Abstract
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is upregulated in a variety of tissues in obesity. It is still unclear as to whether NADPH oxidase upregulation in a specific tissue is part of a systemic response. Here we analyzed the expression pattern of NADPH oxidase in vascular, adipose, and kidney tissues in a rat model of diet-induced obesity. After weaning, rats were fed either a normal or high-fat diet for 12 weeks. The high-fat diet resulted in 20% increased body weight. In the aorta, Nox4 expression was increased by three-fold in obese rats. Upregulations of p22phox and p47phox in adipose, and Nox4, p22phox, and p47phox in kidney were observed in obesity. Marked increases in plasma leptin and insulin were observed, with more modest changes in adiponectin in obese rats. The average systolic blood pressure in the obese group was 11 mmHg higher than that of lean rats (P < 0.005). There was a significant correlation between blood pressure and aortic Nox4 expression (P < 0.01). In cultured vascular smooth muscle cells, adiponectin reduced the expression of Nox4 in a protein kinase A-dependent manner. Our results suggest that upregulation of NADPH oxidase in multiple tissues during obesity appears to be a systemic response. At least in vitro, adiponectin may have a protective antioxidant role by suppressing vascular NADPH oxidase expression. The association between NADPH oxidase Nox4 expression in the vasculature and the elevated blood pressure in obesity requires further investigation.
Keywords: Obesity, NADPH oxidase, Oxidative stress, Blood pressure, Adiponectin
Introduction
Obesity is associated with increased risk of cardiovascular diseases such as atherosclerosis and hypertension.1 Excessive generation of reactive oxygen species (ROS) and resultant oxidative stress in obesity may have an important role in the development of various cardiovascular complications.2 Reduced beta-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is an ROS-generating enzyme.3 This enzyme is a multi-component complex consisting of the cytosolic p47phox and p67phox subunits, the membrane-bound Nox and p22phox subunits, and a small G protein Rac.4 To date, seven Nox isoforms have been found in mammalian cells: namely Nox1 to Nox5 and Duox1 and 2. All Nox proteins have six transmembrane domains, while Nox5 and Duox have 2–4 EF-hand Ca2+-binding domains in the N terminus. Duox has an additional N-terminal transmembrane domain and an extracellular peroxidase homology domain (for a review see5). Activation of this enzyme and subsequent ROS generation is thought to be involved in modulating cell proliferation, apoptosis, hypertrophy, and expression of inflammatory molecules.5 Dysregulated ROS production from NADPH oxidase has been implicated in a variety of cardiovascular disorders including endothelial dysfunction, atherosclerosis, and hypertension.5,6
Increased expression of NADPH oxidase subunits has been observed in animal models with genetic or diet-induced obesity. For example, the mRNA levels of Nox2, p47phox, p22phox, and p67phox are all elevated in the white adipose tissue in a genetic model of severe obesity and type II diabetes in mouse.7 Increased Nox2 and p47phox were also found in the kidney of rats fed a high-fat diet8,9 and in the heart of ob/ob mice.10 Moreover, NADPH oxidase is upregulated during obesity in the aorta,9 coronary arteries,11 and human vascular endothelial cells isolated from obese subjects.12 However, these previous studies employed different models of obesity; thus, it is still unclear as to whether NADPH oxidase upregulation in a specific tissue in obesity is part of a global systemic response or is related to the method used to induce obesity.
In this study, we examined the expression pattern of different NADPH oxidase subunits in multiple tissues in a well-characterized rat model of obesity induced by high-fat diet feeding, where animals voluntarily consume more than double the energy intake of control rats.13–15 Previously, we demonstrated that in these obese rats, the plasma concentrations of insulin, leptin, and adiponectin were all increased as compared to lean controls, whereas the neuropeptide Y concentration in the paraventricular nucleus of the hypothalamus was decreased.13,14 In the present study, we further demonstrate that the diet-induced obesity is associated with a consistent upregulation of NADPH oxidase in the vasculature, kidney, and white fat tissues. Moreover, we provide in vitro evidence that adiponectin may have a role in regulating vascular NADPH oxidase expression.
Methods
Animals and diet treatment
Sprague-Dawley rats were used in accordance with the guidelines from National Health and Medical Research Council of Australia. All procedures were approved by the Animal Ethics Committee of the University of Melbourne. After weaning at 3 weeks, rats were randomly divided into two different diet groups. The normal diet group (n = 22) was fed a standard chow diet consisting of 12% fat, whereas the high-fat diet group (n = 21) was fed a highly palatable cafeteria-style diet consisting of the standard chow supplemented with meat pies, pasta, and cakes as described previously.13–15 This high-fat diet consisted of 30% fat, 56% carbohydrates, and 14% protein.16 At 16 weeks, non-fasted rats were anesthetized by intraperitoneal injection of pentobarbitone sodium (100 mg/kg) and were decapitated after removal of a cardiac blood sample. The thoracic aorta was removed and the fat around the aorta was cleaned in cold phosphate-buffered saline (PBS). The cleaned aorta was preserved in 1 ml of RNAlater (Ambion, Austin, TX, USA) and stored at −20°C. Kidneys and white adipose tissues (collected from the retroperitoneal fat pads) were removed and snap frozen in liquid nitrogen, and then stored at −80°C. In order to ensure a consistency of the samples, all animals were sacrificed within the same time period each morning.
Blood pressure measurement
Systolic blood pressure was measured as described previously using a conscious animal tail-cuff plethysmography system (SDR Clinical Technology, Sydney, Australia). Rats were prewarmed by placing them in a cage under a heat lamp set at 37°C for 5–8 minutes before BP measurements. In each session, values from three to five readings were averaged for analysis.
Plasma hormone levels
Plasma leptin, insulin, and adiponectin concentrations were measured with commercial radioimmunoassays as described previously.14
RNA extraction
Cells or tissues were homogenized in TRI Reagent (Ambion) and the RNA extracted following the manufacturer's instructions.
Real-time polymerase chain reaction
Total RNA was reverse-transcribed to cDNA using random hexamer and TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) at 48°C for 30 minutes followed by 95°C for 5 minutes. The real-time polymerase chain reaction (PCR) reactions were performed in the ABI Prism 7000 system (Applied Biosystems) using the cDNA as template. The 18 seconds RNA was used as a housekeeping gene. Amplification of both target and housekeeping genes was performed in separate tubes using TaqMan Universal PCR master mix (Applied Biosystems), 15 ng cDNA and, the pre-designed probe and primer sets for rat-specific Nox1, Nox4, CYBB (Nox2), p47phox, or CYBA (p22phox) genes (TaqMan Gene Expression Assays, Applied Biosystems). The thermal cycler parameters were 2 minutes at 50°C, 10 minutes at 95°C and 40 cycles of 95°C, 30 seconds and 60°C, 1 minutes. The results of real-time PCR were expressed as 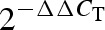 values.
values.
Cell culture and treatment
Rat aortic smooth muscle cells were isolated from the rat aorta by enzymatic digestion as described previously.17 Cells were then cultured in Dulbecco's modification of Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 5% fetal calf serum in 95% O2/5% CO2 at 37°C. Before treatment, cells of ∼90% confluence were arrested with serum-free medium for 24 hours, and then were treated with rat recombinant adiponectin (from BioVision, Mountain View, CA, USA) for 18 hours. Adiponectin was initially reconstituted in PBS, and equal amount of PBS was used as vehicle control in the cell culture.
Data analysis
Results are expressed as mean ± standard error of the mean (SEM). Data were analyzed by one-way analysis of variance (one-way ANOVA followed by Newman–Keuls t-test), paired or unpaired t-tests as appropriate. Statistical significance was accepted at P < 0.05. As the real-time PCR results (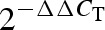 values) in the control group were normalized to 1, correlation analysis with these data was impossible. To overcome this, ΔCT values from half of the control samples were randomly selected and used as calibrators, and
values) in the control group were normalized to 1, correlation analysis with these data was impossible. To overcome this, ΔCT values from half of the control samples were randomly selected and used as calibrators, and 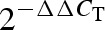 values were calculated for the other half of control samples and all obese samples using these calibrators. These
values were calculated for the other half of control samples and all obese samples using these calibrators. These 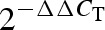 values were used for correlation analyses. All statistic analyses were performed using GraphPad Prism 4.0 (San Diego, CA, USA).
values were used for correlation analyses. All statistic analyses were performed using GraphPad Prism 4.0 (San Diego, CA, USA).
Results
High-fat diet-induced obesity in rats
The body weight of rats in the high-fat diet group was 20% higher compared to the normal diet controls (P < 0.001, Table 1). The average systolic blood pressure in the diet-treated group was 11 mmHg higher than that of the control group (P < 0.005, Table 1). Previous work from our laboratory indicated that adding extra sodium (0.9%) to the drinking water did not influence the blood pressure increase induced by consumption of the high-fat diet (Morris, unpublished observation). When preparing the high-fat food, care was taken to choose foods low in added salt. Therefore, it is unlikely that the increase in blood pressure observed here was caused by changes in sodium intake.
Table 1.
Body weight, systolic blood pressure, and plasma hormone levels in lean and obese rats
| Con | HFD | |
|---|---|---|
| Body weight (g) | 539.0 ± 9.0 | 653.9 ± 11.1* |
| Systolic blood pressure (mmHg) | 135.1 ± 2.5 | 146.0 ± 2.3** |
| Insulin (ng/ml) | 4.5 ± 0.5 | 8.0 ± 0.5* |
| Leptin (ng/ml) | 7.6 ± 0.6 | 24.8 ± 2.4* |
| Adiponectin (μg/ml) | 3.2 ± 0.1 | 4.4 ± 0.2* |
Rats were maintained on a normal diet (Con) or a high-fat diet (HFD) for 12 weeks. Data are mean ± standard error of the mean (SEM). *P < 0.001, **P < 0.005 versus Con, unpaired t-test (n = 21–22).
Consistent with our previous findings,14 the plasma levels of insulin and leptin, were markedly increased, with a more modest increase in adiponectin in obese rats (all P < 0.001, Table 1). Interestingly, when the plasma levels of adiponectin were corrected for white fat mass, the levels in obese animals were half those of the lean rats (0.70 ± 0.05 in control versus 0.38 ± 0.02 µg/ml/g in the high-fat group, P < 0.001, unpaired t-test).
Expression of NADPH oxidase in the aorta, kidney, and adipose tissues
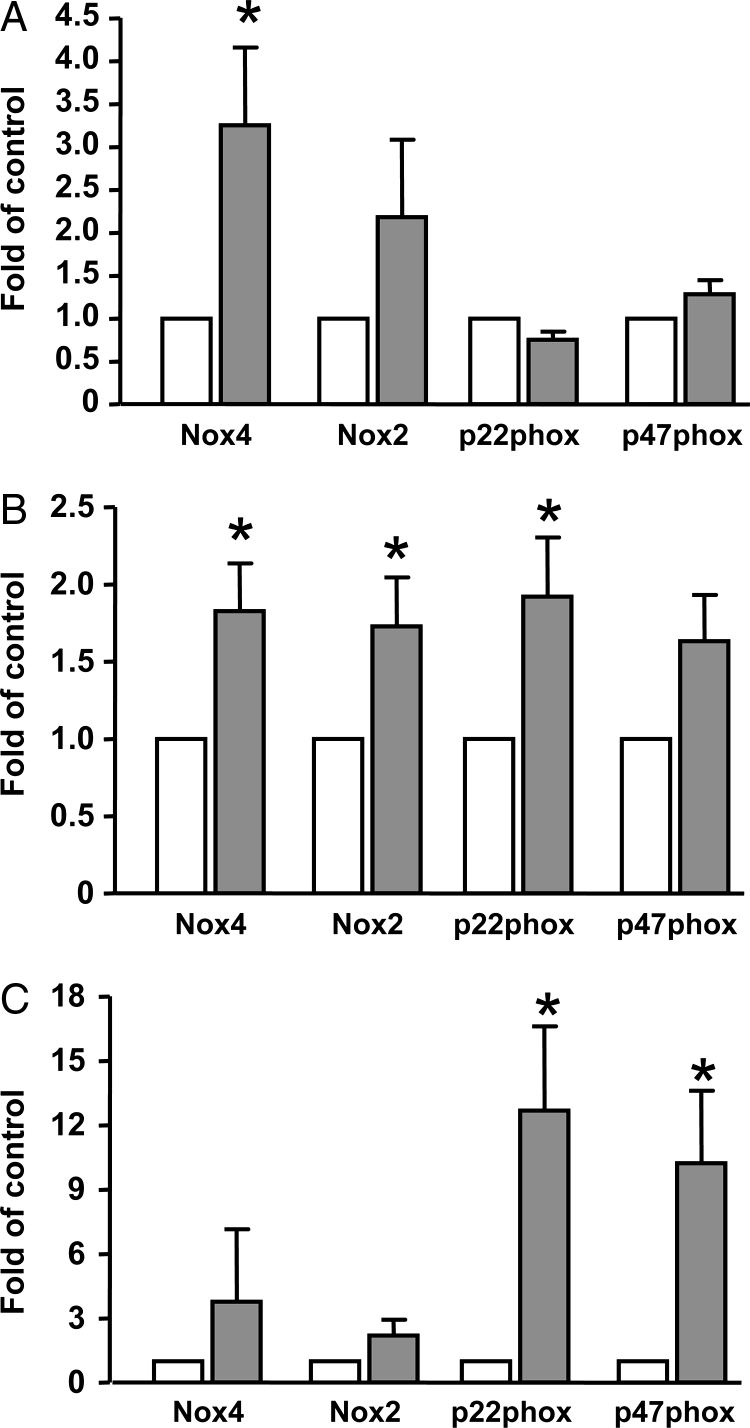
In the aorta, the NADPH oxidase subunits Nox4, Nox2, p22phox, and p47phox were detected by quantitative real-time PCR. We demonstrated that there was a significant increase in the expression of Nox4 in high-fat diet-fed rats, whereas the expressions of Nox2, p22phox, and p47phox were not significantly different between the two groups (Fig. 1A). Nox1 mRNA could not be detected in the aorta samples. However, using the same primer-probe set, we could detect Nox1 expression in cultured vascular smooth muscle cells (see below), indicating that in vivo expression of Nox1 in the aorta was low. To verify whether the upregulation of Nox in blood vessels is associated with increased ROS production, we performed chemiluminescence measurement of superoxide using fresh aortic rings as described previously.17 We found that the basal superoxide release (without exogenous NADPH) was significantly increased in the aorta from the high-fat diet group (35.7 ± 6.6 versus 17.5 ± 3.6 count per second, P < 0.05, n = 4–7). Moreover, we confirmed that this basal superoxide production was inhibited by 99% when the tissues were preincubated with the NADPH oxidase inhibitor diphenyleneiodonium (10 µM), which was consistent with our previous studies.17
Figure 1.
Expression of NADPH oxidase subunits in (A) aorta, (B) kidney, and (C) white adipose tissues from normal diet- (open bars, control) or high-fat-diet-fed (closed bars) rats. Data are mean ± SEM. *P < 0.05 versus control, paired t-test, n = 4–10.
In the kidney, there was a significant increase in the mRNA levels of Nox4, Nox2, and p22phox, but not of p47phox (Fig. 1B). In the white adipose tissue, expression of p22phox and p47phox were significantly elevated, while Nox4 and Nox2 were not significantly altered (Fig. 1C).
Effects of adiponectin on NADPH oxidase expression in smooth muscle cells
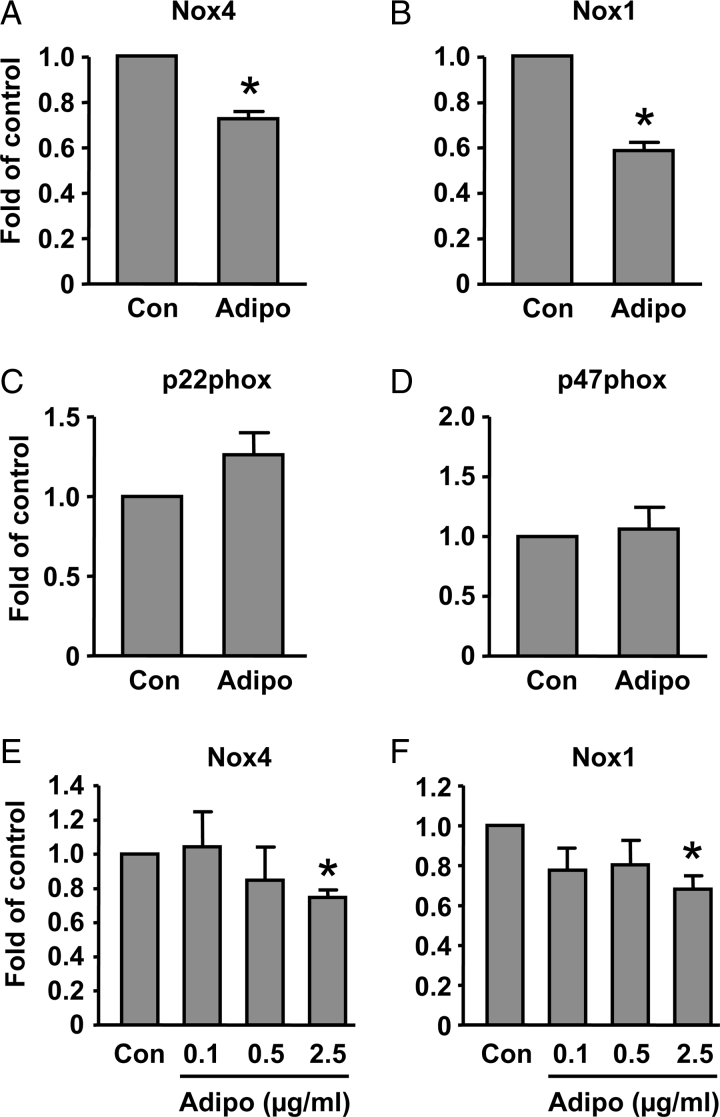
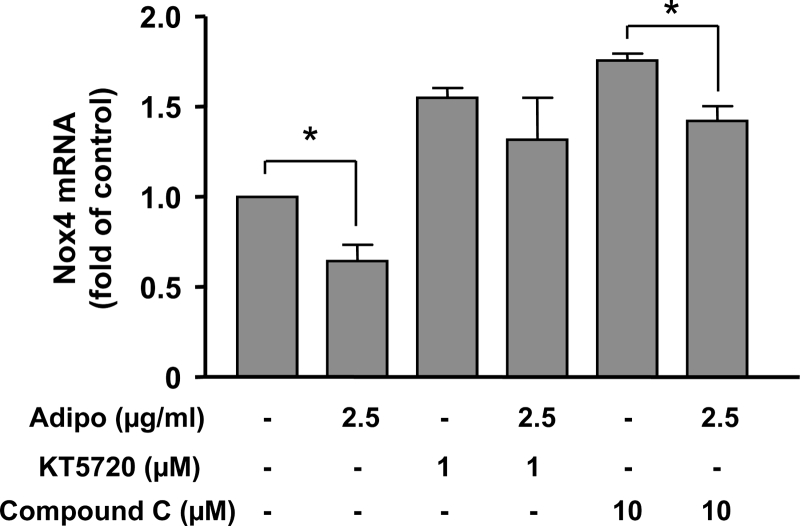
There is evidence that adiponectin can suppress NADPH oxidase-dependent superoxide generation in leukocytes and endothelial cells.18,19 However, little is known about whether adiponectin affects NADPH oxidase expression. We next examined whether adiponectin could affect expression of NADPH oxidase subunits in cultured vascular smooth muscle cells.20 Using real-time PCR, we found that, in contrast to the aorta that expressed Nox4 and Nox2, but not Nox1, rat aortic smooth muscle cells expressed Nox4 and Nox1, while Nox2 was absent. Both p22phox and p47phox were detectable in cultured smooth muscle cells. Treatment of the cells with adiponectin (2.5 µg/ml) for 18 hours significantly reduced the mRNA levels of Nox1 and Nox4, whereas the expression of p22phox and p47phox were not changed (Figs 2A–D). To examine whether the inhibitory effects of adiponectin on Nox expression are concentration dependent, we treated the cells with adiponectin at different concentrations. As shown in Figs 2E and F, adiponectin had little effect on Nox expression at lower concentrations (0.1 or 0.5 µg/ml). To understand the mechanisms of the inhibitory effect of adiponectin on Nox4 expression, we pretreated cells with inhibitors of AMP-activated protein kinase (AMPK) (Compound C) and protein kinase A (PKA) (KT5720). We found that both Compound C and KT5720 increased the basal level of Nox4. However, the inhibitory effect of adiponectin was diminished in the presence of KT5720, but not Compound C (Fig. 3). Moreover, we found that treatment with the cell-permeable AMPK activator AICAR did not reduce the expression level of Nox4 (data not shown).
Figure 2.
Effects of adiponectin (2.5 µg/ml in A–D) treatment for 18 hours on mRNA levels of NADPH oxidase subunits in cultured rat aortic smooth muscle cells. Equal amount of saline was used as vehicle control. Data are mean ± SEM. *P < 0.05 versus control (Con), unpaired t-test, n = 3–6.
Figure 3.
Effects of the PKA inhibitor KT5720 and the AMPK inhibitor Compound C on adiponectin-induced downregulation of Nox4 expression in cultured rat aortic smooth muscle cells. Data are mean ± SEM. *P < 0.05, one-way ANOVA followed by unpaired t-test, n = 5.
Aortic Nox4 expression correlates with blood pressure
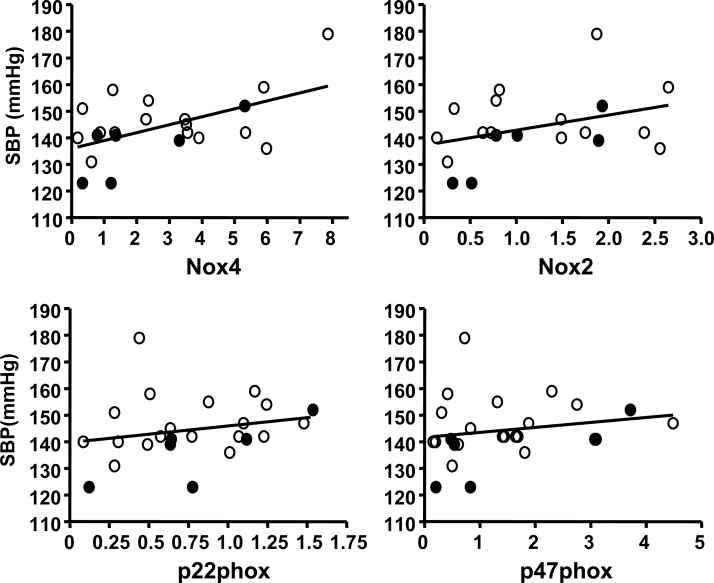
Previous studies have suggested that overexpression of NADPH oxidase may be involved in the development of hypertension.21,22 On the basis of this evidence, we examined whether the upregulation of NADPH oxidase subunits was related to the increased blood pressure in obese rats. We performed correlation analysis and found that there was a significant correlation (r2 = 0.29, P < 0.01) between aortic Nox4 expression and systolic blood pressure (Fig. 4). In contrast, no significant correlation was found between the levels of Nox2, p22phox or p47phox levels, and blood pressure (Fig. 4). Moreover, there was no correlation between blood pressure and the expression of NADPH oxidase subunits in either kidney or adipose tissue.
Figure 4.
Correlation analysis of the mRNA levels of NADPH oxidase subunits in the aorta with the systolic blood pressure. Samples were pooled from both the normal diet (black dots) and the high-fat diet (open dots) groups.
Discussion
In the present study, we systematically analyzed the expression pattern of both the membrane-bound (Nox and p22phox) and the cytosolic (p47phox) subunits of NADPH oxidase in a rat model of high-fat diet-induced obesity.13–15 The insulin level was significantly increased in these obese rats (Table 1). Moreover, the high-fat diet treatment has been shown to result in significant increases in triglycerides (more than doubled) and free fatty acids (by 30%), and a modest increase in blood glucose (data not shown). All these results suggest that a metabolic syndrome-like status (hyperinsulinemia, hyperlipidemia, and increased free fatty acids) was well established. In these obese rats, we found that there was a systemic upregulation of NADPH oxidase in the aorta, kidney, and white fat. In the aorta, we found that there is a significant increase in the expression level of the Nox4 subunit of NADPH oxidase. Moreover, the expression of the Nox2 subunit also tended to be increased by the high-fat diet, but this change was not statistically significant. These data are in agreement with the finding by Roberts et al.9 that vascular NADPH oxidase expression was increased during obesity. We also found that p22phox and p47phox were significantly higher in the white adipose tissue of obese rats, while Nox4, p22phox, and p47phox were increased in the kidney during obesity. Our observations are supported by previous studies showing that NADPH oxidase was upregulated in both of the white fat7 and kidney8,9 in obese animal models. Importantly, by using a single animal model, we have extended previous studies by demonstrating that upregulation of NADPH oxidase in different tissues may represent a systemic response to obesity. The precise molecular mechanisms underlying the tissue-specific pattern of changes in NADPH oxidase subunit expression are not understood. It appears that regulation of NADPH oxidase expression may be highly variable in different cells, even in response to the same stimulus. For example, in kidney cells, it has been shown that angiotensin II upregulated the expression of Nox4 and p22phox,23,24 whereas angiotensin II upregulated p47phox and p22phox, but downregulated Nox4 in vascular smooth muscle cells.25,26 We speculate that two potential mechanisms may explain the current results. First, expression of different NADPH oxidase subunits are likely to be under the control of non-overlapping signaling pathways; and second, different cells may interpret similar neurohormonal signals elicited by obesity differentially by activating diverse signaling cascades.
Adiponectin is a peptide that is specifically and abundantly expressed in adipose tissue, which has a direct sensitizing effect on insulin signaling.27 However, little is known about whether adiponectin affects NADPH oxidase expression. Here we examined the effects of adiponectin in cultured aortic smooth muscle cells, and found that chronic (18 hours) treatment specifically reduced the expression levels of Nox1 and Nox4 (Nox2 could not be detected in cultured smooth muscle cells), while those of p22phox and p47phox were unchanged. Furthermore, the effects of adiponectin on Nox expression appeared to be concentration dependent. Our data suggest that, at least in vitro, adiponectin may exert a protective antioxidant effect by suppressing vascular NADPH oxidase expression. Most studies in obese human subjects report reduced adiponectin levels;27 whether decreased adiponectin is involved in the upregulation of NADPH oxidase in human obesity warrants further examination. In contrast, the plasma level of adiponectin was increased in obese rats, although remaining low relative to fat mass. This phenomenon is consistent with our previous studies14,28 and work of other researchers using mouse and rat models of diet-induced obesity.29,30 Adiponectin may be regulated by factors other than obesity, as others have reported that hypertension increased levels of adiponectin, possibly as an adaptive response.31 Although this seems to be inconsistent with the increased vascular Nox expression in obese rats, we postulate that in situations of reduced adiponectin, the upregulation of Nox in vascular tissues may be more detrimental.
Adiponectin, via its receptors AdipoR1 and AdipoR2, induces intracellular signaling, mainly via activation of AMPK.27 Thus, we examined whether the effect of adiponectin on Nox4 expression was mediated by AMPK. However, we found that adiponectin-induced Nox4 downregulation was not affected by the AMPK inhibitor Compound C, but was diminished by the PKA inhibitor KT5720, indicating that the inhibitory effect of adiponectin on Nox4 expression in vascular smooth muscle cells was unlikely to be mediated by activation of AMPK, but might be related to activation of the cAMP/PKA pathway.32 Furthermore, this result was supported by the finding that activation of AMPK by AICAR did not suppress Nox4 expression in smooth muscle cells.
There is a close relationship between obesity and the development of hypertension.33 Growing evidence shows that oxidative stress in the vasculature,34,35 kidney,36 and adipose tissues7,37 may all contribute to elevated blood pressure in obesity either directly or indirectly. Consistent with our previous studies, feeding with the high-fat diet resulted in an elevated systolic blood pressure.14 Interestingly, we demonstrated that there is a significant correlation between Nox4 expression and blood pressure. Our results are in line with recent evidence demonstrating that there was an upregulation of Nox4 expression in the aorta in spontaneously hypertensive rats.38 Our results indicate that dysregulation of the Nox4-based NADPH oxidase expression and function in the vasculature may be involved in the development of high blood pressure during obesity. This observation is complementary to previous findings showing elevated expression of other Nox isoforms, such as Nox1 and Nox2, may also be involved in causing high blood pressure.21,22 However, Nox1 could not be detected in the rat aorta under present experimental conditions, indicating that Nox1 is unlikely to have a major role in mediating obesity-induced vascular alterations. Moreover, the role of Nox2 in the development of hypertension appears to vary with different etiologies.22,39 However, the correlation we observed does not establish a causal role of increased vascular Nox4 expression in the development of obesity-associated hypertension.
In summary, we provide evidence that the upregulation of NADPH oxidase expression in multiple tissues during diet-induced obesity appears to be a systemic response. At least in vitro, adiponectin may have a protective antioxidant role by suppressing vascular NADPH oxidase expression. The association between Nox4 expression in the vasculature and the elevated blood pressure in obesity requires further investigation.
Acknowledgements
The authors thank Nancy Guo for technical assistance in the real-time PCR assay. This study was partially supported by Project Grants from the National Health and Medical Research Council (NHMRC) of Australia, Grants-in-Aid from the National Heart Foundation of Australia, National 973 Basic Research Program of China (2010CB732605), and NSFC (grant 81070164). G.J.D. receives an NHMRC Principal Research Fellowship.
References
- 1.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 2002;324:127–37. [DOI] [PubMed] [Google Scholar]
- 2.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab 2007;9:813–39. [DOI] [PubMed] [Google Scholar]
- 3.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 4.Babior BM. NADPH oxidase. Curr Opin Immunol 2004;16:42–7. [DOI] [PubMed] [Google Scholar]
- 5.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 6.Jiang F, Drummond GR, Dusting GJ. Suppression of oxidative stress in the endothelium and vascular wall. Endothelium 2004;11:79–88. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al.. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension 2004;43:48–56. [DOI] [PubMed] [Google Scholar]
- 9.Roberts CK, Barnard RJ, Sindhu RK, Jurczak M, Ehdaie A, Vaziri ND. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism 2006;55:928–34. [DOI] [PubMed] [Google Scholar]
- 10.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia 2006;49:1434–46. [DOI] [PubMed] [Google Scholar]
- 11.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, et al.. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol 2007;292:H904–11. [DOI] [PubMed] [Google Scholar]
- 12.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, et al.. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 2007;115:627–37. [DOI] [PubMed] [Google Scholar]
- 13.Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide Y in the face of prolonged high-fat feeding in the rat. J Neurochem 2004;88:909–16. [DOI] [PubMed] [Google Scholar]
- 14.Velkoska E, Cole TJ, Morris MJ. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am J Physiol Endocrinol Metab 2005;288:E1236–43. [DOI] [PubMed] [Google Scholar]
- 15.Morris MJ, Velkoska E, Cole TJ. Central and peripheral contributions to obesity-associated hypertension: impact of early overnourishment. Exp Physiol 2005;90:697–702. [DOI] [PubMed] [Google Scholar]
- 16.Hansen MJ, Ball MJ, Morris MJ. Enhanced inhibitory feeding response to alpha-melanocyte stimulating hormone in the diet-induced obese rat. Brain Res 2001;892:130–7. [DOI] [PubMed] [Google Scholar]
- 17.Jiang F, Guo Y, Salvemini D, Dusting GJ. Superoxide dismutase mimetic M40403 improves endothelial function in apolipoprotein(E)-deficient mice. Br J Pharmacol 2003;139:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalang UJ, Rajappan R, Hunter MG, Kutala VK, Kuppusamy P, Wewers MD, et al.. Adiponectin inhibits superoxide generation by human neutrophils. Antioxid Redox Signal 2006;8:2179–86. [DOI] [PubMed] [Google Scholar]
- 19.Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun 2004;315:264–71. [DOI] [PubMed] [Google Scholar]
- 20.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 2005;65:495–504. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, et al.. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 2005;112:2677–85. [DOI] [PubMed] [Google Scholar]
- 22.Fujii A, Nakano D, Katsuragi M, Ohkita M, Takaoka M, Ohno Y, et al.. Role of gp91phox-containing NADPH oxidase in the deoxycorticosterone acetate-salt-induced hypertension. Eur J Pharmacol 2006;552:131–4. [DOI] [PubMed] [Google Scholar]
- 23.Hannken T, Schroeder R, Stahl RA, Wolf G. Angiotensin II-mediated expression of p27Kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int 1998;54:1923–33. [DOI] [PubMed] [Google Scholar]
- 24.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 2008;283:24061–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, et al.. Novel gp91(phox) homologues in vascular smooth muscle cells : nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 2001;88:888–94. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, et al.. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 2002;90:1205–13. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prior LJ, Velkoska E, Watts R, Cameron-Smith D, Morris MJ. Undernutrition during suckling in rats elevates plasma adiponectin and its receptor in skeletal muscle regardless of diet composition: a protective effect? Int J Obes (Lond) 2008;32:1585–94. [DOI] [PubMed] [Google Scholar]
- 29.Bullen JW, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 2007;292:E1079–86. [DOI] [PubMed] [Google Scholar]
- 30.Naderali EK, Estadella D, Rocha M, Pickavance LC, Fatani S, Denis RG, et al.. A fat-enriched, glucose-enriched diet markedly attenuates adiponectin mRNA levels in rat epididymal adipose tissue. Clin Sci (Lond) 2003;105:403–8. [DOI] [PubMed] [Google Scholar]
- 31.Mallamaci F, Zoccali C, Cuzzola F, Tripepi G, Cutrupi S, Parlongo S, et al.. Adiponectin in essential hypertension. J Nephrol 2002;15:507–11. [PubMed] [Google Scholar]
- 32.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, et al.. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes 2006;55:1840–6. [DOI] [PubMed] [Google Scholar]
- 33.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension 2005;45:9–14. [DOI] [PubMed] [Google Scholar]
- 34.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 2004;122:339–52. [DOI] [PubMed] [Google Scholar]
- 35.Ferroni P, Basili S, Paoletti V, Davi G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis 2006;16:222–33. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 2005;289:R913–35. [DOI] [PubMed] [Google Scholar]
- 37.Kurata A, Nishizawa H, Kihara S, Maeda N, Sonoda M, Okada T, et al.. Blockade of angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int 2006;70:1717–24. [DOI] [PubMed] [Google Scholar]
- 38.Akasaki T, Ohya Y, Kuroda J, Eto K, Abe I, Sumimoto H, et al.. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: involvement of the renin-angiotensin system. Hypertens Res 2006;29:813–20. [DOI] [PubMed] [Google Scholar]
- 39.Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, et al.. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 2005;45:530–7. [DOI] [PubMed] [Google Scholar]