Abstract
Background
Breast cancer is the most common cancer in women and several perioperative factors may account for tumor recurrence and metastasis. The anesthetic agents employed during cancer surgery might play a crucial role in cancer cell survival and patient outcomes. We conducted a retrospective cohort study to investigate the relationship between the type of anesthesia and overall survival in patients who underwent breast cancer surgery performed by one experienced surgeon.
Methods
All patients who underwent breast cancer surgery by an experienced surgeon between January 2006 and December 2010 were included in this study. Patients were separated into two groups according to the use of desflurane or propofol anesthesia during surgery. Locoregional recurrence and overall survival rates were assessed for the two groups (desflurane or propofol anesthesia). Univariable and multivariable Cox regression models and propensity score matching analyses were used to compare the hazard ratios for death and adjust for potential confounders (age, body mass index, American Society of Anesthesiologists physical status classification, TNM stage, neoadjuvant chemotherapy, Charlson Comorbidity Index, anesthesiologists, and functional status).
Results
Of the 976 breast cancer patients, 632 patients underwent breast cancer surgery with desflurane anesthesia, while 344 received propofol anesthesia. After propensity scoring, 592 patients remained in the desflurane group and 296 patients in the propofol group. The mortality rate was similar in the desflurane (38 deaths, 4%) and propofol (22 deaths, 4%; p = 0.812) groups in 5-year follow-up. The crude hazard ratio (HR) for all patients was 1.13 (95% confidence interval [CI] 0.67–1.92, p = 0.646). No significant difference in the locoregional recurrence or overall 5-year survival rates were found after breast surgery using desflurane or propofol anesthesia (p = 0.454). Propensity score-matched analyses demonstrated similar outcomes in both groups. Patients who received propofol anesthesia had a higher mortality rate than those who received desflurane anesthesia in the matched groups (7% vs 6%, respectively) without significant difference (p = 0.561). In the propensity score-matched analyses, univariable analysis showed an insignificant finding (HR = 1.23, 95% CI 0.72–2.11, p = 0.449). After adjustment for the time since the earliest included patient, the HR remained insignificant (HR = 1.23, 95% CI 0.70–2.16, p = 0.475).
Conclusion
In our non-randomized retrospective analysis, neither propofol nor desflurane anesthesia for breast cancer surgery by an experienced surgeon can affect patient prognosis and survival. The influence of propofol anesthesia on breast cancer outcome requires further investigation.
Introduction
Breast cancer is one of the most common malignancies that affect women globally [1]. According to GLOBOCAN 2012, breast cancer is the leading cause of cancer-related deaths. Although the prevention of risk factors, early diagnostic screening, and advances in treatment [2] have improved cancer mortality rates, several perioperative factors may account for recurrence and metastasis, including the selection of anesthetic agents, perioperative regional analgesics, intraoperative opioids and nonsteroidal anti-inflammatory drugs(NSAIDs)/ cyclo-oxygenase (COX) inhibitors, surgical manipulation, and perioperative immunosuppression induced by surgical stress [3].
Recent reports discussed how anesthetics can influence cancer cell survival and progression [3, 4]. An old experimental study revealed that the use of halothane during surgical excision of local tumors strongly accelerated postoperative progression of spontaneous lung metastases produced by the 3LL Lewis lung carcinoma and by the B16 melanoma. Halothane induced the appearance of metastases in organs, such as the liver, in which spontaneous metastases were not usually produced by these tumors [5]. Benzonana et al. [6] reported that isoflurane upregulated the levels of hypoxia-inducible factor (HIF)-1α and HIF-2α and intensified the expression of vascular endothelial growth factor A in renal cell carcinoma cells. In a review article, Tavare et al. [7] concluded that halothane, isoflurane, and sevoflurane upregulated HIF genes in tumor cells resulting in poor prognosis. On the other hand, propofol reduced the levels of HIF-1α protein and was found to reduce the invasion and migration of breast cancer cells (MDA-MB-231) via inhibition of the NF-κB pathway [8]. Melamed et al. [9] demonstrated that propofol did not suppress natural killer (NK) cell activity or promote tumor metastasis in a rat model of breast cancer cells with pulmonary metastasis. Additionally, Kushida et al. [10] reported that propofol suppressed lymphoblast tumor growth in mice, suggesting that propofol enhances anti-tumor immunity. Another study reported that serum from patients who received sevoflurane anesthesia and opioids for primary breast cancer surgery exhibited attenuated apoptosis in estrogen receptor (ER)-negative breast cancer cells compared to serum from patients who received propofol-paravertebral anesthesia [11].
Previous retrospective studies analysed anesthetic type in breast cancer surgery [12–14] and found no association between volatile inhalation and propofol anesthesia with regard to the recurrence-free survival and overall survival of breast cancer, except Lee at al. [12] who suggest propofol-based anesthesia can lower the risk of breast cancer recurrence during the initial 5 years after surgery. However, these studies did not mention that surgeons might be one of the predictors of breast cancer outcome which would be regarded as one of the confounding factors. Chen et al. [15] analysed a pooled population-based database of the 13,360 breast cancer surgery patients and concluded that high surgeon volume is significantly associated with positive patient outcomes in Taiwan.
To the best of our knowledge, there has been limited research on the effects of one of the inhalation agents, desflurane, and one surgeon on breast cancer in vivo. Thus, we conducted a single center retrospective cohort study to assess whether the choice of anesthetics, volatile inhalation agent, desflurane, and propofol anesthesia affects recurrence and overall 5-year survival in patients that underwent breast cancer surgery performed by one experienced surgeon which the surgeon-related confounding could be excluded.
Materials and methods
Study design
This was a retrospective cohort study.
Setting
This study was conducted at the Tri-Service General Hospital (Taipei, Taiwan, Republic of China).
Participants and data sources
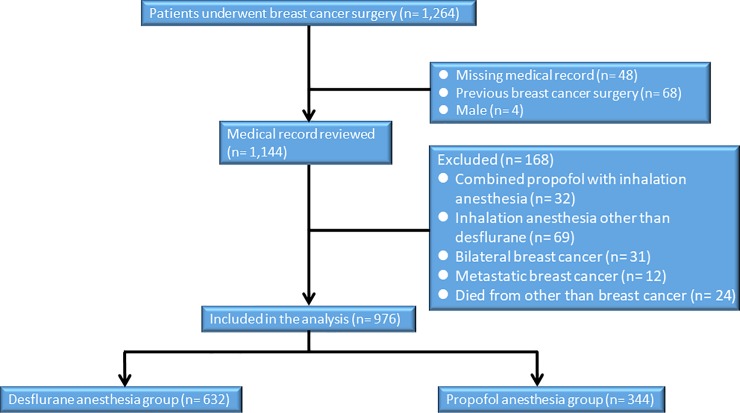
After approval from the ethics committee (TSGHIRB No: 1-104-05-139) of the Tri-Service General Hospital (TSGH), Taipei, Taiwan, Republic of China, relevant information was retrieved from the medical records and the electronic database of TSGH and the requirement for written informed consent was waived by the IRB. This retrospective study included 976 patients treated from January 2006 to December 2010. The patients had an American Society of Anesthesiologists (ASA) score of I–III and had undergone breast cancer surgery by an experienced surgeon (JC Yu) for tumor–node–metastasis (TNM) stage I–IV breast cancer. Six hundred and thirty-two patients were subjected to desflurane anesthesia and 344 underwent surgery under the influence of propofol anesthesia. No combination of propofol and inhalation anesthesia, isoflurane, or sevoflurane was used with our patients. Two hundred and eighty-eight patients were excluded from the analysis. The exclusion criteria were the use of propofol combined with inhalation anesthesia or inhalation agents other than desflurane, missing medical records, bilateral breast cancer, previous breast cancer surgery, metastatic breast cancer, death from other diseases, male gender or age < 20 years. (Fig 1)
Fig 1. Flow diagram of the study population.
All breast cancer surgery was performed by the same surgeon and no prior medications were prescribed before the induction of anesthesia. Hemodynamic monitoring, including non-invasive blood pressure, electrocardiography (lead II), pulse oximetry, and end-tidal carbon dioxide pressure (EtCO2) were performed. The selection of anesthetics was at the discretion of the attending anesthesiologist. Anesthesia was induced with fentanyl (2 μg kg–1), lidocaine (2%, 1.5 mg kg–1), propofol (2–3 mg kg–1), rocuronium (0.6 mg kg–1), or cisatracurium (0.1–0.2 mg kg–1) combined with desflurane in the desflurane group. In the propofol group, anesthesia was induced with fentanyl (2 μg kg–1), lidocaine (2%, 1.5 mg kg–1), a target-controlled infusion (TCI, Fresenius Orchestra Primea; Fresenius Kabi AG, Bad Homburg, Germany) with propofol Ce 4–5 μg mL–1 and rocuronium (0.6 mg kg–1) or cisatracurium (0.1–0.2 mg kg–1). Then, the patients were intubated and maintained with either propofol or desflurane, as well as the analgesic fentanyl. The following data were collected for each patient: gender, age, ASA score, the anesthesiologist, TNM stage, radiotherapy, chemotherapy, hormone therapy, and vital status.
In the desflurane group, anesthesia was maintained with 8–12% desflurane under a 100% oxygen flow of 300 mL min–1 in a closed system. In the propofol group, anesthesia was maintained with propofol Ce 3–4 μg mL–1 and an oxygen flow of 300 mL min–1 with 100% FiO2. Bolus injections of muscle relaxants (rocuronium or cisatracurium) and fentanyl were administered repeatedly as required throughout the procedure.
The anesthesiologists adjusted the Ce using TCI with propofol or desflurane at a range of 0.2–0.5 μg mL–1 or 0.5–2% for maintenance based on hemodynamic changes. The ventilation rate and maximum airway pressure were modulated to maintain EtCO2 at a range between 35–45 mmHg. Bolus dosing of either rocuronium (10 mg) or cisatracurium (2 mg) was prescribed intravenously as required during the recovery of neuromuscular function. During skin closure, desflurane or propofol was discontinued and patients were ventilated with 100% oxygen at a fresh gas flow rate of 6 L min–1. When the patient regained consciousness with spontaneous and smooth breathing, the endotracheal tube was removed. Then the patients were transferred to the post-anesthetic care unit for further care [16, 17].
Variables
Patient data was obtained from the medical records and the electronic database including age at the time of surgery, body mass index (BMI), ASA score, TNM stage, neoadjuvant chemotherapy, the Charlson Comorbidity Index (CCI), anesthesiologists, preoperative functional status regarding metabolic equivalents (METs) to evaluate preoperative cardiorespiratory function and potentially predict perioperative outcomes, histologic grade, ER status, progesterone receptor (PR) status, epidermal growth factor receptor type 2 (HER-2) expression, tri-negative breast cancer (TNBC), and postoperative adjuvant hormonal therapy, chemotherapy or radiotherapy. In addition, we recorded the use of perioperative or postoperative opioids and NSAIDs, the duration of surgery and anesthesia, and the time of first metastasis.
Study sample size
To achieve a power of 80% and a two-tailed type I error rate of α = 0.05, each unmatched group required 213 patients (assuming a mortality rate of 24% in the desflurane anesthesia group and 13.5% in the propofol anesthesia group). Each matched group required 465 patients (assuming a mortality rate of 22.8% in the desflurane anesthesia group and 15.6% in the propofol anesthesia group) [18].
Statistical analysis
The major goal of our study was to identify the influences of different anesthetic agents (desflurane and propofol) on cancer recurrence and overall survival follow-up for 5 years after the surgery. Clinical evidence of locoregional recurrence or distant metastases confirmed by imaging studies or tissue-proved was defined as recurrence. Recurrence-free survival was defined from the date of operation to the date of first recurrence, death due to breast cancer, or the last follow-up, whichever occurred first. Overall survival was defined from the interval between the date of surgery and the date of the final outcome, distant metastasis, or end of follow-up in January 2016.
Patient characteristics and overall survival rates were compared between different anesthetics using the chi-square test, Fisher exact test or Student’s t-test. A propensity score (PS) was constructed to address the differences in baseline characteristics [19] between the two groups using a linear (simple logistic regression) algorithm. Interaction terms did not improve the model fit. In our observational studies, the influence of anesthetic effect on breast cancer outcome may be biased with nonrandomly allocate exposure. To dealing with confounding, an alternative approach, propensity score method is used [19, 20]. In order to minimizing confounding, matching algorithms is prescribed to find best matches between both groups. Since the desflurane group contained more patients than the propofol group did, to maximize statistical power, a greedy nearest-neighbor matching procedure with calipers set at 0.2 SD of the logit of the PS was used to create 1-to-2 matched pairs (296 pairs). The relationship between the choice of anesthetic (desflurane or propofol) and survival was analyzed using the Cox proportional-hazards model and PS-matching with adjustments for age, BMI, ASA physical status classification, anesthesiologists, TNM stage, neoadjuvant chemotherapy, CCI, and preoperative functional status. R (version 3.4.3, available at https://cran-r-project.org/src/base/r-3/r-3.4.3.tar.gz) and SPSS v22 were used for statistical analyses. P-values <0.05 were considered significant.
Results
We reviewed 1,264 breast cancer patients who underwent breast cancer surgery, among which 632 received desflurane and 344 received propofol. Table 1 shows the patient baseline characteristics and treatment. The time since the earliest included patients was significantly longer in the propofol group (3.4 ± 1.2 years) than in desflurane group (2.2 ± 1.4 years; p < 0.001). The patient demographics for the two groups of both overall and matched patients, including age and BMI, were not statistically different. Prognostic factors, such as TNM stage, neoadjuvant chemotherapy, postoperative adjuvant chemotherapy, radiotherapy, hormonal therapy of breast cancer and intraoperative NSAIDs, HER-2 expression, ER expression, PR expression and TNBC were all similar in the desflurane and propofol groups of overall and matched patients. The propofol group of overall patients had significantly more patients with ASA scores ≥ II (p = 0.009) than the desflurane group did, but there was no significant difference with ASA scores ≥ II (p = 0.077) in both groups after matching. The CCI score was significantly higher in the propofol group than in the desflurane group (p = 0.008) in overall patients, but was insignificant after propensity scoring (p = 0.318). More patients in the propofol group received postoperative NSAIDs (p = 0.039); nevertheless, there was no statistical significance in the both groups after matching (p = 0.067). The presence of local recurrence revealed no significant differences between the desflurane group (4%) and the propofol group (4%). Besides, the percentage of patients with distant metastases in the desflurane group (8%) was higher than in the propofol group (6%). But the difference was not statistically significant both in all patients and matched patients (p = 0.454 vs. p = 0.707). The mortality rate was similar in the desflurane (38 deaths, 6%) and propofol (22 deaths, 6%; p = 0.812) groups in overall patients and the finding was also similar in the matched patients (p = 0.561).
Table 1. Patient and treatment characteristics for overall patients and matched patients after propensity scoring.
| Overall patients | Matched patients | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Desflurane N = 632 |
Propofol N = 344 |
p-value | Desflurane N = 592 |
Propofol N = 296 |
p-value | SMD |
| Time since the earliest included patient (yr) | 2.2 ± 1.4 | 3.4 ± 1.2 | < 0.001 | 2.2 ± 1.4 | 3.3 ± 1.2 | < 0.001 | 0.888 |
| Age (yr) | 0.512 | 0.542 | 0.081 | ||||
| < 40 | 64 (10) | 32 (9) | 58 (10) | 27 (9) | |||
| 40–49 | 245 (39) | 117 (34) | 229 (39) | 104 (35) | |||
| 50–59 | 212 (34) | 126 (37) | 203 (34) | 111 (38) | |||
| 60–69 | 82 (13) | 48 (14) | 76 (13) | 35 (12) | |||
| ≥ 70 | 29 (5) | 21 (6) | 26 (4) | 19 (6) | |||
| BMI (kg/ m2) | 23.3 ± 3.5 | 23.3 ± 3.6 | 0.709 | 23.3 ± 3.4 | 23.1 ± 3.6 | 0.397 | 0.060 |
| ASA | 0.009 | 0.077 | 0.157 | ||||
| I | 428 (68) | 199 (58) | 403 (68) | 179 (61) | |||
| II | 175 (28) | 123 (36) | 162 (27) | 99 (33) | |||
| III | 29 (5) | 22 (6) | 27 (5) | 18 (6) | |||
| Functional status | 0.206 | 0.180 | 0.096 | ||||
| ≥ 4 METs | 605 (96) | 323 (94) | 568 (96) | 278 (94) | |||
| < 4 METs | 27 (4) | 21 (6) | 24 (4) | 18 (6) | |||
| CCI | 0.008 | 0.318 | 0.130 | ||||
| 2 | 474 (75) | 228 (66) | 445 (75) | 207 (70) | |||
| 3 | 108 (17) | 67 (20) | 102 (17) | 59 (20) | |||
| 4 | 37 (6) | 37 (11) | 36 (6) | 22 (7) | |||
| ≥ 5 | 13 (2) | 12 (4) | 9 (2) | 8 (3) | |||
| TNM stage of primary tumor | 0.995 | 0.578 | 0.009 | ||||
| 0 | 108 (17) | 58 (17) | 100 (17) | 46 (16) | |||
| I | 235 (37) | 130 (38) | 215 (36) | 120 (41) | |||
| II | 210 (33) | 112 (33) | 203 (34) | 91 (31) | |||
| III | 79 (13) | 44 (13) | 74 (13) | 39 (13) | |||
| HER-2, Negative | 301 (48) | 184 (54) | 0.080 | 282 (48) | 156 (53) | 0.154 | 0.102 |
| ER, Negative | 203 (32) | 88 (26) | 0.039 | 192 (32) | 78 (26) | 0.075 | 0.133 |
| PR, Negative | 136 (22) | 66 (19) | 0.450 | 127 (22) | 57 (19) | 0.516 | 0.053 |
| TNBC | 30 (5) | 14 (4) | 0.745 | 28 (5) | 13 (4) | 0.955 | 0.043 |
| Neoadjuvant chemotherapy | 29(5) | 16(5) | 0.964 | 25(4) | 15(5) | 0.567 | 0.049 |
| Intraoperative NSAIDs | 33 (5) | 14 (4) | 0.422 | 31 (5) | 11 (4) | 0.314 | 0.072 |
| Postoperative NSAIDs | 13 (2) | 15 (4) | 0.039 | 13 (2) | 13 (4) | 0.067 | 0.131 |
| Adjuvant chemotherapy | 369 (58) | 194 (56) | 0.548 | 349 (59) | 170 (57) | 0.665 | 0.031 |
| Adjuvant radiotherapy | 348 (55) | 167 (49) | 0.051 | 326 (55) | 143 (48) | 0.057 | 0.136 |
| Adjuvant hormonal therapy | 473 (75) | 254 (74) | 0.731 | 442 (75) | 215 (73) | 0.516 | 0.046 |
| Relapse | 0.454 | 0.707 | 0.059 | ||||
| No | 555 (88) | 311 (90) | 521 (88) | 266 (90) | |||
| Local recurrence in 5 years | 28 (4) | 13 (4) | 25 (4) | 11 (4) | |||
| Distant metastases in 5 years | 49 (8) | 20 (6) | 46 (8) | 19 (6) | |||
| Survival | |||||||
| 2006–2010 | 594 (94) | 322 (94) | 0.812 | 556 (94) | 275 (93) | 0.561 | |
| 2006 | 138 (92) | 29 (94) | 1.000 | 130 (92) | 26 (93) | 1.000 | |
| 2007 | 167 (95) | 5 (100) | 1.000 | 157 (95) | 4 (100) | 1.000 | |
| 2008 | 126 (91) | 51 (98) | 0.118 | 118 (92) | 47 (98) | 0.184 | |
| 2009 | 51 (94) | 134 (91) | 0.568 | 47 (94) | 114 (90) | 0.562 | |
| 2010 | 112 (98) | 108 (95) | 0.270 | 104 (98) | 84 (94) | 0.249 | |
Data shown as mean ± SD or n (%.)
BMI = body mass index; ASA = American Society of Anesthesiologists; TNM = tumor–node–metastasis; CCI = Charlson comorbidity index; MET = metabolic equivalents; NSAID = nonsteroidal anti–inflammatory drugs; TNBC = triple–negative breast cancer.
Before the surgery, we used the PS from logistic regression to adjust the baseline characteristics and the choice of therapy between the two groups. Since there were more patients in the desflurane group, 1-to-2 matched pairs (296 pairs) was formed to retain statistical power. The standardized mean differences (SMD) for the variables were not all < 0.1, such as time since the earliest included patient, the ASA score, CCI, negative HER-2, negative ER, postoperative NASIDs, and adjuvant radiotherapy.
Data was collected from January 2006 to December 2010 and fewer patients received propofol anesthesia from 2006 to 2008. The number of patients who received propofol increased over 5 years, and the survival rate for the two groups varied each year (Table 1). Therefore, time might be a confounding factor because changes in cancer care over time could have influenced the outcomes. After PS matching, the SMD of time since the earliest included patients remained > 0.1 (Table 1); therefore, matched and unmatched group analyses were adjusted for time since the earliest included patients to avoid any possible confounding effects due to the time factor.
Overall survival from the date of surgery grouped according to anesthesia type and other variables was compared separately in a univariable Cox model and subsequently in a multivariable Cox regression. Multivariable analyses revealed some variables related to the risk of death, including age, ASA score ≥ II, advanced TNM stage, positive ER, positive PR, neoadjuvant chemotherapy, adjuvant chemotherapy, and adjuvant hormonal therapy both in all patients and matched patients (Tables 2 and 3) and intraoperative NSAIDs in matched patients (Table 3). Patients who received propofol anesthesia had a higher mortality rate than those who received desflurane anesthesia in the matched groups (7% vs 6%, respectively) without significant difference (p = 0.561) (Table 1). The crude hazard ratio (HR) for all patients was 1.13 (95% confidence interval [CI] 0.67–1.92, p = 0.646). After adjustment for potential covariates, the HR became 1.17 (95% CI 0.68–2.00, p = 0.577) (Table 2). Similarly, in the propensity score-matched analyses, univariable analysis showed an insignificant finding (HR = 1.23, 95% CI 0.72–2.11, p = 0.449). The adjusted HR remained insignificant (HR = 1.23, 95% CI 0.70–2.16, p = 0.475) (Table 3).
Table 2. Cox regression proportional hazard survival: Univariable and multivariable models for overall patients (n = 976).
| Independent variable | Crude-HR (95% CI) | p-value | Adj-HR (95% CI) | p-value |
|---|---|---|---|---|
| Anesthesia, propofol (ref: Desflurane) | 1.13 (0.67–1.92) | 0.646 | 1.17 (0.68–2.00) | 0.577 |
| Time since the earliest included patient (yr) (ref: < 40) | 0.94 (0.78–1.12) | 0.473 | ||
| Age (yr) | ||||
| 40–49 | 0.32 (0.17–0.73) | 0.005 | 0.45 (0.21–0.94) | 0.034 |
| 50–59 | 0.49 (0.24–0.98) | 0.045 | 0.50 (0.24–1.03) | 0.061 |
| 60–69 | 0.23 (0.08–0.72) | 0.011 | 0.15 (0.05–0.51) | 0.002 |
| ≥70 | 0.82 (0.29–2.32) | 0.701 | 0.43 (0.13–1.44) | 0.171 |
| BMI (kg/ m2) | 1.04 (0.97–1.11) | 0.281 | ||
| ASA (ref: I) | ||||
| II | 1.74 (1.01–3.01) | 0.047 | 0.80 (0.40–1.58) | 0.523 |
| III | 3.69 (1.69–8.09) | 0.001 | 3.55 (1.38–9.15) | 0.009 |
| Functional status, < 4 METs (ref: ≥ 4 METs) | 2.25 (0.97–5.23) | 0.06 | ||
| CCI (ref: 2) | ||||
| 3 | 0.61 (0.28–1.35) | 0.225 | ||
| 4 | 1.58 (0.71–3.51) | 0.260 | ||
| ≥ 5 | 0.65 (0.09–4.72) | 0.672 | ||
| TNM Stage of primary tumor, II + III (ref: 0 + I) | ||||
| II + III | 5.44 (2.58–11.44) | < 0.001 | 6.82 (2.96–15.7) | < 0.001 |
| HER-2 (ref: negative) | 1.28 (0.77–2.14) | 0.339 | ||
| ER (ref: negative) | 0.45 (0.27–0.74) | 0.002 | 0.62 (0.33–1.17) | 0.138 |
| PR (ref: negative) | 0.56 (0.33–0.97) | 0.038 | 1.18 (0.61–2.28) | 0.626 |
| TNBC (ref: no) | 0.35 (0.05–2.50) | 0.294 | ||
| Neoadjuvant chemotherapy (ref: no) | 13.5 (7.86–23.1) | < 0.001 | 10.5 (5.36–20.7) | < 0.001 |
| Intraoperative NSAIDs (ref: no) | 2.26 (0.97–5.26) | 0.059 | ||
| Postoperative NSAIDs (ref: no) | 1.14 (0.28–4.66) | 0.858 | ||
| Adjuvant chemotherapy (ref: no) | 3.14 (1.63–6.03) | < 0.001 | 0.71 (0.32–1.60) | 0.414 |
| Adjuvant radiotherapy (ref: no) | 0.95 (0.57–1.58) | 0.848 | ||
| Adjuvant hormonal therapy (ref: no) | 0.36 (0.22–0.60) | < 0.001 | 0.50 (0.27–0.93) | 0.028 |
| Relapse (ref: no) | 61.4 (29.1–129) | < 0.001 |
All multivariable HRs were adjusted by those variables significant (p < 0.05) in the univariable analyses simultaneously except anesthesia. Relapse status was also excluded from the multivariable model because it was an intermediary variable in the cause path to outcome.
BMI = body mass index; ASA = American Society of Anesthesiologists; TNM = tumor–node–metastasis; CCI = Charlson comorbidity index; MET = metabolic equivalents; NSAID = nonsteroidal anti–inflammatory drugs; ER = estrogen receptor; PR = progesterone receptor; TNBC = triple–negative breast cancer.
Table 3. Cox regression proportional hazard survival: Univariable and multivariable models for matched patients (n = 888).
| Independent variable | Crude-HR (95% CI) | p-value | Adj-HR (95% CI) | p-value |
|---|---|---|---|---|
| Anesthesia, propofol (ref: desflurane) | 1.23 (0.72–2.11) | 0.449 | 1.23 (0.70–2.16) | 0.475 |
| Time since the earliest included patient (yr) (ref: < 40) | 0.95 (0.79–1.15) | 0.617 | ||
| Age (yr) | ||||
| 40–49 | 0.37 (0.17–0.79) | 0.010 | 0.53 (0.24–1.14) | 0.103 |
| 50–59 | 0.49 (0.24–1.01) | 0.054 | 0.52 (0.24–1.10) | 0.087 |
| 60–69 | 0.26 (0.08–0.81) | 0.021 | 0.20 (0.06–0.68) | 0.010 |
| ≥ 70 | 0.69 (0.22–2.18) | 0.531 | 0.37 (0.10–1.41) | 0.144 |
| BMI (kg/ m2) | 1.05 (0.98–1.13) | 0.179 | ||
| ASA (ref: I) | ||||
| II | 1.74 (0.99–3.06) | 0.056 | 0.81 (0.39–1.65) | 0.555 |
| III | 3.91 (1.78–8.59) | 0.001 | 4.50 (1.73–11.7) | 0.002 |
| Functional status, < 4 METs (ref: ≥ 4 METs) | 1.97 (0.79–4.94) | 0.147 | ||
| CCI (ref: 2) | ||||
| 3 | 0.45 (0.18–1.14) | 0.092 | ||
| 4 | 1.82 (0.82–4.05) | 0.141 | ||
| ≥ 5 | 0.87 (0.12–6.31) | 0.890 | ||
| TNM Stage of primary tumor, II + III (ref: 0 + I) | ||||
| II + III | 7.42 (3.52–15.68) | < 0.001 | 6.15 (2.67–14.1) | < 0.001 |
| HER–2 (ref: negative) | 1.23 (0.73–2.87) | 0.444 | ||
| ER (ref: negative) | 0.45 (0.27–0.75) | 0.002 | 0.65 (0.34–1.16) | 0.202 |
| PR (ref: negative) | 0.57 (0.33–1.00) | 0.049 | 1.23 (0.61–2.45) | 0.562 |
| TNBC (ref: no) | 0.36 (0.05–2.61) | 0.313 | ||
| Neoadjuvant chemotherapy (ref: no) | 14.8 (8.49–25.62) | < 0.001 | 11.4 (5.60–23.0) | < 0.001 |
| Intraoperative NSAIDs (ref: no) | 2.50 (1.07–5.82) | 0.034 | 1.01 (0.40–2.54) | 0.978 |
| Postoperative NSAIDs (ref: no) | 1.16 (0.28–4.77) | 0.835 | ||
| Adjuvant chemotherapy (ref: no) | 3.23 (1.63–6.39) | 0.001 | 0.78 (0.34–1.79) | 0.552 |
| Adjuvant radiotherapy (ref: no) | 1.00 (0.60–1.70) | 0.991 | ||
| Adjuvant hormonal therapy (ref: no) | 0.36 (0.21–0.60) | < 0.001 | 0.50 (0.26–0.94) | 0.031 |
| Relapse (ref: no) | 45.8 (25.6–81.8) | < 0.001 |
All multivariable HRs were adjusted by those variables significant (p < 0.05) in the univariable analyses simultaneously except anesthesia. Relapse status was also excluded from the multivariable model due to it was an intermediary variable in the cause path to outcome.
BMI = body mass index; ASA = American Society of Anesthesiologists; TNM = tumor–node–metastasis; CCI = Charlson comorbidity index; MET = metabolic equivalents; NSAID = nonsteroidal anti–inflammatory drugs; ER = estrogen receptor; PR = progesterone receptor; TNBC = triple–negative breast cancer.
The distributions of types of anesthesia among total 20 anesthesiologists were significantly different both in all patients and matched patients (p < 0.001; S2 Table). Nevertheless, after adjustment for anesthesiologists, the adjusted HR for all patients was 0.70 (95% CI 0.31–1.56, p = 0.383) and the adjusted HR for matched patients was 0.67 (95% CI 0.28–1.61, p = 0.369) (S3 Table).
We also found that relapse, ASA scores ≥ III, advanced TNM stage and neoadjuvant chemotherapy contributed to higher mortality. Additionally, age > 40 years and adjuvant hormonal therapy improved survival. (Tables 2 and 3)
Discussion
Our retrospective study demonstrated that propofol and desflurane anesthesia were not associated with overall survival following breast cancer surgery by an experienced surgeon during the initial 5-year follow-up. We found that neither agent had a significant effect on survival rate, metastasis, or recurrence.
Lee et al. [12] suggested that propofol-based anesthesia for breast cancer surgery attenuates the risk of cancer recurrence, but does not improve survival rate during the initial 5 years when compared to sevoflurane-based anesthesia. Kim et al. [13] found there was no difference in breast cancer recurrence between total intravenous anesthesia (2% propofol and remifentanil) and balanced anesthesia (sevoflurane, desflurane, isoflurane, or enflurane with adjuvant intravenous infusion of remifentanil). Yoo et al. [14] compared total intravenous anesthesia with inhalation anesthesia (sevoflurane, desflurane, enflurane, or isoflurane) which also revealed no association between anesthetic type and recurrence-free survival or overall survival. In addition, Wigmore et al. [18] reported that the breast cancer mortality with isoflurane or sevoflurane anesthesia was 8.6% (52/603) while that with propofol anesthesia was 6.6% (103/1560). However, Enlund et al. [21] found that the difference in survival (propofol minus sevoflurane anesthesia) after breast cancer surgery was 0.03 (95% CI 0.01–0.04, p < 0.001) in one-year and 0.02 (95% CI -0.02–0.06, data was not significant) in five years.
Soltanizadeh et al. [22] conducted a systemic review of the outcomes of cancer surgery with inhalational and intravenous anesthesia and concluded that propofol might be the optimal anesthetic choice. In addition, we demonstrated the use of propofol-based anesthesia for colon cancer surgery resulted in better survival than desflurane anesthesia irrespective of TNM stage [23]. These studies suggest an anti-tumor role for propofol [18, 22], but our findings are not in agreement with this hypothesis.
Woo et al. [24] investigated whether desflurane and propofol anesthesia application during breast cancer surgery preserved interleukin (IL)-2/IL-4 and the cluster of differentiation (CD)4(+)/CD8(+) T cell ratio with a favorable immune response. However, the study did not include long-term follow-up for the outcomes of the cancers, or the interactions between the immune system and surrounding factors. Interestingly, we compared propofol with desflurane anesthesia instead of sevoflurane. Nevertheless, the two drugs showed no difference in the survival rate and metastases in breast cancer patients.
Previous research revealed that volatile agents, such as halothane, isoflurane and sevoflurane, inhibited interferon α/β stimulated NK cell cytotoxicity and promoted apoptosis in human T lymphocytes in vivo and in vitro, resulting in a deleterious effect on tumor metastasis [9, 25–28]. Volatile anesthetics act via specific cell signalling mechanisms such as HIF-1α [6, 29, 30] leading to the accommodation and survival of healthy cells. A systemic review revealed that volatile anesthetics can induce tumor dissemination in animal models [31]. Therefore, compared to propofol, volatile agents are less preferable as anesthetics in cancer surgery [12, 21, 22].
Propofol attenuates tumor invasion and dissemination by reducing the expression of matrix metalloproteinases (MMPs) through the inhibition of NF-κB [8]. MMPs are the key enzymes in the breakdown of the basement membrane, and are therefore involved in oncologic outcomes. Experiments showed that propofol induces apoptosis in breast cancer cells by suppressing the miR-24/p27 signal pathway [32] and Kras mutation in breast cancer cells may play a role in propofol-induced apoptosis [33]. However, Meng et al. [34] reported that propofol increased the proliferation of human breast cancer MDA-MB-231 cells and induced cell migration. Despite reports of the anti-cancer effects and benefits of propofol in cancer surgery [12, 21, 22], our data did not support this conclusion.
Our study revealed neoadjuvant chemotherapy to be associated with poor survival after breast cancer surgery which was consistent with several studies of high risk of local recurrence or locoregional recurrence after breast cancer surgery [35–37]. In addition, we found age ≥ 40 years was associated with better survival than age < 40 years in agreement with a previous study that young age was an independent prognostic indicator for locoregional recurrence after breast cancer surgery [38]. Furthermore, we also found adjuvant hormonal therapy improved survival which may imply hormone replacement therapy with a beneficial effect on breast cancer outcome [39].
Surgery plays a crucial role in tumor metastasis during the perioperative period [40]. Manipulation of a tumor and its vasculature releases tumor cells into the host blood and lymphatic circulation, resulting in distant metastasis [41]. Local and systemic release of growth factors and reduced anti-angiogenic factors after surgery may induce the development of micro-metastasis and recurrence [41–43]. Moreover, surgery that induces the stress response can transiently suppress cell-mediated immunity [44–46] and eventually cause the spread of tumor cells. Oh et al. [47] concluded that the effect of anesthetics on the perioperative immune activity may be minimal during breast cancer surgery. Consequently, compared with the resection of other solid tumors, mastectomy was performed subcutaneously and caused less inflammatory reactions which may partially explain the difference between significant outcome with propofol-based anesthesia for colon cancer surgery in our previous study [23] and insignificant results for breast cancer surgery.
In our hospital, the average number of patients who had breast cancer and underwent breast cancer surgery by the specialist surgeon in the last 10 years was more than 350 annually. Sainsbury et al. [48] reported that the treatment strategy employed by surgeons with low caseloads reduced overall survival. A large retrospective population-based analysis by Stefoski-Mikeljevic and his colleagues [49] also showed the relative risk of death was lower for patients managed by surgeons with higher workloads. Moreover, Kingsmore et al. [50] investigated treatment by well-trained specialist surgeons was associated with half the risk of inadequate treatment of the breast cancer, a five-fold lower risk of inadequate axillary staging and nine times lower risk of inadequate definitive axillary treatment, 57% lower local recurrence rates at eight years, and 20% lower risk of death from breast cancer after allowing for case-mix and adjuvant therapies which implied adequate management of breast cancer surgery is essential to ameliorating the prognosis of breast cancer. Skinner et al. [51] also showed surgeons who performed more than 15 breast cancer surgeries per year achieved better 5-year survival than whom performed 1 to 5 breast cancer surgeries per year. A large retrospective study was reported by Chang et al. which analyzed outcomes of 77,971 patients after breast cancer surgery revealed that breast cancer outcomes were significant associated with surgeon seniority and volume in Taiwan [52]. Since all patients with breast cancer received surgery that was performed by an experienced surgeon in our study, the generalizability may not be guaranteed. However, this restriction can minimize the impact of different surgeons on outcome of different anesthetic techniques. The possible explainations on breast cancer outcome may be related to advanced surgical skill and appropriate use of adjuvant therapies. The major difference from the previous reports [12–14, 18, 21] is our multivariable analysis which showed no significant difference in the 5-year survival between desflurane and propofol anesthesia possibly because the patients were treated by an experienced specialist surgeon. Additionally, the low mortality rate of breast cancer may have interfered with our study results, leading to no significant difference in the survival rates for both groups [53]. Moreover, the previous reports in Asian patients with breast cancer were no significant survival [12–14] between anesthetics compared with Western patients with breast cancer [21]. Therefore, the ethnic effects might be considered.
Our study had a few limitations. First, this is a retrospective study. Patients were not randomly allocated and characteristics such as the ASA score and TNM stage may have introduced uncontrolled biases. Second, potential confounding factors and selection bias may exist due to the lack of perioperative anesthesia care standardization. Fifty percentage of the patients received total intravenous anesthesia performed by one anesthesiologist (Anesthesiologist A), therefore, these may cause some selection bias. However, we further adjusted for the effect of anesthesiologists, and figured out that the factor of anesthesiologists was not associated with breast cancer mortality (S3 Table) which was consistent with previous study that anesthesiologist volumes were not risk factors for postoperative mortality or long-term survival after radical cystectomy for bladder cancer in high volume hospital [54]. Third, we only analyzed perioperative factors with one experienced surgeon. Further medical treatment, oncologists, and radiation therapy, which are potential confounding factors, were varied. Fourth, we only investigated desflurane which was the most used volatile anesthetic in our hospital. Fifth, early screening is considered to increase the rate of diagnosis in breast cancer and are related to good prognosis of 5-year follow-up in early stage breast cancer. Long-term follow-up of > 5 years in breast cancer is taken into account to evaluate the difference of both groups. Sixth, this study was conducted in a single center. To investigate our hypothesis further, multicenter studies are required.
In conclusion, propofol and desflurane have no obvious differences in prognosis and survival after breast cancer surgery by an experienced surgeon. Further prospective studies should be conducted to identify the influence of propofol on breast cancer outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank the Cancer Registry Group of Tri-Service General Hospital for the clinical data support.
The authors would like to thank Enago (www.enago.com) for the English language review.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by grants from Tri-Service General Hospital (TSGH-C106-087), Taiwan, Republic of China.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27(5):926–33. 10.1093/annonc/mdw027 [DOI] [PubMed] [Google Scholar]
- 3.Heaney A, Buggy DJ. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth. 2012;109 Suppl 1:i17–i28. [DOI] [PubMed] [Google Scholar]
- 4.Li R, Liu H, Dilger JP, Lin J. Effect of Propofol on breast Cancer cell, the immune system, and patient outcome. BMC Anesthesiol. 2018;18(1):77 10.1186/s12871-018-0543-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro J, Jersky J, Katzav S, Feldman M, Segal S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–85. 10.1172/JCI110303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119(3):593–605. 10.1097/ALN.0b013e31829e47fd [DOI] [PubMed] [Google Scholar]
- 7.Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–50. 10.1002/ijc.26448 [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Zhang L, Han Y, Jiang Z, Wang Q. Propofol reduces MMPs expression by inhibiting NF-kappaB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66(1):52–6. 10.1016/j.biopha.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 9.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–9. 10.1213/01.ane.0000082995.44040.07 [DOI] [PubMed] [Google Scholar]
- 10.Kushida A, Inada T, Shingu K. Enhancement of antitumor immunity after propofol treatment in mice. Immunopharmacol Immunotoxicol. 2007;29(3–4):477–86. 10.1080/08923970701675085 [DOI] [PubMed] [Google Scholar]
- 11.Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. Br J Anaesth. 2014;113 Suppl 1:i63–7. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69(2):126–32. 10.4097/kjae.2016.69.2.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MH, Kim DW, Kim JH, Lee KY, Park S, Yoo YC. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget. 2017;8(52):90477–87. 10.18632/oncotarget.21014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoo S, Lee HB, Han W, Noh DY, Park SK, Kim WH, et al. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology. 2019;130(1):31–40. 10.1097/ALN.0000000000002491 [DOI] [PubMed] [Google Scholar]
- 15.Chen CS, Liu TC, Lin HC, Lien YC. Does high surgeon and hospital surgical volume raise the five-year survival rate for breast cancer? A population-based study. Breast Cancer Res Treat. 2008;110(2):349–56. 10.1007/s10549-007-9715-4 [DOI] [PubMed] [Google Scholar]
- 16.Lai HC, Chan SM, Lu CH, Wong CS, Cherng CH, Wu ZF. Planning for operating room efficiency and faster anesthesia wake-up time in open major upper abdominal surgery. Medicine (Baltimore). 2017;96(7):e6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu TC, Lai HC, Lu CH, Huang YS, Hung NK, Cherng CH, et al. Analysis of anesthesia-controlled operating room time after propofol-based total intravenous anesthesia compared with desflurane anesthesia in functional endoscopic sinus surgery. Medicine (Baltimore). 2018;97(5):e9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. 10.1097/ALN.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 19.Heinze G, Juni P. An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–8. 10.1093/eurheartj/ehr031 [DOI] [PubMed] [Google Scholar]
- 20.Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53(6):1112–7. 10.1093/ejcts/ezy167 [DOI] [PubMed] [Google Scholar]
- 21.Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic—sevoflurane or propofol—and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci. 2014;119(3):251–61. 10.3109/03009734.2014.922649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: A systematic review. J Clin Anesth. 2017;42:19–25. 10.1016/j.jclinane.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 2018;129(5):932–941. 10.1097/ALN.0000000000002357 [DOI] [PubMed] [Google Scholar]
- 24.Woo JH, Baik HJ, Kim CH, Chung RK, Kim DY, Lee GY, et al. Effect of propofol and desflurane on immune cell populations in breast cancer patients: a randomized trial. J Korean Med Sci. 2015;30(10):1503–8. 10.3346/jkms.2015.30.10.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res. 2013;33(10):4255–60. [PubMed] [Google Scholar]
- 26.Markovic SN, Knight PR, Murasko DM. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology. 1993;78(4):700–6. 10.1097/00000542-199304000-00013 [DOI] [PubMed] [Google Scholar]
- 27.Aarts L, van der Hee R, Dekker I, de Jong J, Langemeijer H, Bast A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995;357(1):83–5. 10.1016/0014-5793(94)01337-z [DOI] [PubMed] [Google Scholar]
- 28.Welden B, Gates G, Mallari R, Garrett N. Effects of anesthetics and analgesics on natural killer cell activity. AANA J. 2009;77(4):287–92. [PubMed] [Google Scholar]
- 29.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, et al. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014;111(7):1338–49. 10.1038/bjc.2014.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248(6):909–18. 10.1097/SLA.0b013e31818f3dda [DOI] [PubMed] [Google Scholar]
- 31.Hooijmans CR, Geessink FJ, Ritskes-Hoitinga M, Scheffer GJ. A systematic review of the modifying effect of anaesthetic drugs on metastasis in animal models for cancer. PLoS One. 2016;11(5):e0156152 10.1371/journal.pone.0156152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu B, Gao W, Zhou H, Miao X, Chang Y, Wang L, et al. Propofol induces apoptosis of breast cancer cells by downregulation of miR-24 signal pathway. Cancer Biomark. 2018;21(3):513–9. 10.3233/CBM-170234 [DOI] [PubMed] [Google Scholar]
- 33.Song J, Shen Y, Zhang J, Lian Q. Mini profile of potential anticancer properties of propofol. PLoS One. 2014;9(12):e114440 10.1371/journal.pone.0114440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng C, Song L, Wang J, Li D, Liu Y, Cui X. Propofol induces proliferation partially via downregulation of p53 protein and promotes migration via activation of the Nrf2 pathway in human breast cancer cell line MDA-MB-231. Oncol Rep. 2017;37(2):841–8. 10.3892/or.2016.5332 [DOI] [PubMed] [Google Scholar]
- 35.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001;19(22):4224–37. 10.1200/JCO.2001.19.22.4224 [DOI] [PubMed] [Google Scholar]
- 36.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. 10.1200/JCO.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- 37.Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19(1):27–39. 10.1016/S1470-2045(17)30777-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CH, Lo YF, Tsai HP, Shen SC, Chao TC, Chen MF, et al. Low body mass index is an independent risk factor of locoregional recurrence in women with breast cancer undergoing breast conserving therapy. Chang Gung Med J. 2009;32(5):553–62. [PubMed] [Google Scholar]
- 39.Yu X, Zhou S, Wang J, Zhang Q, Hou J, Zhu L, et al. Hormone replacement therapy and breast cancer survival: a systematic review and meta-analysis of observational studies. Breast Cancer. 2017;24(5):643–57. 10.1007/s12282-017-0789-5 [DOI] [PubMed] [Google Scholar]
- 40.Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–52. 10.1158/0008-5472.CAN-16-1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 2003;4(12):760–8. 10.1016/s1470-2045(03)01282-8 [DOI] [PubMed] [Google Scholar]
- 42.Michelson S, Leith JT. Dormancy, regression, and recurrence: towards a unifying theory of tumor growth control. J Theor Biol. 1994;169(4):327–38. 10.1006/jtbi.1994.1155 [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232(1):58–65. 10.1097/00000658-200007000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–26. 10.1016/j.bbi.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 45.Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114. [DOI] [PubMed] [Google Scholar]
- 46.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–52. 10.1245/s10434-008-9890-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oh CS, Lee J, Yoon TG, Seo EH, Park HJ, Piao L, et al. Effect of equipotent doses of propofol versus sevoflurane anesthesia on regulatory T cells after breast cancer surgery. Anesthesiology. 2018; 129(5):921–931. 10.1097/ALN.0000000000002382 [DOI] [PubMed] [Google Scholar]
- 48.Sainsbury R, Haward B, Rider L, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet. 1995;345(8960):1265–70. 10.1016/s0140-6736(95)90924-9 [DOI] [PubMed] [Google Scholar]
- 49.Stefoski Mikeljevic J, Haward RA, Johnston C, Sainsbury R, Forman D. Surgeon workload and survival from breast cancer. Br J Cancer. 2003;89(3):487–91. 10.1038/sj.bjc.6601148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kingsmore D, Hole D, Gillis C. Why does specialist treatment of breast cancer improve survival? The role of surgical management. Br J Cancer. 2004;90(10):1920–5. 10.1038/sj.bjc.6601846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–15. 10.1245/aso.2003.06.017 [DOI] [PubMed] [Google Scholar]
- 52.Chang HT, Shi HY, Wang BW, Yeh SJ. Breast cancer incidence and predictors of surgical outcome: a nationwide longitudinal study in Taiwan. Clin Oncol (R Coll Radiol). 2017;29(6):362–9. [DOI] [PubMed] [Google Scholar]
- 53.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 54.Jaeger MT, Siemens DR, Wei X, Peng P, Booth CM. Association between anesthesiology volumes and early and late outcomes after cystectomy for bladder cancer: a population-based study. Anesth Analg. 2017;125(1):147–55. 10.1213/ANE.0000000000001781 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.