Abstract
Objective
To investigate the hepatoprotective potential of n-butanolic extract of Astragalus monspessulanus L. (EAM) against in-vitro/in-vivo carbon tetrachloride (CCl4)-induced liver damage in rats. Silymarin was used as a positive control.
Methods and results
The in-vitro experiments were carried out in primary isolated rat hepatocytes first incubated with CCl4 (86 µmol/l). Hepatic injury was discerned by a decrease in cell viability and cell glutathione (GSH) levels, an increase in lactate dehydrogenase leakage into the medium, and an elevation in malondialdehyde (MDA) quantity. Cell pre-incubation with EAM (1 µg/ml and 10 µg/ml) significantly ameliorated the CCl4-induced liver damage. In-vivo rats were challenged orally with CCl4 (10% solution in olive oil) alone and after 7 days pre-treatment with EAM (100 mg/kg body weight per day, oral gavage). CCl4 damage was judged by an increased production of MDA, depletion of cell GSH, and a decrease in cell antioxidant defense system. EAM pre-treatment normalizes the activities of the antioxidant enzymes and the levels of GSH and MDA. These data are supported by the histopathological examination.
Conclusion
These results indicate that EAM has a similar significant protective effect, in vitro and in vivo, against CCl4 induced hepatotoxicity in rat as silymarin.This may be due to its antioxidant and membrane stabilizing properties.
Keywords: Astragalus monspesulans, Carbon tetrachloride, Hepatotoxicity, Oxidative stress, Phytochemical analysis
Introduction
The liver, due to its unique metabolism and relationship to the gastrointestinal tract, is one of the organs that is most highly exposed to many potentially toxic substances. This makes the liver an important target for the toxicity of drugs, xenobiotics, and oxidative stress. Reactive oxygen species (ROS) play an important role in the pathogenesis of various degenerative human diseases and have been implicated in atherosclerosis, liver disorders, lung and kidney damage, aging, and diabetes mellitus.1 Carbon tetrachloride (CCl4) is a well-known hepatotoxic agent used in a number of experimental models to study acute liver failure.2 Liver injuries induced by CCl4 are a result of its oxidative biotransformation to ROS such as trichloromethyl radical (CCl3•) and its derivative trichloromethyl peroxy radical (CCl3OO•), which are thought to react with membrane lipids leading to lipid peroxidation and cellular membrane breakdown.1
Even though there are a wide range of drugs currently employed in the management of hepatic disorders, alternative approaches based on the use of traditional herbal preparations and isolated, biologically active, plant substances have been widely implemented. Astragalus L. (Fabaceae) is a genus distributed in Europe, Asia, and North America. The pharmacological properties of Astragalus spp. are varied and include immunostimulant effects, anti-bacterial and anti-viral properties.3,4 In our laboratory, the protective effects of Astragalus membranaceus L., Astragalus glycyphylloides L., and Astragalus corniculatus Bieb. using different models of hepatotoxicity have been examined.5–7 Astragalus monspessulanus L., Fabaceae (Montpellier Milk Vetch) is native to the Iberian Peninsula, France, Switzerland, the Apennine Peninsula, the Balkan Peninsula, Eastern Europe, and Northwest Africa.8,9 There is no information available regarding the chemical composition or activity of the plant. A previous study by the current authors showed that purified saponin fraction, isolated from A. monspessulanus, had a cytotoxic effect in the HepG2 cell line observed at the highest concentration of 4 mg/ml.10
On the basis of these data, the aim of the following study was to investigate the possible hepatoprotective and antioxidant potential of n-butanolic extract isolated from A. monspessulanus L. (EAM) using in-vitro and in-vivo models of CCl4-induced acute liver injury.
Materials and methods
Plant material and preparation of n-butanol (n-BuOH) extract
The overground parts of A. monspessulanus were collected in May 2010 from Rodopi Mountain, Bulgaria. The plant was identified by Dr D. Pavlova from the Department of Botany, Faculty of Biology, Sofia University, where a voucher specimen has been deposited (N SO 107533).
The air-dried plant material (280 g) was powdered and exhaustively extracted with 80% methanol (CH3OH) under reflux (23 × 750 ml). The extracts were filtrated and concentrated under reduced pressure. The water residue was subjected to extraction with dichloromethane to remove the lipophilic constituents. The defatted water residue was successively extracted with ethyl acetate (EtOAc) and n-butanol(n-BuOH). The n-BuOH extract was evaporated to dryness to give a solid residue of 53.6 g.
The BuOH extract was chromatographed over Diaion® HP-20 (Supelco, Bellefonte, PA, USA) with the system H2O–CH3OH (100 : 0→0 : 100) to give 18 main fractions (A–R). Fraction H was subjected to column chromatography over Sephadex® LH-20 (Pharmacia Fine Chemicals, AB, Uppsala, Sweden) with eluent CH3OH to give four fractions (H1–H4). Fraction H3 was purified again over Sephadex® LH-20 with eluent CH3OH and three fractions were collected (H3−1, H3−2, H3−3). Fraction H3−2 was subjected to low-pressure liquid chromatography over reversed phase C18 with the system CH3OH–H2O (44 : 56) to give a purified flavonoid mixture. Further high performance liquid chromatography (HPLC) analysis of the mixture showed four well-separated peaks corresponding to the main flavonoids, named F1–F4 (Fig. 1).
Figure 1.
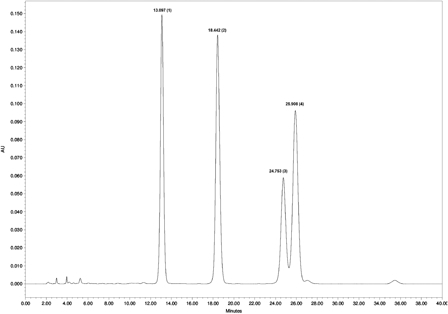
HPLC chromatogram of the flavonoids.
A thin layer chromatography study was carried out on pre-coated silica gel plates (Kieselgel® G, F254) (Merck, Darmstadt, Germany) with solvent system EtOAc–HCOOH–AcOH–H2O (32 : 3 : 2 : 6). The spots were visualized by spraying with Natustoffreagenz. Column chromatography was carried out with Diaion® HP-20 (Supelco, Bellefonte, PA, USA), Sephadex® LH-20 (Pharmacia Fine Chemicals, AB, Uppsala, Sweden) and DAVISIL® C18 (Grace, Davison Discovery Sciences, Hesperia, CA, USA). HPLC analysis was accomplished with Waters® binary HPLC pump, Model 1525 (Milford, MA, USA) and Waters® UV/visible detector, Model 2479 (Milford, MA, USA) over prepacked column Luna® (C18, 100 Å, 250 × 4.6 mm 5 micron, Phenomenex, USA). Isocratic elution was carried out with water-phosphoric acid 0.1%–acetonitrile (83 : 17) at a constant flow rate of 1 ml/min. The chromatogram was monitored at 254 nm.
Animals
Male Wistar rats (body weight (bw) 200–220 g) were used. The rats were housed in plexiglass cages (three per cage) in a 12/12 light/dark cycle, under standard laboratory conditions (ambient temperature 20 ± 2°C and humidity 72 ± 4%) with free access to water and standard pelleted rat food 53–3, produced according to ISO 9001 : 2008. Animals were purchased from the National Breeding Center, Sofia, Bulgaria. A minimum of 7 days acclimatization was allowed before the commencement of the study and their health was monitored regularly by a veterinary physician. Vivarium (certificate of registration of farm № 0072/01.08.2007) was inspected by the Bulgarian Drug Agency in order to check the husbandry conditions (№A-11-1081/03.11.2011). All performed procedures were approved by the Institutional Animal Care Committee (KENIMUS) and the principles stated in the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes ((ETS 123) Council of Europe, 1991) were strictly followed throughout the experiment.11
Chemicals
All the reagents used were of analytical grade. Carbontetrachloride, silymarin as well as collagenase, 1-chloro-2,4-dinitrobenzene (CDNB), beta-Nicotinamide adenine dinucleotide 2-phosphate reduced tetrasodium salt (NADPH), ethylenediaminetetraacetic acid (EDTA), bovine serumalbumin (fraction V), reduced glutathione (GSH), oxidized glutathione (GSSG), glutathione reductase (GR), and cumene hydroperoxide were purchased from Sigma Chemical Co. (Taufkirchen, Germany). 2,2-Dinitro-5,5 dithiodibenzoic acid (DTNB) was obtained from Merck (Darmstadt, Germany).
Isolation and incubation of hepatocytes
Rats were anesthetized with sodium pentobarbital (0.2 ml/100 g). In-situ liver perfusion and cell isolation were performed as described by Fau et al.12 with modifications.13 Cells were counted under the microscope and cell viability was assessed by Trypan blue exclusion (0.05%):12 initial viability averaged 89%.
Incubation of hepatocytes
Liver damage was induced by 1 hour incubation of the isolated hepatocytes with CCl4, applied at a concentration of 86 μmol/l.14 In order to investigate the hepatoprotective activity of EAM, isolated hepatocytes were pre-incubated for 30 minutes with the extract, administered in two concentrations (1 µg/ml and 10 µg/ml) and then incubated with CCl4 (86 μmol/l) for 1 hour. The effect of EAM was compared to those of silymarin (1 µg/ml and 10 µg/ml). To assess the functional status of hepatocytes, the following parameters were measured: cell viability, lactate dehydrogenase (LDH) activity, GSH levels, and malondialdehyde (MDA) quantity. Cell viability was assessed by Trypan blue exclusion method.12 The dye was used at a final concentration of 0.05% and cells were counted under light microscope (×100). At the end of the incubation, the cells were recovered via centrifugation at 400g at 4°C. The supernatant was used for LDH and MDA assessment as described by Bergmeyer et al.15 and Fau et al.,12 respectively. GSH measurement following the method used by Fau et al.12 was assessed in the sediment.
Design of the in-vivo experiment
For the in-vivo experiment, a total of 36 animals were randomly allocated into six experimental groups, each consisting of six animals (n = 6). Group 1 were control animals treated with physiological saline via oral gavage (0.5 ml/g bw). Groups 2 and 3 were rats treated for 7 days with either EAM at 100 mg/kg bw/day or with silymarin at 100 mg/kg bw.16 Group 4 received a single dose (1.25 ml/kg) of 10% CCl4 in olive oil.17 The animals in group 5 were pre-treated with EAM (100 mg/kg/p.o. for7 days), and 90 minutes after the last administration were challenged with CCl4 (10% solution) and group 6 was pre-treated with silymarin (100 mg/kg bw/p.o. for 7 days) and challenged with 10% CCl4 solution 90 minutes after the last treatment.
On day eight of the experiment, blood was taken for biochemical analysis and the animals were sacrificed by cervical dislocation. The livers were removed rapidly, washed with ice-cold saline solution (0.9% NaCl), blotted dry, weighed, and divided into pieces (stored on ice) as follows: 1 g each for assessment of MDA quantity, glutathione (GSH) levels, antioxidant enzyme activities, and histopathology. Tissues were homogenized in ice-cold appropriate buffers using a glass homogenizer (PX-OX 2000).
Serum biochemistry analysis
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) activities were measured, using commercially available standard diagnostic kits (Randox Laboratories Ltd, Ardmore, UK) by an automated optimized spectrophotometrical method (COBOS integra 400 plus Roche Diagnostics). Briefly, blood was taken into a tube containing ethylene glycol tetraacetic acid. Sera were separated by centrifugation in a bench centrifuge (Eppendorf MiniPlus, Hamburg, Germany) at 10 000 rpm for 10 minutes, at 4°C. Enzyme activity in blood serum was evaluated by an autoanalyzer (Mindray, Guamgdong, China).
Biomarkers of oxidative stress
The oxidative damage was evaluated in liver homogenates by estimating the rate of production of thiobarbituric acid reactive substances (TBARS) (expressed as MDA equivalents) and the levels of the cell protector GSH.
MDA quantity was measured spectrophotometrically using the method described by Polizio and Peña.18 The concentration of MDA was calculated using a molar extinction coefficient of 1.56 × 105/M/cm and expressed in nmol/g wet tissue.
GSH was assessed by measuring non-protein sulfhydryls after precipitation of proteins with TCA and using dinitro thio benzoic acid (DTNB).19 The absorbance was determined at 415 nm and the results were expressed as nmol/g wet tissue.
Preparation of liver homogenates for analysis of antioxidant enzymes
The livers were rinsed in ice-cold physiological saline and minced with scissors. Ten percent homogenates were prepared in 0.05 M phosphate buffer (pH = 7.4), centrifuged at 7000g, and the supernatant was used for antioxidant enzymes assay. The protein content of liver homogenate was measured by the method of Lowry.20
Catalase (CAT) activity21
Briefly, 10 µl of homogenate was added to 1990 µl of H2O2 solution (containing 6.8 µl of 30% H2O2 + 1983.2 µl 0.05 M phosphate buffer, pH = 7.4). CAT activity was determined by measuring the decrease in absorbance at 240 nm. The enzyme activity was expressed as µM/mg.
Superoxide dismutase (SOD) activity was measured according to the method of Misura and Fridovich,22 following spectrophotometrical autoxidation of epinephrine at pH = 10.4, 30°C, using the molar extinction coefficient of 4.02 /mM/cm. The incubation mixture contained 50 mM glycine buffer, pH = 10.4. The reaction is started by the addition of epinephrine. SOD activity was expressed as nmol of epinephrine that are prevented from autoxidation after addition of the sample.
Gluthatione peroxidase (GPx) activity was measured by NADPH oxidation, using a coupled reaction system consisting of glutathione, GR, and sumene hydroperoxide.23 Briefly, 100 µl of enzyme sample was incubated for 5 minutes with 1.5 ml 0.05 M phosphate buffer (pH = 7.4), 100 µl 1 mM EDTA, 50 µl 1 mM GSH, 100 µl 0.2 mM NADPH, and 1 unit GR. The reaction was initiated by adding 50 µl cumene hydroperoxide (1 mg/ml) and the rate of disappearance of NADPH with time was determined by monitoring absorbance at 340 nm. Results are expressed in nmol/mg.
GR activity was measured according to the method of Pinto et al.24 by following NADPH oxidation spectrophotometrically at 340 nm and using an extinction coefficient of 6.22 /mM/cm. The incubation mixture contained 0.05 M phosphate buffer, pH = 7.4, 2.5 mM GSSG and 125 µM NADPH at 30°C.
Glutathione-S-transferase activity (GST) was measured using CDNB as substrate.25 The incubation mixture containing 1.6 ml 0.05 M phosphate buffer, 100 µl 1 mM GSH, 100 µl 1 mM EDTA, and 100 µl homogenate, was incubated for 15 minutes at 37°C. After the incubation, 100 µl 1 mM CDNB was added and the increase in absorbance with time was recorded at 340 nm. The enzyme activity is expressed as nmol of CDNB–GSH conjugate formed/min/mg protein.
Histopathological assessment for liver damage
For light microscope evaluation, liver tissues were fixed in 10% buffered formalin and thin sections (4 μm) were subsequently stained with hematoxylin/eosin for general histological feature determination.26 Sections were studied under the NU2 microscope (Carl Zeiss, Jena, Germany) with camera Canon EOS 5D Mark II (Scale bars, 50 µm). Fat deposition was scored by using the Adobe Photoshop's counting tool. Five randomly chosen sections from a sample were used.
Statistical analysis
For the in-vitro experiments, the statistical analysis included analysis of variance and the Student's t-test. The results were presented as mean ± SD of four animals per group. For each of the examined parameters, three parallel samples were used. Statistical programme ‘MEDCALC’ was used for analysis of the in-vivo data. The results are expressed as mean ± SEM for six rats in each group. The significance of the data was assessed using the nonparametric Mann–Whitney test. For both statistical methods, values of P ≤ 0.05 were considered statistically significant.
Results
In-vitro studies in isolated rat hepatocytes
The results are shown in Fig. 2. Compared to control animals, CCl4 incubation led to pronounced toxic effects witnessed by reducing: cell viability by 72% (P < 0.05) (Fig. 2A) and GSH levels by 84% (P < 005) (Fig. 2C); and by increasing: LDH leakage from the cells by 185% (P < 0.05) (Fig. 2B) and MDA production by 83% (P < 0.05) (Fig. 2D). Pre-incubation of the hepatocytes with the EAM partially prevented the liver injury caused by CCl4 and discerned by increased cell viability and restored LDH activity, MDA, and GSH levels. The effect was more pronounced at 10 µg/ml and was comparable to those of silymarine.
Figure 2.
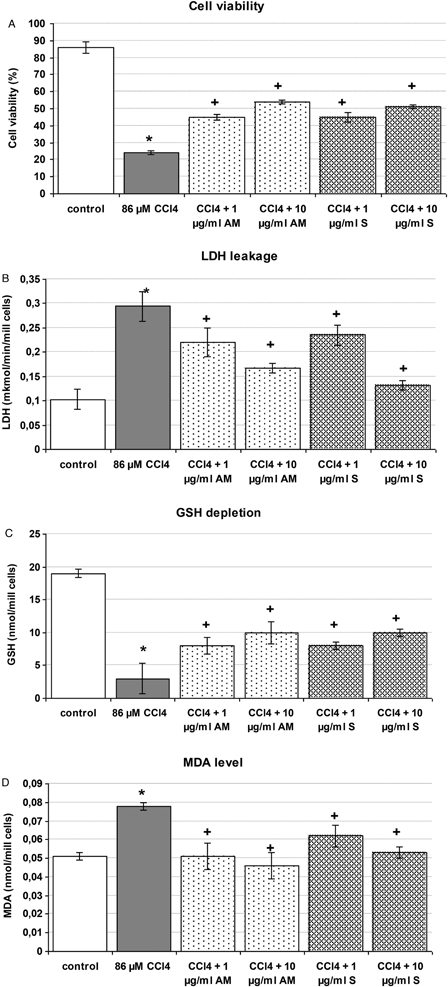
Effect of silymarin and EAM pre-incubation on CCl4-induced model of toxicity in isolated rat hepatocytes. Data are expressed as mean ± SD of four different experiments. *Significant difference from control values (Mann–Whitney test, (<0.05). +Significant difference from CCl4-treated group (Mann–Whitney test, <0.05).
In-vivo studies
There were no observed changes in behavior or in food and water consumption among the animals in either the control or treated groups during treatment. All animals survived until the end of the treatment period.
Serum parameters
Effects of EAM pre-treatment on CCl4-induced changes in serum enzyme activities are shown in Table 1. A significant increase in the activity of serum ALT, AST, and ALP was observed in rats challenged with CCl4 as follows: AST was increased by 56% (P < 0.005), ALT – by 28% (P < 0.05), and ALP – by 73% (P < 0.05). Pre-treatment with EAM resulted in statistically significant (P < 0.005) decreases in enzyme activities, compared to the CCl4 only group. The effect of EAM was comparable to those of silymarin.
Table 1.
Effect of EAM and silymarin pre-treatment on CCl4-induced alterations in serum enzyme activities
| Group | Control | CCl4 | Silymarin | SL + CCl4 | EAM | EAM + CCl4 |
|---|---|---|---|---|---|---|
| AST (IU/L) | 120 ± 20 | 187 ± 17* | 128 ± 15 | 135 ± 13+ | 135 ± 25 | 130 ± 15+ |
| ALT (IU/L) | 61 ± 16 | 78 ± 10* | 58 ± 8 | 60 ± 7+ | 64 ± 10 | 66 ± 11+ |
| ALP(IU/L) | 85 ± 14 | 147 ± 17* | 80 ± 10 | 93 ± 9+ | 79 ± 12 | 99 ± 15+ |
Treatment: CCl4 administered p.o. as 10% solution, alone (CCl4 group) and 2 hours after the last administration of the hepatoprotective agents (EAM + CCl4 and SL + CCl4 groups); Extract from A. monspessulanus (EAM) (100 mg/kg bw/day) and silymarin (SL) (100 mg/kg bw/day) were administered p.o. for 7 days. Data are expressed as mean ± SEM of six rats.
*Significant difference from control values (Mann–Whitney U-test, P < 0.05).
+Significant difference from CCl4-treated group (Mann–Whitney U-test, P < 0.05).
Lipid peroxidation, cell glutathione, and antioxidant enzymes
The effect of EAM and silymarin pre-treatment on hepatic lipid peroxidation and antioxidant enzymes in rats with CCl4-induced toxicity is given in Table 2. Compared to the control group, CCl4 administration induced significant pro-oxidant and hepatotoxic effects, discerned by a marked increase, by 71% (P < 0.05), in MDA quantity and decrease, by 43% (P < 0.05), in GSH level. In addition, a significant (P < 0.05) decrease in antioxidant enzyme activities was observed, i.e. CAT – by 42%, SOD – by 42%, GPx and GR – by 61%, GST – by 43%. EAM and silymarin pre-treatment prevented CCl4-induced oxidative stress by inhibiting lipid peroxidation and restoring the levels of cell glutathione and antioxidant enzymes. Compared to the CCl4 only group, EAM pre-treatment resulted in a significant decrease in MDA quantity (29% (P < 0.05)) and an increase in GSH levels (55% (P < 0.05)), in CAT activity (55% (P < 0.05)), in SOD activity (43% (P < 0.05)), in GPx and GR activities (79% (P < 0.05)), in GST activity (35% (P < 0.05)). EAM hepatoprotective and antioxidant effects were comparable to those of silymarin.
Table 2.
Effect of extract from A. monspessulanus and silymarin pre-treatment on hepatic liver peroxidation and antioxidant profile in rats challenged with CCl4
| Group | Control | CCl4 | Silymarin | SL + CCl4 | EAM | EAM + CCl4 |
|---|---|---|---|---|---|---|
| MDA wet tissue (nmol/g) | 0.70 ± 0.07 | 1.19 ± 0.14* | 0.68 ± 0.09 | 0.84 ± 0.07*+ | 0.75 ± 0.07 | 0.85 ± 0.05+ |
| GSH wet tissue (nmol/g) | 5.19 ± 0.23 | 2.94 ± 0.38* | 5.23 ± 0.33 | 4.84 ± 0.25+ | 4.83 ± 0.31 | 4.55 ± 0.4+ |
| CAT protein (µmol/min/mg) | 79.8 ± 5.9 | 47.7 ± 5.7* | 80.7 ± 6.1 | 75.6 ± 7.6+ | 71.3 ± 7.6 | 65.9 ± 5.8+ |
| SOD protein (µmol/min/mg) | 0.24 ± 0.03 | 0.14 ± 0.02* | 0.23 ± 0.03 | 0.21 ± 0.02+ | 0.22 ± 0.05 | 0.20 ± 0.02+ |
| GPx protein (nmol/min/mg) | 109.3 ± 10 | 42.3 ± 12.8* | 108.9 ± 12.1 | 85.9 ± 7.8*+ | 117.7 ± 22.3 | 75.9 ± 10,2+ |
| GR protein (µmol/min/mg) | 0.23 ± 0.03 | 0.09 ± 0.01* | 0.20 ± 0.01 | 0.14 ± 0.02*+ | 0.17 ± 0,02 | 0.16 ± 0.01*+ |
| GST protein (µmol/min/mg) | 0.30 ± 0.04 | 0.17 ± 0.03* | 0.32 ± 0.04 | 0.26 ± 0.03+ | 0.25 ± 0.02 | 0.23 ± 0.03+ |
Treatment: CCl4 administered p.o. as 10% solution, alone (CCl4 group) and 2 hours after the last administration of the hepatoprotective agents (EAM + CCl4 and SL + CCl4 groups); extract from A. monspessulanus (EAM) (100 mg/kg bw/day) and silymarin (SL) (100 mg/kg bw/day) were administered p.o. for 7 days. Data are expressed as mean ± SEM of six rats.
*Significant difference from control values (Mann–Whitney U-test, P < 0.05).
+Significant difference from CCl4-treated group (Mann–Whitney U-test, P < 0.05).
Histopathological study
The microscopic examination of the livers obtained from control group and groups treated with silymarin and EAM showed normal architecture of the liver parenchyma and hepatocytes, in which microscopically visible changes are absent (Fig. 3A, C and E). Fig. 3B depicts changes expressed as microvesicular accumulations of fats, localized in the center of acini, predominated in the liver of CCl4-treated rats. In the individual cells or group of cells, lytic changes were observed in the nucleus of the hepatocytes also as perivascular, mononuclear aggregations. Pronounced protective effects demonstrated by accumulation of fats in limited number of cells and absence of necrotic changes in the hepatocytes were observed in the liver of rats treated with silymarin and EAM (Fig. 3D and F).
Figure 3.
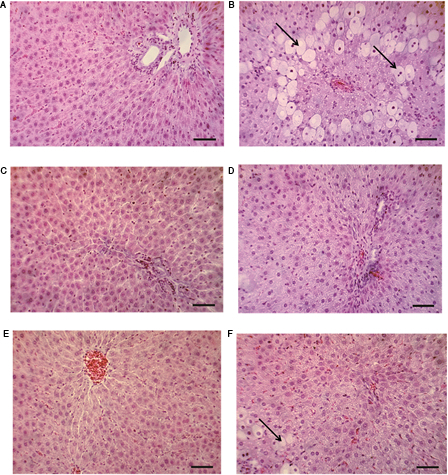
Microscopic observations of untreated and treated livers. (A) Liver tissue from control group of rats – normal cellular architecture next to terminal hepatic venule (scale bars, 50 µm). (B) Section of liver tissue of CCl4-treated group showing microvesicular accumulation of fats located centriacinar (scale bars, 50 µm). (C) Section of liver tissue of silymarin-treated group showing normal hepatocytes (scale bars, 50 µm). (D) Section of liver tissue of silymarin-pre-treated group and then challenged with CCl4 showing protective effect expressed in less accumulation of fats (scale bars, 50 µm). (E) Section of liver tissue of A. monspessulanus extract (EAM)-treated group showing normal hepatocytes (scale bars, 50 µm). (F) Section of liver tissue of A. monspessulanus extract-pre-treated group and then challenged with CCl4 revealing lower fats deposition in the hepatocytes. Hepatic necrosis was not observed (scale bars, 50 µm).
Discussion
Oxidative stress and related lipid peroxidation are regarded as one of the main biological mechanisms responsible for different pathological processes, leading to functional and structural changes in the biological membranes and cell death. The liver is the main metabolic organ where most xenobiotics are metabolized to inactive or less toxic metabolites. However, there are also examples of drugs that undergo bioactivation and are converted to more toxic metabolites than the parent compound. Therefore, the liver, because of its metabolic capacity, is one of the major target organs for injury by a number of exogenous compounds such as environmental toxins, drugs, and chemicals that undergo cytochrome P-450-mediated metabolic activation to ROS or free radicals.27 There is a large body of information revealing the hepatoprotective and antioxidant properties of natural plant-derived products containing phytoconstituents such as saponins, tannins, terpenoids, and flavonoids (isoquercetin, hyperoside, vitexin, myricetin, and kaempherol).28,29 The aim of the current study was to investigate the effect of an EAM using an in-vitro/in-vivo model of CCl4-induced acute liver damage in rats.
In experimental toxicology, CCl4-induced liver injury is used as a model of hepatotoxicity both in vitro and in vivo.2 The basic mechanism of CCl4 toxicity is bioactivation by several cytochrome P-450 isoforms – CYP2E1, CYP2B1, and possibly CYP3A4 – to form the CCl3•, which initiates the chain reaction of lipid peroxidation.30 The CCl3• and its derivative CCl3OO• react with the unsaturated fatty acids in the cellular membranes disturbing a number of cellular processes and leading to a loss of cell protection, discerned by increased formation of TBARS,31 LDH leakage,32 and GSH depletion. The results of this study showed these cellular and biochemical changes both in vitro after 1 hour incubation of the isolated hepatocytes with CCl4 (86 μmol/l) (see Fig. 2) and in vivo after oral gavage administration of 10% CCl4 solution (see Table 2). The pre-incubation of the hepatocytes and pre-treatment of the animals with the EAM showed hepatoprotective activity manifested by restoration of GSH levels and decreased MDA formation (see Fig. 2 and Table 2). In isolated rat hepatocytes, increase in cell viability and decrease in LDH activity is also observed in the combination group (Fig. 2). These effects of EAM were comparable to the effects of silymarin, a well-known and well-used hepatoprotective agent.
Normally, cells defend themselves against ROS damage with enzymes such as SOD, CAT, GPx, and peroxireductase. In liver injury, however, the ability of the natural antioxidant system is impaired and the membrane disintegration of hepatocytes is discerned by a decrease in antioxidant enzyme activities and subsequent release of AST, ALT, ALP, and other serum markers of impaired liver function.1 In the in-vivo part of this study, all these changes were observed after acute administration of CCl4 (Table 1 and 2). However, 7-day pre-treatment with EAM ameliorated CCl4-induced liver damage evidenced by a significant lowering of ALT, AST, and ALP activities, as well as preventing the oxidative damage by preserving antioxidant enzyme activities (CAT, SOD, GST, GPx, GR). Additionally, EAM protection was supported by the microscopical examination that revealed a significant improvement of CCl4-induced histopathological changes – steatosis, lymphocytes infiltration, and centrilobular necrosis (Fig. 3).
A. monspessulanus L. is a species for which there is lack of information about its biological properties. This study demonstrates its antioxidant activity against CCl4-induced liver failure. With regards to the toxic mechanism of action of CCl4, the observed antioxidant and hepatoprotective properties of EAM might be due to its ability to capture free radicals and/or to stabilize the cell membranes. Furthermore, CCl4 intoxication induced a significant increase of hepatic NF-κB and iNOS expression together with significant elevation in tissue TNF-α level33 and TNF-β1 levels.34 In one of their studies, Jeong et al.34 concluded that silymarin prevented CCl4-induced hepatic fibrosis by lowering the expression of TNF-β1 and decreasing the number of α-SMA-positive cells. In another study tracing the protective effects of the flavonoid morin, against CCl4-induced acute hepatic injury in rats, the authors concluded that morin exerted its protection by blocking the expression of inflammatory cytokines and mediators including TNF-α, IL-1β, IL-6, and iNOS.33 Regarding these data, it could be speculated that some of the protective mechanisms of EAM might be due to an interaction with proinflammatory/profibrogenic factors, such as autocrine cytokines.
However, isolation and identification of the exact biologically active compounds, alongside additional studies on their biological properties is an object of further investigation. On the other hand, being the part of genus Astragalus for which hepatoprotective and antioxidant properties35 are due to secondary metabolites such as flavonoids and saponins, it would be expected that the observed protection of EAM against CCl4 toxicity is a result of these biologically active compounds. Moreover, this conclusion is further supported by the fact that the observed EAM protection is comparable to the protection of silymarin due to the complexity of flavonoids.
Conclusion
Under the conditions of this study, it is concluded that the EAM exerts hepatoprotective and antioxidant activity against in-vitro/in-vivo CCl4-induced acute liver damage. The protective effect of the tested extract is comparable to those of silymarin.
Acknowledgement
The authors would like to thank the Medical Science Committee for funding this research (Project no. 18/2013) through Concurs Grant 2013.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest None.
Ethics approval All performed procedures were approved by the Institutional Animal Care Committee (KENIMUS) and the principles stated in the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes ((ETS 123) Council of Europe, 1991) were strictly followed throughout the experiment.
References
- 1.Singh N, Kamath V, Narasimhamurthy K, Rajini PS. Protective effects of potato peel extract against carbon tetrachloride-induced liver injury in rats. Envir Toxicol Pharmacol 2008;26(2):241–1462. [DOI] [PubMed] [Google Scholar]
- 2.Simeonova R, Kondeva-Burdina M, Vitcheva V, Mitcheva M. Some in vitro/in vivo chemically-induced experimental models of liver oxidative stress in rats. BioMed Res Int 2014; Article ID 706302, 10.1155/2014/432647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rios LJ, Waterman PG. A review of the pharmacology and toxicology of Astragalus. Phytoter Res 1997;11(6):411–8. [Google Scholar]
- 4.Pistelli LF. Secondary metabolites of genus Astragalus: structure and biological activity. In: Atta-Ur-Rahman, (ed.) Studies in natural products chemistry; bioactive natural products, vol. 27, part H Karachi: Elsevier B.V; 2002, p. 443–545. [Google Scholar]
- 5.Simeonova R, Krasteva I, Kondeva-Burdina M, Benbassat N. Effects of extract from Astragalus glycyphylloides on carbon tetrachloride-induced hepatotoxicity in Wistar rats. Int J Pharm Bio Sci 2013;4(3):179–86. [Google Scholar]
- 6.Kondeva-Burdina M, Simeonova R, Krasteva I, Benbassat N. Protective effects of extract from Astragalus glycyphylloides on Carbontetrachloride-induced toxicity in isolated rat hepatocytes. Biotechnol Biotech Eq 2013;27(3):3866–9. [Google Scholar]
- 7.Vitcheva V, Simeonova R, Krasteva I, Nikolov S, Mitcheva M. Protective effects of a purified saponin mixture from Astragalus corniculatus Bieb., in vivo hepatotoxicity models. Phytother Res 2013;27(5):731–6. [DOI] [PubMed] [Google Scholar]
- 8.Heywood VH, Ball PW. Leguminosae. In: Tutin TG Heywood VH Burges NA, Mooze DM, Valeutine DH, Walters SM Webb DA, (eds.) Flora Europaea, vol. 2 Cambridge: Cambridge University Press; 1972, p.108–124. [Google Scholar]
- 9.Jordanov D. Flora Reipublicae Popularis Bulgaricae, vol. 6. Sofia: Aedibus Academiae Scientiarum Bulgaricae; 1976, p.167. [Google Scholar]
- 10.Bratkov V, Kondeva-Burdina M, Simeonova R, Tzankova V, Krasteva I. Phytochemical evaluation and effect of saponins’ mixture isolated from Astragalus monspessulanus on HepG2 cell line. Eur J Med Plants 2014;4(5):522–7. [Google Scholar]
- 11. Council of Europe, European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes, 1991; CETS no. 123. [displayed 2007 May 30]. Available from: http://conventions.coe.int/Treaty/Commun/QueVoulezVous.asp?NT=123&CM= 1&CL=ENG.
- 12.Fau D, Berson A, Eugene D, Fromenty B, Fisch C, Pessayre D. Mechanism for the hepatotoxicity of the antiandrogen, nilutamide. Evidence suggesting that redox cycling of this nitroaromatic drug leads to oxidative stress in isolated hepatocytes. J Pharmacol Exp Ther 1992;263(1):69–77. [PubMed] [Google Scholar]
- 13.Mitcheva M, Kondeva M, Vitcheva V, Nedialkov P, Kitanov G. Effect of benzophenone from Hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes. Redox Rep 2006;11(1):3–8. [DOI] [PubMed] [Google Scholar]
- 14.Dianzini MU, Poli G, Gravela E, Chiarpotto E, Albano E. Influence of lipid peroxidation on lipoprotein secretion by isolated hepatocytes. Lipids 1981;16(11):823–9. [DOI] [PubMed] [Google Scholar]
- 15.Bergmeyer HU, Gawehn K, Grassl M. Lactatedehydrogenase, UV-assay with pyruvate and NADH. In: Bergmeyer HU, (ed.) Methods of enzymatic analysis, vol. 1 2nd ed New York, NY, USA: Academic Press; 1974. pp. 481–2. [Google Scholar]
- 16.Habbu PV, Shastry RA, Mahadevan KM, Joshi H, Das SK. Hepatoprotective and antioxidant effects of Argyreia speciosa in rats. Afr J Tradit Complement Altern Med 2008;5(2):158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn TH, Yang YS, Lee JC, Moon CJ, Kim SH, Jun W,. et al. Ameliorative effects of pycnogenol on carbon tetrachloride-induced hepatic oxidative damage in rats. Phytother Res 2007;21(11):1015–9. [DOI] [PubMed] [Google Scholar]
- 18.Polizio AH, Peña C. Effects of angiotensin II type 1 receptor blockade on the oxidative stress in spontaneously hypertensive rat tissues. Regul Pept 2005;128(1):1–5. [DOI] [PubMed] [Google Scholar]
- 19.Bump EA, Taylor YC, Brown MJ. Role of glutathione in the hypoxic cell cytotoxicity of misonidazole. Cancer Res 1983;43(3):997–1002. [PubMed] [Google Scholar]
- 20.Lowry OH. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;239(1):265–75. [PubMed] [Google Scholar]
- 21.Aebi H. Catalase. In: Bergrenyer HU, (ed.) Methods of enzymatic analysis. 2nd ed New York, NY, USA: Academic Press; 1974. p. 673–84. [Google Scholar]
- 22.Misura HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247(10):3170–5. [PubMed] [Google Scholar]
- 23.Tappel AL. Glutathione peroxidase and hydroperoxydes. Method Enzymol 1978;52:506–13. [DOI] [PubMed] [Google Scholar]
- 24.Pinto MC, Mata AM, Lopez-Barea J. Reversible inactivation of Saccharomyces cerevisiae glutathione redictase under reducing conditions. Arch Biochem Biophys 1984;228(1):1–12. [DOI] [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249(22):7130–9. [PubMed] [Google Scholar]
- 26.Bancroft JD, Gamble M. editors Theory and practice of histological techniques. 5th ed Edinburgh, UK: Churchill Livingstone Publications; 2002. pp. 172–5. [Google Scholar]
- 27.Timbrell J. Factors affecting metabolism and disposition. Toxic responses to foreign compounds – direct toxic action: tissue lesions. In. Timbrel J, (ed.) Principles of biochemical toxicology. 3rd ed New York, NY, USA: Taylor and Francis; 2000. pp. 176–80. [Google Scholar]
- 28.Etuk EU, Agaie BM, Ladan MJ, Garba I. The modulatory effect of Cochlospermum tinctorium a rich aqueous root extract on liver damage induced by carbon tetrachloride in rats. Afr J Pharm Pharmacol 2009;3(4):151–7. [Google Scholar]
- 29.Sahreen S, Khan MR, Khan RA. Evaluation of antioxidant activities of various solvent extracts of Carissa opaca fruits. Food Chem 2010;122(4):1205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 2003;33(2):105–36. [DOI] [PubMed] [Google Scholar]
- 31.Bhadauria M, Nirala SK, Shukla S. Propolis protects CYP 2E1 enzymatic activity and oxidative stress induced by carbon tetrachloride. Mol Cell Biochem 2007;302(1–2):215–24. [DOI] [PubMed] [Google Scholar]
- 32.Sahreen S, Khan MR, Khan RA. Hepatoprotective effects of methanol extract of Carissa opaca leaves on CCl4-induced damage in rat. BMC Complement Altern Med 2011;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeba DH, Mahmoud ME. Therapeutic potential of morin against liver fibrosis in rats: modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ Toxicol Pharmacol 2014;37(2):662–71. [DOI] [PubMed] [Google Scholar]
- 34.Jeong DH, Lee GP, Jeong WI, Do SH, Yang HJ, Yuan DW,. et al. Alterations of mast cells and TGF-β1 on the silymarin treatment for CCl4-indiced hepatic fibrosis. World J Gastroenterol 2005;11(8):1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simeonova R, Vitcheva V, Kondeva-Burdina M, Krasteva I, Nikolov S, Mitcheva M. Effect of purified saponin mixture from Astragalus corniculatus on enzyme- and nonenzyme-induced lipid peroxidation in liver microsomes from spontaneously hypertensive rats and normotensive rats. Phytomedicine 2010;17(5):346–9. [DOI] [PubMed] [Google Scholar]


