub-chronic low dose testicular injury in the Fischer 344 rat. PLoS ONE 2012;7:e44280. doi: 10.1371/journal.pone.0044280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uygur R, Aktas C, Caglar V, Uygur E, Erdogan H, Ozen OA. Protective effects of melatonin against arsenic-induced apoptosis and oxidative stress in rat testes. Toxicol Ind Health 2013; doi: 10.1177/0748233713512891 [DOI] [PubMed] [Google Scholar]
- 37.Prabakaran D, Ashokkumar N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie 2013;95:366–73. doi: 10.1016/j.biochi.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 38.Adedara IA, Farombi EO. Influence of kolaviron and vitamin E on ethylene glycol monoethyl ether-induced haematotoxicity and renal apoptosis in rats. Cell Biochem Funct 2014;32:31–38. doi: 10.1002/cbf.2968 [DOI] [PubMed] [Google Scholar]
- 39.Dhanabalan S, Mathur PP. Low dose of 2,3,7,8 tetrachlorodibenzo-p-dioxin induces testicular oxidative stress in adult rats under the influence of corticosterone. Exp Toxicol Pathol 2009;61:415–23. doi: 10.1016/j.etp.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 40.Tremellen K. Oxidative stress and male infertility-a clinical perspective. Human Reprod Update 2008;14:243–58. doi: 10.1093/humupd/dmn004 [DOI] [PubMed] [Google Scholar]
- 41.Chapin RE, Norton RM, Popp JA, Bus JS. The effects of 2,5-hexanedione on reproductive hormones and testicular enzyme activities in the F-344 rat. Toxicol Appl Pharmacol 1982;62:262–72. doi: 10.1016/0041-008X(82)90124-7 [DOI] [PubMed] [Google Scholar]
- 42.Chapin RE, Morgan KT, Bus JS. The morphogenesis of testicular degeneration induced in rats by orally administered 2,5-hexanedione. Exp Mol Pathol 1983;38:149–69. doi: 10.1016/0014-4800(83)90082-5 [DOI] [PubMed] [Google Scholar]


