Abstract
Objectives: The goal of this study was to determine the redox activity of iron (ethylenebis[2-(o-hydroxyphenyl)glycine]) (EHPG) and (ethylenebis[2-(o-hydroxybenzyl)glycine]) (EHBG) (N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid) derivative complexes and of some N,O–salan complexes of iron. The hexadentate chelate (EHPG and EHBG) ligands varied in their substituents (polar OMe, NHAc, or lipophilic Ph), while the latter had different charge and lipophilicity. The low redox activity of these complexes is important in their potential applications as magnetic resonance imaging contrast agents.
Methods: Redox activity was assessed in the entire Haber–Weiss cycle and separately in the Fenton reaction. The spin-trapping method with 5,5-dimethyl-1-pyrroline-N-oxide monitored in electron paramagnetic resonance was used. The standard Mn marker was applied as a reference for quantitative analysis. Additionally, ascorbate oxidation was analyzed with UV–Vis spectrophotometry.
Results: Both the Haber–Weiss cycle and in particular the Fenton reaction showed low redox activity of the studied complexes, which did not exceed 30% of [Fe(EDTA)]− or FeCl3 activity. The N,O–salan complexes expressed even lower activity, i.e. 10–20% activity of [Fe(EDTA)]−.
Discussion: For the EHPG and EHBG complexes, it is likely that hydrophobicity and the possibility of H-bond formation play a major role in the resulting redox effects. For this reason, chelates equipped with phenyl groups in the majority belong to less redox-active complexes. For N,O–salan complexes, activity is not correlated with the charge of the coordination sphere, but again, the highly hydrophobic character of the groups and the non-pendant substituents capable of H-bonding that are present in these ligands limit the affinity of hydrophilic species.
Keywords: Fe3+ complexes, Salan ligand, EHPG, N, N′-Bis(2-hydroxybenzyl)ethylenediamine-N, N′-diacetic acid, Redox activity, Fenton reaction, Haber-Weiss cycle
Introduction
Magnetic resonance imaging (MRI) has gained particular interest as a medical diagnostic technique due to its non-invasive character, high spatial resolution, and extended applicability in soft tissues.1 Another key feature of MRI is the possibility of image altering by using contrast agents.2 Recently published N,O models based on Fe3+ complexes could potentially serve as complementary or even alternative agents to the commonly used gadolinium compounds.3–5 These belong to (ethylenebis[2-(o-hydroxyphenyl)glycine]) (EHPG) and (ethylenebis[2-(o-hydroxybenzyl)glycine]) (EHBG) or N,N′-bis(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid derivatives as well as pentadentate aminophenol chelates. Despite the many advantages of iron systems,6,7 i.e. their endogenous character, well-known physiological pathways, and elimination of the ecological and side effect issues regarding gadolinium, there are concerns about the potential deleterious effects.8 The major concern is redox activity, mostly proceeding via one-electron exchange. A classic example of such a process is the Haber–Weiss cycle (equations (1) and (2)).9 The second reaction closing the short series of one-electron transformations and making the process catalytic from the point of view of iron is well known as the Fenton reaction.
| (1) |
| (2) |
Both radicals, and ·OH, belong to reactive oxygen species (ROS).10 Although is relatively unreactive in aqueous media, the product of the Fenton reaction ·OH expresses tremendous reactivity with a half-life in the cells of 10−9 seconds.11 A series of Fe complexes with common chelates has already been tested. Fe–EDTA deserved the inglorious first place in the hall of fame of ‘OH producers’, followed by the nitrilotriacetic acid complexes, citrate, and phosphates.12,13 On the other hand, ATP and deferoxamine (DFO) turned out to be very poor Haber–Weiss catalysts.14 It was also demonstrated that Fe–EDTA is a very good Haber–Weiss catalyst expressing high activity in each of the component reactions. Contrarily, Fe–DTPA (diethylenetriaminepentaacetic acid) was found to catalyze the Fenton reaction even more effectively than EDTA, while Fe–DTPA was rarely involved in the reaction with .15 For this reason, we investigated both the entire Haber–Weiss cycle by the generation of , analyzing the relative quantity of ·OH and the Fenton reaction separately. We came up with several factors which have the most important influence on the entire Haber–Weiss cycle and discussed them with respect to the gathered data.
It is worth noting that there are also many beneficial effects of redox activity, as active iron N,O-complexes serve as a catechol dioxygenase model16,17 and catalyze the desired oxidation of aromatic compounds.18
Materials and methods
The synthesis of the iron(III) complexes of EHPG, EHBG, and ligands L1–L3 was described in our papers.3–5 Iron(II) complexes were obtained in situ by mixing an equimolar (for L2:Fe(II)/L2 the molar ratio was 1/2) amount of freshly recrystallized FeSO4 and the appropriate ligand, reaching a final molarity of 1 mM as used in the Fenton test. The complexes were tested directly after preparation. The literature data indicate that such Fe2+ complexes are stable to auto-oxidation in the presence of air.19
Sample preparations
Phosphate buffer, pH = 7.4, 150 mM was prepared from 24 ml of 1 M K2HPO4 and 60 ml 1 M KH2PO4 with distilled water to a final volume of 200 ml. Acetate buffer, pH = 6.0, 202 mM was prepared from 0.48 ml of glacial acetic acid and 16.11 g sodium acetate with water to a final volume of 1 l. 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) 0.6 mM stock solution was prepared from 20 μl commercial DMPO (Sigma-Aldrich) with 280 μl acetate buffer and stored in a refrigerator. Ascorbic acid (Asc) was recrystallized from ethanol and diethyl ether (1:1 v/v). Hydrogen peroxide aqueous 0.056 M solution was used; its molarity was determined by permanganate titration. Xanthine-saturated solution was prepared by dissolving 0.1 g xanthine in 50 ml phosphate buffer. The solution was stored in the dark. Xanthine oxidase 0.4 U/ml aqueous solution was prepared from a commercial enzyme (Sigma-Aldrich) and stored in a refrigerator. The tested iron complexes were prepared according to the literature reports.3–5
Electron paramagnetic resonance (EPR) measurements were performed on an EPR JEOL JES-FA 300 spectrometer at room temperature with the following parameters: central field 345 ± 7.5 mT, power 1 mW, modulation width 0.025 mT, field modulation 0.2 mT, time constant 0.3 seconds, amplitude 160, measurement time 4 minutes, and Mn standard marker Mn2+ in MgO. The data were retrieved using ESR DATA PROCESSING 3.3.35 E XB and processed with Microcal® Origin 6.0. Exemplary EPR spectra are presented in the Supporting Materials.
Haber–Weiss cycle tests
Samples of 50.5 μl volume were measured in a glass capillary mounted in a quartz EPR tube. The molarities given in parentheses refer to the stock solutions. The samples were prepared as follows: 22 μl of DMPO solution (0.6 mM as described above), 3 μl of the tested iron compound (1 mM), 17 μl of saturated xanthine solution in phosphate buffer, and 0.5 μl of xanthine oxidase solution (0.4 U/ml) were introduced into a glass vessel with 5 μl deionized water and incubated for 1.5 hours at 25°C. After incubation, 3 μl of hydrogen peroxide aqueous solution (56 mM) was added, shaken, transferred into a capillary, and measured immediately. Exemplary EPR spectra are presented in the Supporting Materials. The [Fe(EHPG)]− and [Fe(EHBG–Ph)]− complexes were measured in the same conditions, but the stock solutions of the complexes were prepared in a dimethyl sulfoxide (DMSO)/water mixture (1:1 v/v) due to their lower solubility in water. According to Li et al.'s studies, DMSO does not affect the measurements in the spin-trapping method.20
Fenton reaction tests
Samples of 38 μl volume were measured in a glass capillary mounted in a quartz EPR tube. The molarities given in parentheses refer to the stock solutions. The samples were prepared as follows: 34 μl of DMPO solution (0.6 mM as described above) and 2 μl of the tested iron compound (1 mM) were added. Finally, 2 μl of hydrogen peroxide aqueous solution (56 mM) was introduced, shaken, transferred into a capillary, and measured immediately.
Ascorbic acid oxidation procedure
Samples (5 ml) with final concentrations of Asc 25 μM and tested iron compound 7.5 μM were measured at 265 nm immediately after mixing the following components: 2.5 ml Asc aqueous solution (50 μM), 2.462 ml phosphate buffer (pH = 7.4; 115 mM), and 38 μl iron compound (1 mM).
Results
We applied the spin-trapping method to conduct quantitative measurements of the ·DMPO–OH adduct on EPR for the Haber–Weiss cycle. The Fenton reaction was studied in a similar manner. Additional investigations were undertaken by spectrophotometric determination of ascorbate oxidation. The analyzed complexes are presented in Scheme 1.
Scheme 1.
Structures of the analyzed iron complexes. The geometries are based on X-ray structures of closely related complexes.
Haber-Weiss cycle
The activity tests of the iron compounds in the Haber–Weiss cycle were done to determine the range of participation in one of the most well-known processes that potentially occur in the body, as the possibility of producing hydroxyl radicals in oxidative stress would be extremely undesirable. The application of DMPO to assess ·OH formation was based on Li et al.'s20 and Buettner et al.'s15 papers. However, the conditions described by Buettner et al. (20 μM iron complex, 80 μM H2O2, and 50 mM DMPO at 15 G, recalculated as 150 mT) led us to obtaining sharp signals of paramagnetic iron electron transitions instead of the typical quartet of DMPO adducts. The latter were found at 345 mT, and the conditions adapted after Li with increased molarity (final concentrations: 60 μM iron complex, 3.3 mM H2O2, and 260 μM DMPO) revealed a classical DMPO signal among the signals of the manganese standards. The conditions of generation were adapted based on Richmond et al.'s studies: xanthine oxidase generated the superoxide radical from xanthine, which reduced Fe3+ to the second oxidation state according to equation (1).21 After the incubation period, H2O2 was introduced, and here the Fenton reaction could take place, forming the hydroxyl radical ·OH (equation (2)) recorded immediately as the ·DMPO–OH adduct. Since the ·DMPO–O2 adduct as a diradical is much less stable,22 the Haber–Weiss cycle could be reliably monitored by recording the more stable ·DMPO–OH.
The quantitative measurements of the latter were performed with reference to an external standard Mn marker in EPR.20 The calculations were based on the integral ratio of the formed ·DMPO–OH adduct and the signals of the standard. Therefore, they have a relative character showing their activity in the redox process. Nevertheless, appropriate measurements for free Fe3+ ions and the [Fe(EDTA)]− complex were also registered, and these have already been studied thoroughly in the past15,23,24 (Fig. 1).
Figure 1.
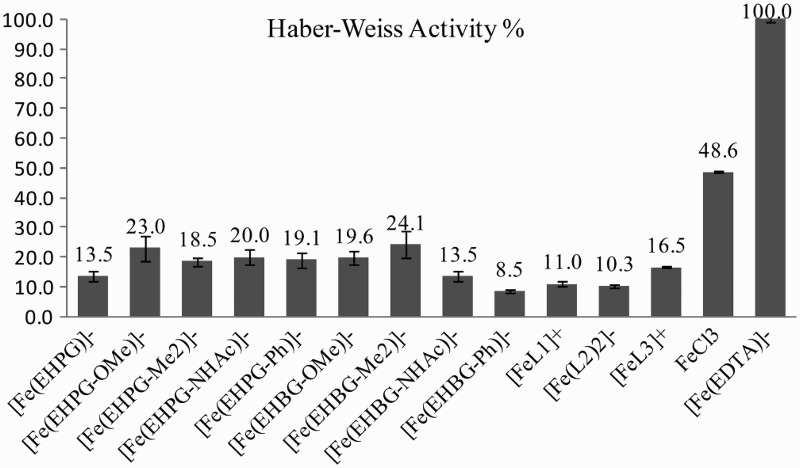
Percentage activity of iron complexes in the Haber–Weiss cycle.
Fenton reaction
The studies were done on respective Fe2+ complexes formed in situ with appropriate ligands and Fe(II) ions. Thus the goal of the Fenton tests was to assess how the complexes would behave if they were reduced to Fe2+ species in the reducing conditions of the cells. The second goal was to investigate, just as in the case of DTPA, whether the low activity in the Haber–Weiss cycle of the complexes is caused by both reactions (equations (1) and (2)) or whether only reduction (equation (1)) is the limiting step. Again, the intensity (integrals) of the DMPO signals was referenced to the signals of the Mn standard marker that is usually used in EPR (Fig. 2).
Figure 2.
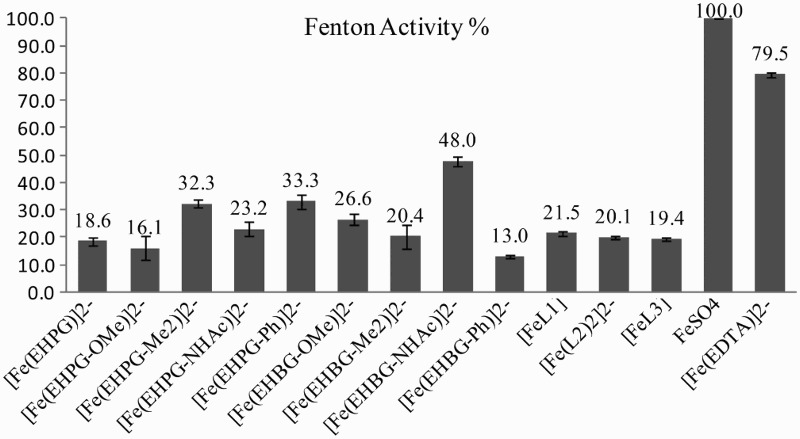
Percentage activity of iron complexes in the Fenton reaction.
Additional studies
We investigated the influence of atmospheric oxygen on the measurements. It was necessary to determine whether the presence of oxygen would disturb studies on easily oxidizing Fe2+ compounds in the Fenton reaction. For this purpose, we prepared samples with deoxygenated solvents by cold degassing in low temperature (liquid nitrogen, threefold repetition) and by argon saturation (10 minutes). We registered the ·DMPO–OH signal for a Fe2+ sample (Fig. 3).
Figure 3.
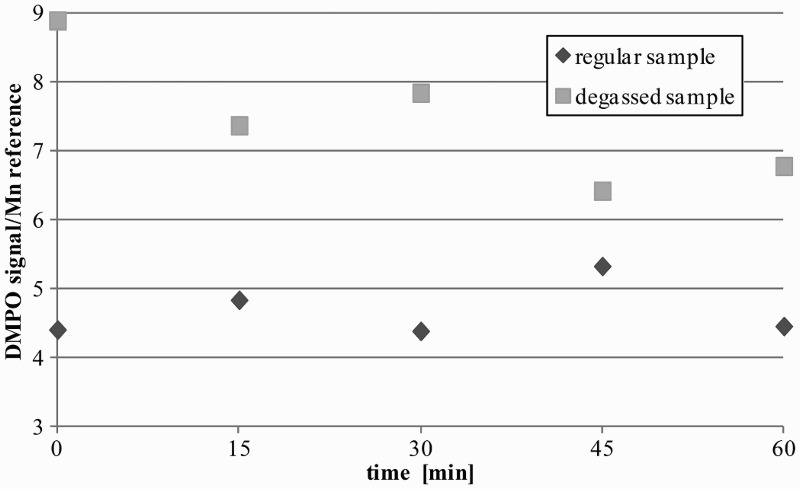
Result of sample degassing: FeSO4·7H2O (0.1 M), DMPO (5 M), and H2O2 (0.5 M).
The samples with degassed media gave an elevated signal of ·DMPO–OH. This could be explained by the fact that in ‘regular’ sample preparations in atmospheric conditions, some hydroxyl radicals ·OH react with O2 and are not trapped with DMPO, or only DMPO is partially deactivated with diradical oxygen molecules. However, there are positive observations from the tests: even in regular media ·DMPO–OH adducts could be easily observed at a slightly decreased level; moreover, in the ‘regular sample’ the ·DMPO–OH signals were stable enough to be registered within an hour (much longer than a regular experiment), which is consistent with the literature report.25 This is contrary to the degassed sample, whose intensity diminished during the measurement time – probably due to saturation with oxygen. Taking into account the high O2 content in the blood (21 ml O2/100 ml blood, 1.34 ml O2 on each gram of hemoglobin – Hüfner's constant26) and the complexity of the sample preparations, we decided to carry out the studies without sample deoxygenation.
Another issue was slow DMPO decomposition. Therefore, we applied DMPO purification according to Buettner and Oberley27 and we compared the Fenton reaction tests and the stored and purified reagent. The results were within standard error, thus we performed all of the measurements on a commercial DMPO without additional purification.
Ascorbic acid oxidation
Asc in pH 7 was deprotonated to (AscH−) and could be oxidized with Fe3+ according to equation (3).
| (3) |
The redox potentials of the pairs are ·Asc·−/AscH− = 0.28 and Fe3+/Fe2+ = 0.77 V, however, in vivo they are lower since a high ratio [AscH−/Asc·−] is maintained.12
Studies on Asc oxidation with Fe3+ compounds could shed additional light on their activity as undesired oxidizing agents. The process could easily be followed with UV–Vis by the disappearance of one of the Asc absorption maximums at 265 nm. The absorption bands of the complexes did not interfere with the strong signal of Asc. We modified Dean and Nicholson's conditions for tests recording the absorptions immediately after mixing the iron compounds with Asc.23 Based on a calibration curve we could calculate the absolute progress of oxidations as a result of the undesired activity of the iron(III) compounds (Fig. 4).
Figure 4.
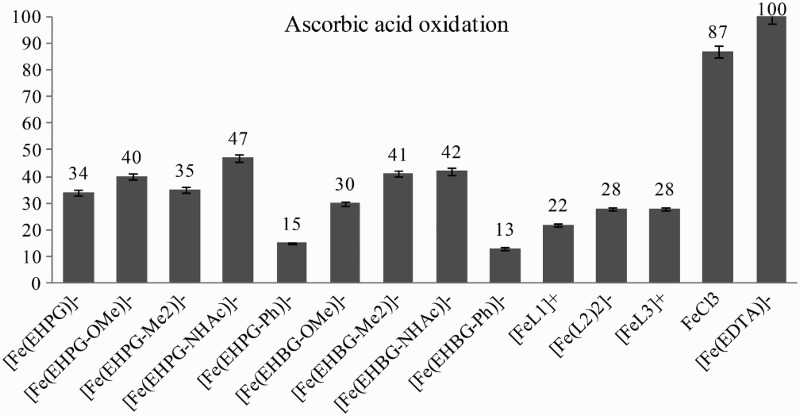
Ascorbic acid oxidation with various Fe3+ compounds.
Discussion
The N,O-complexes presented here were investigated with regard to their redox activity. Low effectiveness of ·OH formation was desired for their potential application as MRI contrast agents. Both the Haber–Weiss cycle and in particular the Fenton reaction showed low redox activity of the studied complexes which did not exceed 30% of [Fe(EDTA)]− or FeCl3 activity. Only [Fe(EHBG–NHAc)]2− turned out to have half the activity as compared to free Fe2+ in the Fenton tests. It is interesting to point to the relatively high diversity among the complexes of the same ligand group of EHPG or EHBG. To our knowledge, such studies have not been presented yet. This effect is particularly evident in the Fenton reaction, in which the [Fe(EHBG–NHAc)]2− complex could express triple activity of its phenyl counterpart, i.e. [Fe(EHBG–Ph)]2−. On the other hand, [Fe(EHBG–NHAc)]2− did not catalyze the entire Haber–Weiss cycle efficiently. Thus this case seems to be similar to the well-known behavior of [Fe(DTPA)]2−.12 The redox activity could be suppressed to some extent by the addition of the ligand excess, as we and others have observed for EDTA/Fe studies.23 Based on the literature and on our studies, we can conclude that the redox activity of iron chelates is a superposition of the following, interdependent factors:
-
1.
Stability of the complex, expressed in a stability constant, however, this parameter does not take into account competitive coordination by different metals or transmetalations.
-
2.
Ligand fitting the metal center and the consequences in coordination geometry could be observed in the X-ray structures as high distortion from an ideal polyhedron and resulted in a new coordination site.
-
3.
Accessibility to the coordination spheres, both steric and electronic (hydrophilicity of the surrounding ligands14), is closely related to factor (2), however, it takes into account additional interactions via the second coordination sphere of H-bonding ligands and π-electron interactions. Factors (2) and (3) have a key impact on the mechanism of the potential redox reaction. While direct coordination enables the inner-sphere mechanism assigned for EDTA, a tight and saturated coordination sphere practically excludes this route and forces an alternative, tunneling outer-sphere mechanism, which is additionally supported by ligand contribution.23
-
4.
The electron-donating/-withdrawing character of the ligand and its substituents has a direct impact on the redox potential but also influences the activity of the metal center as a Lewis acid. This last behavior is particularly undesired in medical applications and, in some cases, is observed in protein or DNA damage.28,29
Most of these factors are already well known for complexes tested in this work. Considering point (1), the stability constants of EHPG- and EHBG-type Fe3+ complexes are very high, with pK 30–40. They favor Fe3+ over Fe2+ with 10 order higher stability constants.30 While the stability constants of the L1–L3 Fe3+ complexes were not determined specifically, similar complexes with a tripodal O,N,O-moiety have overall stability constants ranging from 21 to 48.31 Such a high stability of the complexes reduces the possibility of transmetalation to other coordinating species (e.g. phosphates), unless some extremely specific chelates, e.g. siderophores, appear in the medium. Ligand fitting, in point (2), has been studied via calculations and X-ray crystallography. There are several examples of structures containing a water molecule coordinated directly in the first coordination sphere, such as EDTA,30 but also the tetradentate aminophenols closely related to our L1 and L3 ligands.16 Such coordination of the water molecule and its replacement with another oxygen ligand is used as the explanation for the high redox activity of EDTA by an inner-sphere electron transfer mechanism.32 Dean and Nicholson23 suggest that water molecule replacement with ascorbate is the most efficient way for oxidation of the latter. As was mentioned before, this factor could be important for models L1 and L3, however, since only low redox activity is observed for these systems, we conclude that in solution water molecules do not directly coordinate to the metal center. This supposition is additionally supported by our observation of their behavior as proton relaxation accelerators, i.e. the recorder relaxivities would have been much higher if they could coordinate the water molecules directly.5 The ligands within the groups EHPG or EHBG differ in substituent character according to their polarity, H-bond formation, and hydrophilicity. As was shown, their acid–base character is based on the acidity of the phenol group (point (4)).3 However, here the resulting activity is not directly linked with this feature, since although EHPG–Me2 is the weakest acid and EHPG–NHAc is the strongest one, they both represent moderate redox activity and do not belong to the extreme cases. The redox potential of these complexes is highly dependent on the measurement conditions, i.e. on the concentration, solvent, electrolyte, or electrode.33 There are also indications in the literature of discrepancies in the redox potential and relative activity of the complexes. These are explained by inadequacy of the measurements in reference to the in vivo conditions12 or by the existence of other, unexpected parallel processes.15 Thus it is likely that hydrophobicity and the possibility of H-bond formation play a major role in the resulting redox effects (point (3)). For this reason, chelates equipped with phenyl groups from the EHPG and EHBG chelates mostly belong to the less redox-active complexes. The only serious derogation is observed for ascorbate oxidation, where the phenylic derivatives are 40–50% more active than the NHAc counterparts. In this test the latter derivatives expressed the lowest activity for all of the studied complexes. It is important to note that phenyl moieties were found to express a protective effect against oxidative damage to mammalian cells due to their lipophilic character promoting access to the intracellular environment.14
The ascorbate test is a method of studying direct oxidation effects. Moreover, ascorbate could reach very high concentrations in certain cells, such as eye cells or pneumocytes.28 The trend for the relative activity for all complexes in the ascorbate test is consistent with the other studies in this work excluding NHAc derivatives. This exceptional behavior of NHAc derivatives requires further investigation. A positive effect in the sense of potential applications as MRI contrast agents is observed for complexes with L1–L3 ligands. Despite their actual pentadentate character, they exhibit decreased redox activity with reference to all other complexes in the studies. This activity is not correlated with the charge of the coordination sphere but again with the highly hydrophobic character of the groups (factor (3)) and with non-pendant substituents capable of H-bonding that are present in these ligands and which limit the affinity of hydrophilic species. Since aminophenol ligands are regarded to be good π–electron donors (factor (4)), the metal center does not behave as a strong Lewis acid, thus its affinity to ROS is limited.33 Moreover, the basic character of alkyl amine groups favors the third oxidation state of iron, which decreases its potential for reduction.30
We performed measurements of iron complexes with reference to the Fe–EDTA complex and free iron ions. This set of results allowed us to compare them to the other important iron chelates (DFO, DTPA, and citrate) as described in the literature (Table 1).
Table 1. Relative activity of iron complexes with selected ligands.
| Ligand | Fenton | Method, Ref. | Haber-Weiss | Method, Ref. | Ascorbate, Ref. |
|---|---|---|---|---|---|
| EDTA | 100% | 100% | 100% | ||
| DFO | 10% | C23 | 11% | C21 | 5%23 |
| 7% | C12 | 1% | B24 | ||
| 15% | C23 | ||||
| 13% | C12 | ||||
| DTPA | 84% | A13 | 3% | A13 | |
| 670% | B24 | <1% | B24 | ||
| 50% | C12 | 33% | C12 | ||
| 1% | |||||
| Citrate | 60% | A13 | 4% | A13 | |
| 58% | C12 | 14% | C12 |
Methods: A, xanthine oxidase followed by oxidation of formate to CO2; B, pulse radiolysis followed by oxidation of formate to CO2; C, hydroxylation of salicylate.
The complexes under study in this work express lower activity in the Fenton reaction than DTPA and citrate complexes. This could be explained by higher affinity to a higher oxidation state as discussed above for factors (1) and (4). However, the overall Haber–Weiss cycle is elevated for the majority of EHPG and EHBG series, while [Fe(EHBG–Ph)]− and the complexes of L1–L3 ligands fall into the common, desirable low activity that is similar to citrate or DFO complexes. In this case, factors (2) and (3) could have a higher impact on hampering interactions with ROS in both equations (1) and (2). It is difficult to compare ascorbate oxidation, however, DFO as a saturated, deprived of any hydrophilic pendant, moiety expresses lower activity than the complexes presented in the work. Attention should be given to the EHPG and EHBG complexes with NHAc substituents, where this polar, pendant fragment could serve as an auxiliary group for electron transfer or could potentially enhance interaction with the hydrophilic ascorbate anion via H-bonding (factor (3)), thus resulting in a more effective oxidation process.
The overall analysis with respect to potential applications of these complexes as contrast agents is positive, especially as their activity approaches that of citrate. The citrate ligand is an active compound in ferric ammonium citrate, a T1-contrast agent applied in the gastric tract, which does not express any serious deleterious effects due to its redox activity.34 The problem of redox activity is one of the most important issues in considering a compound for medical application. On the other hand there are some reports claiming that the cytotoxic effect of the most redox-active complexes (like [Fe(EDTA)]−) is lower than expected.8,32 This is explained by the apparent behavior of the ·OH radical. It was shown that the free ·OH radical is most damaging, while in the presence of [Fe(EDTA)]− the ·OH radical not ‘totally free’ in solution, thus its tremendous reactivity is silenced. Therefore, assessing potential deleterious effects necessitates further investigations in vivo.
Conclusions
Most (ethylenebis[2-(o-hydroxyphenyl)glycine])- and (ethylenebis[2-(o-hydroxyphenyl)glycine])-substituted iron complexes express less than 30% of EDTA activity in the Fenton reaction, the entire Haber–Weiss cycle, and ascorbate oxidation. Pentadentate ligands L1–L3 have even lower activity due to their lipophilic character and limited capability of H-bonding. Some of the complexes, i.e. [Fe(EHBG)]2−, are responsible for forming ·OH from H2O2 in the Fenton reaction but are still low efficient in the catalytic transformation in the Haber–Weiss cycle. These studies have shed some light on their limited activity in potential redox processes and are promising in limiting their side effects upon their introduction into a living organism.
Acknowledgments
We thank Mrs M. Łupicka for several of the UV–Vis measurements. We gratefully acknowledge Dr W. Domagała's assistance in the EPR measurements, data processing, and numerous valuable discussions.
Disclaimer statements
Contributors UCh performed EPR measurements of Fe3+ EHPG and EHBG complexes, while NK is responsible for the rest of the work.
Funding This work was supported by the National Science Centre (NCN) in Poland; Decision No.: DEC-2012/07/B/ST5/03157.
Conflict of interest There are no conflicts of interest in the submitted work.
Ethics approval None.
Supplementary material
Supplementary material for this article can be found at: www.maneyonline.com/doi/suppl/10.1179/1351000215Y.0000000017.
References
- 1.Weishaupt D, Kӧchli VD, Marinček B. How does MRI work? An introduction to the physics and function of magnetic resonance imaging. 2nd ed Berlin: Springer Verlag; 2006. [Google Scholar]
- 2.Bellin MF. MR contrast agents, the old and the new. Eur J Radiol 2006;60(3):314–23. [DOI] [PubMed] [Google Scholar]
- 3.Kuźnik N, Jewuła P, Oczek L, Kozłowicz S, Grucela A, Domagała W. EHPG iron(III) complexes as potential contrast agents for MRI. Acta Chim Slov 2014;61:87–93. [PubMed] [Google Scholar]
- 4.Kuźnik N, Szafraniec-Gorol G, Oczek L, Grucela A, Jewuła P, Kuźnik A, et al. . A study on the synthesis and properties of substituted EHBG–Fe (III) complexes as potential MRI contrast agents. J Organomet Chem 2014;769:100–5. [Google Scholar]
- 5.Kuźnik N, Wyskocka M, Jarosz M, Oczek L, Goraus S, Komor R, et al. . Amino-phenol complexes of Fe(III) as promising T1 accelerators. Arab J Chem 2014; doi: 10.1016/j.arabjc.2014.11.009. [DOI] [Google Scholar]
- 6.Hasserodt J. Magnetogenic probes that respond to chemical stimuli in an off–on mode. New J Chem 2012;36(9):1707–12. [Google Scholar]
- 7.Dorazio SJ, Morrow JR. The development of iron(II) complexes as paraCEST MRI contrast agents. Eur J Inorg Chem 2012;(12):2006–14. [DOI] [PubMed] [Google Scholar]
- 8.Welch KD, Davis TZ, Van Eden ME, Aust SD. Deleterious iron-mediated oxidation of biomolecules. Free Radic Biol Med 2002;32(7):577–83. [DOI] [PubMed] [Google Scholar]
- 9.Koppenol WH. The Haber–Weiss cycle – 70 years later. Redox Rep 2001;6(4):229–34. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 1984;219(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur H, Halliwell B. Detection of hydroxyl radicals by aromatic hydroxylation. Methods Enzymol 1994;233:67–82. [DOI] [PubMed] [Google Scholar]
- 12.Burkitt MJ, Gilbert BC. Model studies of the iron-catalysed Haber–Weiss cycle and the ascorbate-driven Fenton reaction. Free Radic Res Commun 1990;10(4–5):265–80. [DOI] [PubMed] [Google Scholar]
- 13.Sutton HC. Efficiency of chelated iron compounds as catalysts for the Haber–Weiss reaction. J Free Radic Biol Med 1985;1(3):195–202. [DOI] [PubMed] [Google Scholar]
- 14.Samuni AM, Afeworki M, Stein W, Yordanov AT, DeGraff W, Krishna MC, et al. . Multifunctional antioxidant activity of HBED iron chelator. Free Radic Biol Med 2001;30(2):170–7. [DOI] [PubMed] [Google Scholar]
- 15.Buettner GR, Doherty TP, Patterson LK. The kinetics of the reaction of superoxide radical with Fe (III) complexes of EDTA, DETAPAC and HEDTA. FEBS Lett 1983;158(1):143–6. [DOI] [PubMed] [Google Scholar]
- 16.Velusamy M, Palaniandavar M, Gopalan RS, Kulkarni G. Novel iron (III) complexes of tripodal and linear tetradentate bis (phenolate) ligands: close relevance to intradiol-cleaving catechol dioxygenases. Inorg Chem 2003;42(25):8283–93. [DOI] [PubMed] [Google Scholar]
- 17.Safaei E, Sheykhi H, Wojtczak A, Jaglicic Z, Kozakiewicz A. Synthesis and characterization of an iron (III) complex of glycine derivative of bis (phenol) amine ligand in relevance to catechol dioxygenase active site. Polyhedron 2011;30:1219–24. [Google Scholar]
- 18.Ménage S, Galey JB, Dumats J, Hussler G, Seité M, Luneau IG, et al. . O2 activation and aromatic hydroxylation performed by diiron complexes. J Am Chem Soc 1998;120(51):13370–82. [Google Scholar]
- 19.Cohen G, Sinet PM. The Fenton reaction between ferrous-diethylenetriaminepentaacetic acid and hydrogen peroxide. FEBS Lett 1982;138(2):258–60. [Google Scholar]
- 20.Li L, Abe Y, Kanagawa K, Usui N, Imai K, Mashino T, et al. . Distinguishing the 5,5-dimethyl-1-pyrroline N-oxide (DMPO)–OH radical quenching effect from the hydroxyl radical scavenging effect in the ESR spin-trapping method. Anal Chim Acta 2004;512(1):121–4. [Google Scholar]
- 21.Richmond R, Halliwell B, Chauhan J, Darbre A. Superoxide-dependent formation of hydroxyl radicals: detection of hydroxyl radicals by the hydroxylation of aromatic compounds. Anal Biochem 1981;118(2):328–35. [DOI] [PubMed] [Google Scholar]
- 22.Shoji T, Li L, Abe Y, Ogata M, Ishimoto Y, Gonda R, et al. . DMPO–OH radical formation from 5,5-dimethyl-1-pyrroline N-oxide (DMPO) in hot water. Anal Sci 2007;23(2):219–21. [DOI] [PubMed] [Google Scholar]
- 23.Dean RT, Nicholson P. The action of nine chelators on iron-dependent radical damage. Free Radic Res 1994;20(2):83–101. [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Halliwell B. Reaction of iron–EDTA chelates with the superoxide radical. Arch Biochem Biophys 1982;218(1):174–8. [DOI] [PubMed] [Google Scholar]
- 25.Kuppusamy P, Zweier JL. Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J Biol Chem 1989;264(17):9880–4. [PubMed] [Google Scholar]
- 26.Deshpande VM, Pilbeam SP, Dixon RJ. A comprehensive review in respiratory care. East Norwalk, CT: Appleton & Lange; 1988. [Google Scholar]
- 27.Buettner GR, Oberley LW. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun 1978;83(1):69–74. [DOI] [PubMed] [Google Scholar]
- 28.Cragg L, Hebbel RP, Miller W, Solovey A, Selby S, Enright H. The iron chelator L1 potentiates oxidative DNA damage in iron-loaded liver cells. Blood 1998;92(2):632–8. [PubMed] [Google Scholar]
- 29.Saberikia I, Safaei E, Kowsari MH, Lee YI, Cotic P, Bruno G, et al. . A new iron (III) complex of glycine derivative of amine-chloro substituted phenol ligand: synthesis, characterization and catechol dioxygenase activity. J Mol Struct 2012;1029:60–7. [Google Scholar]
- 30.Martell AE, Motekaitis RJ, Chen D, Hancock RD, McManus D. Selection of new Fe (III)/Fe (II) chelating agents as catalysts for the oxidation of hydrogen sulfide to sulfur by air. Can J Chem 1996;74(10):1872–9. [Google Scholar]
- 31.Steinhauser S, Heinz U, Bartholomä M, Weyhermüller T, Nick H, Hegetschweiler K. Complex formation of ICL670 and related ligands with FeIII and FeII. Eur J Inorg Chem 2004;2004(21):4177–92. [Google Scholar]
- 32.Salem IA, El-Maazawi M, Zaki AB. Kinetics and mechanisms of decomposition reaction of hydrogen peroxide in presence of metal complexes. Int J Chem Kinet 2000;32(11):643–66. [Google Scholar]
- 33.Dean RK, Fowler CI, Hasan K, Kerman K, Kwong P, Trudel S, et al. . Magnetic, electrochemical and spectroscopic properties of iron (III) amine–bis (phenolate) halide complexes. Dalton Trans 2012;41(16):4806–16. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DL, Muller HH, Young SW. Formulation of radiographically detectable gastrointestinal contrast agents for magnetic resonance imaging: effects of a barium sulfate additive on MR contrast agent effectiveness. Magn Reson Med 1992;23(1):154–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


