Abstract
Background
It has been demonstrated that oxidative stress can induce red blood cell rigidity and haemolysis, which in turn can cause hyperviscosity and hyperbilirubinaemia, respectively. However, haemolysis may be associated with a low level of haemoglobin, which reduces whole blood viscosity (WBV). Bilirubin can behave as antioxidant or oxidant, and one uncharted course for diagnostic pathology is how or whether bilirubinaemia and viscosity are associated. Further, oxidative stress is now being assessed using lipoprotein-a (Lp(a)), among other things but whether it is associated with blood viscosity has not been established.
Aim
This study investigates the association and correlation of haemoglobin level and WBV with serum Lp(a) and bilirubin levels in a general population of patients.
Materials and methods
Sixty-eight cases that were tested for Lp(a), concomitantly with full blood count and liver function, in our archived clinical pathology database were used in this study. WBV levels were determined using a validated formula. Multivariate and univariate analyses as well as correlation were performed.
Results
WBV was found to be significantly associated with bilirubin (P < 0.02), but not with Lp(a). Haemoglobin concentration was inversely correlated with Lp(a) (P < 0.04), but not with bilirubinaemia.
Conclusion
This pilot study suggests that hyperbilirubinaemia and hyperviscosity are associated and positively correlated. Consideration of whether serum bilirubin (as an indirect index of oxidative stress) can be used in combination with WBV (as index of macrovascular effect of oxidative stress) to assess oxidative damage is recommended.
Keywords: Clinical assessment, Diagnostic pathology, Haemolysis, Hyperbilirubinaemia, Hyperviscosity, Oxidative stress
Introduction
In clinical diagnostic practice, bilirubin measurement is an important component of the test panel for liver functions. Total bilirubin is commonly used for monitoring of patients with anaemia who are suspected of disorders associated with haemolysis. Hyperbilirubinaemia has been associated with circadian rhythm and oxidative stress.1 Circadian rhythm has been linked to several physiological processes including antioxidant activities vis-à-vis oxidative stress and blood viscosity.2–4 Interestingly, and typical of paradoxical properties of antioxidants, bilirubin can behave as an antioxidant or oxidant depending on its concentration.5 Particularly, bilirubin at low concentration is an antioxidant in neonatal jaundice,5 but during haemolysis it is possible that bilirubin free radicals such as lumirubin may be generated;6,7 thereby conferring oxidant properties on bilirubin.
The concept of bilirubin being associated with oxidative stress is interesting with potential for its use in laboratory medicine diagnosis, because it is an established routine laboratory index. Given that oxidative stress may be associated with serum bilirubin level on one hand, and exacerbates whole blood viscosity (WBV) on the other, it is worth investigating whether bilirubinaemia could be associated with WBV. Further, evidence of oxidative stress and a concomitant demonstration of any of the effective vascular events including increase in WBV is necessary to establish oxidative damage.8 If bilirubinaemia is associated with WBV; it would be worth investigating how or whether the two parameters are correlated, given the antioxidant and pro-oxidant properties of bilirubin. A determination of association and correlation could mean a possibility that the two parameters can be used to assess oxidative damage.
Lipoprotein(a) (Lp(a)) is a laboratory marker that is emerging as a possible risk factor for cardiovascular diseases (CVD). Studies have provided support to the hypothesis that Lp(a) attenuates fibrinolysis and promotes coagulation,9 thus affirming the notion that high-level plasma Lp(a) is a risk factor for CVD.10,11 Lp(a) is attributed to have a role as an acute phase inflammatory reactant and is associated with oxidative stress.12–14 It is believed that high plasma Lp(a) level does not translate to causality of CVD.15 Instead, it is thought to confer additional risk only in the presence of traditional factors.12 However, haemolysis, Lp(a) and oxidative stress constitute markers of lipoprotein metabolism.16 One of the problems in diagnostic pathology, is that Lp(a) measurement is in urgent need of standardization.17,18 Lp(a) is yet to be an established biomarker, and only very few clinicians request the test. It is the intention in this study to investigate how Lp(a) is related to anaemia, bilirubinaemia, and WBV.
The theory that oxidative stress is a major contributing factor to blood viscosity dates back more than four decades.19–23 An estimate of WBV can now be determined from haematocrit (HCT) and serum total proteins (TP) levels thus:24,25
 |
where HCT = haematocrit (%) and TP = serum total proteins (g/l).
Low WBV, which is related to anaemia, complicates low shear rate and constitutes part of the abnormalities that are associated with a decreased vasodilating response to endothelial mechanical stimulation.26 However, the level of WBV increases with HCT and underpins the principle of treating hyperviscosity in patients with polycythemia. Conversely, anaemia if due to haemolysis is associated with high serum bilirubin level and low blood viscosity.
Hypotheses
Oxidative stress can induce red cell rigidity and haemolysis (Fig. 1), which in turn can cause increased blood viscosity and serum bilirubin, respectively. Given that bilirubin can be antioxidant or pro-oxidant, it would therefore be a factor of predominance. That is, (a) pro-oxidant bilirubinaemia and blood viscosity could be associated and positively correlated; or (b) reducing WBV due to haemolysis-induced anaemia and increasing antioxidant bilirubin could cause negative correlation. Second, oxidative stress may be assessed using Lp(a), but whether the latter is associated with blood viscosity is not yet established. Thus, we hypothesize that an increase in plasma Lp(a) level as an indirect index of oxidative stress is associated and positively correlates with WBV.
Figure 1.
Hypothetical association between bilirubinaemia, haemoglobin, Lp(a), and blood viscosity – callouts indicating known points of association in the pathophysiology.
Objective
This study investigated whether decreased haemoglobin level or an increase in WBV is associated with increase in bilirubinaemia and/or plasma Lp(a) concentration in a general population of patients. Of particular interest was whether bilirubinaemia and/or Lp(a) level should be investigated for use as indirect indices of oxidative stress in clinical practice.
Assumption
There is problem regarding variation in reference values for Lp(a), especially due to racial differences.27–29 It was assumed that this variation in reference values in different populations does not impact upon the study. The use of hyperbilirubinaemia and/or Lp(a) as indirect indices of oxidative stress is yet to be established.
Material and methods
This work is part of a clinical laboratory-based Biomedical Science Research supported materially by Albury South West Pathology – a unit of the Western Pathology Cluster of NSW Health, Australia. The Ethics Committee of the Area Health Service approved the use of the de-identified data. The database comprised archived clinical pathology data from January 1999 to December 2008.30
All tests were performed at the Albury laboratory of South West Pathology, except for Lp(a) which were sent to Newcastle Hunter Area Pathology Service, Newcastle. Bilirubin measurement in this study refers to total conjugated and unconjugated bilirubin. Lp(a) test results in the 10-year period were obtained from the laboratory information system and audited. Sixty-eight cases, comprising only adults, that had data for full blood counts (FBCs) and liver function test (LFT) parameters were selected.
HCT and TP were used to determine WBV at high shear rate by extrapolation method,25 as follows:
 |
where HCT = haematocrit (%) and TP = serum total proteins (g/l).
The data (n = 68) were first ranked and categorized into quartiles based on haemoglobin levels, and re-categorized based on WBV. In order to evaluate possible association with serum Lp(a) and bilirubin, multivariate (MANOVA) and univariate (ANOVA) analyses were performed to determine difference between subgroups using S-Plus.
Correlation analysis was performed using the CORREL function in Microsoft Excel. Parameters that make up the LFTs and haematological indices were included. The intention was to ascertain whether any of the parameters would be correlated and/or statistically significantly related. In order to visualize possible changes in haemoglobin level and WBV associated with serum bilirubin and Lp(a) levels observable in any follow-up of individuals, three cases that had two sets of results each, were reviewed.
Results
The description of the data is presented in Table 1. Among the four parameters of interest, distrubtions are accepted to be normal (kurtosis < 3), but Lp(a) concentration is very widely distributed (Table 1). Therefore, Lp(a) values were transformed into the log inverse for analysis.
Table 1.
Data description for LFTs and FBC
| Liver function tests | Full blood count | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lp(a) | WBV | TP | BILI | ALP | ALT | AST | HGB | HCT | MCV | MCH | MCHC | RDW | PLT | WBCC | RBC | |
| Mean | 525 | 17.40 | 73 | 11 | 85 | 31 | 22 | 147 | 0.44 | 88.98 | 29.75 | 334 | 13.36 | 244 | 6.84 | 4.95 |
| SD | 596 | 0.97 | 4 | 5 | 33 | 28 | 10 | 13 | 0.04 | 5.63 | 2.02 | 12 | 1.00 | 66 | 1.78 | 0.49 |
| Median | 223 | 17.35 | 73 | 10 | 79 | 24 | 21 | 145 | 0.44 | 89.60 | 30.30 | 334 | 13.30 | 235 | 6.70 | 4.91 |
| Kurtosis | 1.72 | −0.58 | −0.47 | 1.18 | 8.37 | 29.30 | 2.23 | −0.25 | 0.60 | 2.55 | 5.34 | 0.20 | 2.66 | −0.23 | 1.79 | 1.22 |
| Maximum | 2627 | 19.31 | 83 | 26 | 250 | 216 | 61 | 181 | 0.56 | 101.90 | 34.10 | 362 | 16.80 | 392 | 13.20 | 6.31 |
| Minimum | 47 | 15.14 | 64 | 4 | 45 | 8 | 5 | 121 | 0.38 | 70.20 | 21.70 | 304 | 11.90 | 120 | 4.00 | 3.77 |
In the evaluation of changes in various parameters with incremental changes in (ranked) haemoglobin levels, MANOVA presented statistical significance between subpopulations in 1st vs. 4th quartile (P = 0). Univariate analysis (ANOVA fixed factor) of Lp(a) and serum bilirubin (SB) presented statistically significant difference in the former, but not in the latter (Fig. 2).
Figure 2.

Changes in bilirubinaemia and Lp(a) with increment in haemoglobin.
In the evaluation of changes in various parameters with incremental changes in (ranked) WBV, MANOVA demonstrated statisitcal significance (P = 0). Univariate analysis (ANOVA fixed factor) gave a statistically significant difference in SB (P < 0.02), but not in Lp(a) (Fig. 3).
Figure 3.
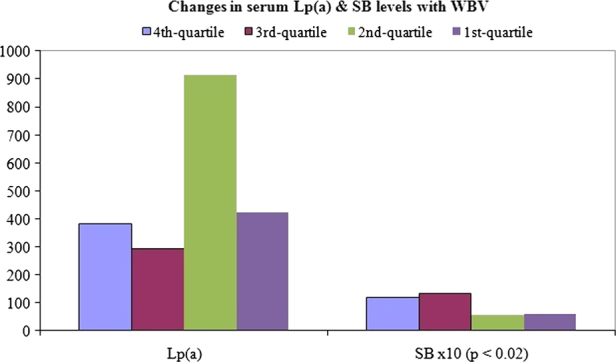
Changes in bilirubinaemia and Lp(a) with increment in blood viscosity.
The outcome of correlation analysis shows a moderate positive correlation between blood viscosity and bilirubinaemia, but not with Lp(a); while haemoglobin showed negative correlation with Lp(a), but not with bilirubinaemia (Table 2).
Table 2.
Output of correlations analysis
| WBV | Lp(a) | SB | ALP | ALT | AST | |
|---|---|---|---|---|---|---|
| WBV | 1.000 | |||||
| Lp(a) | −0.005 | 1.000 | ||||
| SB | 0.309 | −0.016 | 1.000 | |||
| ALP | 0.163 | 0.108 | −0.066 | 1.000 | ||
| ALT | 0.069 | 0.073 | 0.120 | 0.290 | 1.000 | |
| AST | 0.067 | −0.130 | 0.161 | 0.091 | 0.547 | 1.000 |
| HGB | 0.330 | −0.258 | 0.161 | 0.038 | 0.204 | 0.019 |
| HCT | 0.389 | −0.164 | 0.111 | 0.004 | 0.190 | 0.024 |
| MCV | 0.105 | −0.052 | −0.187 | 0.014 | 0.048 | 0.174 |
| MCH | 0.075 | −0.181 | −0.088 | 0.070 | 0.089 | 0.149 |
| MCHC | −0.065 | −0.268 | 0.147 | 0.088 | 0.077 | 0.000 |
| RDW | 0.184 | 0.060 | 0.214 | 0.176 | 0.238 | 0.233 |
| PLT | −0.371 | 0.015 | −0.171 | 0.203 | 0.026 | −0.020 |
| WBCC | −0.336 | −0.012 | −0.176 | 0.179 | 0.052 | 0.218 |
| RBC | 0.248 | −0.089 | 0.230 | −0.012 | 0.127 | −0.088 |
Case evaluations
The three cases reviewed indicates that when Lp(a) levels were higher, all three patients had lower levels of serum bilirubin, while two-thirds had higher WBV and lower haemoglobin (Table 3).
Table 3.
Absolute value results of three cases that had follow-up tests
| Lp(a) | WBV | SB | HGB | ||
|---|---|---|---|---|---|
| Case 1 | 1st record | 379 | 17.05 | 12 | 146 |
| 2nd record | 708 | 17.15 | 9 | 143 | |
| Case 2 | 1st record | 911 | 19.04 | 22 | 141 |
| 2nd record | 1078 | 18.53 | 10 | 135 | |
| Case 3 | 1st record | 1107 | 17.58 | 5 | 154 |
| 2nd record | 885 | 16.15 | 6 | 152 |
Discussion
It is known that oxidative stress is associated with increase in hyperviscosity, but not necessarily with low HCT/haemoglobin. We have investigated how serum Lp(a) level and bilirubinaemia, as potential indirect indicators of oxidative stress, would be associated and/or correlated with WBV and haemoglobin. Our results show that hyperbilirubinaemia, but not Lp(a), is associated and positively correlated with WBV. It also shows albeit conversely that Lp(a) is associated and negatively correlated with haemoglobin concentration.
It suggests that as oxidative stress is more associated with increasing WBV and not decreasing HCT/haemoglobin, so also is bilirubinaemia. Further, the results show that among all components of the LFT panel, bilirubinaemia is most correlated and statistically significantly related to WBV. There is a report indicating that patients with cholestatic jaundice have increased WBV.31 Therefore, the observation reported here affirms that hyperbilirubinaemia is positively correlated to WBV. Bearing in mind the antioxidant potential of bilirubin, the positive correlation with WBV could also suggest a possibility of pro-oxidant bilirubin overwhelming the antioxidant property.
Another interesting aspect of the study is whether Lp(a), as an indirect index of oxidative stress, is more associated with either anaemia or WBV. The pertinent observation is a statistically significant inverse relationship between haemoglobin and Lp(a). It could be inferred that the statistically significant negative relationship is due to oxidative stress concomitantly inducing membrane lipoprotein metabolism leading to increased Lp(a); and haemolysis causing a reduction in haemoglobin level (Fig. 1). However, we note that the hypothesis of correlation between Lp(a) and WBV failed.
Further, a critical visual review shows that there is an increase in bilirubin level associated with increase in haemoglobin concentration (Fig. 2), but this was not significant. This observation is in line with the report that erythroid apoptosis is not correlated with bilirubinaemia.32 In two of the three cases, which were reviewed to check the assumption that the racial differences in normal level of Lp(a) may not impact on changes to be observed in any follow-up of individuals, it is observed that WBVs were higher and haemoglobin concentrations lower when the serum Lp(a) concentration increased. Thus, the anecdotal cases indicate that increase in Lp(a) may be associated with increase in WBV, but correlation analysis in the general population did not corroborate.
Correlation does not always imply causation and some studies have shown that haemolysis may not contribute significantly to other pathophysiology associated with oxidative stress.33 Conversely, non-significant correlation does not always imply lack of relationship. In this study, Lp(a) levels showed negative correlation with haemoglobin, but not significantly related to bilirubin or WBV. However, the propensity of oxidative stress to cause haemolysis-induced hyperbilirubinaemia and hyperviscosity is demonstrated by the positive association as well as correlation between bilirubin and WBV. Further, the statistically significant inverse correlation between haemoglobin and Lp(a) in the study tends to lend weight. Therefore, each of the four variables has demonstrated an explainable correlation with at least one of the other three.
Study limitations
There are several limitations in this study. First, the participants were de-identified. As the outcome of this study provides them no direct or immediate benefit, contact with patients or their clinicians was not made. Information on disease condition, clinical management, drugs, and presence of metabolic disorders, malignancy, or coagulation disorders were not accessed. Second, serum bilirubin measured in this study was the total fraction with no differentiation between unconjugated (USB) or conjugated bilirubin. The data do not include neonates who are typically not tested for Lp(a) and we acknowledge this as a limitation especially, due to issues such as (i) USB crossing the blood–brain barrier and affecting the integrity of microvascular endothelial cell monolayers through oxidative stress,34 and (ii) hyperbilirubinaemia vs. anaemia with regard to causation. High bilirubin from haemolytic, pre-hepatic processes has a high unconjugated content and bilirubin from post-hepatic or disturbed bilirubin transport processes would be mostly conjugated. This becomes confounded when there is haemolysis from anaemia with concomitant liver disease. This study has other limitations in that there were no obvious cases of anaemia or hyperviscosity in the study population. Further, a small fraction of females were tested and their haemoglobin levels are generally lower in comparison with that of males.
Conclusion
This study has determined that bilirubinaemia and blood viscosity are associated and positively correlated, while haemoglobin and Lp(a) are associated and negatively correlated. The implication is that the pro-oxidant property of serum bilirubin (as an indirect index of oxidative stress) can be used in combination with WBV (as an index of concomitant macrovascular effect of oxidative stress) to assess oxidative damage. Further investigation to corroborate this report is recommended.
References
- 1.Larsson A, Hassan M, Ridefelt P, Axelsson J. Circadian variability of bilirubin in healthy men during normal sleep and after an acute shift of sleep. Chronobiol Int 2009;25:1613–21. [DOI] [PubMed] [Google Scholar]
- 2.Subramanian P, Dakshayani KB, Pandi-Perumal SR, Trakht I, Cardinali DP. 24-hour rhythms in oxidative stress during hepatocarcinogenesis in rats: effect of melatonin or alpha-ketoglutarate. Redox Rep 2008;13:78–86. [DOI] [PubMed] [Google Scholar]
- 3.Rekhviashvili A, Tsinamdzgvrishvili B, Chkhetia M, Labakhua G. Relationship of 24-hour blood pressure rhythm with endothelial function and blood rheology. Georgian Med News 2008;159:21–6. [PubMed] [Google Scholar]
- 4.Richards RS, Nwose EU, Bwititi P. Biochemical basis of circadian rhythms and diseases: with emphasis on post-traumatic stress disorder. Med Hypotheses 2011;77:605–9. [DOI] [PubMed] [Google Scholar]
- 5.Friel JK, Friesen RW, Miller AC. Bilirubin: friend or foe? Society for Free Radical Biology and Medicine; 2006. [Google Scholar]
- 6.Agati G, Fusi F, Donzelli GP, Pratesi R. Quantum yield and skin filtering effects on the formation rate of lumirubin. J Photochem Photobiol B 1993;18:197–203. [DOI] [PubMed] [Google Scholar]
- 7.Roll EB, Christensen T. Formation of photoproducts and cytotoxicity of bilirubin irradiated with turquoise and blue phototherapy light. Acta Paediatr 2005;94:1448–54. [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev 2004;84:1381–478. [DOI] [PubMed] [Google Scholar]
- 9.von Depka M, Nowak-Göttl U, Eisert R, Dieterich C, Barthels M, Scharrer I, et al.. Increased lipoprotein (a) levels as an independent risk factor for venous thromboembolism. Blood 2000;96:3364–8. [PubMed] [Google Scholar]
- 10.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: a meta-analysis of observational studies. Stroke 2007;38:1959–66. [DOI] [PubMed] [Google Scholar]
- 11.Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, et al.. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J 2006;20:559–61. [DOI] [PubMed] [Google Scholar]
- 12.Barghash NA, Elewa SM, Hamdi EA, Barghash AA, El Dine R. Role of plasma homocysteine and lipoprotein (a) in coronary artery disease. Br J Biomed Sci 2004;61:78–83. [DOI] [PubMed] [Google Scholar]
- 13.Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, et al.. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation 2004;109:3164–70. [DOI] [PubMed] [Google Scholar]
- 14.Antonicelli R, Testa R, Bonfigli AR, Sirolla C, Pieri C, Marra M, et al.. Relationship between lipoprotein(a) levels, oxidative stress, and blood pressure levels in patients with essential hypertension. Clin Exp Med 2001;1:145–50. [DOI] [PubMed] [Google Scholar]
- 15.Danesh J, Collins R, Peto R. Lipoprotein(a) and coronary heart disease. Meta-analysis of prospective studies. Circulation 2000;102:1082–5. [DOI] [PubMed] [Google Scholar]
- 16.Matteucci E, Giampietro O. Oxidative stress in families of type 1 diabetic patients. Diabetes Care 2000;23:1182–6. [DOI] [PubMed] [Google Scholar]
- 17.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, et al.. Use of a reference material proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to evaluate analytical methods for the determination of plasma lipoprotein(a). Clin Chem 2000;46:1956–67. [PubMed] [Google Scholar]
- 18.Jialal I. Evolving lipoprotein risk factors: lipoprotein(a) and oxidized low-density lipoprotein. Clin Chem 1998;44:1827–32. [PubMed] [Google Scholar]
- 19.Merrill EW, Gilliland ER, Cokelet G, Shin H, Britten A, Wells REJ. Rheology of human blood, near and at zero flow. Effects of temperature and hematocrit level. Biophys J 1963;3:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards RS, Roberts TK, Mathers D, McGregor NR, Dunstan RH, Butt HL. Investigation of erythrocyte oxidative damage in rheumatoid arthritis and chronic fatigue syndrome. J Chron Fatigue Syndr 2000;6:37–46. [Google Scholar]
- 21.Yang ZC, Xia K, Wang L, Jia SJ, Li D, Zhang Z, et al.. Asymmetric dimethylarginine reduced erythrocyte deformability in streptozotocin-induced diabetic rat. Microvasc Res 2007;73:131–6. [DOI] [PubMed] [Google Scholar]
- 22.Huang CR, Chen HQ, Pan WD, Shih T, Kristol DS, Copley AL. Effects of hematocrit on thixotropic properties of human blood. Biorheology 1987;24:803–10. [DOI] [PubMed] [Google Scholar]
- 23.Reinke W, Johnson P, Gaehtgens P. Effect of shear rate variation on apparent viscosity of human blood in tubes of 29 to 94 microns diameter. Circ Res 1986;59:124–32. [DOI] [PubMed] [Google Scholar]
- 24.Tamariz LJ, Young JH, Pankow JS, Yeh HC, Schmidt MI, Astor B, et al.. Blood viscosity and hematocrit as risk factors for type 2 diabetes mellitus: the atherosclerosis risk in communities (ARIC) study. Am J Epidemiol 2008;168:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwose EU. Whole blood viscosity assessment issues I: extrapolation chart and reference values. North Am J Med Sci 2010;2:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbeke FH, Agharazii M, Boutouyrie P, Pannier B, Guerin AP, London GM. Local shear stress and brachial artery functions in end-stage renal disease. J Am Soc Nephrol 2007;18:621–8. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan S, Dahlen G, Jarpa R, Webber L, Berenson G. Racial (black-white) differences in serum lipoprotein (a) distribution and its relation to parental myocardial infarction in children. Bogalusa Heart Study. Circulation 1991;84:160–7. [DOI] [PubMed] [Google Scholar]
- 28.Knapp RG, Schreiner PJ, Sutherland SE, Keil JE, Gilbert GE, Klein RL, et al.. Serum lipoprotein(a) levels in elderly black and white men in the Charleston Heart Study. Clin Genet 1993;44:225–31. [DOI] [PubMed] [Google Scholar]
- 29.Banerjee D, Wong EC, Shin J, Fortmann SP, Palaniappan L. Racial and Ethnic Variation in Lipoprotein (a) Levels among Asian Indian and Chinese Patients. J Lipids 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwose EU, Richards RS, Butkowski E, Cann N. Position paper for health authorities: archived clinical pathology data-treasure to revalue and appropriate. Afr J Med Med Sci 2010;39:311–5. [PubMed] [Google Scholar]
- 31.Mark M, Walter R, Contesse J, Reinhart WH. Impairment of blood rheology by cholestatic jaundice in human beings. J Lab Clin Med 2003;142:391–8. [DOI] [PubMed] [Google Scholar]
- 32.Ozkan H, Oren H, Tatli M, Ates H, Kumral A, Duman N. Erythroid apoptosis in idiopathic neonatal jaundice. Pediatrics 2008;121:e1348–51. [DOI] [PubMed] [Google Scholar]
- 33.Jalloh S, Van Rostenberghe H, Yusoff NM, Ghazali S, Nik Ismail NZ, Matsuo M, et al.. Poor correlation between hemolysis and jaundice in glucose 6-phosphate dehydrogenase-deficient babies. Pediatr Int 2005;47:258–61. [DOI] [PubMed] [Google Scholar]
- 34.Palmela I, Cardoso FL, Bernas M, Correia L, Vaz AR, Silva RF, et al.. Elevated levels of bilirubin and long-term exposure impair human brain microvascular endothelial cell integrity. Curr Neurovasc Res 2011;8:153–69. [DOI] [PubMed] [Google Scholar]



