Abstract
Objectives
This study was focused on the monitoring how the anti-inflammatory substance, N1-methylnicotinamide (MNA), could influence oxidation and glycooxidation stress markers in rats under conditions of streptozotocin (STZ)-induced diabetes mellitus.
Methods
Diabetes mellitus was induced in 60 male Wistar rats by intraperitoneal injection of STZ and after 7 days diabetic animals were allocated to five groups according to the dose of MNA administered for 7 weeks. The degree of DNA damage in lymphocytes, as well as advanced glycation endproducts (AGEs), protein carbonyls, lipid peroxides, and total antioxidant capacity (TEAC) in plasma were measured.
Results
Glycation damage to proteins (represented by AGEs level) was significantly increased in all diabetic groups compared to untreated non-diabetic animals. MNA did not affect TEAC of plasma in any group of diabetic rats. Supplementation of diabetic rats with MNA at the dose of 200 mg/kg resulted in decreased protein carbonyls (from 0.0818 ± 0.0091 to 0.0558 ± 0.0044 nmol/mg proteins; P < 0.05, n = 15) and DNA oxidation, reflected by the levels of 8-oxoG (0.6302 ± 0.085 vs. 0.9213 ± 0.108 8-oxoG/106 G; P < 0.05, n = 15), compared to untreated diabetic animals.
Discussion
Our results demonstrated that MNA at suitable concentrations could influence oxidative modifications of proteins and DNA.
Keywords: Diabetes mellitus, Glycooxidative stress, Methylnicotinamide, Oxidative stress, Protein carbonyls
Introduction
The oxidative damage provoked by reactive free radicals has been demonstrated to play a significant role in aging, diabetes, and several other pathological events.1 Diabetes mellitus is associated with an increased flux of reactive oxygen species into the extracellular space and the impaired cellular redox system that results in a gradual loss of reducing capacity, with later implications on enzyme activities and antioxidant defense system.2–4
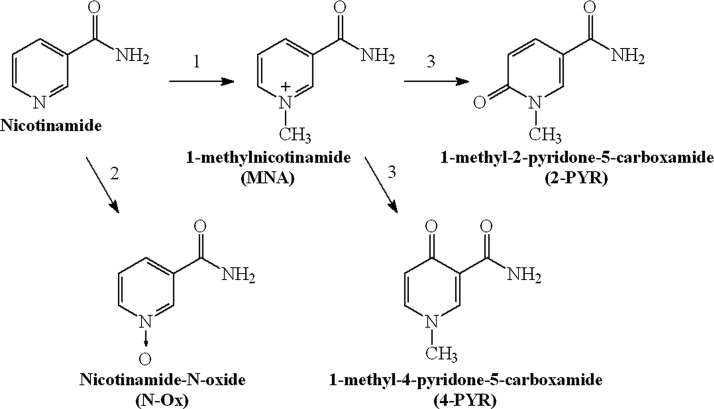
With regard to the presence of free radicals and oxidative steps in the glycooxidation process, compounds with antioxidative effects have been tested in order to slow down or to stop glycooxidation.5,6 Nicotinamide (NA) is known to be an essential nutrient (vitamin B3), as it is the precursor of nicotinamide adenine dinucleotide (NAD+) that participates in a wide range of biological processes including energy production, redox state of cells, cellular resistance to injury, as well as longevity.7,8 Indeed, NA has been reported to exert a number of physiological and pharmacological effects, including the prevention of ATP depletion, inhibition of poly(ADP ribose) polymerase (PARP), prevention of apoptosis, and suppression of lipid peroxidation.9 The results derived of animal studies indicate that NA may prevent experimental diabetes similar to type 1 onset diabetes in humans.10 NA is metabolized in the liver by cytochrome P450 to nicotinamide-N-oxide (N-Ox), but mainly is transformed by nicotinamide N-methyltransferase to 1-methylnicotinamide (MNA) that is further metabolized to 1-methyl-2-pyridone-5-carboxamide (2-PYR) or 1-methyl-4-pyridone-5-carboxamide by aldehyde oxidase (Fig. 1).11
Figure 1.
Major pathways of nicotinamide metabolism (1, nicotinamide N-methyltransferase; 2, cytochrome P450; 3, aldehyde oxidase).
For many years, MNA has been considered inactive marker of NA metabolic pathway. In in vitro studies MNA has been shown to be a potent anti-inflammatory agent.12,13 It appears to be more protective than NA in the prevention of the onset of alloxan-induced diabetes in mice.14 Thus, we cannot exclude the fact that in vivo some biological effects of NA are mediated by MNA.
MNA therapy was not effective in cases with accompanying bacterial infection, which indicates that therapeutic effectiveness of MNA is related to its anti-inflammatory, but not antibacterial, properties.15 Moreover, it was demonstrated that MNA displays an antithrombotic potential.16 All these observations contradict the common view that MNA is a biologically inactive metabolite of NA. MNA anti-inflammatory activity could be associated with scavenging of reactive forms of oxygen, in particular superoxide radical anion and hydroxyl radical. However, in contrast to NA, the mechanism of this anti-inflammatory activity is still unclear.17
This study was focused on monitoring the markers of oxidative stress and glycooxidation under the conditions of streptozotocin (STZ)-induced diabetes mellitus in rats and possible beneficial effect of MNA on these markers in plasma or serum.
Materials and methods
Chemicals
MNA chloride salt was synthesized by alkylation of NA with methyl chloride in methanol solution, as described earlier.18 MNA chloride used in our experiments was of high purity (>99.8%), and NA was identified as a major impurity (<0.2%). STZ, 2,4-dinitrophenylhydrazine and glycerol were purchased from Fluka (Buchs, Switzerland); EDTA, o-phenylenediamine, guanidine hydrochloride, Tween 20, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), Trolox, bovine serum albumin (BSA), Histopaque 1083, low melting point (LMP) agarose, normal melting point (NMP) agarose, and Triton X-100 were purchased from Sigma-Aldrich Chemical (Taufkirchen, Germany); DAPI (4,6-diamidino-2-fenylindol dihydrochlorid) was purchased from Merck (Darmstadt, Germany), formamidopyrimidine-DNA glycosylase (Fpg) was received from Prof. A.R. Collins from the Department of Nutrition, Faculty of Medicine, University of Oslo in Norway.
Experimental animals
In our study 280–320 g Wistar male rats were used (n = 75). Diabetes mellitus was induced with STZ (45 mg/kg of body weight dissolved in 0.1 mol/l citrate buffer, pH 4.5), injected via the tail vein. Diabetes was diagnosed after 48 hours based on fasting blood glucose level. Animals with blood glucose concentrations higher than 16 mmol/l after injection of STZ were considered diabetic and were included in our study. Control rats were injected with a corresponding volume of citrate buffer. Insulin was not administered to any group of animals.
Animals were randomly divided into five groups (n = 15) according to the administration and doses of MNA: controls (non-diabetic) rats (C), untreated diabetic rats (D), diabetic rats treated with MNA at the doses of 20 (DB), 100 (DC), or 200 mg/kg body weight per day (DD), respectively. MNA was given to animals in selected groups in drinking water as a vehicle, while control animals (diabetic or non-diabetic) were treated with vehicle only (pure drinking water).
The duration of animal treatment was 7 weeks, starting on the seventh day after induction of diabetes. After that time the animals were sacrificed by the intramuscular administration of ketamine, 100 mg/kg and xylazine, 15 mg/kg of body weight.
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the USA National Institute of Health (NIH Publication No. 85–23, revised 1985), as well as with the guidelines formulated by the European Community for the Use of Experimental Animals (L358-86/609/EEC). The experimental procedures used in the present study were approved by the local Ethics Committee on Animal Experiments.
Blood collection
Blood was withdrawn by dripping from abdominal aorta (intravenous cannula 0.8 × 25 mm, B. Braun Melsungen AG [Melsungen, Germany]) and anticoagulated with lithium heparin. Plasma levels of basic biochemical parameters (glucose, uric acid, total cholesterol, and triacylglycerols) were determined using commercial kits from Roche Diagnostics (Rotkreuz, Switzerland) with Hitachi 911 automatic analyzer (Tokyo, Japan).
Determination of advanced glycation endproducts in plasma
The level of advanced glycation endproducts (AGEs) was measured with a modified method19 as the total fluorescence recorded at wavelengths (exc/emis.) 370 nm/450 nm in plasma diluted with 0.01 mol/l phosphate-buffered saline and expressed in arbitrary units per gram of plasma proteins. Fluorescence spectrometer PERKIN-ELMER LS45 was used.
Determination of lipid peroxides in serum
Serum lipid peroxides were assayed spectrophotometrically (PharmaSpec UV-1700 Shimadzu). The analysis was based on the ability of lipid peroxides to convert iodide to iodine, which in assay mixture consequently reacts with iodide to form I3. The reaction was initiated by addition of serum (100 µl) to the assay mixture prepared according to the El-Saadani's protocol.20 After 30 minutes the total amount of lipid peroxides was monitored as absorbance of formed I3 at 365 nm. Concentrations were calculating using molar extinction coefficient for I3 (ɛ = 24 600 lmol−1 cm−1 and presented in nmol per ml of serum.
Determination of protein carbonyls in plasma
Concentration of protein carbonyls was measured by enzyme-linked immunosorbent assay method.21 Briefly carbonyls of protein samples were derivatized with 2,4-dinitrophenylhydrazine. A biotin-conjugated antidinitrophenyl-rabbit-IgG antiserum was used as a primary antibody and a monoclonal goat antirabbit-IgG antibody peroxidase conjugate as secondary antibody with spectrophotometric detection at 490 nm. Results were calculated from calibration curve using oxidated BSA as the standard and expressed in nmol of carbonyls per mg of proteins.
Determination of 8-oxoG (detection of DNA damage – comet assay) in lymphocytes
Oxidative damage to DNA was determined by the enzymatic modified comet assay using Fpg for cleavage of oxidatively modified purines. DNA damage is expressed as a number of 8-oxoG per 106 of guanines.22–24
DNA breaks are represented by total damage (TD) values, where i is a class of damage and N is the number of cells in each class:
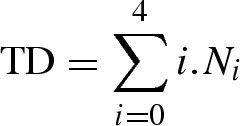 |
TD is linearly related to DNA break frequency over a wide range of damage. The levels of 8-oxoG per 106 were calculated using TD values and calibration curve y = 134.97x + 7.0612, where y represents TD and x represents DNA breaks expressed as 8-oxoG per 106 of guanines according to ESCODD et al.24
Determination of total antioxidant capacity in plasma
A method for the determination of antioxidant activity (Trolox equivalent antioxidant capacity (TEAC) assay) is a decolorization assay25 applicable to both lipophilic and hydrophilic antioxidants. The preformed radical monocation of 2,2′-azino-bis(-3-ethylbenzothiazoline-6-sulfonic acid) (ABTS.+) is generated by oxidation of ABTS with potassium persulfate and is reduced in the presence of hydrogen-donating antioxidants into colorless form. Quantification was performed using the dose-response curve for reference antioxidant Trolox – a water-soluble form of vitamin E (Sigma-Aldrich, St. Louis, USA) Results are presented as mmol of Trolox per liter.
Statistical analysis
Descriptive statistics were obtained for all variables using mean ± SD for normally distributed continuous variables. The data without deviation from normality were evaluated using one-way analysis of variance, in another case Kruskal–Wallis test was used. For statistical analysis, we employed the statistical program StatsDirect® v.2.3.7 (StatsDirect Sales, Sale, UK). Graphical representation of data was made using Excel 2000 (Microsoft Co.). A P-value less than 0.05 was considered statistically significant. Correlations were characterized with Pearson's correlation coefficient.
Results
The biochemical parameters measured in experimental animals are shown in Table 1. Biochemical parameters (glucose, uric acid, total cholesterol, and triacylglycerols) were not influenced with MNA administration.
Table 1.
Selected biochemical parameters in plasma of experimental animals (mean ± SD)
| Animal group | Glycemia (mmol/l) | Cholesterol (mmol/l) | Triacylglycerols (mmol/l) | Uric acid (μmol/l) |
|---|---|---|---|---|
| C | 14.12 ± 0.7 | 1.03 ± 0.038 | 0.902 ± 0.161 | 50.680 ± 8.234 |
| n = 15 | n = 15 | n = 15 | n = 15 | |
| D | 28.02 ± 1.6*** | 1.642 ± 0.07*** | 0.947 ± 0.188 | 49.357 ± 2.738 |
| n = 14 | n = 14 | n = 14 | n = 14 | |
| DB | 28.82 ± 2.5*** | 1.561 ± 0.050*** | 0.825 ± 0.123 | 77.460 ± 21.727 |
| n = 15 | n = 15 | n = 15 | n = 15 | |
| DC | 30.69 ± 2.2*** | 1.577 ± 0.128** | 0.976 ± 0.147 | 62.947 ± 11.301 |
| n = 15 | n = 15 | n = 15 | n = 15 | |
| DD | 31.24 ± 1.9*** | 1.681 ± 0.078*** | 1.002 ± 0.180 | 44.307 ± 4.019 |
| n = 14 | n = 14 | n = 14 | n = 14 |
C, untreated controls (non-diabetic); D, untreated diabetic animals; DB, diabetic animals treated with 20 mg MNA/kg of body weight; DC, diabetic animals treated with 100 mg MNA/kg of body weight; DD, diabetic animals treated with 200 mg MNA/kg of body weight.
Significant difference compared to controls: **P < 0.01, ***P = 0.001.
Glycation of proteins
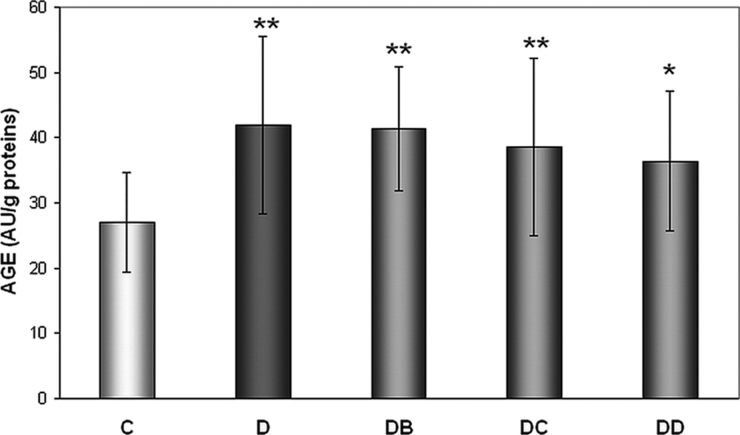
In all diabetic groups AGEs levels were significantly higher compared to group C – by 55.2% (group D), 53% (DB), 42.9% (DC), and 34.6% (DD) (Fig. 2). Although no individual MNA dose directly influenced AGEs level, there was found negative MNA dose dependence (r = −0.9946, P = 0.0054).
Figure 2.
Effect of MNA treatment on AGE-derived fluorescence. C, untreated controls (non-diabetic) (n = 14); D, untreated diabetic animals (n = 14); DB, diabetic animals treated with 20 mg MNA/kg of body weight (n = 14); DC, diabetic animals treated with 100 mg MNA/kg of body weight (n = 15); DD, diabetic animals treated with 200 mg MNA/kg of body weight (n = 14). DM was induced with single dose of STZ (45 mg/kg in 0.5 mol/l citrate buffer, pH 4.5) into the tail vein. Significant difference compared to controls: *P < 0.05, **P = 0.01. Values are expressed in mean ± SD.
Oxidation of proteins
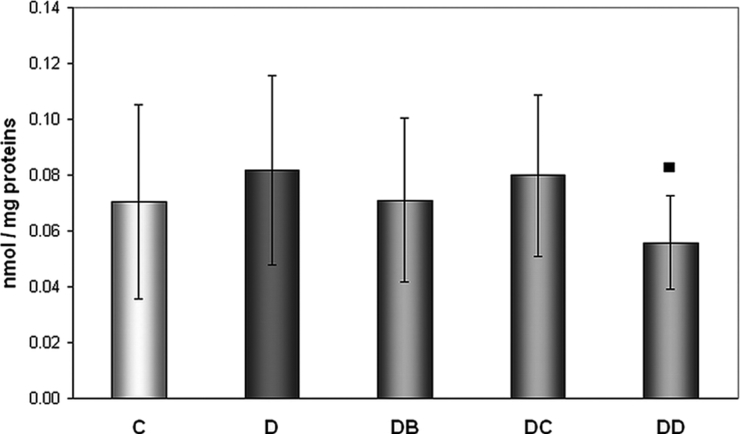
We did not find higher levels of protein carbonyls in diabetics compared to controls, but the positive effect of MNA was observed at the highest dose administered (Fig. 3). MNA given at the dose of 200 mg/kg of body weight for 7 weeks resulted in significantly decreased concentration of protein carbonyls compared to diabetic animals without treatment (0.0818 ± 0.0339 vs. 0.0558 ± 0.0166 nmol/mg proteins).
Figure 3.
Effect of MNA treatment on formation of protein carbonyls. C, untreated controls (non-diabetic) (n = 15); D, untreated diabetic animals (n = 14); DB, diabetic animals treated with 20 mg MNA/kg of body weight (n = 14); DC, diabetic animals treated with 100 mg MNA/kg of body weight (n = 15); DD, diabetic animals treated with 200 mg MNA/kg of body weight (n = 14). DM was induced with single dose of STZ (45 mg/kg in 0.5 mol/l citrate buffer, pH 4.5) into the tail vein. Significant difference compared to untreated diabetic controls: ▪P < 0.05. Values are expressed in mean ± SD.
Peroxidation of lipids
Significant effect of diabetes on peroxidation of lipids was not observed (23.83 ± 13.35 nmol/ml in controls vs. 34.99 ± 25.01 nmol/ml in diabetics; P = 0.1562) as well as no effect of MNA treatment in any investigated groups of animals was observed.
Oxidative damage to DNA
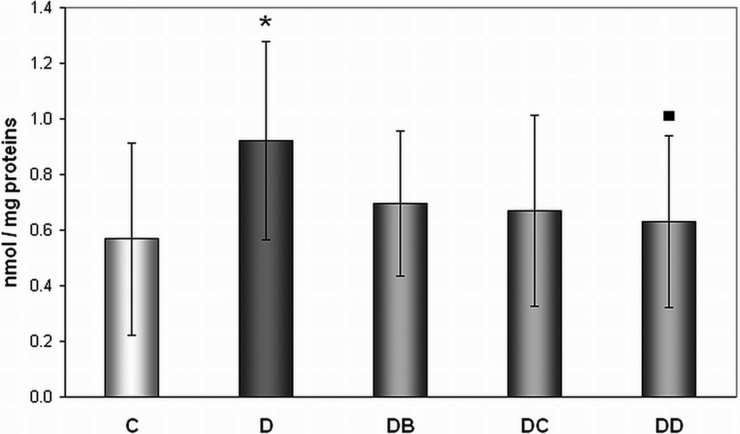
In our study significantly increased levels (62.2%) of TD to DNA in untreated diabetic rats (group D) in comparison with healthy controls were observed (0.9213 ± 0.3577 vs. 0.5680 ± 0.3471 8-oxoG/106 G) (Fig. 4). The dose of 200 mg/kg of body weight (group DD) significantly (by 31%) decreased DNA oxidation, represented by levels of 8-oxoG levels, when compared with untreated diabetic animals (group D) (0.6302 ± 0.3079 vs. 0.9213 ± 0.3577 8-oxoG/106 G).
Figure 4.
Effect of MNA treatment on DNA oxidation in lymphocytes. C, untreated controls (non-diabetic) (n = 13); D, untreated diabetic animals (n = 11); DB, diabetic animals treated with 20 mg MNA/kg of body weight (n = 14); DC, diabetic animals treated with 100 mg MNA/kg of body weight (n = 15); DD, diabetic animals treated with 200 mg MNA/kg of body weight (n = 13). DM was induced with single dose of STZ (45 mg/kg in 0.5 mol/l citrate buffer, pH 4.5) into the tail vein. Significant difference compared to controls: *P < 0.05; significant difference compared to untreated diabetic controls: ▪P < 0.05. Values are expressed in mean ± SD.
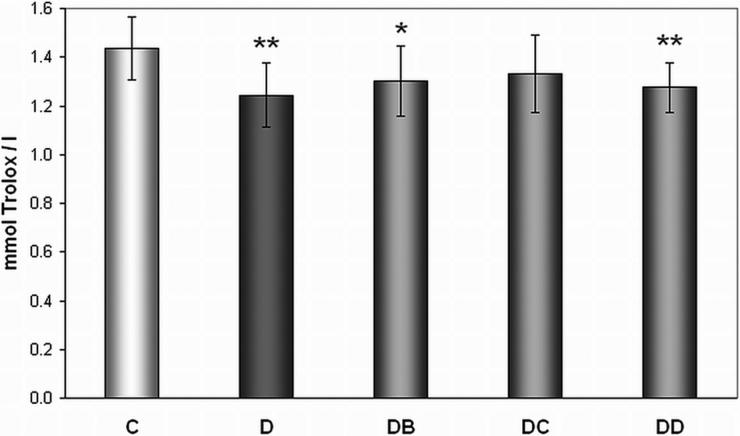
TEAC of plasma
We have observed lower TEAC in plasma of diabetic rats (group D) when compared with control animals (1.244 ± 0.132 vs. 1.434 ± 0.129 mmol Trolox/l). Effect of MNA was not observed (Fig. 5).
Figure 5.
Effect of MNA treatment on level of Trolox equivalent antioxidant capacity of plasma. C, untreated controls (non-diabetic) (n = 15); D, untreated diabetic animals (n = 14); DB, diabetic animals treated with 20 mg MNA/kg of body weight (n = 14); DC, diabetic animals treated with 100 mg MNA/kg of body weight (n = 15); DD, diabetic animals treated with 200 mg MNA/kg of body weight (n = 14). DM was induced with single dose of STZ (45 mg/kg in 0.5 mol/l citrate buffer, pH 4.5) into the tail vein. Significant difference compared to controls: *P < 0.05, **P = 0.01. Values are expressed in mean ± SD.
Correlations between the parameters
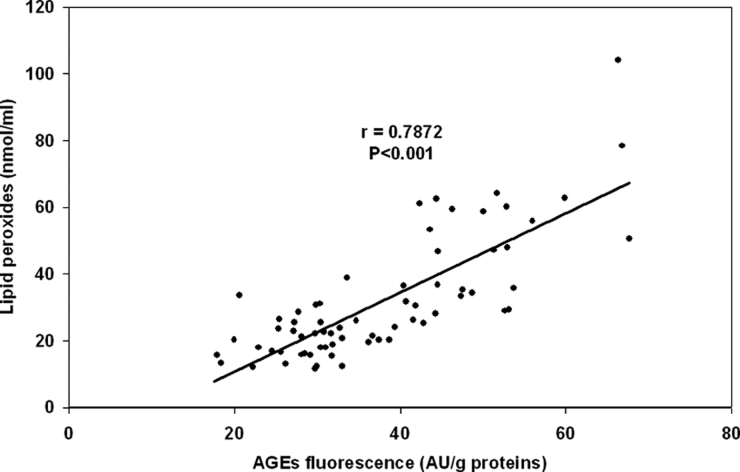
In the group of 7-week administration of low dose of MNA (20 mg/kg of body weight) we have observed the association between the extent of DNA damage and AGEs level (Table 2). We have found significant correlations between concentrations of lipid peroxides and AGEs levels in all studied groups. Since individual groups have shown similar correlation, Fig. 6 presents joined correlation for all experimental animals. Under the conditions of diabetes mellitus lipid peroxidation significantly negatively correlated with antioxidative capacity.
Table 2.
Correlations between the selected parameters (ns – not significant)
| Experimental group | AGE vs. lipid peroxides | TEAC vs. lipid peroxides | AGE vs. 8-oxoG | |||
|---|---|---|---|---|---|---|
| R | P | r | P | R | P | |
| C | 0.7224 | 0.0053 | 0.2304 | ns | −0.0080 | ns |
| D | 0.8336 | 0.0004 | −0.6222 | 0.0232 | −0.4099 | ns |
| DB | 0.5922 | 0.0256 | 0.2103 | ns | 0.6323 | 0.0203 |
| DC | 0.8610 | <0.0001 | −0.1435 | ns | 0.1822 | ns |
| DD | 0.8830 | <0.0001 | 0.1616 | ns | −0.0787 | ns |
Figure 6.
Correlation between the AGEs fluorescence and concentration of lipid peroxides in serum in joined group of all experimental animals.
Discussion
Although NA has been shown to possess a variety of anti-inflammatory properties and other biologically important effects, its main metabolite MNA has been studied as a biologically active biomarker only during the last couple of years. Extensive trial evaluating some of the therapeutic effects of NA, i.e. the prevention of the onset of type 1 diabetes, ended with a disappointment.26 Interestingly, there is an evidence in the literature that its metabolite – MNA could be more potent than its parent compound. It can be expected that MNA when introduced into blood circulation will interact with glycosaminoglycans located on a surface of vascular endothelium cells without effective penetration inside the tissue, thus increasing its local concentration on the surface. In principle, MNA can exert its therapeutic function through multiple mechanisms; however, it seems likely that MNA anti-inflammatory properties can be associated with its ability to reduce adherence of pro-inflammatory cells and molecules to a surface of vascular endothelium.13 Also in in vitro conditions MNA has been shown to be a potent anti-inflammatory agent.13,15 It is more protective than NA in the prevention of the onset of alloxan-induced diabetes in mice.14 Results from the in vitro study27 indicate that NA and MNA may also affect reactive oxygen species generation – they have shown the dose-dependent scavenging properties.
In the present study we have tried to find out whether indicated scavenging properties of MNA influence the levels of markers of oxidative stress and oxidative modification of proteins and nucleic acids. We have treated our animals with different doses of MNA – 20, 100 and 200 mg/kg of body weight for 7 weeks.
One of the studied parameters was the level of advanced glycation end products using fluorescent properties of some of their molecules. Under the conditions of hyperglycemia early glycation products are accumulated and later modified resulting in formation of AGEs.28 This is in agreement with our results. A negative correlation found between MNA dose and AGEs fluorescence level could suggest the possibility of better effect if higher dose of MNA is used. In our previous work29 we have published the decrease in plasma lipid peroxides influenced by administration of MNA at the dose of 100 mg/kg of body weight, Here we have found significant correlations between concentrations of lipid peroxides29 and AGEs levels in all studied groups. It is apparent under the conditions of diabetes mellitus that glycation and oxidation are closely related and they damage concurrently more types of biomolecules.
Protein oxidation is of basic importance in many degenerative diseases. Free radicals induce several oxidative modifications, from which the protein carbonyls are most studied and they are a good marker in evaluation of oxidative stress in vivo. Unlike some studies30,31 we did not confirm higher levels of protein carbonyls in diabetics compared to controls, but the positive effect of MNA was found when the highest dose was administered.
In the cells mainly the enzyme systems represent antioxidative capacity, while in plasma low-molecular weight antioxidants are predominating. We have observed lower TEAC in diabetic plasma in comparison with control animals thus confirming the assumed oxidative stress in diabetes. Depressed antioxidant status in diabetes mellitus type I was discussed in several studies.4,32 On the other hand, the other studies have not demonstrated any differences between diabetic patients and controls.33,34
According to our results effect of MNA on total plasma antioxidant capacity has been only mild in the group DC (100 mg MNA/kg of body weight), which could have been caused by the fact that MNA probably works at different levels of protection against oxidative stress. Under the conditions of diabetes mellitus antioxidative capacity negatively correlated with lipid peroxidation, which could mean that ‘depletion’ of antioxidants may increase susceptibility of diabetics to oxidative damage.
As the DNA damage is efficiently repaired by cell repair enzymes, its measurement provides a current picture of the level of damage to DNA in contrast to measurement of oxidation of other biomolecules such as lipids or proteins, with slower turnover. Thus, oxidation of DNA may be of important value in the study of progression of disease dependent on oxidative stress. Using the comet assay we have found a total increase of damage to DNA in diabetic animals confirming results of Dandona et al.35 and Collins et al.,36 who have studied correlations with other markers. In their work the frequency of Fpg locations, representing damage to a DNA chain, in diabetic samples was pretty related to blood glucose concentration, which we have not found. High concentrations of 8-hydroxydeoxyguanosine as a typical product of DNA oxidation, were found by high-performance liquid chromatography method in lymphocytes of insulin-dependent diabetes mellitus as well as non-insulin-dependent diabetes mellitus patients.35
We have observed significantly increased levels of TD to DNA in untreated diabetic rats in comparison with healthy controls but in the case of treatment of animals with MNA this difference has disappeared. In the group of rats with 7-weeks administration of subdose of MNA (20 mg/kg of body weight) an association between DNA damage and AGEs level was observed. 8-oxoG level results from balance between oxidative DNA damage and the activity of repair systems as well as activity of antioxidative systems and it remains unclear as to which component of the protection system preferentially works.
In the earlier study Watala et al.29 have observed a significant decrease in non-fasting glucose after treatment with MNA and a mild but significant decrease in HbA1c and for the first time demonstrated that MNA possessed profound antidiabetic effect that could be linked to its vasoprotective activity.
Our presented results also show that MNA may have potential therapeutic application in prevention and treatment of various (mainly cardiovascular) complications accompanying diabetes mellitus through affecting oxidative and glycooxidative processes, which increase susceptibility to lipid peroxidation, responsible for increased incidence of atherosclerosis in diabetes mellitus. However, potential therapeutic application of MNA needs further studies with experimental animals as well as in humans.
Acknowledgements
This work was supported by VEGA Grant No. 1/1133/11 and NATO collaborative linkage grant LST.CLG.980106. We are thankful to Health and Mind, civil association, for partial financial support. We thank to Dr Jan Adamus for providing us with MNA.
References
- 1.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–20. [DOI] [PubMed] [Google Scholar]
- 2.Van Dam PS, Van Asbek BS, Erkelens BW, Marx JJM, Gispen WH, Brevenboer B. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes Metab Rev 1995;11:181–92. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des 2008;14(10):962–8. [DOI] [PubMed] [Google Scholar]
- 4.Muchová J, Liptáková A, Országhová Z, Garaiová I, Tisoň P, Čársky J, et al.. Antioxidant systems in polymorphonuclear leucocytes of type 2 diabetes mellitus. Diabet Med 1999;16(1):74–8. [DOI] [PubMed] [Google Scholar]
- 5.Boušová I, Martin J, Jahodář L, Dušek J, Palička V, Dršata J. Evaluation of in vitro effects of natural substances of plant origin using a model of protein glycoxidation. J Pharm Biomed Anal 2005;37:957–62. [DOI] [PubMed] [Google Scholar]
- 6.Baynes JW, Thorpe SR. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med 2000;28:1708–16. [DOI] [PubMed] [Google Scholar]
- 7.Sartini D, Santarelli A, Rossi V, Goteri G, Rubini C, Ciavarella D, et al.. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol Med 2007;13:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AC, Ramsden DB. Nicotinamide homeostasis: a xenobiotic pathway that is key to development and degenerative diseases. Med Hypotheses 2005;65:353–62. [DOI] [PubMed] [Google Scholar]
- 9.Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD. Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 2003;69:150–7. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien BA, Harmon BV, Cameron DP, Allan DJ. Nicotinamide prevents the development of diabetes in the cyclophosphamide-induced NOD mouse model by reducing beta-cell apoptosis. J Pathol 2000;191:86–92. [DOI] [PubMed] [Google Scholar]
- 11.Aoyama K, Matsubara K, Okada K, Fukushima S, Shimizu K, Yamaguchi S, et al.. N-methylation ability for azaheterocyclic amines is higher in Parkinson's disease: nicotinamide loading test. J Neural Transm 2000;107:985–95. [DOI] [PubMed] [Google Scholar]
- 12.Wozniacka A, Wieczorkowska M, Gebicki J, Sysa-Jedrzejowska A. Topical application of 1-methylnicotinamide in the treatment of rosacea: a pilot study. Clin Exp Dermatol 2005;30:632–5. [DOI] [PubMed] [Google Scholar]
- 13.Gebicki J, Sysa-Jedrzejowska A, Adamus J, et al.. 1-Methylnicotinamide: a potent anti-inflammatory agent of vitamin origin. Pol J Pharmacol 2003;55:109–12. [PubMed] [Google Scholar]
- 14.Fischer LJ, Falany J, Fisher R. Characteristics of nicotinamide and N1-methylnicotinamide protection from alloxan diabetes in mice. Toxicol Appl Pharmacol 1983;70:148–55. [DOI] [PubMed] [Google Scholar]
- 15.Adamiec M, Adamus J, Ciebiada I, Denys A, Gebicki J. Search for drugs of the combined anti-inflammatory and anti-bacterial properties: 1-methyl-N’-(hydroxymethyl) nicotinamide. Pharmacol Rep 2006;58:246–9. [PubMed] [Google Scholar]
- 16.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, et al.. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts antithrombotic activity mediated by COX-2/PGI2 pathway. Br J Pharmacol 2007;152:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata S, Takeuchi M, Teradaira S, Yamamoto N, Iwata K, Okumura K, et al.. Radical scavenging activities of niacin-related compounds. Biosci Biotechnol Biochem 2002;66(3):641–5. [DOI] [PubMed] [Google Scholar]
- 18.Shaw EN. Quaternary pyridinium compounds. In: , Klingsberg E (ed.) Pyridine and its derivatives. New York: Interscience Publishers; 1961. p. 1–31. [Google Scholar]
- 19.Kalousová M, Škrha J, Zíma T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res 2002;51:597–604. [PubMed] [Google Scholar]
- 20.El-Saadani M, Esterbauer H, El-Sayed M, Goher M, Nassar AY, Jürgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res 1989;30:627–30. [PubMed] [Google Scholar]
- 21.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn ChC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med 1997;23(3):361–6. [DOI] [PubMed] [Google Scholar]
- 22.Collins AR, Duthie SJ, Dobson VL. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 1993;14:1733–5. [DOI] [PubMed] [Google Scholar]
- 23.Collins AR, Dobson VL, Dušinská M, Kennedy G, Štetina R. Comet assay: what can it really tell us? Mutat Res 1997;375:183–93. [DOI] [PubMed] [Google Scholar]
- 24., ESCODD Gedik CM, Collins A. Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J 2004;18:1–23. [DOI] [PubMed] [Google Scholar]
- 25.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26(9/10):1231–7. [DOI] [PubMed] [Google Scholar]
- 26.Gale EA, Bingley PJ, Emmett CL, Collier T, European Nicotinamide Diabetes Intervention Trial (ENDIT) Group . European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363(9413):925–31. [DOI] [PubMed] [Google Scholar]
- 27.Biedroń R, Ciszek M, Tokarczyk M, Bobek M, Kurnyta M, Słominska EM, et al.. 1-Methylnicotinamide and nicotinamide: two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch Immunol Ther Exp 2008;56:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed N, Thornalley PJ. Quantitative screening of protein biomarkers of early glycation, advanced glycation, oxidation and nitrosation in cellular and extracellular proteins by tandem mass spectrometry multiple reaction monitoring. Biochem Soc Trans 2003;31(6):1417–22. [DOI] [PubMed] [Google Scholar]
- 29.Watała C, Kaźmierczak P, Dobaczewski M, Przygodzki T, Bartus M, Lomnicka M, et al.. Anti-diabetic effects of 1-methylnicotinamide (MNA) in streptozocin-induced diabetes in rats. Pharmacol Rep 2009;61:86–98. [DOI] [PubMed] [Google Scholar]
- 30.Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol 2008;92:548–51. [DOI] [PubMed] [Google Scholar]
- 31.Cakatay U. Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes Metab 2005;31:551–7. [DOI] [PubMed] [Google Scholar]
- 32.Vessby J, Basu S, Mohsen R, Berne C, Vessby B. Oxidative stress and antioxidant status in type 1 diabetes mellitus. J Intern Med 2002;251:69–76. [DOI] [PubMed] [Google Scholar]
- 33.Willems D, Dorchy H, Dufrasne D. Serum antioxidant status and oxidized LDL in well-controlled young type 1 diabetic patients with and without subclinical complications. Atherosclerosis 1998;137:61–4. [DOI] [PubMed] [Google Scholar]
- 34.Feillet C, Roche B, Tauveron I, Bayle D, Rock E, Borel P, et al.. Susceptibility to oxidation and physicochemical properties of LDL in insulin-dependent diabetics. Atherosclerosis 1998;136:405–7. [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Thusu K, Cook S, Snyder B, Makowski J, Armstrong D, et al.. Oxidative damage to DNA in diabetes mellitus. Lancet 1996;347:444–5. [DOI] [PubMed] [Google Scholar]
- 36.Collins AR, Rašlová K, Somorovská M, Petrovská H, Ondrušová A, Vohnout B, et al.. DNA damage in diabetes: correlation with a clinical marker. Free Radic Biol Med 1998;25(3):373–7. [DOI] [PubMed] [Google Scholar]