ABSTRACT
Background: Ischemia-modified albumin (IMA) is an altered type of serum albumin that forms under conditions of oxidative stress and an independent predictor of major adverse cardiovascular events.
Objectives: To measure the levels of IMA in 45 children and adolescents with β-thalassemia major (β-TM) compared with 30 healthy controls and assess its relation to lipid peroxidation, vascular complications and subclinical atherosclerosis.
Methods: β-TM patients without symptoms of heart disease were studied focusing on transfusion history, chelation therapy, serum ferritin, malondialdehyde (MDA) and IMA levels. Echocardiography was performed and carotid intima media thickness (CIMT) was assessed.
Results: IMA and MDA levels were significantly higher in β-TM patients compared with controls (p < 0.001). IMA was higher among patients with heart disease, pulmonary hypertension risk and serum ferritin ≥2500 µg/l than those without. TM patients compliant to chelation had significantly lower IMA levels. IMA levels were positively correlated to MDA and CIMT while negatively correlated to ejection fraction and fractional shortening.
Conclusion: Our results highlight the role of oxidative stress in the pathophysiology of vascular complications in thalassemia. IMA could be useful for screening of β-TM patients at risk of cardiopulmonary complications and atherosclerosis because its alteration occurs in early subclinical disease.
KEYWORDS: Thalassemia, oxidative stress, ischemia-modified albumin, malondialdehyde, CIMT, vascular dysfunction
Introduction
Patients with β-thalassemia major (β-TM) are under significant iron-driven oxidative stress [1]. Many studies reported increased blood levels of the redox active fractions of non-transferrin bound iron and labile plasma iron in patients with β-thalassemia [2,3]. It has also been demonstrated that such patients experience decreased antioxidant capacity and increased products of peroxidative damage [4].
Enhanced oxidation status in β-TM is reflected by increased malondialdehyde (MDA), as a result of excess α-chains in erythrocytes and erythroblasts being unstable and prone to denaturation and oxidation, peroxidation of tissues that leak MDA into the blood, as well as depleted antioxidant capacity lowering defense to oxidants [5,6]. Liver lipid peroxidation also increases leakage of ferritin into the circulation. Levels of MDA have been reported to be correlated with iron concentrations in the liver as well as serum ferritin of thalassemia patients [5,7,8].
Human serum albumin (HSA) is composed of 585 amino acids (66,438 Da), containing 17 pairs of intra-molecular disulfide bridges and one free cysteine (Cys34) [9]. HSA has the unique capability of binding reversibly or covalently to a wide diversity of ligands including water, cations (such as Ca2+, Na+ and K+), fatty acids, hormones, bilirubin and drugs [10].
Ischemia-modified albumin (IMA) is an altered type of serum albumin that is formed under conditions of oxidative stress [11]. Reactive oxygen species resulting from conditions such as ischemia, hypoxia, acidosis, free radicals and free iron can decrease the ability of the N-terminus to bind with transition metals [12,13]. When myocardial ischemia (MI) occurs, hypoxia followed by acidosis and destruction of Na+–K+ pump leads to formation of IMA by cleavage of the first two amino acids (Asp-Ala) of the HSA N-terminus. IMA is known to have low binding affinities toward transition metals [14,15].
IMA is currently used as an early marker for myocardial ischemia and acute coronary syndrome [16]. It is also increased in diabetes mellitus, hyperlipidemia, chronic renal disease and obesity [17–20]. The constriction of vessels due to atherosclerotic lesions causes hypoxia/ischemia and oxidative changes resulting in transformation of free albumin to IMA in the circulation [21,22]. Recently, increased levels of IMA have been reported in patients with β-TM [11]. However, the potential relation between IMA and vascular dysfunction among this population has not been explored.
In this work, we aimed to measure the levels of IMA in children and adolescents with β-TM as a marker of oxidative stress, and to assess its relation to lipid peroxidation as well as the effect on cardiovascular complications, subclinical atherosclerosis and compliance to iron chelation therapy.
Materials and methods
This cross-sectional study included 45 patients with transfusion-dependent β-TM (≤18 years); 25 males and 20 females who were randomly recruited from the regular attendants of the Pediatric Hematology Clinic, Pediatric Hospital, Ain Shams University. They were compared to 30 age- and sex-matched healthy individuals enrolled as controls. The control group did not receive any medication and were proven to be healthy after full clinical examination and laboratory investigations. The age of the patients ranged from 11 to 20 years with a mean age of 15.4 ± 3.9 years, while the control group had a mean age of 14.7 ± 2.1 years. An informed consent was obtained from the guardian of each patient or control before participation. The procedures applied in this study were approved by the Ethical Committee of Human Experimentation of Ain Shams University and are in accordance with the Helsinki Declaration of 1975.
All patients were diagnosed with β-TM based on complete blood picture, reticulocyte count and markers of hemolysis as well as qualitative and quantitative of analysis of hemoglobin using high performance liquid chromatography (HPLC) [23]. Exclusion criteria were any evidence of infection, α-thalassemia, sickle β-thalassemia, symptomatic heart disease, renal disease, diabetes mellitus, hypothyroidism, rheumatoid arthritis or other autoimmune diseases.
All included patients were subjected to detailed medical history and thorough clinical examination with special emphasis on disease duration, anthropometric measures, pubertal stage determined according to Tanner classification [24], blood pressure, evidence of cardiac disease, history of splenectomy, viral hepatitis, transfusion history and chelation therapy. The transfusion received was calculated as the transfusion index: volume of transfused packed red cells in ml per kg body weight per year (expressed as the mean value in the last 2 years).
Patients with β-TM received either mono or combined chelation therapy. Monochelation was in the form of deferoxamine (DFO) infused subcutaneously in a dose that ranged from 30 to 45 mg/kg/d given 5 days/week, oral deferiprone in a daily dose that ranged from 50 to 100 mg/kg/d, or oral deferasirox in a dose 40 mg/kg/d once daily. For combined chelation therapy, deferoxamine was given for 5 days/week with either daily deferiprone or deferasirox. Compliance to chelation therapy was assessed by reviewing patient self-report of dose-taking and the appropriate number of doses taken during each day was checked by prescription refills and pill count; a cutoff point below 80% was considered as poor compliance to the regimen [25].
Sample collection
Peripheral venous blood samples were collected on potassium-ethylene diamine tetra-acetic acid (K2-EDTA) (1.2 mg/ml) for complete blood count (CBC) and hemoglobin analysis. For chemical analysis and enzyme linked immunosorbent assay (ELISA), clotted samples were obtained and serum was separated by centrifugation for 15 min then stored at −80°C till subsequent use. Samples with gross hemolysis or lipemia were discarded ‘to minimize the possibility of sample adventitious oxidation post collection’.
Laboratory analysis
Laboratory investigations included CBC using Sysmex XT-1800i (Sysmex, Kobe, Japan), examination of Leishman-stained smears for differential white blood cell (WBC) count, hemoglobin analysis by HPLC using D-10 (BioRad, Marnes La Coquette, France), liver function tests (serum albumin, total and direct bilirubin, alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), markers of hemolysis (lactate dehydrogenase [LDH] and indirect bilirubin) as well as serum ferritin on Cobas Integra 800 (Roche Diagnostics, Mannheim, Germany).
Serum ferritin level was measured at the start of the study with calculation of the mean value of the last year prior to the study in order to know the ferritin trend. According to the literature, the cutoff value 2500 µg/l was used to classify patients into two groups as it was defined to be the best for prediction of thalassemia complication [26] and has been used for prognosis with higher incidence of cardiac complication and shortened survival in patients with levels >2500 µg/l compared with lower levels [27,28].
Serum MDA levels were measured by the production of thiobarbituric acid reactive substances (TBARS) according to the method of Buege and Aust [29] using colorimetric kit (Bio-Diagnostics, Egypt, Cat. No., MD 2529). Determination of serum IMA levels was done by ELISA using kit supplied by GSCIENCE (Glory science Co., Ltd, USA) Catalog # 11169. In brief, the kit adopts purified Human IMA to coat microtiter plate and make solid-phase antibody. Standards or samples were then added to the microtiter plate wells and IMA if present, was bound to the antibody pre-coated wells. A standardized preparation of horseradish peroxidase-conjugated polyclonal antibody, specific for IMA was added to each well and incubated to form antibody–antigen–enzyme–antibody complex. The TMB substrate was added to each well. Only those wells that contain IMA and enzyme-conjugated antibody exhibited a change in color. The enzyme–substrate reaction was terminated by the addition of a stop solution and the color change was measured spectrophotometrically at a wavelength of 450 nm within 15 min. A standard curve was plotted relating the intensity of the color (O.D.) to the concentration of standards. The IMA concentration in each sample is interpolated from this standard curve.
Echocardiography
All studied patients were clinically asymptomatic for pulmonary hypertension (PH) and cardiovascular abnormalities. Screening for PH was performed by the non-invasive Doppler echocardiography with different modalities using Vivid E9 (GE Healthcare, Oslo, Norway) to evaluate right and left ventricle function, pulmonary artery pressure and tricuspid regurgitant jet velocity (TRV). A TRV ≥2.5 m/s was used as a proxy for patients at risk for PH [30,31]. Heart disease was defined by at systolic left ventricle (LV) dysfunction (LV shortening fraction <30% or LV ejection fraction < 55%) [32].
Measurement of carotid intima media thickness
All of the carotid scans were done by B mode using carotid Doppler ultrasound scanner (Toshiba Ultrasonography machine [Xario], Japan) with a 7–10.0-MHz linear array transducer following a predetermined standardized scanning protocol [33]. All ultrasound scans were performed by an experienced radiologist in vascular Doppler who was unaware of children’s clinical details. Patients were placed in supine position having the neck in hyperextension. Common carotid artery (CCA) was scanned bilaterally (in the longitudinal and transverse views). The left and right CCAs were imaged in a standardized magnification (2 × 2 cm). The location of measurement was standardized at 1 cm before carotid bulb over a length of 1 cm at the far wall of both CCAs. Images were captured when both the anterior and posterior wall margins were clearly seen, to ensure the images were taken perpendicular to the vessel. A minimum of four images of each CCA were taken. All images were taken at end-diastole, incident with the R-wave on a continuously recorded electrocardiogram and then digitally stored for later analysis. The three best quality images for each of the carotid arteries were selected and analyzed. Best quality was defined with those images that produced the most number of points for analysis. For each image, the greatest distance between the lumen–intima interface and media–adventitia interface (intima media thickness [IMT]) was measured at a minimum of 100 points. Three measurements were taken from each side and averaged; then, the mean carotid intima media thickness (CIMT) was calculated as the average of measurements obtained from both CCAs.
Statistical analysis
Analysis of data was done using Statistical Program for Social Science version 21 (SPSS Inc., Chicago, IL, USA). Quantitative variables were described in the form of mean and standard deviation or median and interquartile range (IQR; 75th and 25th percentiles). Qualitative variables were described as number and percent. Kolmogrov Smirnov test was used for testing the distribution of normality. In order to compare parametric quantitative variables between two groups, Student’s t test was applied. For comparison of non-parametric quantitative variables between two groups, Mann–Whitney test was used. Qualitative variables were compared using Chi-square (χ2) test or Fischer’s exact test when frequencies were below five. Pearson correlation coefficients were used to assess the association between two normally distributed variables. When a variable was not normally distributed, a Spearman correlation test was performed. Clustering analysis was performed on the complete dataset. Akaike’s Information Criterion was used and was not supportive of a linear model. Quadratic non-linear regression analysis was employed to determine the relation between IMA and clinical, laboratory and radiological variables. Receiver operating characteristic (ROC) curve was used to determine the best cut-off value of IMA that best combined sensitivity and specificity. The area under the curve (AUC) was calculated for each plot. A p value <0.05 was considered significant in all analyses.
Results
Clinical characteristics of patients with β-TM
The mean disease duration was 12.9 ± 3.2 years. Transfusion index was 391.6 ± 118.1 ml/kg/year (range 120–480 ml/kg/year). The laboratory and radiological data of β-TM patients were listed in Table 1. When patients with β-TM were classified according to ferritin cutoff 2500 µg/l as reported in the literature, comparison between patients with mean serum ferritin ≥2500 µg/l and those with serum ferritin <2500 µg/l (Table 2) revealed a significant difference in transfusion index being elevated in patients with mean serum ferritin ≥ 2500 µg/l. Moreover, β-TM patients with mean serum ferritin ≥ 2500 µg/l had significant lower ejection fraction as well as higher TRV and CIMT than those with lower ferritin levels.
Table 1. Laboratory and radiological data of the studied patients with β-TM and healthy controls.
| Variable | β-TM (n = 45) | Healthy controls (n = 30) | p value |
|---|---|---|---|
| WBC count (×109/l), median (IQR) | 10.7 (8.1–17.5) | 7.1 (5.3–8.6) | <0.001 |
| Hemoglobin (g/dl), mean ± SD | 7.62 ± 1.14 | 12.4 ± 3.4 | <0.001 |
| HbF (%), median (IQR) | 62.0 (27.3–80.0) | 0.6 (0.4–0.9) | <0.001 |
| Serum albumin (mg/dl), mean ± SD | 3.97 ± 0.45 | 4.5 ± 0.51 | <0.001 |
| Total bilirubin (mg/dl), mean ± SD | 1.34 ± 0.62 | 0.6 ± 0.15 | <0.001 |
| Indirect bilirubin (mg/dl), median (IQR) | 0.8 (0.5–1.0) | 0.4 (0.3–0.5) | 0.002 |
| ALT(IU/l), median (IQR) | 33 (20–65) | 21 (13–30) | <0.001 |
| AST(IU/l), median (IQR) | 38 (30–65) | 18 (10–35) | <0.001 |
| Lactate dehydrogenase (IU/l), median (IQR) | 446 (195–604) | 330 (280–410) | 0.01 |
| Serum ferritin (µg/l), median (IQR) | 2291 (1263–4045) | 45 (27–60) | <0.001 |
| IMA (U/ml), median (IQR) | 18 (13–40) | 4 (3–5) | <0.001 |
| MDA (nmol/ml), median (IQR) | 30 (20–45) | 10 (7–15) | <0.001 |
| EF (%), mean ± SD | 64.5 ± 4.5 | – | – |
| FS (%), mean ± SD | 35.2 ± 3.3 | – | – |
| LVESD (mm), mean ± SD | 3.0 ± 0.47 | – | – |
| LVEDD (mm), mean ± SD | 4.58 ± 0.64 | – | – |
| TRV (m/s), mean ± SD | 2.48 ± 0.42 | – | – |
| Mean CIMT (mm), mean ± SD | 0.47 ± 0.1 | 0.33 ± 0.02 | <0.001 |
β-TM: β-thalassemia major; WBC: white blood cell; Hb: hemoglobin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; IMA: ischemia-modified albumin; MDA: malondialdehyde; EF: ejection fraction; FS: fractional shortening; LVESD: left ventricle end systolic diameter; LVEDD: left ventricle end diastolic diameter; TRV: tricuspid regurgitant jet velocity; CIMT: carotid intima media thickness; IQR: interquartile range.
Table 2. Clinical and radiological data of β-TM patients with mean serum ferritin above or lower than 2500 µg/l.
| Variable | Ferritin <2500 (µg/l) (n = 24) |
Ferritin ≥2500 (µg/l) (n = 21) |
p value |
|---|---|---|---|
| Age (years) | 14.8 ± 1.9 | 13.7 ± 3.6 | 0.055 |
| Sex, n (%) | |||
| Males | 14 (58.3) | 11 (52.4) | 0.688 |
| Females | 10 (41.7) | 10 (47.6) | |
| BMI SDS | 0.32 (0.13–0.56) | 0.28 (0.17–0.39) | 0.581 |
| Transfusion index (ml/kg/year) | 320 (240–480) | 470 (380–530) | 0.012 |
| Disease duration (years) | 13.6 ± 2.7 | 12.2 ± 3.7 | 0.168 |
| EF (%) | 66.7 ± 4.5 | 60.0 ± 4.3 | 0.009 |
| FS (%) | 36.0 ± 3.9 | 34.6 ± 2.6 | 0.208 |
| LVESD | 3.06 ± 0.50 | 2.91 ± 0.42 | 0.373 |
| LVEDD | 4.64 ± 0.59 | 4.51 ± 0.71 | 0.578 |
| TRV (m/s) | 2.33 ± 0.42 | 2.58 ± 0.4 | 0.047 |
| Mean CIMT (mm) | 0.45 ± 0.09 | 0.49 ± 0.11 | 0.023 |
BMI: body mass index; SDS: standard deviation score; EF: ejection fraction; FS: fractional shortening; LVESD: left ventricle end systolic diameter; LVEDD: left ventricle end diastolic diameter; TRV: tricuspid regurgitant jet velocity; CIMT: carotid intima media thickness. Data were expressed as mean ± SD using Student’s t test for comparisons or as median (IQR) using Mann–Whitney test for comparisons unless specified as number (percentage) where Chi-square test (χ2) was used.
Levels of IMA and MDA in patients with β-TM and healthy controls
Median serum IMA levels were significantly higher in β-TM patients (median [IQR], 18 [13–40] U/ml) with a range of 10–130 U/ml compared with the control group (median [IQR], 4 [3–5] U/ml; range 1–8 U/ml); p < 0.001. MDA levels were also elevated in β-TM patients (median [IQR], 30 [20–45] nmol/ml) with a range of 10–112 nmol/ml compared with the control group (median [IQR], 10 [7–15] nmol/ml; range 5–18 nmol/ml); p < 0.001 (Table 1 and Figure 1).
Figure 1.
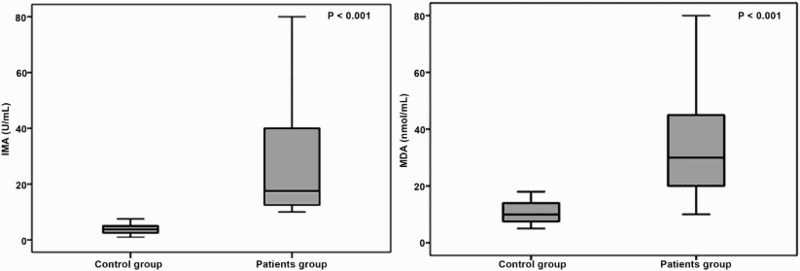
Ischemia modified albumin (IMA) and malondialdehyde (MDA) levels in patients with β-thalassemia major and controls.
Analysis of IMA in relation to the clinical characteristics of patients with β-TM (Table 3) revealed significantly higher levels among patients with heart disease than those without. Patients with PH risk had significantly higher IMA levels than PH risk-negative patients. Serum IMA and MDA levels were elevated among patients with serum ferritin ≥2500 µg/l compared with patients below this cutoff while patients compliant to chelation had significantly lower IMA and MDA levels than non-compliant ones.
Table 3. IMA and MDA levels in relation to clinical characteristics of β-TM patients.
| Variable | Patients number | IMA (U/ml) | MDA (nmol/ml) | ||
|---|---|---|---|---|---|
| Median (IQR) | p value | Median (IQR) | p value | ||
| Sex | |||||
| Male | 25 (55.6) | 15 (12.5–25) | 0.081 | 30 (20–50) | 0.918 |
| Female | 20 (44.4) | 17.5 (15–60) | 30 (20–45) | ||
| Family history | |||||
| Positive | 27 (60) | 17.5 (15–80) | 0.232 | 30 (20–55) | 0.675 |
| Negative | 18 (40) | 16.25 (12.5–30) | 30 (15–45) | ||
| Puberty | |||||
| Delayed | 30 (66.7) | 18.75 (15–80) | 0.064 | 32.5 (20–60) | 0.344 |
| Appropriate | 15 (33.3) | 15 (12.5–17.5) | 25 (20–35) | ||
| Splenectomy | |||||
| Positive | 24 (53.3) | 17.5 (15–77.5) | 0.097 | 30 (20–45) | 0.982 |
| Negative | 21 (46.7) | 15 (1.5–20) | 30 (20–45) | ||
| Viral hepatitis | |||||
| Positive | 23 (51.1) | 17.5 (13.75–80) | 0.231 | 32.5 (25–55) | 0.081 |
| Negative | 22 (48.9) | 17.5 (12.5–20) | 23 (18–40) | ||
| Heart disease | |||||
| Positive | 9 (20) | 17.5 (15.0–77.5) | 0.017 | 30 (20–50) | 0.385 |
| Negative | 36 (80) | 12.5 (12.5–17.5) | 23 (20–45) | ||
| PH risk | |||||
| Positive | 10 (22.2) | 80 (17.5–100) | <0.001 | 30 (20–65) | 0.913 |
| Negative | 35 (77.8) | 15 (12.5–20) | 27.5 (20–45) | ||
| Serum ferritin (µg/l) | |||||
| ≥2500 | 24 (53.3) | 60 (2–90) | 0.001 | 40 (30–65) | 0.001 |
| <2500 | 21 (64.7) | 15 (12.5–17.5) | 20 (16.5–35) | ||
| Compliance to chelation | |||||
| Good | 18 (40) | 15 (12.5–17.5) | 0.009 | 20 (20–35) | 0.02 |
| Poor | 27 (60) | 20 (12.5–80) | 40 (25–65) | ||
β-TM: β-thalassemia major; PH risk: pulmonary hypertension risk. Data were expressed as median (IQR) using Mann–Whitney test for comparisons.
ROC curve analysis revealed that a cutoff value of IMA at 75 U/ml could differentiate β-TM patients with PH risk with 90% sensitivity, 91.4% specificity and positive predictive value of 75% and negative predictive value 97%; AUC 0.883 (95% confidence interval 0.752–0.959). In addition, the cutoff value of IMA at 17.5 U/ml could differentiate β-TM patients with heart disease with 80.5% sensitivity, 88.9% specificity and positive predictive value of 96.7% and negative predictive value 73.3%; AUC 0.887 (95% confidence interval 0.758–0.962) (Figure 2).
Figure 2.
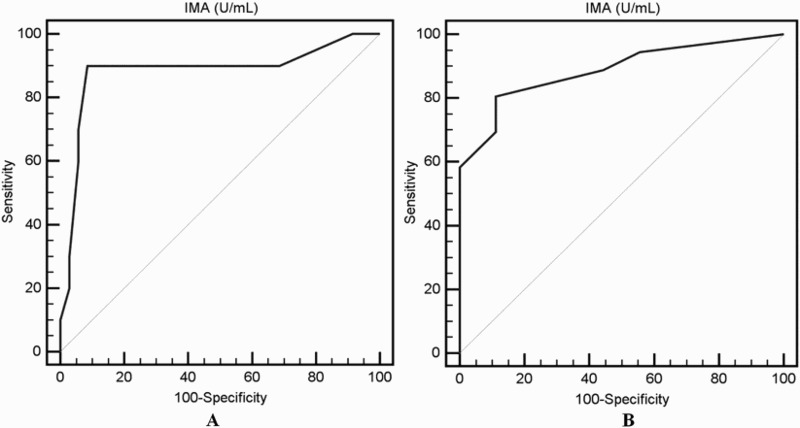
Receiver operating characteristic (ROC) curve analysis of the cut off value of IMA for detection of PH risk (A) and for heart disease (B) among patients with β-thalassemia major.
Correlation between IMA and MDA levels and clinical, laboratory and radiological parameters among β-TM patients
Significant positive correlations were found between IMA levels and disease duration (r = 0.311, p = 0.045), WBC count (r = 0.322, p = 0.031), serum ALT (r = 0.388, p = 0.01) and AST (r = 0.382, p = 0.037). MDA was positively correlated to disease duration (r = 0.297, p = 0.048). Both IMA and MDA levels were positively correlated (r = 0.503, p = 0.001) and there was a significant positive correlation between these two markers and mean serum ferritin (IMA; r = 0.645, p < 0.001 and MDA; r = 0.567, p < 0.001) among TM patients (Figure 3).
Figure 3.
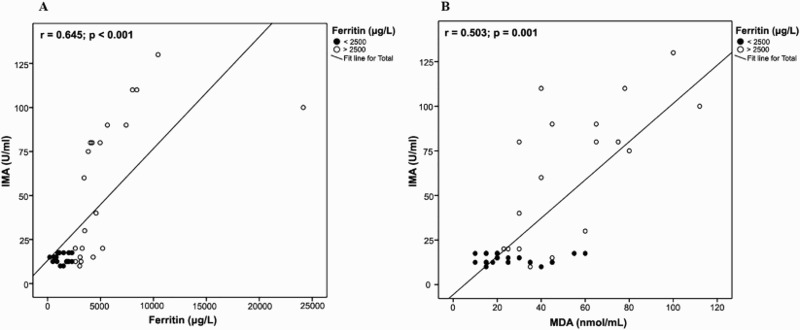
Correlation between IMA and serum ferritin (A) and MDA levels (B) among patients with β-thalassemia major.
As regards radiological data, there was a significant positive correlation between IMA levels and TRV. In addition, significant negative correlations were found between both IMA and MDA levels and each of ejection fraction and fractional shortening while both markers were positively correlated to CIMT (Figures 4 and 5). Quadratic non-linear regression analysis revealed that WBC count, serum ferritin, serum MDA, ejection fraction and CIMT were independently related to IMA levels among thalassemia patients (Table 4).
Figure 4.
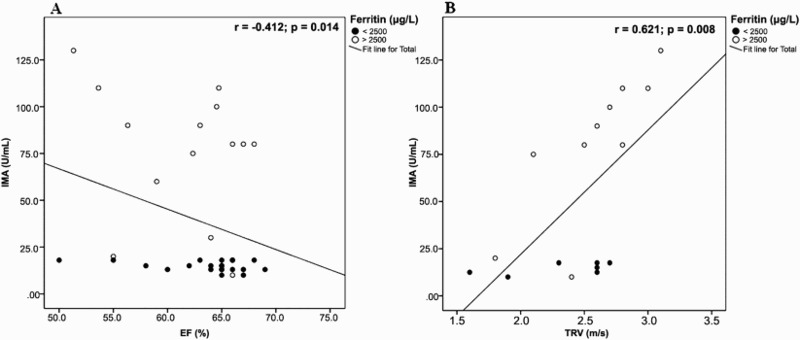
Correlation between IMA echocardiographic parameters among patients with β-thalassemia major. (A) IMA and ejection fraction. (B) IMA and tricuspid regurgitant jet velocity.
Figure 5.
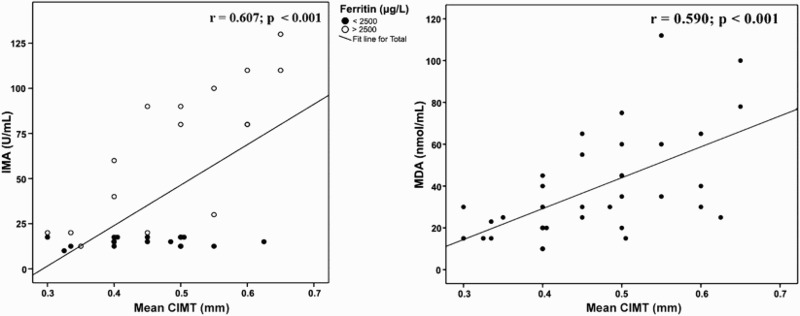
Correlation between IMA and MDA and carotid intima media thickness among patients with β-thalassemia major.
Table 4. Quadratic non-linear regression analysis for variables related to increased IMA levels in patients with β-thalassemia major.
| Variable | Unstandardized | Standardized | p value | |
|---|---|---|---|---|
| Coefficients | Coefficients | |||
| B | Standard Error | Beta | ||
| Disease duration (year) | 1.135 | 8.242 | 0.107 | 0.891 |
| WBC (×109/l) | 1.803 | 0.603 | 1.396 | 0.005 |
| ALT (IU/l) | 0.236 | 0.207 | 0.483 | 0.261 |
| AST (IU/l) | 0.433 | 0.287 | 1.007 | 0.144 |
| Serum ferritin (µg/l) | 0.016 | 0.002 | 1.844 | <0.001 |
| MDA (nmol/ml) | 0.891 | 0.396 | 0.665 | 0.03 |
| EF (%) | −70.374 | 23.004 | −8.485 | 0.004 |
| TRV (m/s) | 317.218 | 215.864 | 2.988 | 0.164 |
| Mean CIMT (mm) | 647.511 | 141.315 | 1.670 | 0.023 |
WBC: white blood cell; ALT: alanine aminotransferase; AST: aspartate aminotransferase; MDA: malondialdehyde; EF: ejection fraction; TRV: tricuspid regurgitant jet velocity; CIMT: carotid intima media thickness.
Discussion
Bar-Or et al. [34] first established an albumin cobalt binding (ACB) test to measure IMA levels in the patient’s blood through measuring the binding of cobalt to NH2 terminus of human albumin. ACB assay has been used in previous studies [35–37]. Lee et al. [38] developed a cobalt albumin binding assay to correct the flaws of the ACB test with improving the sensitivity and precision. Recently, an ELISA has been used in detecting IMA levels in various studies and was faster, less expensive and comparably reliable with the ACB assay [39–41].
In this study, we used ELISA as a sensitive and reliable method and found significantly higher serum IMA levels in β-TM patients compared with healthy controls (Table 1 and Figure 1) and a positive correlation between IMA and serum ferritin (Figure 3). Additionally, serum IMA was elevated among patients with serum ferritin ≥2500 µg/l compared with patients below this cutoff (Table 2).
As hypoxia and oxidative stress are considered in the pathophysiology of thalassemia, serum IMA levels were found to be elevated among those patients. Our results are in agreement with other reports [11,41,42]. Awadallah et al. [11] studied IMA levels in TM patients using ACB assay and found higher serum IMA levels in β-TM patients compared with controls and a significant correlation between IMA and serum ferritin. Similar resulted were obtained in TM patients by Mousa et al. [41] using ELISA. In contrast, De et al. [43] showed that ACB was not significantly higher in thalassemia cases when compared to controls and concluded that ACB is not superior to other markers of oxidative stress in identifying oxidative stress in thalassemia patients receiving repeated transfusions
MDA is considered as a marker of tissue injury and lipid peroxidation from oxidative stress. Increased MDA levels have been demonstrated in patients with β-TM and correlated with serum ferritin [5,44]. The presence of high MDA levels in our studied β-TM patients and the positive correlation between MDA and IMA (Figure 3) as well as serum ferritin further supported these data. This coincides with Awadallah and coworkers [11] who found significantly higher serum MDA levels in β-TM patients than in controls which significantly correlated with serum ferritin. Similar results were recently obtained by Chiou et al. [8]. Moreover, Dash et al. [45] reported that plasma IMA positively correlated with serum MDA, a marker of oxidative stress and free radical generation. In another study by Awadallah et al. [42], IMA levels were higher in β-TM patients compared with controls and there was a significant inverse correlation between total antioxidant capacity (TAC) and IMA. The authors concluded that this further argues for the inclusion of IMA as one of the oxidative stress markers in thalassemic patients.
Bilirubin (a potent antioxidant) is elevated in patients with β-thalassemia due to hemolysis. In addition, iron overload (due to excessive blood transfusion and also from excess intestinal absorption) could potentially induce hepatic toxicity [46,47], and consequently increases bilirubin level, that arises from decrease in the activity of cytochrome c oxidase disrupting mitochondrial respiration [46]. However, in our studied patients with thalassemia major, bilirubin was mildly elevated most likely because of treatment with iron chelating agents, particularly DFO. Moreover, there was no correlation between bilirubin levels and each of IMA or MDA.
It has been observed that patients with β-TM had lower bilirubin levels than those with HbE β-thalassemia [47]. Moreover, oxidative stress and elevated MDA levels in TM has been documented in various reports even with elevated bilirubin [48,49]. In addition, a significant decrease of TAC has been reported in thalassemia patients compared to controls [42,50]. TAC was also lower in thalassemia patients with DFO chelation therapy compared to those without DFO [50] while Cakmak et al. [51] reported no significant differences in TAC between thalassemia and control groups, in spite of increased level of oxidant status and oxidative stress index. These findings imply that oxidant–antioxidant balance was disturbed in β-TM and there is enhanced oxidative stress with decreased antioxidant defense irrespective of bilirubin levels.
Heart failure related to iron-induced toxicity remains the main leading cause of morbidity and mortality in β-TM patients, although life expectancy in this population has improved in the last years [52]. Mokhtar and associates [53] reported the presence of cardiovascular morbidities in 41.3% of β-thalassemia patients with cardiomyopathy being the most frequent (6.9%), followed by arrhythmia (1.6%) and PH (0.4%). Another study done by Bejaoui and Guirat [54] showed that cardiac disorders occurred in 19.8% of the studied β-TM patients with higher incidence of PH (12.5%) and heart failure (39%) being the major leading cause of death.
In our study, 9 (20%) thalassemia patients had heart disease. β-TM patients with mean serum ferritin ≥2500 µg/l had significantly lower EF and higher TRV than those with serum ferritin <2500 µg/l (Table 2). In agreement with our findings, Silvilairat et al. [27] reported a preserved systolic and diastolic LV function in patients who have serum ferritin <2500 ng/ml. Hamdy and colleagues [55] showed that the presence of right ventricle systolic dysfunction in patients with β-TM is related to high levels of serum ferritin.
We found significantly higher IMA levels among β-TM patients with heart disease and IMA cutoff value of 17.5 U/ml could detect cardiac disease with high sensitivity and specificity (Figure 2). Falkensammer et al. [56] reported that IMA was a better independent predictor of major adverse cardiovascular events than N-terminal pro-B-type natriuretic peptide or high-sensitivity cardiac troponin T in patients with peripheral arterial occlusive disease. Moreover, Açıkgöz et al. [57] revealed that increased serum IMA may be related to myocardial stress after subarachnoid hemorrhage. On the other hand, Sbarouni et al. [58] investigated whether IMA is also elevated in patients with compensated heart failure due to dilated cardiomyopathy (DCM) and concluded that IMA does not differ in patients with clinically stable DCM compared with normal subjects, but varies significantly in relation to the severity of the disease.
Hemoglobinopathies, including thalassemias, have been recognized as one of the most common causes of PH worldwide. The pathophysiology is multifactorial, characterized by chronic hemolysis, loss of splenic function (or splenectomy), hypercoagulability, vascular inflammation, liver dysfunction, hypoxemia, iron overload, left ventricular dysfunction and increased cardiac output [59–61].
Meloni et al. [62] reported echocardiography results from 60 TM patients and evaluated the association between TRV, iron stores, and serologic markers of hemolysis. They concluded that well-transfused TM patients have a lower risk for PH. However, they had vascular stressors (chronic hemolysis, loss of splenic function (or splenectomy), hypercoagulability, vascular inflammation, liver dysfunction, hypoxemia, iron overload, left ventricular dysfunction, and increased cardiac output) that raise their lifetime PH risk to levels higher than for the general population. All these factors could contribute to increased IMA levels found among the 22.2% of our patients with PH risk (Table 3).
Furthermore, IMA levels correlated positively with TRV and negatively with both ejection fraction and fractional shortening (Figure 4). As our patients were asymptomatic for heart disease and PH, these findings could be of value for early diagnosis of cardiopulmonary complications among thalassemia patients and allow modification of treatment strategies. In concordance with our results, Can and colleagues [63] found a positive correlation between PH risk and IMA levels in chronic obstructive pulmonary disease patients.
Iron-loading has been linked with endothelial function, circulating cholesterol oxidation products, and possibly coronary artery disease, whereas a procoagulant milieu in thalassemia may participate in accelerated atherogenesis [64–66]. In addition, activated leucocytes circulate in the peripheral blood in β-TM patients; and enhanced apoptosis, which is considered to participate in carotid plaque pathology [67,68].
The most frequently used methods for assessment of vascular dysfunction in the pediatric population are the quantification of flow-mediated, endothelium-dependent dilation of the brachial artery and measurement of the intima media thickening of the carotid artery. Increased intima media thickness was correlated with late cardiovascular events and/or mortality in adults [69]. In addition to its association with known cardiovascular risk factors and both prevalent and incident coronary heart disease, the rate of CIMT progression is directly related to the risk for future cardiovascular events [70].
The mean CIMT in our β-TM patients with serum ferritin ≥2500 µg/dl was significantly increased compared with those below this level (Table 2) and there were significant correlations between CIMT and each of mean serum ferritin, IMA (Figure 5) and MDA. Also, multivariable linear regression analysis (Table 4) revealed that the mean serum ferritin, serum MDA, TRV, ejection fraction and CIMT were the independent predictors of increased IMA levels among thalassemia patients. Kucuk et al. [71] investigated the reliability of IMA in evaluating atherosclerosis in patients with familial Mediterranean fever and found that IMA levels and CIMT were significantly higher in patients than controls with a positive correlation with IMA and CIMT in those patients.
Iron chelation therapy may protect against pulmonary damage by sequestering catalytic iron and improving oxidative status. It may be beneficial in the prevention of pulmonary complications in thalassemia [72]. In this study, patients compliant to chelation had lower IMA and MDA levels than non-compliant ones, proving the fact that intensification of chelation in patients with β-TM results in improvements in LV ejection fraction and endothelial function [73].
Our study may have clinical implications besides using IMA as a marker for cardiovascular complications. It has been reported that supplements of the antioxidant compounds (vitamins C and E, selenium, and zinc) can prevent some of the damage in the thalassemic red blood cell membrane and ameliorate pathophysiological complications [42,74]. Recently, Yanpanitch et al. [75] investigated the effects of antioxidant cocktails for the treatment of β-thalassemia/hemoglobin E (HbE), which is the most common form of β-thalassemia in Southeast Asia. Antioxidant cocktails improved anemia, iron overload, oxidative stress, and hypercoagulable state in β-thalassemia/HbE. In addition, the efficacy and safety of oral vitamin C supplementation as an adjuvant therapy to the three available iron chelators in moderately iron-overloaded pediatric patients with β-TM has been explored in a prospective study by Elalfy et al. [76] and showed promising results. Thus, intervention studies using IMA/MDA as a primary outcome measure would provide additional information and prove our findings.
Study limitations
One limitation of this study includes the presence of a relatively small number of patients which may have implications upon the significance of some data. It is also worth to mention that we only correlated the levels of IMA to MDA, serum ferritin, echocardiographic parameters and CIMT. However, investigating the relation between IMA and liver iron concentration, cardiac MRI T2*, as well as different chelation therapies may provide additional information.
In conclusion, IMA levels were elevated in β-TM patients due to iron overload and oxidative stress mechanisms. High IMA levels in patients with asymptomatic cardiac affection and the significant correlation with echocardiographic parameters might indicate that it could be used for screening of patients at risk since this alteration occurs in early stage cardiac disease. The positive correlation between CIMT and IMA suggests that it could be used as a marker of subclinical atherosclerosis in β-TM patients. Further larger longitudinal studies are warranted to verify the cutoff values of IMA levels for detection of cardiopulmonary complications in β-TM and to evaluate the relation between IMA and cardiac and liver MRI before it could be incorporated into patients’ risk stratification to guide the current therapeutic plan in patients with in β-TM. Assessment of vascular dysfunction in thalassemia using other methods and studying its relation with altered cardiac function represents an interesting area of future research.
Notes on contributors
Amira Abdel Moneam Adly, MD Pediatrics, is a professor of Pediatrics, Pediatrics Department, Ain Shams University.
Nayera Hazaa Khalil ElSherif, MD Pediatrics, is an assistant professor of Pediatrics, Pediatrics Department, Ain Shams University.
Eman Abdel Rahman Ismail, MD, is a clinical pathology consultant of Clinical Pathology, Clinical Pathology Department, Ain Shams University.
Yosra Abdelzaher Ibrahim, MD Radiology, is an assistant professor of Radiology, Radiology Department, Ain Shams University.
Gamal Niazi, MD Radiology, is an assistant professor of Radiology, Radiology Department, Ain Shams University.
Sara Hamed Elmetwally, M.B., B.Ch., is a researcher in Pediatrics Department, Ain Shams University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Hahalis G, Kremastinos DT, Terzis G, et al. Global vasomotor dysfunction and accelerated vascular aging in β-thalassemia major. Atherosclerosis. 2008;198:448–457. [DOI] [PubMed] [Google Scholar]
- [2].Chakraborty D, Bhattacharyya M. Antioxidant defense status of red blood cells of patients with β-thalassemia and Eβ-thalassemia. Clin Chim Acta. 2001;305:123–129. [DOI] [PubMed] [Google Scholar]
- [3].Cabantchik ZI, Breuer W, Zanninelli G, et al. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. [DOI] [PubMed] [Google Scholar]
- [4].Kassab-Chekir A, Laradi S, Ferchichi S, et al. Oxidant, antioxidant status and metabolic data in patients with beta-thalassemia. Clin Chim Acta. 2003;338:79–86. [DOI] [PubMed] [Google Scholar]
- [5].Walter PB, Fung EB, Killilea DW, et al. Oxidative stress and inflammation in iron overloaded patients with β-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Adhiyanto C, Hattori Y, Yamashiro Y, et al. Oxidation status of β-thalassemia minor and Hb H disease, and its association with glycerol lysis time (GLT50). Hemoglobin. 2014;38:169–172. [DOI] [PubMed] [Google Scholar]
- [7].Cheng ML, Ho HY, Tseng HC, et al. Antioxidant deficit and enhanced susceptibility to oxidative damage in individuals with different forms of alpha-thalassaemia. Br J Haematol. 2005;128:119–127. [DOI] [PubMed] [Google Scholar]
- [8].Chiou SS, Tsao CJ, Tsai SM, et al. Metabolic pathways related to oxidative stress in patients with hemoglobin h disease and iron overload. J Clin Lab Anal. 2014;28:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dugaiczyk A, Law SW, Dennison OE. Nucleotide sequence and the encoded amino acids of human serum albumin mRNA. Proc Natl Acad Sci U S A. 1982;79:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].He XM, Carter DC. Atomic structure and chemistry of human serum albumin. Nature. 1992;358:209–215. [DOI] [PubMed] [Google Scholar]
- [11].Awadallah SM, Atoum MF, Nimer NA, et al. Ischemia modified albumin: an oxidative stress marker in β-thalassemia major. Clin Chim Acta. 2012;413:907–910. [DOI] [PubMed] [Google Scholar]
- [12].Bar-Or D, Curtis G, Rao N, et al. Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. Eur J Biochem. 2001;268:42–48. [DOI] [PubMed] [Google Scholar]
- [13].Roy D, Quiles J, Gaze DC, et al. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart. 2006;92:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morrow DA, de Lemos JA, Sabatine MS, et al. The search for a biomarker of cardiac ischemia. Clin Chem. 2003;49:537–539. [DOI] [PubMed] [Google Scholar]
- [15].Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24:333–341. [DOI] [PubMed] [Google Scholar]
- [16].Sbarouni E, Georgiadou P, Voudris V. Ischemia modified albumin changes – review and clinical implications. Clin Chem Lab Med. 2011;49:177–184. [DOI] [PubMed] [Google Scholar]
- [17].Borderie D, Allanore Y, Meune C, et al. High ischemia-modified albumin concentration reflects oxidative stress but not myocardial involvement in systemic sclerosis. Clin Chem. 2004;50:2190–2193. [DOI] [PubMed] [Google Scholar]
- [18].Kaefer M, Piva SJ, De Carvalho JA, et al. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clin Biochem. 2010;43:450–454. [DOI] [PubMed] [Google Scholar]
- [19].Valle Gottlieb MG, da Cruz IB, Duarte MM. Associations among metabolic syndrome, ischemia, inflammatory, oxidatives, and lipids biomarkers. J Clin Endocrinol Metab. 2010;95:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Piva SJ, Duarte MM, Da Cruz IB. Ischemia-modified albumin as an oxidative stress biomarker in obesity. Clin Biochem. 2011;44:345–347. [DOI] [PubMed] [Google Scholar]
- [21].Kazanis K, Dalamaga M, Nounopoulos C, et al. Ischemia modified albumin, high-sensitivity c-reactive protein and natriuretic peptide in patients with coronary atherosclerosis. Clin Chim Acta. 2009;408(1–2):65–69. [DOI] [PubMed] [Google Scholar]
- [22].Dominguez-Rodriguez A, Abreu-Gonzalez P. Current role of ischemia-modified albumin in routine clinical practice. Biomarkers. 2010;15:655–662. [DOI] [PubMed] [Google Scholar]
- [23].Giardina PJV, Forget BG. Thalassemia syndromes. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H, editor. Hematology: basic principles and practice. 5th ed. Philadelphia: Elsevier Churchill Livingstone; 2008. p. 535–563. [Google Scholar]
- [24].Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity and weight velocity: British children, 1965.II. Arch Dis Child. 1966;41:613–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- [26].Vermylen C. What is new in iron overload? Eur J Pediatr. 2008;167:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Silvilairat S, Sittiwangkul R, Pongprot Y, et al. Tissue Doppler echocardiography reliably reflects severity of iron overload in pediatric patients with beta thalassemia. Eur J Echocardiogr. 2008;9:368–372. [DOI] [PubMed] [Google Scholar]
- [28].Tantawy AA, Adly AA, Ismail EA, et al. Flow cytometric assessment of circulating platelet and erythrocytes microparticles in young thalassemia major patients: relation to pulmonary hypertension and aortic wall stiffness. Eur J Haematol. 2013;90:508–518. [DOI] [PubMed] [Google Scholar]
- [29].Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. [DOI] [PubMed] [Google Scholar]
- [30].Pashankar FD, Carbonella J, Bazzy-Asaad A, et al. Prevalence and risk factors of elevated pulmonary artery pressures in children with sickle cell disease. Pediatrics. 2008;121:777–782. [DOI] [PubMed] [Google Scholar]
- [31].Morris CR, Kim HY, Trachtenberg F, et al. Risk factors and mortality associated with an elevated tricuspid regurgitant jet velocity measured by Doppler-echocardiography in thalassemia: a thalassemia clinical research network report. Blood. 2011;118:3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127:1523–1530. [DOI] [PubMed] [Google Scholar]
- [33].Dalla Pozza R, Ehringer-Schetitska D, Fritsch P, et al. Association for European paediatric cardiology working group cardiovascular prevention. Intima media thickness measurement in children: A statement from the association for European paediatric cardiology (AEPC) working group on cardiovascular prevention endorsed by the association for European paediatric cardiology. Atherosclerosis. 2015;238:380–387. [DOI] [PubMed] [Google Scholar]
- [34].Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J Emerg Med. 2000;19:311–315. [DOI] [PubMed] [Google Scholar]
- [35].Bhagavan NV, Lai EM, Rios PA, et al. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin Chem. 2003;49:581–585. [DOI] [PubMed] [Google Scholar]
- [36].Peacock F, Morris DL, Anwaruddin S, et al. Meta-analysis of ischemia-modified albumin to rule out acute coronary syndromes in the emergency department. Am Heart J. 2006;152:253–262. [DOI] [PubMed] [Google Scholar]
- [37].Sinha MK, Vazquez JM, Calvino R, et al. Effects of balloon occlusion during percutaneous coronary intervention on circulating ischemia modified albumin and transmyocardial lactate extraction. Heart. 2006;92:1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee E, Eom JE, Jeon KH, et al. Evaluation of albumin structural modifications through cobalt-albumin binding (CAB) assay. J Pharm Biomed Anal. 2014;91:17–23. [DOI] [PubMed] [Google Scholar]
- [39].Kountana E, Tziomalos K, Semertzidis P, et al. Comparison of the diagnostic accuracy of ischemia-modified albumin and echocardiography in patients with acute chest pain. Exp Clin Cardiol Spring. 2013;18:98–100. [PMC free article] [PubMed] [Google Scholar]
- [40].Vyakaranam S, Bhongir AV, Patlolla D, et al. Maternal serum ischemia modified albumin as a marker for hypertensive disorders of pregnancy: a pilot study. Int J Reprod Contracept Obstet Gynecol. 2015;4:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mousa S, Afifi M, Saedii A, et al. Ischemia modified albumin in children with transfusion-dependent β-thalassemia: a new marker for an old problem. Egyptian J Haematol. 2016;41:45–49. [Google Scholar]
- [42].Awadallah S, Al Arrayed K, Bahareth E, et al. Total antioxidant capacity and ischemia modified albumin in beta thalassemia. Clin Lab. 2013;59:687–691. [DOI] [PubMed] [Google Scholar]
- [43].De A, Chowdhury N, Sen P, et al. Correlation of cobalt binding activity of albumin with the common markers of oxidative stress in thalassemia syndrome patients. Oxid Antioxid Med Sci. 2013;2:297–301. [Google Scholar]
- [44].Naithani R, Chandra J, Bhattacharjee J, et al. Peroxidative stress and antioxidant enzymes in children with β-thalassemia major. Pediatr Blood Cancer. 2006;46:780–785. [DOI] [PubMed] [Google Scholar]
- [45].Dash P, Mangaraj M, Ray S, et al. Ischaemia modified albumin-an indicator of widespread endothelial damage in diabetes mellitus. J Physiobiochem Metab. 2014;3:1. [Google Scholar]
- [46].Bazvand F, Shams S, Borji Esfahani M, et al. Total antioxidant status in patients with major β-thalassemia. Iran J Pediatr. 2011;21:159–165. [PMC free article] [PubMed] [Google Scholar]
- [47].Sultana N, Sadiya S, Rahman MH. Correlation between serum bilirubin and serum ferritin level in thalassaemia patients. Bangladesh J Med Biochem. 2011;4:6–12. [Google Scholar]
- [48].Cighetti G, Duca L, Bortone L, et al. Oxidative status and malondialdehyde in beta-thalassaemia patients. Eur J Clin Invest. 2002;32:55–60. [DOI] [PubMed] [Google Scholar]
- [49].Walter PB, Macklin EA, Porter J, et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: an ancillary study of the novartis CICL670A0107 trial. Haematologica. 2008;93:817–825. [DOI] [PubMed] [Google Scholar]
- [50].Hamed EA, ElMelegy NT. Renal functions in pediatric patients with beta-thalassemia major: relation to chelation therapy: original prospective study. Italian J Pediatr. 2010;36:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cakmak A, Soker M, Koc A, et al. Prolidase activity and oxidative status in patients with thalassemia major. J Clin Lab Analysis. 2010;24:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Pepe A, Meloni A, Capra M, et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: cardiac iron and function comparison determined by quantitative magnetic resonance imaging. Hematologica. 2011;96:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mokhtar GM, Gadallah M, El Sherif NH, et al. Morbidities and mortality in transfusion-dependent beta-thalassemia patients (single-center experience). Pediatr Hematol Oncol. 2013;30:93–103. [DOI] [PubMed] [Google Scholar]
- [54].Bejaoui M, Guirat N. Beta thalassemia major in a developing country: epidemiological, clinical and evolutionary aspects. Mediterr J Hematol Infect Dis. 2013;5:e201–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hamdy AM, Zein El-Abdin MY, Abdel-Hafez MA. Right ventricular function in patients with beta thalassemia: relation to serum ferritin level. Echocard. 2007;24:795–801. [DOI] [PubMed] [Google Scholar]
- [56].Falkensammer J, Frech A, Duschek N, et al. Prognostic relevance of ischemia-modified albumin and Nt-proBNP in patients with peripheral arterial occlusive disease. Clin Chim Acta. 2015;438:255–260. [DOI] [PubMed] [Google Scholar]
- [57].Açıkgöz Ş, Edebali N, Barut F, et al. Ischemia modified albumin increase indicating cardiac damage after experimental subarachnoid hemorrhage. BMC Neurosci. 2014;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sbarouni E, Georgiadou P, Koutelou M, et al. Ischaemia-modified albumin in dilated cardiomyopathy. Ann Clin Biochem. 2009;46(Pt 3):241–243. [DOI] [PubMed] [Google Scholar]
- [59].Wood JC. Cardiac complications in thalassemia major. Hemoglobin. 2009;33(Suppl 1):S81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Morris CR, Vichinsky EP. Pulmonary hypertension in thalassemia. Ann N Y Acad Sci. 2010;1202:205–213. [DOI] [PubMed] [Google Scholar]
- [61].Farmakis D, Aessopos A. Pulmonary hypertension associated with hemoglobinopathies: prevalent but overlooked. Circulation. 2011;123:1227–1232. [DOI] [PubMed] [Google Scholar]
- [62].Meloni A, Detterich J, Pepe A, et al. Pulmonary hypertension in well-transfused thalassemia major patients. Blood Cells Mol Dis. 2015;54:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Can U, Yerlikaya FH, Yosunkaya S. Role of oxidative stress and serum lipid levels in stable chronic obstructive pulmonary disease. J Chin Med Assoc. 2015;78:702–708. [DOI] [PubMed] [Google Scholar]
- [64].Rooyakkers TM, Stroes ES, Kooistra MP. Ferric saccharate induces oxygen radical stress and endothelial dysfunction in vivo. Eur J Clin Invest. 2002;32:9–16. [DOI] [PubMed] [Google Scholar]
- [65].Day SM, Duquaine D, Mundada LV. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107:2601–2606. [DOI] [PubMed] [Google Scholar]
- [66].Aggeli C, Antoniades C, Cosma C. Endothelial dysfunction and inflammatory process in transfusion-dependent patients with beta-thalassemia major. Int J Cardiol. 2005;105:80–84. [DOI] [PubMed] [Google Scholar]
- [67].Tricot O, Mallat Z, Heymes C. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. [DOI] [PubMed] [Google Scholar]
- [68].Kyriakou DS, Alexandrakis MG, Kyriakou ES. Activated peripheral blood and endothelial cells in thalassemia patients. Ann Hematol. 2001;80:577–583. [DOI] [PubMed] [Google Scholar]
- [69].Aggoun Y, Szezepanski I, Bonnet D. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events in children. Pediatr Res. 2005;58:173–178. [DOI] [PubMed] [Google Scholar]
- [70].Devine PJ, Carlson DW, Taylor AJ. Clinical value of carotid intima-media thickness testing. J Nucl Cardiol. 2006;13:710–718. [DOI] [PubMed] [Google Scholar]
- [71].Kucuk A, Uslu AU, Arslan S, et al. Ischemia-modified albumin and atherosclerosis in patients with familial Mediterranean fever. Angiology. 2015;17(3):23–33. [DOI] [PubMed] [Google Scholar]
- [72].Yatmark P, Morales NP, Chaisri U, et al. Effects of iron chelators on pulmonary iron overload and oxidative stress in beta-thalassemic mice. Pharmacology. 2015;96:192–199. [DOI] [PubMed] [Google Scholar]
- [73].Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–1884. [DOI] [PubMed] [Google Scholar]
- [74].Srichairatanakool S, Fucharoen S. Antioxidants as complementary medication in thalassemia. In: Atroshi F, editor. Pharmacology and nutritional intervention in the treatment of disease. 2014. InTech, DOI: 10.5772/57372 Available from: http://www.intechopen.com/books/pharmacology-and-nutritional-intervention-in-the-treatment-of-disease/antioxidants-as-complementary-medication-in-thalassemia. [DOI] [Google Scholar]
- [75].Yanpanitch OU, Hatairaktham S, Charoensakdi R, et al. Treatment of β-thalassemia/hemoglobin E with antioxidant cocktails results in decreased oxidative stress, increased hemoglobin concentration, and improvement of the hypercoagulable state. Oxid Med Cell Longev. 2015;2015:537–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Elalfy MS, Saber MM, Adly AA, et al. Role of vitamin C as an adjuvant therapy to different iron chelators in young β-thalassemia major patients: efficacy and safety in relation to tissue iron overload. Eur J Haematol. 2016;96:318–326. [DOI] [PubMed] [Google Scholar]


