Abstract
Metabolic stresses associated with disease, ageing, and exercise increase the levels of reactive oxygen species (ROS) in skeletal muscle. These ROS have been linked mechanistically to adaptations in skeletal muscle that can be favourable (i.e. in response to exercise) or detrimental (i.e. in response to disease). The magnitude, duration (acute versus chronic), and cellular origin of the ROS are important underlying factors in determining the metabolic perturbations associated with the ROS produced in skeletal muscle. In particular, insulin resistance has been linked to excess ROS production in skeletal muscle mitochondria. A chronic excess of mitochondrial ROS can impair normal insulin signalling pathways and glucose disposal in skeletal muscle. In contrast, ROS produced in skeletal muscle in response to exercise has been linked to beneficial metabolic adaptations including mitochondrial biogenesis and muscle hypertrophy. Moreover, unlike insulin resistance, exercise-induced ROS appears to be primarily of non-mitochondrial origin. The present review summarizes the diverse ROS-targeted metabolic outcomes associated with insulin resistance versus exercise in skeletal muscle, thus, presenting two contrasting perspectives of pathologically harmful versus physiologically beneficial ROS. Here, we discuss the key sites of ROS production during exercise and the effect of ROS in skeletal muscle of people with type 2 diabetes.
Keywords: Reactive oxygen species, Mitochondria, Skeletal muscle, Exercise
Introduction
Skeletal muscle is a primary site of adaptation to metabolic insults that can occur as a result of disease or physical stressors such as exercise.1,2 Individuals with type 2 diabetes (T2D) have an impaired capacity of skeletal muscle to transport glucose and store it as glycogen in response to hyperinsulinaemia.3 On the other hand, skeletal muscle becomes more efficient at glucose disposal in response to a programme of endurance exercise training;4 and mitochondrial content and function also become enhanced.5 As polarized as the skeletal muscle adaptations to insulin resistance/T2D and exercise training are, one likely mediating factor in both biological cases in point is an excess of reactive oxygen species (ROS).
ROS are produced at multiple cellular sites including in the mitochondria during oxidative phosphorylation,6 in the endoplasmic reticulum in response to protein folding stresses,7 in membrane-bound NADPH oxidases,8 through xanthine oxidase (XO) activation,9 and via phospholipase A2 (PLA2)-dependent processes.10
In basal conditions, ROS produced in the mitochondria constitute a major source of cellular ROS.6 During oxidative phosphorylation, reducing equivalents (NADH, FADH2) formed during energy metabolism provide electrons to be transferred along a series of electron carriers within the mitochondrial respiratory chain. The transfer of electrons generates increased membrane potential, increased oxygen consumption, and ultimately the conversion of ADP to ATP.11 However, the process is not perfect and a small percentage (∼0.15%) of electrons leak from the respiratory chain complexes (I and III), resulting in the production of ROS including superoxide (·O2−) and hydrogen peroxide (H2O2).12
Interestingly, an excess of ROS has been implicated as both physiologically beneficial and pathologically harmful in the body. ROS have been shown to act as important intracellular signalling molecules for insulin signalling transduction in healthy tissues.13,14 In addition, exercise is known to increase the levels of ROS in skeletal muscle and other tissues.15 ROS produced during exercise are also thought to play a key role in skeletal muscle adaptations associated with exercise training.16
In contrast to this physiologically beneficial ROS, a chronic over-production of ROS systemically and in skeletal muscle promotes oxidative stress and might contribute to the pathogenesis of T2D,2,17 ageing,18,19 cancer cachexia,20 and sarcopenia.21 Excess ROS produced during metabolic and cellular processes can oxidatively damage macromolecules including DNA, lipids, and proteins, modify cellular redox status, and alter cellular functions.22 Furthermore, by acting as signalling molecules,23 excess ROS might act as important causative secondary messengers in the impaired insulin signalling pathways associated with insulin resistance in T2D.11
The seemingly paradoxical role of excess ROS in physiological versus pathological states in skeletal muscle could be a function of magnitude, duration, and/or cellular origin of ROS produced.9 In the present review, we focus on two divergent but key areas that appear to shed some light on this apparent paradox: insulin resistance-related ROS in T2D and contraction-induced ROS.
Linking mitochondrial ROS to skeletal muscle insulin resistance
Skeletal muscle is quantitatively the most important tissue in peripheral insulin resistance in vivo.24 In fact, in T2D, impairments in skeletal muscle glucose disposal account for ∼80% of the defective insulin mediated whole-body glucose disposal.25
Possible causative factors in the pathophysiology of skeletal muscle insulin resistance include ectopic lipid- or lipid intermediate (notably diacylglycerol and ceramide)-induced impairments in insulin signalling pathways via protein kinase C activation26,27 and mitochondrial dysfunction.28 Different experimental models have also linked excess ROS production with the development of skeletal muscle insulin resistance. These models include (a) excess ROS production by the mitochondria in experimental murine models of excess nutrient intake and T2D;2,23,28–30 (b) excess ROS production by NADPH oxidases due to the overactivity of the renin–angiotensin system and increased angiotensin II levels;31 and more recently (c) excess ROS production by XO in experimental diabetes models.32 The present review will focus only on excess ROS production according to model (a) above given the abundance of research that has focused on mitochondrial ROS as being implicated in skeletal muscle insulin resistance.
The relation between increased mitochondrial ROS and skeletal muscle insulin resistance has been well established in vitro and in vivo.17,23,28,29 In humans, it was demonstrated that both acute and chronic high-fat dietary intakes can increase mitochondrial H2O2 production and oxidative stress in the skeletal muscle of healthy, insulin-sensitive individuals.23 Moreover, obese, insulin-resistant humans have increased the levels of mitochondrial skeletal muscle H2O2 emission compared with lean insulin-sensitive individuals.23,33 Hoehn et al.29 demonstrated an increased mitochondrial ·O2− production in palmitate-treated L6-myotubes in vitro and in high-fat-fed experimental mice in vivo. Furthermore, approaches aimed at the selective quenching of mitochondrial ·O2− including the genetic overexpression of manganese superoxide dismutase (MnSOD) or supplementation with the mitochondrial ·O2− targeted antioxidant Mn(III)-tetrakis (4-benzoic acid) porphyrin were shown to improve or prevent skeletal muscle insulin resistance in the high-fat-fed mice.29 In addition, Anderson et al.23 employed the use of both a small antioxidant peptide (SS31) targeted to the mitochondrial inner membrane and the genetic overexpression of mitochondrial-targeted human catalase (MCAT) in experimental rats and mice respectively, fed high-fat diets. All the treatments resulted in a reduction of mitochondrial H2O2 emission by >50%. Moreover, chronic intake of SS31 or genetic overexpression of MCAT prevented the onset of high-fat-diet-induced insulin resistance in skeletal muscle in the experimental animals.23 Together, these and other17 studies implicate excess mitochondrial ROS as both a causative factor in skeletal muscle insulin resistance and a key target for antioxidant-based prevention and treatment strategies.
In contrast to these findings, two recent studies found no benefit of mitochondrial-targeted antioxidant treatments in C57BL/6 mice28,30 for improving skeletal muscle insulin resistance, despite marked reductions in mitochondrial ROS and improvements in skeletal muscle oxidative stress levels. These findings thus raise the possibility that targeting elevated mitochondrial ROS levels associated with skeletal muscle insulin resistance might not in fact be a fruitful treatment for improving insulin sensitivity in disease states (such as T2D) characterized by skeletal muscle insulin resistance. The failure of large-scale antioxidant supplementation trials for T2D prevention34–36 appears to support the futility of this approach for diabetes prevention. However, it should be noted that many of these large studies were limited by study design and subject selection factors.37 Moreover, most studies lacked insight into the mode of action of specific antioxidants and their biological targets,37,38 thus making the interpretation of the findings difficult.
Pathways through which ROS might impair insulin signalling have not been well defined, but ROS have been shown to activate stress-sensitive molecules including mitogen-activated protein kinase (MAPK)39 and c-Jun N-terminal kinase (JNK).40 JNK can phosphorylate the insulin receptor substrate (IRS) subunit of the insulin signalling cascade at serine residues, thus attenuating key metabolic pathways of the insulin signalling cascade (Fig. 1).41 Moreover, antioxidant supplementation in rats was shown to inhibit high-fat-diet-induced activation of JNK and IRS-1 serine phosphorylation in skeletal muscle, thus preventing a reduction in insulin sensitivity.42 Mechanisms through which ROS activate JNK in tissues are unclear.43 However, it has been shown that ROS can activate JNK via oxidation and inactivation of specific JNK-inactivating phosphatases.43 Furthermore, it has been shown that tumour necrosis factor-α-induced mitochondrial ROS can activate JNK through activation of the apoptosis signal-regulating kinase -1 in human hepatoma cells.44
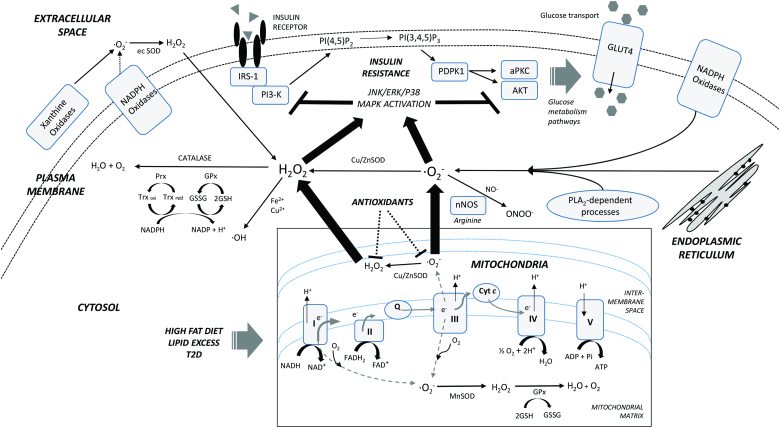
Figure 1.
Cellular origins and effects of ROS in skeletal muscle during insulin resistance. Excess ROS produced in the mitochondria in response to a high-fat diet, excess lipid levels, or T2D promotes activation of major stress pathways that can impair normal insulin signalling, skeletal muscle glucose transport, and intracellular glucose metabolism. A network of endogenous antioxidants acts to scavenge these ROS, but the levels are relatively deficient when burdened with a chronic excess of ROS. Exogenous antioxidant therapy targeting mitochondrial ROS might attenuate this insulin resistance in skeletal muscle (refer to the text for a detailed discussion). Thick arrows indicate primary cellular ROS sources. Broken lines indicate some uncertainty. NADH, reduced nicotinamide adenine dinucleotide; NAD+, oxidized nicotinamide adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; FAD+, oxidized flavin adenine dinucleotide; I–V, complexes of electron transport/oxidative phosphorylation; Cyt c, cytochrome C; GSH, reduced glutathione; GSSG, glutathione disulphide; GPx, glutathione peroxidase; Prx, peroxiredoxin reductase; Trx oxi, oxidized thioredoxin; Trx red, reduced thioredoxin; MnSOD, manganese superoxide dismutase; Cu/ZnSOD, copper/zinc superoxide dismutase; ec SOD, extracellular superoxide dismutase; nNOS, neuronal nitric oxide synthase; PLA2, phospholipase A2; ·O2−, superoxide anion; H2O2, hydrogen peroxide; NO·, nitric oxide; ONOO−, peroxynitrite; ·OH, hydroxyl radical; IRS-1, insulin receptor substrate 1; PI3-K, phosphoinositide 3-kinase; PI(4,5)P2, phosphatidylinositol (3,4)-bisphosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5)-triphosphate; PDPK1, 3-phophoinositide-dependent protein kinase-1; aPKC, atypical protein kinase C; AKT, serine/threonine protein kinase B; GLUT4, glucose transporter type 4; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p38 MAPK, p38 mitogen-activated protein kinase.
Do people with T2D have increased levels of ROS in skeletal muscle?
Studies that have directly measured ROS production in human T2D skeletal muscle have been scant, limited methodologically by ex vivo measurement techniques, and have produced uncertain findings.45–47 Abdul-Ghani et al.45 reported no difference in overall mitochondrial ROS production in skeletal muscle of people with T2D versus healthy controls, although the individuals with T2D had an increased mitochondrial H2O2 generation per unit of ATP production. Given that ATP production is driven by cellular energy demand in vivo, these findings suggest an increased in vivo ROS production in the skeletal muscle of people with T2D.45 Another study46 reported a tendency for increased mitochondrial ROS production in individuals with T2D when compared with control participants matched for age, body mass index (BMI), and physical fitness (and mitochondrial content). In contrast to these studies, Minet and Gaster47 found reduced absolute mitochondrial H2O2 production in primary myotubes from individuals with T2D and no difference in the ratio of mitochondrial H2O2 production per unit of ATP production when compared with the control participants. However, as discussed by the authors, different findings in the latter in vitro study compared with the previous studies could relate to the absence of in vivo metabolic conditions relevant to ROS production in T2D.47 Overall, these studies might also be limited by the accuracy of assays used to assess ROS production from isolated mitochondria. In particular, commonly used redox-sensitive probes such as Amplex Red (10-acetyl-3,7-dihydroxyphenoxazine) could be cross-reactive with lipid hydroperoxides and/or other redox-related metabolites11,48 and are prone to artefactual ROS formation.49 Thus, a degree of caution is required in the interpretation of these findings.11
In addition to the direct measurements of ROS production, indirect oxidative stress measurements reflective of excess ROS including protein carbonyls and 8-hydroxy-2′-deoxyguanosine were shown to be increased in skeletal muscle of people with T2D when compared with non-diabetics.32,50,51 In contrast to these findings, Brinkmann et al.52 reported reduced levels of F2-isoprostanes in the skeletal muscle of people with T2D when compared with age and BMI-matched controls.
Oxidative stress is defined broadly as a suprathreshold imbalance between the production and the scavenging of ROS by the body's antioxidant defence network, favouring ROS production.23 Thus, a limitation of using these oxidative stress markers as indicators of ROS production is that reduced antioxidant levels could promote oxidative stress without a concomitant increase in ROS production. Studies investigating the levels of antioxidant enzymes in the skeletal muscle of people with T2D have produced mixed findings when compared with the levels of insulin-sensitive controls. The protein levels of MnSOD were found to be reduced in the skeletal muscle of individuals with T2D in some studies32,53 but not others.52 On the other hand, Brinkmann et al.52 reported increased levels of peroxiredoxins (PRDX2 and PRDX6) but unaltered levels of glutathione peroxidase (GPx) in the skeletal muscle of people with T2D by using immunohistochemical methods. Collectively, these findings suggest that, compared with the healthy controls, the skeletal muscle antioxidant status in people with T2D is unclear and further studies are required that closely match age, body mass, and physical activity levels.
A lack of comprehensive data on the oxidative stress milieu in the skeletal muscle of people with T2D coupled with the absence of currently available accurate in vivo measurement techniques for the probing of ROS11 makes it difficult to establish a clear conclusion on alterations in the levels of ROS in the skeletal muscle of people with T2D. Moreover, considering potentially complicating factors between studies such as metabolic heterogeneity and the possibility of confounding factors such as the presence of diabetic complications, comparisons between current and future investigations will require some caution.
The importance of ROS in normal skeletal muscle insulin signalling
It was a goal of the above discussion to review the detrimental effects of excess ROS production on skeletal muscle insulin sensitivity in T2D. However, it is apparent that ROS are not inherently detrimental to insulin action. In fact, some evidence tends to the contrary, at least in healthy tissues.13,14
In contrast to potentially harmful excess mitochondrial ROS production, the generation of ROS from non-mitochondrial sources has been shown to be important in relation to healthy insulin signalling in insulin-sensitive tissues.8,54 In particular, H2O2 production by plasma membrane-bound NADPH oxidases has been shown to increase in response to insulin stimulation both in adipocytes and skeletal muscle.8,54 A recent study also implicated XO as a key ROS generator in response to insulin stimulation in skeletal muscle.9 Increased insulin-stimulated H2O2 in skeletal muscle might occur via a phosphoinositide 3-kinase (PI3-K)- and/or protein kinase C-induced calcium release mechanism of action.54 Moreover, H2O2 has been shown to oxidatively modify and inactivate key protein tyrosine phosphatases (PTEN and PTP1B) in adipocytes and skeletal muscle, thus resulting in enhanced insulin signalling.8,14 Although this physiological H2O2 appears to be primarily of NADPH oxidase8,54 or XO9 origin, it was demonstrated that the levels of H2O2, PI3-K/Akt pathway activation, and insulin sensitivity were increased in the skeletal muscle of high-fat-fed transgenic mice lacking the antioxidant enzyme GPx.14 Moreover, the treatment of GPx−/− mice with the antioxidant N-acetyl cysteine promoted increased insulin resistance in the skeletal muscle.14 GPx is ubiquitously expressed both in the mitochondria and the cytosol, thus implying the possible involvement of mitochondrial ROS in the maintenance of normal insulin sensitivity also.
The apparent paradoxical role of ROS in the improvement versus impairment of insulin sensitivity in skeletal muscle could be related to the magnitude and/or duration (acute versus chronic) of excess ROS produced9,14 as well as the cellular origin of ROS (i.e. mitochondrial ROS versus XO-derived ROS).9 To further our discussion on physiologically beneficial ROS, we now proceed with a detailed review of exercise and skeletal muscle ROS production. A discussion of the major cellular sites of ROS production during muscular contraction is followed by a review of several key potential ROS-mediated adaptive responses to exercise.
Sites of skeletal muscle ROS production during contraction
Since oxygen consumption increases during exercise, it is often assumed that the mitochondria are the major source of ROS production during contraction. However, the mitochondria make a large contribution to ROS production at rest, but not during contraction in mouse myotubes.55 Also, mitochondrial oxygen consumption can increase up to 100-fold during contraction, whereas ROS production only increases 2- to 4-fold.55–57 Indeed, recent work suggests that the majority of ROS production during contraction is from non-mitochondrial sources and includes NADPH oxidase, nitric oxide synthase (NOS), calcium-dependent 14 kDa isoform of PLA2 and XO.55–58 Contracting rat and mouse muscle cells have been shown by several authors by using the ROS-sensitive fluorescent probe dichloro-dihydro-fluorescein diacetate (DCFH-DA) to increase intracellular ROS levels, with the fluorescence being detected throughout the muscle fibre and not localized to the mitochondria.55–57 Thus, the increase in ROS during contraction is probably not from the mitochondria and involves other potential sites of ·O2−/H2O2 production (Fig. 2).
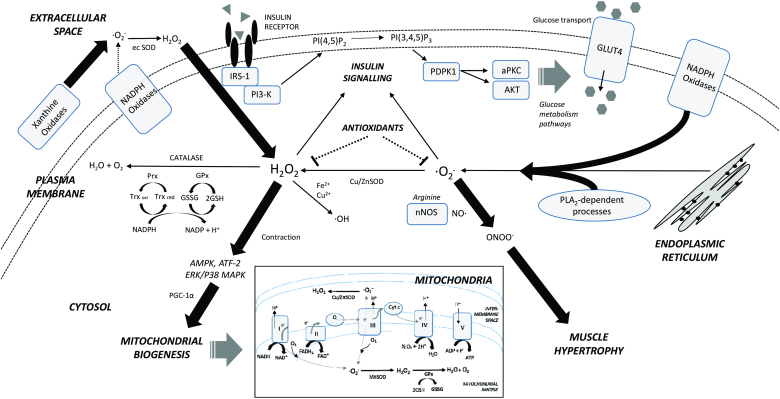
Figure 2.
Cellular origins and effects of ROS in skeletal muscle during exercise contraction. Excess ROS produced in non-mitochondrial sources in response to exercise can promote adaptations including mitochondrial biogenesis through stress kinase activation and muscle hypertrophy via peroxynitrite-related signalling pathways. ROS (primarily from non-mitochondrial sources) also promotes insulin signalling transduction. A network of endogenous antioxidants acts to scavenge these ROS, but an excess intake of exogenous antioxidants might impair exercise adaptations (refer to the text for a detailed discussion). Thick arrows indicate primary cellular ROS sources. Broken lines indicate some uncertainty. NADH, reduced nicotinamide adenine dinucleotide; NAD+, oxidized nicotinamide adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; FAD+, oxidized flavin adenine dinucleotide; I–V, complexes of electron transport/oxidative phosphorylation; Cyt c, cytochrome C; GSH, reduced glutathione; GSSG, glutathione disulphide; GPx, glutathione peroxidase; Prx, peroxiredoxin reductase; Trx oxi, oxidized thioredoxin; Trx red, reduced thioredoxin; MnSOD, manganese superoxide dismutase; Cu/ZnSOD, copper/zinc superoxide dismutase; ec SOD, extracellular superoxide dismutase; nNOS, neuronal nitric oxide synthase; PLA2, phospholipase A2; ·O2−, superoxide anion; H2O2, hydrogen peroxide; NO·, nitric oxide; ONOO−, peroxynitrite; ·OH, hydroxyl radical; IRS-1, insulin receptor substrate 1; PI3-K, phosphoinositide 3-kinase; PI(4,5)P2, phosphatidylinositol (3,4)-bisphosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5)-triphosphate; PDPK1, 3-phophoinositide-dependent protein kinase-1; aPKC, atypical protein kinase C; AKT, serine/threonine protein kinase B; GLUT4, glucose transporter type 4; JNK, c-Jun N-terminal kinase; ERK, extracellular signal-regulated kinase; p38 MAPK, p38 mitogen-activated protein kinase; AMPK, AMP-activated protein kinase; ATF-2, activating transcription factor-2; PGC-1α, peroxisome proliferator-activated receptor (PPAR)-γ coactivator-1α.
Potential site(s) of ROS production during contraction
NADPH oxidase
Of the potential sources of ROS production during contraction, it appears that NADPH oxidase (NOX) plays a key role, since blocking its activity in rat primary muscle cells with the NOX inhibitor, apocynin, or in isolated muscle fibres with NOX inhibitors such as diphenyleneiodonium chloride, apocynin, or a specific peptide inhibitor of NOX, gp91ds-tat, completely prevents the electrically stimulated increase in ROS.56,59,60
Nitric oxide synthase
The free radical, nitric oxide (NO), is produced in the skeletal muscle fibres during contraction59 from the neuronal NOS isoform.61 Furthermore, the highly reactive ROS peroxynitrite (ONOO−) is produced from the reaction of NO with ·O2−.62 Interestingly, the production of NO and ROS do not always occur concomitantly. NO production is only detected with fluorescent probes in muscle fibres at moderate-to-high contractile intensities, whereas ROS production can be detected throughout low-to-high contractile intensities.59 This would imply that ONOO− production is also not likely to occur until contraction intensities reach at least moderate levels.
Xanthine oxidase
The production of ROS from XO is well matched with exercise intensity, since the precursors of hypoxanthine, such as inosine monophosphate, only accumulate following moderate-to-high intensity exercise.63 However, the contribution of XO to skeletal muscle ROS production in humans during exercise is probably small due to XO being localized in the endothelial cells of the skeletal muscle rather than within the muscle fibre.64
Phospholipase A2
Inhibitors of the calcium-dependent PLA2 during in vitro contraction of rat diaphragm blocks ROS formation in muscle homogenates.10 However, selective inhibitors of PLA2 recently have been shown to be ineffective at preventing contraction-induced ROS formation in single muscle fibres, suggesting a limited role for PLA2 in skeletal muscle ROS production during contraction.59
Exercise intensity and ROS production
Over 30 years ago, Davies et al.15 published the first evidence that free radical levels were elevated in rat skeletal muscle following exhaustive exercise. However, it was only recently that Bailey et al.65 provided the first direct evidence in humans that maximal exercise increases intramuscular free radical accumulation by using electron paramagnetic resonance spectroscopy. Exhaustive exercise is well documented to increase markers of oxidative stress in skeletal muscle, such as oxidized glutathione (GSSG) and lipid peroxidation.66–68 However, the actual exercise intensity whereby ROS are increased in skeletal muscle is difficult to precisely quantify. Nevertheless, submaximal exercise has been shown to increase the markers of skeletal muscle oxidative stress. Treadmill running in rodents at ∼70% of maximal oxygen uptake (VO2max) significantly increases skeletal muscle GSSG.69,70 In humans, cycling exercise at ∼85% VO2max significantly increases skeletal muscle GSSG.71 By using interstitial dialysis techniques, Karamouzis et al.72 have shown cycling at ∼70% VO2max is sufficient to significantly increase skeletal muscle F2-isoprostane levels. Thus, it would appear that moderate-to-high intensity endurance exercise is sufficient to induce skeletal muscle oxidative stress.
Regulation of contraction-induced mitochondrial biogenesis by ROS
Although pathologically high chronic levels of ROS are cytotoxic, it is also now clear that at low (physiological) levels they play an important role in cell signalling in normal healthy skeletal muscle.73 Increasing mitochondrial content in cultured cells reduces oxygen consumption per unit of mitochondria,19 thus reducing mitochondrial ROS production and oxidative stress.74 Increasing mitochondrial content also protects mice against diet-induced obesity and insulin resistance and increases lifespan in a number of organisms.18 Endurance training increases skeletal muscle mitochondrial biogenesis (the synthesis of new mitochondria)75,76 and antioxidant enzymes.70,77 It was Davies et al. in 1982,15 who initially proposed that this could be a stimulus for the mitochondrial biogenesis observed following endurance training. However, it has been only in the past several years that evidence has emerged that the small, temporary, and perhaps localized ROS production during exercise is involved in regulating these beneficial effects involving the mitochondria,73 which could potentially act to reduce mitochondrial ROS production.
Increasing the ROS levels in skeletal muscle cells activates several redox-sensitive kinases such as AMP-activated protein kinase (AMPK), activating transcription factor-2 (ATF-2), and the members of the MAPK family including p38 MAPK, JNK, and extracellular signal-regulated kinase (ERK, also called p44/42 MAPK).78,79 All these kinases are activated in skeletal muscle during exercise70 and are at least partly involved in the regulation of mitochondrial biogenesis,75,80 primarily via the transcriptional coactivator, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), which is a key regulator of mitochondrial biogenesis.81,82 Increasing ROS levels in skeletal muscle cells activates AMPK, resulting in elevated PGC-1α,78 with these ROS effects being blocked by co-treatment with antioxidants.78 Silveira et al.73 published the first clear evidence linking the regulation of contraction-induced mitochondrial biogenesis to ROS. Electrostimulation that induces contraction of rat primary muscle cells increased ROS and also PGC-1α mRNA that could be prevented by treatment with antioxidants.73
The regulatory role for ROS in contraction-induced mitochondrial biogenesis has led to considerable controversy in the literature regarding the potential for antioxidant supplements to prevent these skeletal muscle adaptations to endurance training. There are some training studies that support such a role16,83 and others that do not.84–86 Furthermore, other studies have reported that antioxidants can inhibit the gene expression response of specific metabolic genes following training, but they also find no effect on functional muscle adaptations such as increased peak power or mitochondrial enzyme activity.77 There are also several acute exercise studies showing that inhibition of XO-derived ROS with the XO inhibitor, allopurinol, can inhibit redox-sensitive kinases known to regulate mitochondrial biogenesis, such as the exercise-induced phosphorylation of p38 MAPK and ERK.67,70,87 However, long-term treatment with allopurinol does not prevent the increases in skeletal muscle mitochondrial proteins or antioxidant enzymes following endurance training.70 The lack of the effect of allopurinol on skeletal muscle adaptations following endurance training suggests considerable redundancy in the mitochondrial biogenesis pathways. These findings also highlight that exercise studies need to examine both acute (a single bout) and chronic (i.e. training) exercise to obtain a full understanding of the adaptive responses in skeletal muscle.
Regulation of contraction-induced antioxidant enzymes by ROS
The gene expression of MnSOD, extracellular SOD, and GPx are all increased following a single bout of exercise70 with several weeks of endurance training known to increase skeletal muscle antioxidant enzymes, particularly MnSOD and GPx.70,85,88 Furthermore, although still largely undefined, mitochondrial biogenesis appears to be involved in the regulation of antioxidant enzymes, since PGC-1α is required for the induction of Cu/ZnSOD, MnSOD, GPx1, and catalase in response to oxidative stress.89
Regulation of muscle hypertrophy by ROS
Recently, the highly reactive oxidant ONOO−, which is formed by the reaction of O2− with NO, has been shown to regulate skeletal muscle hypertrophy induced by overload.90 The signalling pathway appears to be via the ONOO− stimulated activation of the transient receptor potential cation channel, subfamily V, member 1 (Trpv1) to release intracellular Ca2+, which then activates mammalian target of rapamycin (mTOR) to increase protein synthesis. A role for ROS in the regulation of protein synthesis is supported by Makanae et al.,91 who found that a high daily oral dose of the antioxidant vitamin C not only attenuated skeletal muscle oxidative stress but also the hypertrophy normally observed following the mechanical overloading of the plantaris by hindlimb ablation. Understanding the molecular pathways regulating skeletal muscle protein synthesis are important for the treatment of muscle disorders, particularly the age-related loss of muscle mass, known as sarcopenia. Indeed, in elderly subjects, the normal increase in muscle protein synthesis is blunted in response to resistance exercise when compared with young subjects.92 Studies are now required in humans to determine whether ONOO− is involved in the muscle hypertrophy response to resistance training, as this may provide novel targets for pharmacological interventions that augment the training response for the elderly.
Conclusion
While solid experimental data mechanistically links excess mitochondrial ROS production to the pathogenesis of insulin resistance and T2D, some recent findings in rodents interject some uncertainty into the once promising approach of targeting mitochondrial ROS as a means to improving insulin resistance. Moreover, it is not clear from the studies undertaken to date if in fact mitochondrial ROS production is increased in the skeletal muscle of people with T2D.
In contrast to insulin resistance/T2D, non-mitochondrial sites of ROS are primarily responsible for the ROS produced during exercise. These sites include NADPH oxidase, NOS, and perhaps to a lesser extent XO and are probably playing a role in the regulation of key adaptations to exercise, such as mitochondrial biogenesis, increased antioxidant defences, and perhaps muscle hypertrophy. However, further research is required to resolve the effect, if any, that antioxidant supplementation might have on preventing beneficial endurance training adaptations.
Acknowledgements
Shaun Mason is a recipient of the Australian Postgraduate Award (APA) at Deakin University.
References
- 1.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 1976;38:273–291. [DOI] [PubMed] [Google Scholar]
- 2.Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B,. et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 2008;118:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cline GW, Petersen KF, Krssak M, Shen J, Hundal RS, Trajanoski Z,. et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med 1999;341:240–246. [DOI] [PubMed] [Google Scholar]
- 4.Koivisto VA, Yki-Jarvinen H, DeFronzo RA. Physical training and insulin sensitivity. Diabetes Metab Rev 1986;1:445–481. [DOI] [PubMed] [Google Scholar]
- 5.Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem 2011;286:10605–10617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009;417:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One 2013;8:e54059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadev K, Zilbering A, Zhu L, Goldstein BJ. Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem 2001;276:21938–21942. [DOI] [PubMed] [Google Scholar]
- 9.Barazzoni R, Zanetti M, Gortan Cappellari G, Semolic A, Boschelle M, Codarin E,. et al. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia 2012;55:773–782. [DOI] [PubMed] [Google Scholar]
- 10.Nethery D, Stofan D, Callahan L, DiMarco A, Supinski G. Formation of reactive oxygen species by the contracting diaphragm is PLA2 dependent. J Appl Physiol 1999;87:792–800. [DOI] [PubMed] [Google Scholar]
- 11.Fisher-Wellman KH, Neufer PD. Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 2012;23:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 2002;277:44784–44790. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BJ, Mahadev K, Wu X, Zhu L, Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal 2005;7:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S,. et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab 2009;10:260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochemical and Biophys Res Commun 1982;107:1198–1205. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV,. et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 2008;87:142–149. [DOI] [PubMed] [Google Scholar]
- 17.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944–948. [DOI] [PubMed] [Google Scholar]
- 18.Guarente L. Mitochondria–A Nexus for Aging, Calorie Restriction, and Sirtuins. Cell 2008;132:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S,. et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 2006;103:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sukhanov S, Semprun-Prieto L, Yoshida T, Michael Tabony A, Higashi Y, Galvez S,. et al. Angiotensin II, oxidative stress and skeletal muscle wasting. Am J Med Sci 2011;342:143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang YC, Liu Y, Hayworth CR, Bhattacharya A, Lustgarten MS, Muller FL,. et al. Dietary restriction attenuates age-associated muscle atrophy by lowering oxidative stress in mice even in complete absence of CuZnSOD. Aging Cell 2012;11:770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisbal C, Lambert K, Avignon A. Antioxidants and glucose metabolism disorders. Curr Opin Clin Nutr Metab Care 2010;13:439–446. [DOI] [PubMed] [Google Scholar]
- 23.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT,. et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 1985;76:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonadonna RC, Del Prato S, Saccomani MP, Bonora E, Gulli G, Ferrannini E,. et al. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest 1993;92:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC,. et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004;53:25–31. [DOI] [PubMed] [Google Scholar]
- 27.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005–2011. [DOI] [PubMed] [Google Scholar]
- 28.Boudina S, Sena S, Sloan C, Tebbi A, Han YH, O'Neill BT,. et al. Early mitochondrial adaptations in skeletal muscle to diet-induced obesity are strain dependent and determine oxidative stress and energy expenditure but not insulin sensitivity. Endocrinology 2012;153:2677–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ,. et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 2009;106:17787–17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paglialunga S, van Bree B, Bosma M, Valdecantos MP, Amengual-Cladera E, Jorgensen JA,. et al. Targeting of mitochondrial reactive oxygen species production does not avert lipid-induced insulin resistance in muscle tissue from mice. Diabetologia 2012;55:2759–2768. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE,. et al. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 2006;281:35137–35146. [DOI] [PubMed] [Google Scholar]
- 32.Bravard A, Lefai E, Meugnier E, Pesenti S, Disse E, Vouillarmet J,. et al. FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 2011;60:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, De Filippis EA,. et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 2010;59:2444–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P,. et al. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr 2006;84:395–399. [DOI] [PubMed] [Google Scholar]
- 35.Heart Protection Study Collaborative G MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:23–33. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Ajani U, Chae C, Hennekens C, Buring JE, Manson JE. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA 1999;282:1073–1075. [DOI] [PubMed] [Google Scholar]
- 37.Pashkow FJ. Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflam 2011;2011:514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biesalski HK, Grune T, Tinz J, Zollner I, Blumberg JB. Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients 2010;2:929–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Liu JZ, Hu JX, Wu H, Li YL, Chen HL,. et al. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radic Biol Med 2011;51:539–551. [DOI] [PubMed] [Google Scholar]
- 40.Ge X, Yu Q, Qi W, Shi X, Zhai Q. Chronic insulin treatment causes insulin resistance in 3T3-L1 adipocytes through oxidative stress. Free Radic Res 2008;42:582–591. [DOI] [PubMed] [Google Scholar]
- 41.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K,. et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333–336. [DOI] [PubMed] [Google Scholar]
- 42.Vinayagamoorthi R, Bobby Z, Sridhar MG. Antioxidants preserve redox balance and inhibit c-Jun-N-terminal kinase pathway while improving insulin signaling in fat-fed rats: evidence for the role of oxidative stress on IRS-1 serine phosphorylation and insulin resistance. J Endocrinol 2008;197:287–296. [DOI] [PubMed] [Google Scholar]
- 43.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005;120:649–661. [DOI] [PubMed] [Google Scholar]
- 44.Imoto K, Kukidome D, Nishikawa T, Matsuhisa T, Sonoda K, Fujisawa K,. et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes 2006;55:1197–1204. [DOI] [PubMed] [Google Scholar]
- 45.Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 2009;52:574–582. [DOI] [PubMed] [Google Scholar]
- 46.Hey-Mogensen M, Hojlund K, Vind BF, Wang L, Dela F, Beck-Nielsen H,. et al. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 2010;53:1976–1985. [DOI] [PubMed] [Google Scholar]
- 47.Minet AD, Gaster M. Hydrogen peroxide production is not primarily increased in human myotubes established from type 2 diabetic subjects. J Clin Endocrinol Metab 2011;96:E1486–1490. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharya A, Lustgarten M, Shi Y, Liu Y, Jang YC, Pulliam D,. et al. Increased mitochondrial matrix-directed superoxide production by fatty acid hydroperoxides in skeletal muscle mitochondria. Free Radic Biol Med 2011;50:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Summers FA, Mason RP. Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Free Radic Biol Med 2012;53:1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheede-Bergdahl C, Penkowa M, Hidalgo J, Olsen DB, Schjerling P, Prats C,. et al. Metallothionein-mediated antioxidant defense system and its response to exercise training are impaired in human type 2 diabetes. Diabetes 2005;54:3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres SH, De Sanctis JB, de LBM, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 2004;181:419–427. [DOI] [PubMed] [Google Scholar]
- 52.Brinkmann C, Chung N, Schmidt U, Kreutz T, Lenzen E, Schiffer T,. et al. Training alters the skeletal muscle antioxidative capacity in non-insulin-dependent type 2 diabetic men. Scand J Med Sci Sports 2012;22:462–470. [DOI] [PubMed] [Google Scholar]
- 53.Larsen S, Stride N, Hey-Mogensen M, Hansen CN, Andersen JL, Madsbad S,. et al. Increased mitochondrial substrate sensitivity in skeletal muscle of patients with type 2 diabetes. Diabetologia 2011;54:1427–1436. [DOI] [PubMed] [Google Scholar]
- 54.Espinosa A, Garcia A, Hartel S, Hidalgo C, Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J Biol Chem 2009;284:2568–2575. [DOI] [PubMed] [Google Scholar]
- 55.Vasilaki A, Csete M, Pye D, Lee S, Palomero J, McArdle F,. et al. Genetic modification of the manganese superoxide dismutase/glutathione peroxidase 1 pathway influences intracellular ROS generation in quiescent, but not contracting, skeletal muscle cells. Free Radic Biol Med 2006;41:1719–1725. [DOI] [PubMed] [Google Scholar]
- 56.Espinosa A, Leiva A, Pena M, Müller M, Debandi A, Hidalgo C,. et al. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol 2006;209:379–388. [DOI] [PubMed] [Google Scholar]
- 57.McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med 2005;39:651–657. [DOI] [PubMed] [Google Scholar]
- 58.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 2008;88:1243–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A,. et al. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 2013;18:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michaelson LP, Shi G, Ward CW, Rodney GG. Mitochondrial redox potential during contraction in single intact muscle fibers. Muscle Nerve 2010;42:522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirschfield W, Moody MR, O'Brien WE, Gregg AR, Bryan RM Jr., Reid MB. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am J Physiol Regul Integr Comp Physiol 2000;278:R95–100. [DOI] [PubMed] [Google Scholar]
- 62.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahlin K, Broberg S, Ren JM. Formation of inosine monophosphate (IMP) in human skeletal muscle during incremental dynamic exercise. Acta Physiol Scand 1989;136:193–198. [DOI] [PubMed] [Google Scholar]
- 64.Hellsten-Westing Y. Immunohistochemical localization of xanthine oxidase in human cardiac and skeletal muscle. Histochemistry 1993;100:215–222. [DOI] [PubMed] [Google Scholar]
- 65.Bailey DM, Lawrenson L, McEneny J, Young IS, James PE, Jackson SK,. et al. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radic Res 2007;41:182–190. [DOI] [PubMed] [Google Scholar]
- 66.Veskoukis AS, Nikolaidis MG, Kyparos A, Kokkinos D, Nepka C, Barbanis S,. et al. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl Physiol Nutr Metab 2008;33:1140–1154. [DOI] [PubMed] [Google Scholar]
- 67.Kang C, O'Moore KM, Dickman JR, Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med 2009;47:1394–1400. [DOI] [PubMed] [Google Scholar]
- 68.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol 1999;87:465–470. [DOI] [PubMed] [Google Scholar]
- 69.Wadley GD, McConell GK. High-dose antioxidant vitamin C supplementation does not prevent acute exercise-induced increases in markers of skeletal muscle mitochondrial biogenesis in rats. J Appl Physiol 2010;108:1719–1726. [DOI] [PubMed] [Google Scholar]
- 70.Wadley GD, Nicolas MA, Hiam D, McConell GK. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am J Physiol Endocrinol Metab 2013;304:E853–E862. [DOI] [PubMed] [Google Scholar]
- 71.Zhang SJ, Sandstrom ME, Lanner JT, Thorell A, Westerblad H, Katz A. Activation of aconitase in mouse fast-twitch skeletal muscle during contraction-mediated oxidative stress. Am J Physiol Cell Physiol 2007;293:C1154–1159. [DOI] [PubMed] [Google Scholar]
- 72.Karamouzis I, Christoulas K, Grekas D, Giannoulis K, Vamvakoudis E, Mandroukas K. The response of muscle interstitial F2-isoprostane (8-ISO-PGF2alpha) during dynamic muscle contractions in humans. Prostaglandins Leukot Essent Fatty Acids 2004;71:87–90. [DOI] [PubMed] [Google Scholar]
- 73.Silveira LR, Pilegaard H, Kusuhara K, Curi R, Hellsten Y. The contraction induced increase in gene expression of peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1alpha), mitochondrial uncoupling protein 3 (UCP3) and hexokinase II (HKII) in primary rat skeletal muscle cells is dependent on reactive oxygen species. Biochim Biophys Acta 2006;1763:969–976. [DOI] [PubMed] [Google Scholar]
- 74.Mitsuishi M, Miyashita K, Itoh H. cGMP rescues mitochondrial dysfunction induced by glucose and insulin in myocytes. Biochem Biophys Res Commun 2008;367:840–845. [DOI] [PubMed] [Google Scholar]
- 75.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 2007;282:194–199. [DOI] [PubMed] [Google Scholar]
- 76.Wadley GD, Choate J, McConell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol 2007;585:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meier P, Renga M, Hoppeler H, Baum O. The impact of antioxidant supplements and endurance exercise on genes of the carbohydrate and lipid metabolism in skeletal muscle of mice. Cell Biochem Funct 2013;31:51–59. [DOI] [PubMed] [Google Scholar]
- 78.Irrcher I, Ljubicic V, Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1{alpha} transcription in skeletal muscle cells. Am J Physiol Cell Physiol 2009;296:C116–123. [DOI] [PubMed] [Google Scholar]
- 79.Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H,. et al. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol 2006;575:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007;104:12017–12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 2003;24:78–90. [DOI] [PubMed] [Google Scholar]
- 82.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V,. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999;98:115–124. [DOI] [PubMed] [Google Scholar]
- 83.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M,. et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 2009;106:8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 2011;301:E779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yfanti C, Akerstrom T, Nielsen S, Nielsen AR, Mounier R, Mortensen OH,. et al. Antioxidant supplementation does not alter endurance training adaptation. Med Sci Sports Exerc 2010;42:1388–1395. [DOI] [PubMed] [Google Scholar]
- 86.Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc 2011;43:1017–1024. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 2005;567:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hollander J, Fiebig R, Gore M, Bejma J, Ookawara T, Ohno H,. et al. Superoxide dismutase gene expression in skeletal muscle: fiber-specific adaptation to endurance training. Am J Physiol 1999;277:R856–862. [DOI] [PubMed] [Google Scholar]
- 89.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S,. et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 2006;127:397–408. [DOI] [PubMed] [Google Scholar]
- 90.Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nature Med 2013;19:101–106. [DOI] [PubMed] [Google Scholar]
- 91.Makanae Y, Kawada S, Sasaki K, Nakazato K, Ishii N. Vitamin C administration attenuates overload-induced skeletal muscle hypertrophy in rats. Acta Physiologica 2013;208:57–65. [DOI] [PubMed] [Google Scholar]
- 92.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W,. et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 2009;587:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]