ABSTRACT
Objectives: To improve understanding of the preclinical stage of colonic inflammation by exploring the existence of a link between early inflammatory changes in the colonic mucosa and the systemic redox balance.
Methods: Clinical characteristics, a fasting blood draw, and mucosal biopsies from the right, left, and sigmoid-rectum colonic tracts collected from 28 healthy individuals (14/14 males/females) who underwent colonoscopy. Myeloperoxidase (MPO) positive cells infiltrating colonic mucosa specimens were assessed by immunohistochemistry, and patients divided into high or low MPO expressing cells/optical field groups (MPOhigh or MPOlow, respectively).The systemic oxidative balance has been studied through derived-Reactive Oxygen Metabolites (d-ROMs), Biological Antioxidant Potential (BAP), and Lipoperoxide-cholesterol Oxidizing (LP-CHOLOX) tests on serum.
Results: MPOhigh patients demonstrated an increased systemic oxidative stress compared to MPOlow individuals (P = 0.035), especially when MPO is referred to the left-sided colonic mucosa (P = 0.007). MPOlow subjects in the sigmoid-rectum showed a significant higher antioxidant capacity in the serum (P < 0.02). Sex-specific differences in MPO expression (male and female: 4.6 ± 3.2 and 2.6 ± 1.5 MPO-positive cells/optical field, respectively, P = 0.044), and a decreasing gradient in MPO expression moving from the cecum to the rectum (ascendant, descendant, and sigmoid-rectum: 3.7 ± 2.8, 3.1 ± 1.7, and 1.4 ± 0.5, respectively, P = 0.012) were also found and discussed.
Discussion: The study is the first demonstrating a connection between systemic redox balance and MPO expression in the colonic mucosa, according to the colonic tract and patient gender. Further research evaluating the MPO expression in the human colon and its relationship with pathological conditions could benefit from these results.
KEYWORDS: Myeloperoxidase, MPO, oxidative stress, colonic mucosa, redox balance, antioxidant capacity, colonic inflammation
Introduction
Subclinical pro-inflammatory status of the colorectal mucosa is a fundamental early determinant of many severe conditions, such as inflammatory bowel diseases (IBDs) [1–4] and colorectal carcinogenesis [5–8]. We know that the effects of these pathological conditions spread out of the colonic mucosa, thus affecting the whole body [9] and producing alterations in systemic inflammatory markers and distant organs [10,11], also mediated by substantial changes in the systemic redox balance [8,12–17].
In this process, myeloperoxidase (MPO) is emerging as a key player. Expressed in the tissues as well as in circulating leukocytes [18–20], MPO is a heme-containing peroxidase released extracellularly and into phagosomal compartments by neutrophils, monocytes, and some tissue macrophages, where it catalyzes the reaction of halide and pseudohalide ions with hydrogen peroxide (H2O2) to generate hypohalous acids [21]. Indeed, its activity is tightly related to local and systemic redox balance, as demonstrated by previous research [22,23]. Although MPO has been increasingly recognized for its role in promoting and maintaining IBDs and colorectal carcinogenesis, conflicting evidence remains. In particular, an apparent dichotomy concerning the role of MPO expression on carcinogenetic processes exists [24]. On the one hand, MPO seems to promote colorectal carcinogenesis [25–27], while on the other hand higher MPO expression has shown to repress cancer progression and thus improve patient prognosis [28,29]. Other findings that remain to be clarified concern the different epidemiology of colorectal carcinogenesis in relation to gender (right-sided colorectal cancer is more frequent in females) as well as to the different colonic tracts [30,31].
Prompted by this background, we hypothesized that MPO expression is not uniformly represented in the large bowel, and varying levels of expression may relate to the systemic oxidative balance, thereby in turn affecting the early steps of inflammation-related carcinogenesis. Thus, in this study, the presence and entity of systemic oxidative stress have been investigated in relation to the distribution of MPO-positive cell infiltrates in the normal-appearing colorectal mucosa.
Material and methods
Ethics statement
All patients enrolled in the present study were asked to provide written informed consent, and the study protocol was specifically approved by the local Ethics Committee (‘Comitato Etico Provinciale di Modena’ Prot. No. 4396/C.E.).
Study population and sample processing
Twenty-eight consecutive patients (M/F 14/14) who underwent colonoscopy for non-specific abdominal symptoms (recurrent abdominal pain, constipation without body weight loss) as prescribed by their primary care physician were enrolled in the study after joining by written informed consent. Two samples of normal mucosa for each colonic segment (right colon and left colon) and one sample for the sigmoid-rectum were collected from each of the 28 patients during colonoscopy; they were fixed in formalin and embedded in paraffin for immunohistochemistry. All patients had a negative colonoscopy. Before the colonoscopy, a blood draw for serological analysis was collected and immediately processed. Sera were stored frozen at −80°C until the execution of oxidative stress tests. Glycemia has been measured directly from the patients with a glucometer for clinical use (Breeze 2 Glucometer, Bayer AG, Germany). For each patient, we collected demographic (age and sex) and anthropometric characteristics (height, waist circumference, body weight, BMI (body weight in kg/height in m2), waist-to-hip ratio, and blood pressure). Patients were negative for significant events in past medical history and drug assumption. Serological and immunohistochemistry tests were performed in blind for anthropometric data and other clinical records.
Immunohistochemistry and scoring for MPO expression
MPO expression has been calculated as the mean number of positive cells per optical field (cells/OF). Detailed procedures for the immunohistochemistry technique are reported in our previous study [27]. Before immunohistochemistry, a routine histology of tissue samples was carried out after hematoxylin and eosin staining of the sections. To assess the number of MPO-positive cells expressed in the colorectal mucosa, a quantitative score was used, counting the number of stained cells by immunohistochemistry under a light microscope. Two slides for each sample (about three samples for each patient) were scored by the analysis of at least 25 microscopic fields (magnification ×100). Cells located in capillary vessels were excluded by the observers. Slides were scored by two independent observers. Then, the mean number of MPO-positive cells (positive cells/OF) for each patient was calculated for the entire colon-rectum, and for each large bowel segment: right colon (cecum to proximal half of the transverse colon), left colon (distal half of the transverse colon and descending colon), and the sigmoid-rectum. Increased MPO expression has been defined for patients that express higher values than the mean number of positive cells/OF according to a specific colonic tract (and defined as MPOhigh subjects), whereas low MPO expression patients corresponded to lower values than mean positive cells/OF in a particular colonic tract (and defined as MPOlow subjects).
Oxidative stress assessment
Three tests have been used to assess the redox properties of the serum samples: d-ROMs test, LP-CHOLOX test, and BAP test produced by Diacron International®, Inc. (Grosseto, Italy), and performed using the dedicated platform FREE Carpe Diem©. The platform consists in a photometer with six interference filters to measure the absorbance of the solution (serum plus reagents) in accordance with the specific test procedures. FREE Carpe Diem© platform and tests are approved for in vitro diagnostic use.
d-ROMs (derived-Reactive Oxygen Metabolites) assay is used to measure the oxidant ability of a serum sample towards 4-amino-N,N-diethylaniline sulfate used as chromogen. Especially alkoxyl e hydroperoxyl radicals, derived from hydroperoxides (ROOH), help to determine the oxidizing capacity measured by d-ROMs test. Units are expressed as Carratelli units (CARR U) whose 1 CARR U corresponds to 0.08 mg hydrogen peroxide/dl. Reference values for d-ROMS test are as follows: normal, up to 299 CARR U; borderline values, between 300 and 320 CARR U; low-level oxidative stress between 321 and 340 CARR U; middle level oxidative stress between 341 and 400 CARR U; high level oxidative stress between 401 and 500 CARR U; very high level 500 CARR U or more [32–34].
LP-CHOLOX (Lipoperoxide-cholesterol Oxidizing) test measures photometrically the level of lipoperoxides in the serum, resulting mainly from cholesterol, by means of their ability to facilitate the oxidation of Fe2+ ion to Fe3+. The produced Fe3+ bounds to thiocyanate developing a color complex photometrically measurable. The increase in absorbance is directly proportional to the concentration of lipoperoxides present in the serum. Units are expressed in μEq/L. Reference values are the following: normal, up to 599 μEq/L; slight alteration, between 600 and 799 μEq/L; moderate alteration, 800–999 μEq/L; strong alteration, 1000 μEq/L or more [35].
BAP (Biological Antioxidant Potential) test has been used to assess antioxidant properties of the sera. BAP allows determining the concentration of antioxidants through the ability to reduce the iron ion from ferric (Fe3+) to the ferrous (Fe2+) form in an iron chloride solution (2%). BAP test provides an overall measure of many antioxidants such as bilirubin, uric acid, vitamins C and E, and proteins. Units are expressed in μmol L−1. Reference values: optimal, 2200 μmol L−1 or more; borderline condition, 2200–2000 μmol L−1; slight reduction, 1999–1.800 μmol L−1; moderate reduction, 1.799–1.600 μmol L−1; strong reduction, 1.599–1.400 μmol L−1; very strong reduction, 1.400 μmol L−1 or less [36,37].
Statistical analysis
Statistical analysis was performed using SigmaPlot 12, Systat Software, Inc., London (UK). Continuous variables were expressed as mean ± standard deviation (SD). Normality test (Shapiro–Wilk) and Equal Variance Test were used to assess data distribution. Kruskal–Wallis ANOVA on Ranks test has been used to evaluate differences in data among non-normal distributed values. One-way ANOVA has been used for normally distributed data. Pearson Moment Correlation test was used for measuring the strength of association between pairs of variables expressed by continuous numeric values. The level of statistical significance was set at P < 0.05.
Results
Demographic and anthropometric characteristics of the patients are shown in Table 1. Mean BMI was 26.7 ± 4.2 kg m−2, waist circumference and waist-to-hip ratio resulted slightly increased as well (98.8 ± 14.3 and 0.94 ± 0.08 cm, respectively), indicating that our sample of patients was on average overweight. Mean age, BMI, systolic blood pressure, waist circumference, and glycemia were not significantly different between male and female patients, while remarkable was the difference in the mean values for the waist-to-hip ratio (male and female, 1.00 ± 0.05 and 0.89 ± 0.08, respectively, P < 0.001), and diastolic blood pressure (male and female, 81.8 ± 4.9 and 71.6 ± 9.9 mmHg, respectively, P = 0.003). A significant covariation has been found between d-ROMs and LP-CHOLOX test results, as showed in Figure 1.
Table 1. Demographic and anthropometric features of the enrolled subjects.
| Variables | Age | BMI | Waist† | Hip† | W/H ratio | SBP‡ | DBP‡ | Glycemia£ |
|---|---|---|---|---|---|---|---|---|
| All, n = 28 | 61.9 (11.9) | 26.7 (4.2) | 98.8 (14.3) | 104.3 (9.4) | 0.94 (0.08) | 142.9 (22.8) | 76.5 (9.3) | 108.0 (20.2) |
| Male, n = 14 | 62.3 (11.5) | 27.3 (3.0) | 103.3 (11.4) | 103.4 (7.3) | 1.00 (0.05) | 147.7 (18.8) | 81.8 (4.9) | 108.1 (14.7) |
| Female, n = 14 | 61.4 (12.6) | 26.1 (5.2) | 94.3 (15.8) | 105.1 (11.3) | 0.89 (0.08) | 138.5 (25.9) | 71.6 (9.9) | 107.9 (25.2) |
| P value M/F | 0.84 | 0.46 | 0.09 | 0.64 | < 0.001 | 0.06 | 0.003 | 0.98 |
Mean (standard deviation). †in centimeters; ‡in mmHg; £in mg/dl; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; W/H ratio: waist-to-hip ratio.
Figure 1.
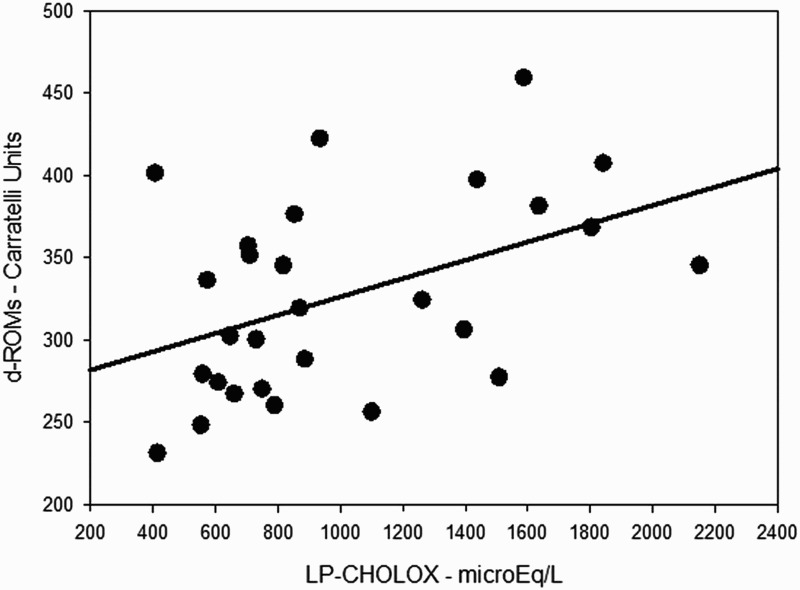
Simple scatter plot with regression line indicating a direct correlation between d-ROMs and LP-CHOLOX test results (ρ 0.45; P = 0.017).
MPO expression was 2.7 ± 1.7 cells/OF for the entire colon-rectum; 3.7 ± 2.8 cells/OF for the right colon; 3.1 ± 1.7 cells/OF for the left colon; 1.4 ± 0.5 cells/OF for the sigmoid-rectum. Significant differences between male and female patients have been observed only for MPO expression in the right colon (P = 0.044), while for the other variables such as d-ROMs and LP-CHOLOX, differences did not reach statistical significance. MPO expression decreased moving from proximal colon to sigmoid-rectum with a statistically significant trend, as reported in Table 2 and Figure 2. Different levels of MPO immunostaining are illustrated in Figure 3, and on Supplementary online materials (Figures A to J).
Table 2. Serological oxidative balance and colonic mucosa MPO expression.
| Variables | d-ROMs | LP-CHOLOX | BAP | MPO-overall colon | MPO-right colon | MPO-left colon | MPO-sigmoid-rectum | P value MPO expression |
|---|---|---|---|---|---|---|---|---|
| All | 326.6 (59.4) | 1008.4 (479.8) | 1649.0 (347.4) | 2.7 (1.7) | 3.7 (2.8) | 3.1 (1.7) | 1.4 (0.5) | 0.012 |
| Males | 305.1 (51.5) | 837.1 (476.5) | 1710.5 (228.9) | 2.8 (2.0) | 4.6 (3.2) | 3.0 (2.0) | 1.6 (1.2) | 0.024 |
| Females | 348.2 (60.6) | 1179.6 (433.4) | 1587.4 (436.0) | 2.6 (1.3) | 2.6 (1.5) | 3.2 (1.3) | 1.3 (0.4) | 0.011** |
| P value M/F | 0.053 | 0.057 | 0.35 | 0.75 | 0.044* | 0.75 | 0.38 |
Mean (standard deviation). *significant difference between males and females; **left colon vs. sigmoid-rectum.
Figure 2.
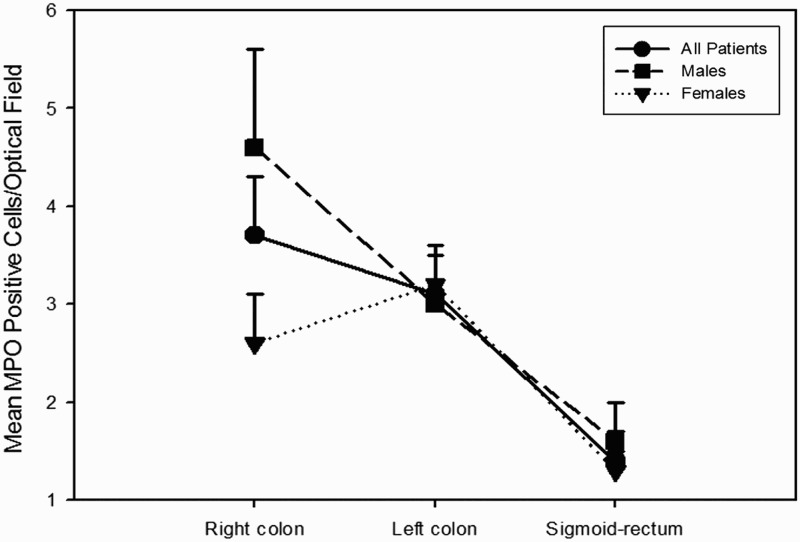
MPO expression varied along the colorectum, decreasing from the ascending colon to the sigmoid-rectum.
Figure 3.
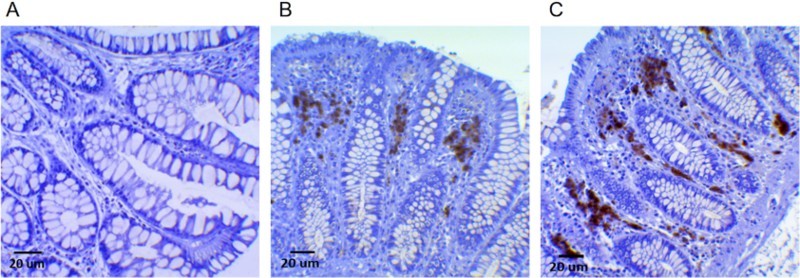
Immunohistochemical staining of MPO-positive cells in healthy human colonic mucosa samples. In (A) the colonic mucosa does not show a significant presence of MPO-positive cells, whereas in (B) a low/moderate expression is evident as dark-brown staining between crypts, particularly at the luminal pole. In (C), MPO-positive cells are well-recognizable as dark-brown staining also more deeply in the stroma at the base of the crypts, where epithelial stem-cell niches are present. Additional Figures (A to J) are available online.
Oxidative stress in relation to MPO expression
Considering the whole colon, oxidative stress in the serum resulted higher (d-ROMs: 357.9 ± 41.2 CARR U) in MPOhigh (>2.7 cells/OF) subjects compared to MPOlow subjects (d-ROMs: 309.3 ± 61.7 CARR U, P = 0.035). Considering each large bowel segment, differences in d-ROMs values in relation to MPO expression were especially significant in the left colon, in which mean d-ROMs for MPOhigh subjects was 358.2 ± 46.4 U CARR compared to 294.9 ± 46.7 CARR U in the MPOlow subjects (P = 0.007). In the right colon as well as in the sigmoid-rectum the trend was similar but without reaching statistical significance (Figure 4).
Figure 4.
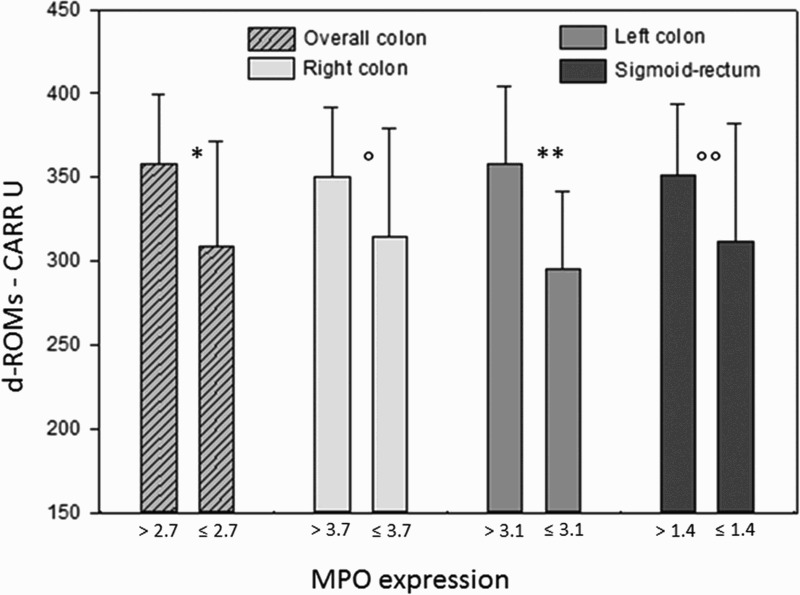
Bars represent mean ± SD. Mean MPO values on the X-axis were different according to the different MPO expression along the large bowel. Differences were statistically significant between higher/lower than mean MPO expression for the overall colon (*P = 0.035) and for the left colon (**P = 0.007), while in the right colon and sigmoid-rectum differences did not reach the statistical significance (P = 0.18; P = 0.17).
Adjusting for sex, MPOhigh males showed significant higher values of systemic oxidative stress compared to MPOlow males (d-ROMs: MPOhigh and MPOlow, 356.0 ± 42.7 and 284.7 ± 40,1 CARR U, respectively, P = 0.012), whereas considering the right colon (d-ROMs: MPOhigh and MPOlow, 340.6 ± 50.5 and 288.5 ± 48.3, respectively, P = 0.115), the left colon (d-ROMs: MPOhigh and MPOlow, 340.2 ± 51.1 and 286.1 ± 44.6, respectively, P = 0.079), and the sigmoid-rectum (d-ROMs: MPOhigh and MPOlow, 322.0 ± 21.6 and 287.2, respectively, P = 0.330) separately, a statistical significance is not achieved. For females, a trend with higher d-ROMs values for increased MPO expression has been found but without reaching statistical significance.
LP-CHOLOX test resulted slightly increased in MPOhigh patients but this was not statistically significant considering the overall colon (MPOhigh and MPOlow, 1086.0 ± 571.0 and 965.3 ± 432.9 μEq/L, respectively; P = 0.53) as well as for each large bowel segment (right colon: MPOhigh and MPOlow, 1115.8 ± 622.6 and 990.4 ± 430.6 μEq/L, respectively, P = 0.60; left colon: MPOhigh and MPOlow, 1082.9 ± 561.9 and 856.2 ± 351.1 μEq/L, respectively, P = 0.27; sigmoid-rectum: MPOhigh and MPOlow, 1105.1 ± 519.4 and 981.8 ± 493.3 μEq/L, respectively, P = 0.57). Also adjusting for sex, there were not significant differences.
Biological antioxidant power (BAP) test resulted decreased in MPOhigh patients, though a statistical significance was observed only in the sigmoid-rectum segment (MPOhigh and MPOlow, 1428.9 ± 493.2 and 1799.8 ± 162.6 umol L−1, respectively, P < 0.02, Figure 5). Adjusting for sex, male and female groups separately did not show significant differences.
Figure 5.
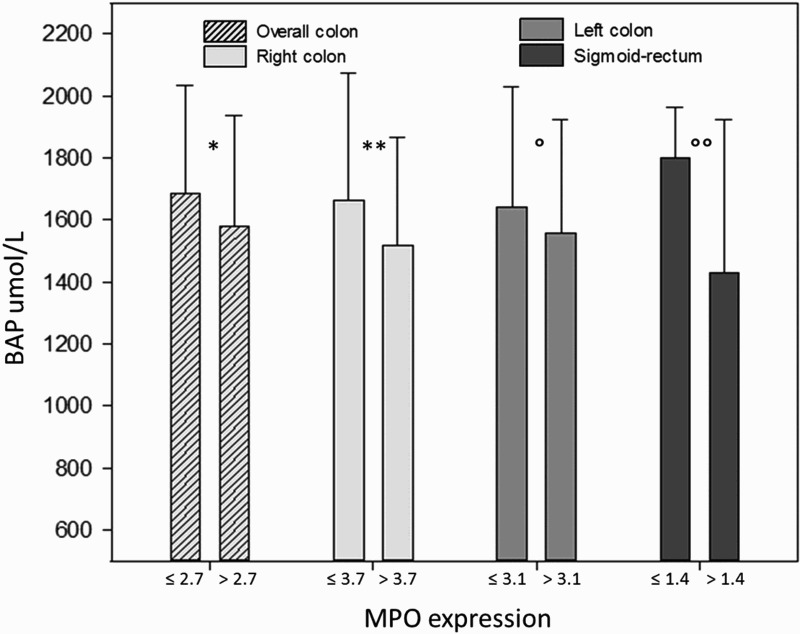
Biological antioxidant power (BAP) test tended to be higher in patients with lower MPO-positive cells in the colonic mucosa. A statistically significant difference is appreciable in the sigmoid-rectum (*P = 0.45; **P = 0.42; P = 0.62; P < 0.02).
MPO expression in relation to the other variables
A significant difference in MPO expression according to sex was found in the right colon, where males expressed higher MPO values than females (see Table 2). Moreover, men showed a significant difference in glycemic values between high expressing vs. low expressing MPO in the right colon (MPOhigh and MPOlow, 116.8 ± 11.8 and 99.7 ± 11.7 mg/dl, respectively, P = 0.04). Females showed increased mean diastolic blood pressure in the high expressing MPO group for sigmoid-rectum only (MPOhigh and MPOlow, 79.0 ± 6.1 and 66 .7 ± 5.2 mmHg, respectively; P = 0.015).
Oxidative stress and clinical metabolic profiles
d-ROMs values in the sera showed no correlation with anthropometric and metabolic profiles in our subjects, whereas patients with normal to slightly increased ( < 800 μEq/L) LP-CHOLOX test significantly differ from patients with higher (≥800 μEq/L) LP-CHOLOX test values for mean diastolic blood pressure (72.2 ± 9.5 and 81.1 ± 6.8 mmHg, respectively; P < 0.02, Figure 6). No other significant difference between groups was found in our patients for LP-CHOLOX test and metabolic/anthropometric parameters.
Figure 6.
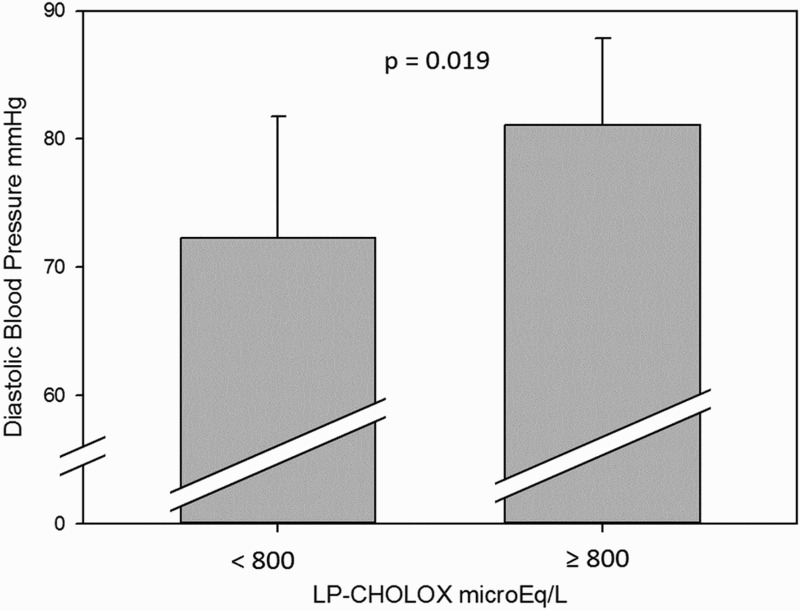
Mean diastolic blood pressures were higher in subjects with high LP-CHOLOX results (≥ 800 μEq/L), P = 0.019.
Significant correlations were found between BAP test results and several metabolic variables. BAP inversely covariated with glycemic values with a statistically significant trend, as illustrated in Figure 7, which shows that increases in glycemic values were associated with lower antioxidant power (ρ −0.45; P = 0.015). Moreover, BAP showed significant relationships with BMI (BAP decreased when BMI increased, ρ −0.41; P = 0.029, see Figure 8), and waist circumference (higher scores in BAP were associated with lower mean values for waist circumference 103.3 ± 14.1 and 89.3 ± 9.5 cm, patients with BAP ≤ 1800 and BAP > 1800, respectively, P = 0.012).
Figure 7.
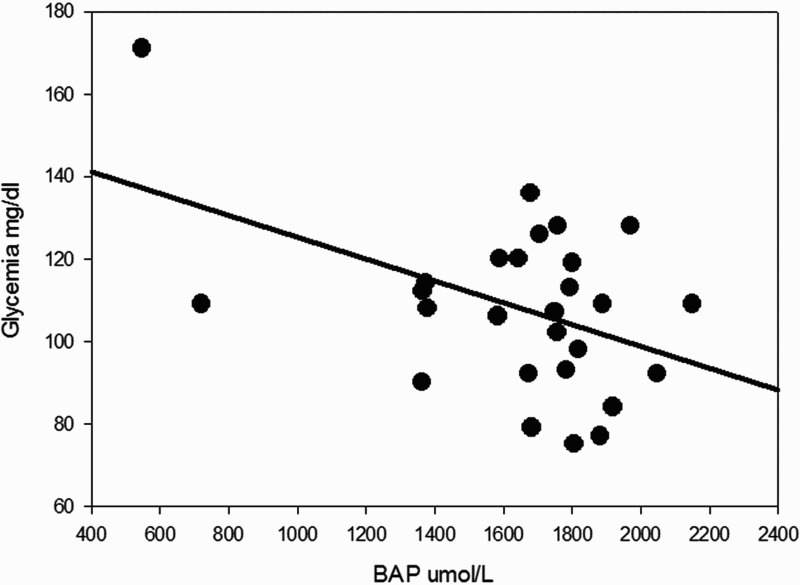
Simple scatter plot with regression line indicates that a higher glycemia was associated with a lower antioxidant power (ρ −0.45; P = 0.015).
Figure 8.
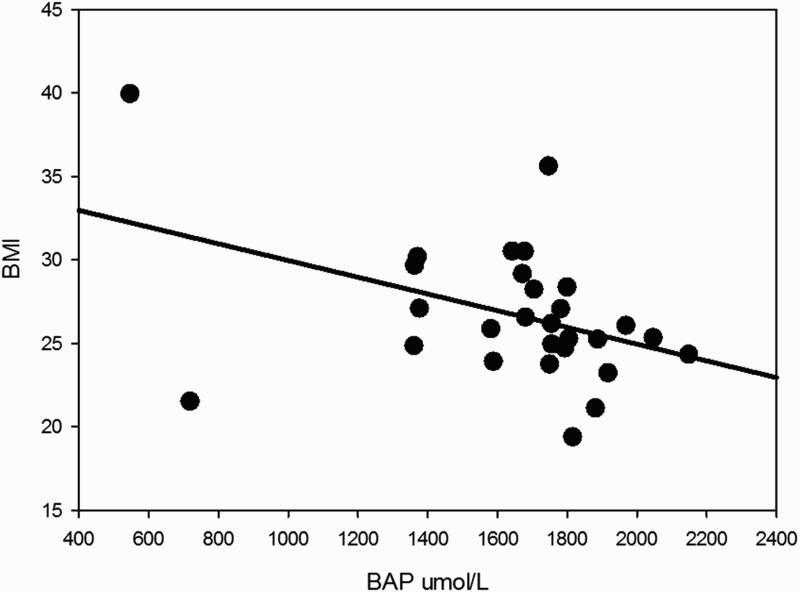
BAP results inversely correlated with BMI, indicating a lower antioxidative potential in the sera of subjects with higher BMI (ρ −0.41; P = 0.029).
Discussion
This is the first study demonstrating a connection between MPO expression in the colorectal mucosa and systemic oxidative balance in the blood of healthy subjects. Moreover, our data show that systemic redox balance is related in a different manner to different colonic segments and gender.
Subjects with a higher number of MPO-positive cells in the colonic mucosa presented higher values at the d-ROMs test in the serum, indicating that a stronger oxidative capacity (equivalent to an excess of about 3.9 mg of hydrogen peroxide/dl) characterizes MPOhigh subjects. The finding was especially significant considering MPO expression in the left colon. In fact, MPOhigh individuals for this segment expressed a marked difference in d-ROMs values compared to MPOlow patients (358.2 ± 46.4 vs. 294.9 ± 46.7 CARR U, respectively, P = 0.007). An explanation of this finding could reside in the emerging fact that the left colon seems to be less subject to immunosurveillance and subclinical inflammation than the right one. We can hypothesize that when an inflammatory response is triggered in the left colon, the impact on systemic oxidative balance is stronger than it would be in the right colon. This interpretation is in accordance with other findings. Indeed, Paski et al. demonstrated that physiologically there is an increased inflammatory activity (such as epithelial injury, crypt architecture distortion, lamina propria cellularity, and cryptitis) in the cecum compared to the rectum [38]. Also, intraepithelial T-cells were shown to be higher in the proximal colon compared to the distal in healthy adults [39]. Thus, immune surveillance seems greater in the right colon than in left colon, and we would expect that a hyper-expression of MPO in the left colon may reflect a more severe alteration of the colonic mucosa compared to an excess of MPO expression in the right colon. Accordingly, higher serum antioxidant capacity resulted significantly associated with MPOlow expression in the sigmoid-rectum. In other words, the more MPO is expressed in the sigmoid-rectum, the less antioxidant capacity is present in the bloodstream.
These findings point up that proximal and distal colon are physiologically different, furnishing novel insight to the existing piece of evidence [30,40–42]. In fact, our data also demonstrate a decreasing trend in the expression of MPO in the colonic mucosa moving from the proximal colon to the rectum (Figure 2). As mentioned before, this probably reflects a physiological reduced immune surveillance in the distal colon-rectum. Studies have shown that the human colon contains complex and diverse microbial colonies of approximately 1013 to 1014 bacteria [43], and these numbers increase with a positive gradient from the proximal colon to distal colorectum [44]. Lee et al. [45] suggested that mucosal immune cells in the colorectum have to maintain a delicate balance between immunogenicity against pathogens and tolerance for the commensal microbiota, and the distal colorectum would require immune cells to promote tolerance rather than immunogenicity. Therefore, since MPO expression is a marker of aspecific immune activity able to recruit other immune cells [19] our study supports this hypothesis.
Interestingly, our data show also differences according to gender in relation to MPO expression and systemic oxidative balance. We observed a stronger correlation between higher d-ROMs values and MPO expression in men than in women. Again, we demonstrate that MPO expression in the right colon is significantly lower in females than in males. The reason could consist in the different hormonal status. It is well established that gender is related to oxidative balance in both humans and animals [46–48], and estrogens could actively modulate sex differences in MPO expression with the evidence of a higher MPO activity in estrogen deficient animal models [49].
Basing on these considerations, we can attempt to explain some of the epidemiologic characteristics concerning inflammatory-related colorectal carcinogenesis. First of all, right-sided CRCs are more frequent in females than in males, and we can hypothesize that the MPOlow status of female’s right colon predisposes this sex to develop CRC in that colonic segment compared to men. Further studies are however necessary to elucidate whether other immunological differences exist between male and female right colon. Moreover, left colon is considered more prone to develop CRC [50], even though left-sided CRCs present a better prognosis than right-sided ones [51]. Along with other evidence of reduced immunosurveillance, lower MPO expression in the descendant could explain the higher incidence of CRC in this site. Finally, a higher MPO expression in CRC tissues may relate to a better prognosis indicating a stronger immune response against the tumor in cancer patients [28,29], while we can suggest that conflicting evidence in the literature should be reconsidered keeping in mind the varying expression of MPO according to the cancer site.
We are aware that the study has some significant limitations. First, this is a descriptive study, so it cannot define causal-effect relationships relying solely on our data. Moreover, we cannot exclude that the relationships between MPO-positive cells expression inside the colonic mucosa and the systemic redox balance discussed in this study are due to some concomitant factors that we were not able to discriminate (e.g. differences in the diet or lifestyle).
In conclusion, this study represents the first evidence that the expression of MPO in the large bowel is related to systemic oxidative balance in healthy individuals. A higher MPO expression in the colonic mucosa was associated with a higher oxidative power in the sera, whereas higher antioxidant potential (BAP) levels in the sera paralleled a lower MPO expression in the sigmoid-rectum. Noteworthy, an interesting decreasing gradient in MPO expression moving from proximal to distal colon was evident. These findings strongly support the hypothesis that systemic oxidative balance in the peripheral blood could reflect colorectal mucosa pro-inflammatory alterations, opening a new perspective on the factors possibly involved in the colorectal carcinogenesis.
Supplementary Material
Notes on contributors
Stefano Mancini graduated in Medicine and Surgery from the University of Bologna, Italy (2004), and then specialized in Internal Medicine at the University of Modena and Reggio Emilia where he is currently completing his PhD degree in ‘Clinical and Experimental Medicine.’ His research has focused initially on the cardiometabolic risk factors and then focused on mechanisms of carcinogenesis mediated by the immune system and inflammation in the colorectum. His PhD project concerns the definition of a global risk model for the development of colorectal cancer.
Francesco Mariani graduated in Chemistry and Pharmaceutical Technology, and he is PhD in ‘Experimental and Clinical Oncology’ from the University of Modena and Reggio Emilia. His field of research has focused on the molecular and metabolic mechanisms leading to the development of colorectal cancer. He works as a researcher at the Department of Diagnostics, Clinical, and Public Health Medicine, and also he is obtaining a second degree in Medicine and Surgery, at the University of Modena and Reggio Emilia.
Paola Sena had a degree in Biology from the University of Modena and Reggio Emilia and then obtained a PhD in ‘Oncological Surgery of the Digestive System’. He is currently Assistant Professor at the Department of Biomedical Sciences, Metabolic and Neuroscience at the University of Modena and Reggio Emilia, where he teaches Anatomy and Histology. Her scientific work covers the molecular biology and signaling in inflammatory foci of aberrant crypts of the human colon, and she is an expert in developmental biology.
Marta Benincasa earned a degree in Chemistry from the University of Modena and Reggio Emilia and then a Master degree in Methodology of Laboratory Research (1992). She is scientific laboratory technician in the Department of Biomedical, Metabolic and Neural Sciences since 1995. Technical Skills: maintenance of cell line cultures in adhesion and suspension; mono- and bi-dimensional gel-electrophoresis; immunohistochemistry; immunofluorescence; bidimensional HPLC in proteomics analysis.
Luca Roncucci, MD, PhD, specialized in Internal Medicine, is Associate Professor in the Department of Internal Medicine of the University of Modena and Reggio Emilia. He trained as Research Fellow at the Ontario Cancer Institute of Toronto, Canada with a grant from the National Research Council of Italy, and obtained his PhD degree at the University of Modena in Human Pathology in 1995. Scientific research work focused mainly on colorectal neoplastic diseases, starting since 1983. He developed particular expertise on cell kinetics in normal colorectal mucosa and neoplastic colorectal lesions, chemoprevention of adenoma recurrence, the familiarity of colorectal cancer, and early events of human colorectal carcinogenesis (first report of aberrant crypt foci and mucin-depleted foci in the human colon).
Funding Statement
This work was supported by Associazione Ricerca Tumori Intestinali - ARTI.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Stefano Mancini http://orcid.org/0000-0002-7350-5603
References
- [1].Thjodleifsson B, Sigthorsson G, Cariglia N, et al. . Subclinical intestinal inflammation: an inherited abnormality in Crohn’s disease relatives? Gastroenterology. 2003;124:1728–1737. doi: 10.1016/S0016-5085(03)00383-4 [DOI] [PubMed] [Google Scholar]
- [2].Keohane J, O’Mahony C, O’Mahony L, et al. . Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterology. 2010;105:1788, 1789–1794. doi: 10.1038/ajg.2010.156 [DOI] [PubMed] [Google Scholar]
- [3].Zhulina Y, Hahn-Strömberg V, Shamikh A, et al. . Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1725–1731. doi: 10.1097/MIB.0b013e318281f2d3 [DOI] [PubMed] [Google Scholar]
- [4].Masoodi I, Kochhar R, Dutta U, et al. . Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig Dis Sci. 2012;57:1336–1340. doi: 10.1007/s10620-012-2027-5 [DOI] [PubMed] [Google Scholar]
- [5].Mariani F, Sena P, Roncucci L. Inflammatory pathways in the early steps of colorectal cancer development. World J Gastroenterol. 2014;20:9716–9731. doi: 10.3748/wjg.v20.i29.9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].John BJ, Abulafi AM, Poullis A, et al. . Chronic subclinical bowel inflammation may explain increased risk of colorectal cancer in obese people. Gut. 2007;56:1034–1035. doi: 10.1136/gut.2007.125955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Biasi F, Leonarduzzi G, Oteiza PI, et al. . Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711–1747. doi: 10.1089/ars.2012.4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guina T, Biasi F, Calfapietra S, et al. . Inflammatory and redox reactions in colorectal carcinogenesis. Ann N Y Acad Sci. 2015;1340:95–103. doi: 10.1111/nyas.12734 [DOI] [PubMed] [Google Scholar]
- [9].Westbrook AM, Wei B, Braun J, et al. . Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–4834. doi: 10.1158/0008-5472.CAN-08-4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singh S, Kullo IJ, Pardi DS, et al. . Epidemiology, risk factors and management of cardiovascular diseases in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:26–35. doi: 10.1038/nrgastro.2014.202 [DOI] [PubMed] [Google Scholar]
- [11].Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44 [DOI] [PubMed] [Google Scholar]
- [12].Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–1345. doi: 10.3109/10715762.2012.717692 [DOI] [PubMed] [Google Scholar]
- [13].Roessner A, Kuester D, Malfertheiner P, et al. . Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011 [DOI] [PubMed] [Google Scholar]
- [14].Mangerich A, Dedon PC, Fox JG, et al. . Chemistry meets biology in colitis-associated carcinogenesis. Free Radic Res. 2013;47:958–986. doi: 10.3109/10715762.2013.832239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tüzün A, Erdil A, İnal V, et al. . Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem. 2002;35:569–572. doi: 10.1016/S0009-9120(02)00361-2 [DOI] [PubMed] [Google Scholar]
- [16].van der Logt EMJ, Roelofs HMJ, Wobbes T, et al. . High oxygen radical production in patients with sporadic colorectal cancer. Free Radic Biol Med. 2005;39:182–187. doi: 10.1016/j.freeradbiomed.2005.03.003 [DOI] [PubMed] [Google Scholar]
- [17].Reuter S, Gupta SC, Chaturvedi MM, et al. . Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van der Veen BS, de Winther MPJ, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538 [DOI] [PubMed] [Google Scholar]
- [19].Hickey MJ. MPO and neutrophils: a magnetic attraction. Blood. 2011;117:1103–1104. doi: 10.1182/blood-2010-11-317479 [DOI] [PubMed] [Google Scholar]
- [20].Masoodi I, Tijjani BM, Wani H, et al. . Biomarkers in the management of ulcerative colitis: a brief review. Ger Med Sci. 2011;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rayner BS, Love DT, Hawkins CL. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radic Biol Med. 2014;71:240–255. doi: 10.1016/j.freeradbiomed.2014.03.004 [DOI] [PubMed] [Google Scholar]
- [22].Davies MJ. Myeloperoxidase-derived oxidation: mechanisms of biological damage and its prevention. J Clin Biochem Nutr. 2010;48:8–19. doi: 10.3164/jcbn.11-006FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arnhold J, Furtmuller PG, Obinger C. Redox properties of myeloperoxidase. Redox Rep. 2003;8:179–186. doi: 10.1179/135100003225002664 [DOI] [PubMed] [Google Scholar]
- [24].Al-Salihi M, Reichert E, Fitzpatrick FA. Influence of myeloperoxidase on colon tumor occurrence in inflamed versus non-inflamed colons of ApcMin/+ mice. Redox Biol. 2015;6:218–225. doi: 10.1016/j.redox.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Terzić J, Grivennikov S, Karin E, et al. . Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058 [DOI] [PubMed] [Google Scholar]
- [26].Garrity-Park M, Loftus EV, Sandborn WJ, et al. . Myeloperoxidase immunohistochemistry as a measure of disease activity in ulcerative colitis: association with ulcerative colitis-colorectal cancer, tumor necrosis factor polymorphism and RUNX3 methylation. Inflamm Bowel Dis. 2012;18:275–283. doi: 10.1002/ibd.21681 [DOI] [PubMed] [Google Scholar]
- [27].Roncucci L, Mora E, Mariani F, et al. . Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2291–2297. doi: 10.1158/1055-9965.EPI-08-0224 [DOI] [PubMed] [Google Scholar]
- [28].Däster S, Eppenberger-Castori S, Hirt C, et al. . Absence of myeloperoxidase and CD8 positive cells in colorectal cancer infiltrates identifies patients with severe prognosis. Oncoimmunology. 2015;4:e1050574–1. doi: 10.1080/2162402X.2015.1050574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Droeser RA, Hirt C, Eppenberger-Castori S, et al. . High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One. 2013;8:e64814. doi: 10.1371/journal.pone.0064814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Park JH, Kim MJ, Park SC, et al. . Difference in time to locoregional recurrence between patients with right-sided and left-sided colon cancers. Dis Colon Rectum. 2015;58:831–837. doi: 10.1097/DCR.0000000000000426 [DOI] [PubMed] [Google Scholar]
- [31].DeCosse JJ, Ngoi SS, Jacobson JS, et al. . Gender and colorectal cancer. Eur J Cancer Prev. 1993;2:105–116. doi: 10.1097/00008469-199303000-00003 [DOI] [PubMed] [Google Scholar]
- [32].Cesarone MR, Belcaro G, Carratelli M, et al. . A simple test to monitor oxidative stress. Int Angiol. 1999;18:127–130. [PubMed] [Google Scholar]
- [33].Trotti R, Carratelli M, Barbieri M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002;44:37–40. [PubMed] [Google Scholar]
- [34].Cornelli U, Belcaro G, Cesarone MR, et al. . Analysis of oxidative stress during the menstrual cycle. Reprod Biol Endocrinol. 2013;11:74. doi: 10.1186/1477-7827-11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Macri A, Scanarotti C, Bassi AM, et al. . Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15 % and vitamin B12 eye drops. Graefe’s Arch Clin Exp Ophthalmol. 2015;253:425–430. doi: 10.1007/s00417-014-2853-6 [DOI] [PubMed] [Google Scholar]
- [36].Fukuda T, Kurano M, Fukumura K, et al. . Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress. Korean Circ J. 2013;43:481–487. doi: 10.4070/kcj.2013.43.7.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fukui T, Yamauchi K, Maruyama M, et al. . Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens Res. 2011;34(9):1041–1045. doi: 10.1038/hr.2011.76 [DOI] [PubMed] [Google Scholar]
- [38].Paski SC, Wightman R, Robert ME, et al. . The importance of recognizing increased cecal inflammation in health and avoiding the misdiagnosis of nonspecific colitis. Am J Gastroenterol. 2007;102:2294–2299. doi: 10.1111/j.1572-0241.2007.01389.x [DOI] [PubMed] [Google Scholar]
- [39].Kirby JA, Bone M, Robertson H, et al. . The number of intraepithelial T cells decreases from ascending colon to rectum. J Clin Pathol. 2003;56:158. doi: 10.1136/jcp.56.2.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Azzoni C, Bottarelli L, Campanini N, et al. . Distinct molecular patterns based on proximal and distal sporadic colorectal cancer: arguments for different mechanisms in the tumorigenesis. Int J Colorectal Dis. 2007;22:115–126. doi: 10.1007/s00384-006-0093-x [DOI] [PubMed] [Google Scholar]
- [41].Horii J, Hiraoka S, Kato J, et al. . Age-related methylation in normal colon mucosa differs between the proximal and distal colon in patients who underwent colonoscopy. Clin Biochem. 2008;41:1440–1448. doi: 10.1016/j.clinbiochem.2008.08.089 [DOI] [PubMed] [Google Scholar]
- [42].Ponz de Leon M, Sacchetti C, Sassatelli R, et al. . Evidence for the existence of different types of large bowel tumor: suggestions from the clinical data of a population-based registry. J Surg Oncol. 1990;44:35–43. doi: 10.1002/jso.2930440109 [DOI] [PubMed] [Google Scholar]
- [43].Macfarlane GT, Macfarlane LE. Acquisition, evolution and maintenance of the normal gut microbiota. Dig Dis. 2009;27 Suppl 1:90–98. doi: 10.1159/000268127 [DOI] [PubMed] [Google Scholar]
- [44].Gill SR, Pop M, Deboy RT, et al. . Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lee GH, Malietzis G, Askari A, et al. . Is right-sided colon cancer different to left-sided colorectal cancer? - A systematic review. Eur J Surg Oncol. 2014;41:300–308. doi: 10.1016/j.ejso.2014.11.001 [DOI] [PubMed] [Google Scholar]
- [46].Giergiel M, Lopucki M, Stachowicz N, et al. . The influence of age and gender on antioxidant enzyme activities in humans and laboratory animals. Aging Clin Exp Res. 2012;24:561–569. [DOI] [PubMed] [Google Scholar]
- [47].Perez-Torres I, Roque P, El Hafidi M, et al. . Association of renal damage and oxidative stress in a rat model of metabolic syndrome. Free Radic Res. 2009;43:761–771. doi: 10.1080/10715760903045296 [DOI] [PubMed] [Google Scholar]
- [48].Miller AA, De Silva TM, Jackman KA, et al. . Effect of gender and sex hormones on vascular oxidative stress. Clin Exp Pharmacol Physiol. 2007;34:1037–1043. doi: 10.1111/j.1440-1681.2007.04732.x [DOI] [PubMed] [Google Scholar]
- [49].Pósa A, Szabó R, Csonka A, et al. . Endogenous estrogen-mediated heme oxygenase regulation in experimental menopause. Oxid Med Cell Longev. 2015;2015:429713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheng L, Eng C, Nieman LZ, et al. . Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011;34:573–580. doi: 10.1097/COC.0b013e3181fe41ed [DOI] [PubMed] [Google Scholar]
- [51].Yahagi M, Okabayashi K, Hasegawa H, et al. . The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg. 2016;20:648–655. doi: 10.1007/s11605-015-3026-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


