Abstract
Our purpose was to characterize changes in paraoxonase 1 (PON1) activity and concentration after single aerobic exercise sessions conducted before and after 6 weeks of niacin therapy in men with metabolic syndrome (MetS). Twelve men with MetS expended 500 kcal by walking at 65% of VO2max before and after a 6-week regimen of niacin. Niacin doses were titrated by 500 mg/week from 500 to 1500 mg/day and maintained at 1500 mg/day for the last 4 weeks. Fasting blood samples were collected before and 24 hours after each exercise session and analyzed for PON1 activity, PON1 concentration, myeloperoxidase (MPO), apolipoprotein A1, oxidized low-density lipoprotein (oLDL), lipoprotein particle sizes and concentrations. PON1 activity, PON1 concentration, MPO, and oLDL were unaltered following the independent effects of exercise and niacin (P > 0.05 for all). High-density lipoprotein particle size decreased by 3% (P = 0.040) and concentrations of small very low-density lipoprotein increased (P = 0.016) following exercise. PON1 activity increased 6.1% (P = 0.037) and PON1 concentrations increased 11.3% (P = 0.015) with the combination of exercise and niacin. Exercise and niacin works synergistically to increase PON1 activity and concentration with little or no changes in lipoproteins or markers of lipid oxidation.
Keywords: Paraoxonase, Lipoprotein particle size, Lipid oxidation, Exercise, Niacin
Introduction
The metabolic syndrome (MetS) is characterized by a cluster of cardiovascular disease (CVD) risk factors that include abdominal obesity, dyslipidemia, hypertension, insulin resistance, and prothrombotic and proinflammatory states.1 An individual may be classified with MetS if they have three of the following risk factors: increased waist circumference; high triglycerides (TGs); low high-density lipoprotein cholesterol (HDLc); hypertension; and impaired fasting glucose.1 It has been estimated that one out of three US adults over the age of 20 can be classified as having MetS.2
Oxidative stress may play an important role in the development of CVD in individuals with MetS.3 HDL particles protect low-density lipoprotein (LDL) against oxidative stress in peripheral tissues.4 However, oxidation of LDL and HDL can be attenuated by an important enzyme, paraoxonase 1 (PON1).5,6 PON1 may help to prevent or slow the atherosclerotic process by reducing the formation of oxidized LDL (oLDL) and decreasing the uptake of oLDL by macrophages.7 Thus, PON1 helps to prevent the inflammatory process and oxidative stress involved in atherosclerosis.7,8 PON1 is synthesized and secreted by the liver and circulates exclusively with the HDL particle.8,9 More recently, studies have focused primarily on PON1's antioxidative properties, which are inversely related to CVD.10
Health benefits of regular exercise include decreased blood pressure (BP), improved insulin sensitivity, improved glucose regulation, decreased body weight, and improved lipid profile.11 Aerobic exercise improves the lipid profile by increasing plasma HDLc and lowering plasma TG levels.12,13 Sedentary individuals participating in acute aerobic exercise at an intensity of 70–80% maximal oxygen uptake (VO2max) and a caloric expenditure between 350 and 500 kcal have improved lipid profiles by increasing plasma HDLc and decreasing TG concentrations.12,14 Acute bouts of exercise have been reported to transiently increase PON1 activity.15,16 The means by which exercise increases PON1 activity remains largely unknown; however, the increase in oLDL and ROS following exercise are likely to play roles in post-exercise PON1 changes.17 The initial protective action of PON1 to prevent oxidation of lipids is rapidly followed by inactivation.18 The interaction between ROS and the free sulfhydryl group at cysteine-284 in the PON1 structure are thought to inactivate the arylesterase activity of PON1 following oxidative stress.18,19
Niacin is an essential B vitamin (B3) that has lipid-lowering effects when used in prescription doses.20,21 Niacin reduces TG concentrations up to 30% and may increase HDLc by 25%.20,21 Niacin increases the production of apolipoprotein A1 (Apo A1) and Apo A1 is integral for the activity of PON1.22,23 Moreover, niacin has demonstrated anti-inflammatory properties and has been shown to reduce vascular oxidative stress.24 It is possible that attenuated vascular oxidative stress with niacin therapy is partially due to enhanced HDL antioxidant activity mediated by PON1.
The purpose of this investigation was to determine the independent and combined effects of exercise and extended-release niacin on the concentration and activity of PON1 in men with MetS.
Subjects and methods
Participants
Twelve obese male participants, meeting the NCEP-ATP III criteria for MetS, completed a single session of exercise on a treadmill at 60–70% of VO2max to expend 500 kcal. Participants who met the following criteria were included in the study: sedentary, non-smoking males between the ages of 30 and 65, obese (body mass index (BMI) ≥ 30 kg/m), hypertriglyceridemic (TG ≥ 150 mg/dl), and no contraindications to aspirin and niacin therapy. A fasting blood sample was obtained prior to exercise and 24 hours after exercise. Next, extended-release niacin was physician-prescribed and participants completed 6 weeks of niacin therapy. Following the niacin intervention, all participants returned to complete a single session of exercise and blood sampling as before. Diet and physical activity records were self-reported during the blood sampling period.25
This study was approved by our university's Institutional Review Board for research involving human subjects. All participants were fully informed regarding all aspects of the study and provided voluntary written consent. The methods used for recruitment and screening participants are published elsewhere.25
Exercise intervention
Participants completed a single session of laboratory-based treadmill walking 1 week following a maximal graded exercise to expend 500 kcal at 60–70% VO2max. The caloric expenditure was estimated by measuring expired gas (oxygen and carbon dioxide) fractions sampled at the mouth using a pneumotach, and breath-by-breath gas analysis system (Medical Graphics, St Paul, MN, USA).
Extended-release niacin administration
Participants were prescribed extended-release niacin by a physician. The extended-release niacin was titrated from 500 to 1500 mg/day over a 3-week period. Participants were asked to begin taking 500 mg/day right before bed for the first week. Dosage increased to 1000 mg/day for the second week. Dosage was increased to 1500 mg/day from week 3 to the end of the study (week 6). Participants were asked to take a 300 mg aspirin per day along with the niacin to reduce the risk of flushing.25 There were only 2 of the original 15 participants that reported more than one episode of flushing due to the niacin therapy.
Blood sampling
Blood samples were drawn 10–12 hours following an overnight fast. Blood samples were drawn prior to the exercise session and 24 hours post-exercise and these two were repeated following 6 weeks of niacin therapy. Blood samples were centrifuged at 3000 rpm for 15 minutes. An aliquot of serum was prepared and placed into a −80°C ultralow freezer until testing.
Biochemical analysis
PON1 activity
PON1 activity (arylesterase) was determined by using a commercially available enzymatic kit (Catalog# 0801199, ZeptoMetrix Corporation; Buffalo, NY, USA). The samples and standards were analyzed in duplicate. The absorbance was measured at 270 nm using a Spectronic UV1 spectrophotometer by Thermo Scientific. The PON1 activity was reported in kU/l. The inter-assay and intra-assay variation was 2.94 and 2.53%, respectively.
Enzyme-linked immunosorbent assay
PON1 concentration, Apo A1, oLDL, and myeloperoxidase (MPO) were determined using an enzyme-linked immunosorbent assay: (Catalog# E0243HU, USCN Life Sciences Inc., Wuhan, China; Catalog# EA5201-1, AssayPro LLC., St Charles, MO, USA; Catalog# BI20042, ALPCO Diagnostics, Salem, NH, USA; and Catalog# K6631A, ALPCO Diagnostics). The samples and standards were analyzed in duplicate. A 96 well-plate washer and plate reader (Biotek Instruments Inc.; Winooski, VT, USA) was used and programmed according to manufacturer's instructions. The inter-assay for each assay was <4.88, 4.94, 4.11, and 3.65%, respectively. The intra-assay variation for each was <4.55, 5.01, 4.96, and 4.34%, respectively.
Lipoprotein particle size
Frozen serum samples were sent to Liposcience Inc. for nuclear magnetic resonance spectroscopy testing. Very LDL (VLDL), LDL, and HDL particle numbers and sizes were determined by nuclear magnetic resonance (NMR; Liposcience Inc., Raleigh, NC, USA).26,27 The coefficient of variation (CV) for particle numbers was <4%. The CV for particle sizes was <2%.
Statistical analysis
Group means and standard deviations were determined for the following descriptive statistics: age (years); height (cm); weight (kg); BMI (kg/m); waist hip ratio; VO2max (ml/kg/minute). The dependent variables of interest are PON1 concentration, PON1 activity, MPO, Apo A1, oLDL, and lipoprotein particle size and number for HDL and LDL. The Shapiro–Wilks test for normality was used to determine if the baseline variables of interests were normally distributed. A multiple 1 group (group) × 4 (sampling point) repeated measures analysis of variance was used to determine significant changes in variables of interest. A comparison-wise alpha level was set at P < 0.05. Duncan's NMR tests were used to follow-up the global tests. Pearson product-moment correlations were determined to characterize relationships between baseline concentrations and changes in variables of interest.
Results
Participants
The baseline physiological characteristics are provided in Table 1. The participant's baseline variables are presented in Table 2. All participates maintained their body weight throughout the study.25
Table 1.
Baseline physiological characteristics (means ± SEM)
| Variable | Units | Mean ± SEM | Minimum | Maximum |
|---|---|---|---|---|
| Age | years | 44±2 | 37 | 51 |
| Height | cm | 175.6±2.8 | 165.5 | 185.7 |
| Body weight | kg | 106.9±5.1 | 88.4 | 125.4 |
| BMI | kg/m2 | 34.5±0.9 | 31.1 | 37.9 |
| Waist circumference | cm | 108.8±8.2 | 100.6 | 117.0 |
| % Fat | % of body weight | 35±1 | 30 | 40 |
| VO2max | ml/minute/kg | 27.5±1.6 | 21.9 | 33.1 |
| Glucose | mmol/l | 5.99±0.28 | 3.83 | 8.16 |
| NEFA | mmol/l | 0.49±0.03 | 0.39 | 0.59 |
| HDLc | mmol/l | 1.06±0.05 | 0.70 | 1.35 |
| Triglyceride | mmol/l | 3.31±0.32 | 2.01 | 5.13 |
BMI, body mass index; NEFA, non-esterified fatty acids; VO2max, maximal oxygen consumption; HDLc, high-density lipoprotein cholesterol. Table modified from Plaisance et al.25
Table 2.
Response to exercise (means ± SEM)
| Variable | Units | Baseline | 24 hours post-exercise | P value |
|---|---|---|---|---|
| PON1a | kU/l | 125.9±4.9 | 131.5±4.0 | 0.269 |
| PON1c | µg/ml | 112.4±8.2 | 118.9±8.5 | 0.391 |
| Apo A1 | mmol/l | 28.0±9.1 | 28.1±7.4 | 0.983 |
| Total HDL particles | µmol/l | 31.5±1.3 | 32.1±1.7 | 0.469 |
| Large HDL particles | µmol/l | 3.5±0.5 | 3.1±0.5 | 0.068 |
| Medium HDL particles | µmol/l | 5.1±1.5 | 4.2±1.3 | 0.496 |
| Small HDL particles | µmol/l | 22.9±1.4 | 24.9±1.5 | 0.214 |
| HDL particle size | nm | 8.6±0.1 | 8.5±0.1 | 0.040* |
| HDLc | mmol/l | 1.06±0.05 | 1.04±0.05 | 0.403 |
| TG | mmol/l | 3.31±0.32 | 2.61±0.32 | 0.006* |
| TG: HDLc ratio | 3.12±0.12 | 2.51±0.08 | <0.001* | |
| Large VLDL particles | nmol/l | 5.7±1.0 | 5.5±1.0 | 0.321 |
| Medium VLDL particles | nmol/l | 31.5±5.3 | 28.2±3.9 | 0.222 |
| Small VLDL particles | nmol/l | 48.6±3.8 | 56.0±4.9 | 0.016* |
| VLDL particle size | nm | 58.4±2.4 | 56.4±2.1 | 0.102 |
| Total LDL particles | nmol/l | 1788.3±105.1 | 1829.2±111.3 | 0.272 |
| Intermediate LDL particles | nmol/l | 43.6±13.1 | 49.8±9.7 | 0.581 |
| Large LDL particles | nmol/l | 264.7±47.5 | 273.0±47.0 | 0.722 |
| Small LDL particles | nmol/l | 1480.0±111.2 | 1506.3±107.5 | 0.534 |
| Medium small LDL particles | nmol/l | 312.4±25.0 | 307.7±24.2 | 0.755 |
| Very small LDL particles | nmol/l | 1167.6±86.9 | 1198.4±85.3 | 0.355 |
| LDL particle size | nm | 20.1±0.1 | 20.1±0.1 | 0.385 |
| oLDL | mmol/l | 8.2±0.5 | 6.9±2.6 | 0.490 |
Baseline, before exercise (control); 24 hours post-exercise; PON1a, paraoxonase 1 activity; PON1c, paraoxonase 1 concentration; Apo A1, apolipoprotein A1; HDL, high-density lipoprotein; HDLc, high-density lipoprotein cholesterol; TG, triglyericide concentration. VLDL, very low-density lipoprotein; LDL, low-density lipoprotein, oLDL, oxidized low-density lipoprotein.
Significance compared to baseline (control): *P < 0.05.
PON1 response to exercise
Exercise decreased TG concentrations (P = 0.006) and reduced HDL particle size (P = 0.040). Furthermore, the session of exercise resulted in a significantly reduced ratio of TG: HDLc (P < 0.001). However, PON1 concentration, PON1 activity, oLDL, MPO, and all other lipoprotein particle sizes and concentrations remained unchanged with exercise (Table 2).
At baseline, PON1 concentration was correlated with Apo A1 (P = 0.041). Changes in dependent variables that occurred with exercise were computed (Change = 24 hours post-exercise − baseline) and relationships among these change variables were explored. The exercise-induced change in PON1 activity was significantly correlated with exercise-induced changes in HDLc (r = 0.740, P = 0.006). No other correlations between PON1 concentration, PON1 activity, TG: HDLc ratio, and lipoprotein characteristics were observed.
Six weeks of niacin therapy
Six weeks of niacin therapy did not alter PON1 activity (P = 0.143), PON1 concentration P = 0.536), or Apo A1 (P = 0.198). Six weeks of niacin significantly reduced TG (P < 0.001) and medium small LDL particle concentration (P = 0.018). Six weeks of niacin therapy significantly reduced the ratio of TG: HDL (P < 0.001). However, no other lipid lipoprotein changes were observed with niacin (see Table 3).
Table 3.
Effects of niacin alone and niacin + exercise (means ± SEM)
| Variable | Units | Baseline | 6 weeks of niacin | 6 weeks of niacin + 1 exercise session |
|---|---|---|---|---|
| PON1a | kU/l | 125.9±4.9 | 115.9±6.2 | 122.9±5.5‡ |
| PON1c | µg/ml | 112.4±8.2 | 105.9±8.5 | 121.9±9.3‡ |
| Apo A1 | mmol/l | 28.0±9.1 | 24.1±5.9 | 21.5±15.8 |
| Total HDL particles | µmol/l | 31.5±1.3 | 32.0±1.1 | 33.2±1.3 |
| Large HDL particles | µmol/l | 3.5±0.5 | 3.8±0.8 | 3.8±0.6 |
| Medium HDL particles | µmol/l | 5.1±1.5 | 2.9±0.9 | 3.9±0.7 |
| Small HDL particles | µmol/l | 22.9±1.4 | 25.3±1.1 | 25.5±1.4 |
| HDL particle size | nm | 8.6±0.1 | 8.6±0.1 | 8.6±0.1 |
| HDLc | mmol/l | 1.06±0.05 | 1.06±0.05 | 1.11±0.05 |
| TG | mmol/l | 3.31±0.32 | 2.21±0.21† | 1.80±0.14‡ |
| TG: HDLc ratio | 3.12±0.12 | 2.08±0.05† | 1.62±0.04‡ | |
| Large VLDL particles | nmol/l | 5.7±1.0 | 5.4±0.8 | 4.2±1.0‡ |
| Medium VLDL particles | nmol/l | 31.5±5.3 | 25.4±3.7 | 25.9±4.0 |
| Small VLDL particles | nmol/l | 48.6±3.8 | 44.6±5.8 | 49.4±4.8 |
| VLDL particle size | nm | 58.4±2.4 | 59.9±3.0 | 55.5±2.9‡ |
| Total LDL particles | nmol/l | 1788.3±105.1 | 1626.1±131.6 | 1678.0±121.1 |
| Intermediate LDL particles | nmol/l | 43.6±13.1 | 32.1±13.7 | 38.9±12.8 |
| Large LDL particles | nmol/l | 264.7±47.5 | 349.4±65.7 | 352.1±51.8 |
| Small LDL particles | nmol/l | 1480.0±111.2 | 1244.6±128.8 | 1287.4±110.1 |
| Medium small LDL particles | nmol/l | 312.4±25.0 | 244.6±26.3† | 266.4±24.7 |
| Very small LDL particles | nmol/l | 1167.6±86.9 | 1000.0±103.5 | 1021.1±86.3 |
| LDL particle size | nm | 20.1±0.1 | 20.3±0.2 | 20.4±0.1 |
| oLDL | mmol/l | 8.2±0.5 | 5.9±2.0 | 8.11±2.51 |
Baseline, before exercise (control); Niacin baseline, 6 weeks of niacin before exercise; PON1a, paraoxonase 1 activity; PON1c, paraoxonase 1 concentration; Apo A1, apolipoprotein A1; HDL, high-density lipoprotein; HDLc, high-density lipoprotein cholesterol; TG, triglyericide concentration; VLDL, very low-density lipoprotein; LDL, low-density lipoprotein; oLDL, oxidized low-density lipoprotein concentration.
Significance compared to baseline, †P < 0.05; significance compared to niacin baseline, ‡P < 0.05.
Changes in dependent variables of interest occurring with niacin therapy (Change = values obtained after 6 weeks of niacin therapy − values obtained at baseline) were calculated and the relationships between baseline PON1 activity and concentration and change variables were determined. Niacin-induced changes in PON1 concentration were significantly correlated with changes in LDL particle number (r = 0.775, P = 0.005) and changes in small LDL particle numbers (r = 0.878, P < 0.001). Niacin-induced changes in PON1 status were not correlated with niacin-induced changes in the ratio of TG: HDLc.
Combined effects of exercise and niacin therapy
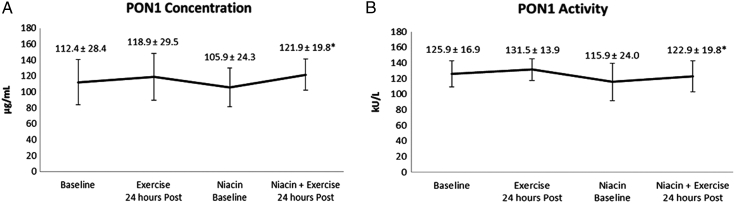
PON1 concentration and PON1 activity responses to the combined effects of acute exercise and 6 weeks of extended-release niacin therapy are presented in Fig. 1A and B. PON1 concentration was significantly increased (P = 0.015) (Fig. 1A). PON1 activity was significantly increased following the combined effects of acute exercise and extended-release niacin (P = 0.037) (Fig. 1B). TG were significantly reduced following the combined effects of exercise and niacin therapy (P = 0.006). Exercise and niacin therapy resulted in a significantly lower ratio of TG: HDLc as compared to niacin alone (P < 0.001). Likewise, the combined effects of acute exercise and extended-release niacin significantly reduced large VLDL particle numbers (P = 0.043). All other lipoprotein changes were observed (see Table 3).
Figure 1.
PON1 concentration and activity response to exercise and niacin therapy, means ± SD. (A) PON1 concentration is presented in micrograms per milliliter of sample; Baseline (control), before exercise; Exercise, 24 hours post-exercise; Niacin Baseline, 6 weeks of niacin therapy; Niacin + Exercise, 24 hours post-exercise plus niacin. (B) PON1 activity is presented in kU/l, kilo international units per liter of sample; significance between niacin baseline and niacin + exercise, *P < 0.05.
Changes in dependent variables of interest occurring with exercise and niacin therapy (Change = values obtained following 6 weeks of niacin therapy plus exercise − values obtained at baseline following niacin therapy alone) were calculated and the relationships between baseline PON1 activity and concentration and change variables were determined.
Exercise and niacin-induced changes in PON1 activity were correlated with changes in HDL particle size (r = −0.627, P = 0.039). Changes in PON1 concentration was not correlated with any change variable following the combined effects of exercise and niacin therapy.
Discussion
The primary and novel findings of this study are that exercise and niacin together increased PON1 concentration and activity, but PON1 characteristics were not altered by either intervention alone. Our results indicate for the first time that these therapeutic interventions have additive or complementary effects on PON1 concentration and activity in those with metabolic dyslipidemia. Furthermore, PON1 concentration and activity can be elevated in the absence of changes in markers of oxidative stress and changes in lipoprotein lipids that are often reported with either exercise or niacin.
Dynamic continuous exercise has been previously shown to increase PON1 activity15,16 with some exceptions.28,29 The exercise effects on PON1 activity appear to be transient as we and others do not observe significant elevations 24 hours after completing exercise.15,16,29 Tomas et al.15 found that PON1 activity and oLDL were significantly increased following 30 minutes of cycle ergometry in previously sedentary individuals who completed 16 weeks of aerobic exercise training. The post-training results of the exercise session were different from what was observed prior to training. It may be argued, based on the findings of Tomas et al.,15 that long-term exercise training may be necessary for PON1 changes to take place. In other words, systemic, cellular, and molecular changes induced by regular practiced exercise may be necessary for increasing PON1. Limited support for this position may be found from cross-sectional results where adolescents that participated in sports had a higher PON1 activity than their sedentary counterparts.30,31 In our study, the independent effects of exercise did not alter PON1 concentration or activity.
Increases in HDLc and decreases in TG are common following a single session of exercise at intensities between 70 and 80% while expending 300–500 kcal. These changes are maintained up to 48 hours post-exercise.12–14 However, HDLc was not altered in this current investigation.
Exercise resulted in smaller HDL particles without changes in Apo A1 or LDL characteristics and in the absence of changes in PON1 concentration or activity. In addition, PON1 concentration and activity were not correlated with exercise-induced changes in HDL particle size. Our findings may be interpreted to mean that the reduction in HDL particle size does not necessarily result in characteristic changes in PON1 activity. Our results are in conflict with those of Bergmeier et al.32 that provide evidence that a reduction in HDL particle size contributes to subsequent increases in PON1 concentration and activity.
To our knowledge, ours is the first study designed to examine PON1 changes with niacin therapy. Our results indicate that niacin doses up to 1500 mg/day for 6 weeks does not appear to affect PON1, HDL, Apo A1, or blood markers of lipid peroxidation.
Niacin has been used as an effective therapy for increasing HDLc and lowering TG concentrations.20,21,33 Our results are consistent with those published previously regarding the TG-lowering effects of niacin.20,21,33 We found that HDL particle sizes, HDL particle numbers, and HDLc were not altered in our investigation. There are several groups that have examined particle size distribution following niacin therapy.34–36 In contrast, several investigators reported significant increases in large HDL particle numbers and significant decreases in small HDL particle numbers following 12–16 weeks of niacin therapy with dosages ranging from 1000 to 2000 mg/day in individuals with coronary artery disease and MetS.34–36
Niacin increases the production of Apo A1 as compared to placebo.23 Apo A1 plays a vital role at increasing the activity of PON1.22 Lamon-Fava et al.23 found that extended-release niacin titrated over 12 weeks at a dosage of 2000 mg/day would cause a significant increase in Apo A1. However, in our current investigation, Apo A1 was not altered following 6 weeks of niacin therapy at a dosage of 1500 mg/day.
MPO is a byproduct of lipoprotein lipid oxidation, and MPO may reduce the protective effects of HDL to maintain vascular function of the endothelium.24 On the other hand, niacin is thought to improve vascular health by reducing oxidative stress.24 Sorrentino et al.24 found a significant reduction in MPO following 12 weeks of niacin therapy titrated to 1500 mg/day in individuals with type 2 diabetes mellitus and meeting criteria for MetS. MPO was not altered following 6 weeks of niacin therapy in the present study.
Although, extended-release niacin therapy reduced total and small LDL particle numbers by 10–18% and increased large LDL particles by 32% in our study, these changes were not significant. Kuvin et al.34 found that extended-release niacin significantly reduced small LDL particles by 12% and significantly increased large LDL particles by 82%. We did not observe changes in PON1 concentration or activity, but changes in lipoprotein metabolism, decreased TG and decreased LDL particle distribution, are consistent with previously reported changes with niacin. The reduction in small LDL particles and the increase in the large LDL particles are consistent with a shift towards a less atherogenic LDL profile following 6 weeks of niacin therapy.
Finally, it is possible that the dual interventions of exercise and niacin therapy complement one another to increase PON1 concentration and activity because we observed significant increases in both after combining these strategies. Interestingly, transient changes were observed 24 hours after exercise. This has not been observed in previous studies. These statistically significant changes in PON1 that we reported may or may not have clinical or physiological significance. We interpret this to mean that niacin exerts an influence on exercise response that persists beyond what has been shown for exercise alone. However, these postulated mechanisms have yet to be confirmed by experimental data.
The combination of exercise and niacin therapy on lipoprotein particle size distribution has not been studied. Large VLDL particle size are considered to be a major contributor to abnormal lipoprotein metabolism.37 The combination of exercise and extended-release niacin therapy significantly decreased VLDL particle numbers by 8%. The greatest change was found with VLDL particle size, which decreased by 28% following the dual intervention. Independently, exercise and niacin have been shown to decrease the availability of hepatic TG to incorporate into VLDL and results in a reduction of VLDL particle size.37 The results from these studies may help us understand how exercise and niacin work together. Our results suggest that exercise and niacin appear to have a complementary effect on VLDL particle distribution.
Several limitations of this current study must be considered. First, this study only included obese male participants that were not on lipid altering or BP medications, non-smokers, or sedentary. Second, the sample size was small and included only one exercise session before and after 6 weeks of niacin. Third, PON1 status may be affected by a variety of factors, which include pharmacological agents, physical activity, smoking, and diet. Fourth, we did not phenotype or look at PON1-192 and PON1-55 polymorphisms. Fifth, the participants were asked to self-report physical activity and dietary logs for 3 days prior to the investigation. Finally, our intent was to observe changes in PON1 using a physiological hypothesis but nothing to address mechanisms.
In conclusion, PON1 concentration and/or activity may be important and often overlooked characteristics of HDL to decrease an individual's risk for CVD either through lifestyle modification or through pharmacological intervention. The independent actions of exercise did not alter PON1 concentration and activity. Similarly, 6 weeks of extended-release niacin therapy did not alter PON1 concentration and activity. However, when exercise was completed after 6 weeks of extended-release niacin therapy, PON1 concentration and activity significantly increased without observed changes in oLDL and MPO. Our findings may suggest that several factors are working together to elicit changes in PON1 following exercise and niacin therapy. Therefore, additional research is needed to investigate the potential mechanisms responsible to increase PON1.
Disclaimer statements
Contributors The author responsibilities were as follows - J. Kyle Taylor: study design, data collection, data analysis, and writing of the manuscript; Eric P. Plaisance: study design, data collection, and data analysis; Jack Mahurin: physician oversight and screening; Michael L. Mestek: data collection and data analysis; Jose Moncada-Jimenez: data collection; and Peter W. Grandjean: study design, data collection, study oversight, and assistance with writing of the manuscript.
Funding Supported by a Global Pharmaceuticals Research & Development Grant from Abbott Laboratories.
Conflict of interest None.
Ethics approval The research as approved by Auburn University's IRB.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2010;2(3):180–93. [DOI] [PubMed] [Google Scholar]
- 3.Yubero-Serrano EM, Delgado-Lista J, Pena-Orihuela P, Perez-Martinez P, Fuentes F, Marin C,. et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp Mol Med 2013;45:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kontush A, Chapman MJ. Antiatherogenic small, dense HDL – guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med 2006;3(3):144–53. [DOI] [PubMed] [Google Scholar]
- 5.Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis 1993;104(1–2):129–35. [DOI] [PubMed] [Google Scholar]
- 6.Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest 1998;101(8):1581–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackness MI, Durrington PN, Mackness B. The role of paraoxonase 1 activity in cardiovascular disease: potential for therapeutic intervention. Am J Cardiovasc Drugs 2004;4(4):211–7. [DOI] [PubMed] [Google Scholar]
- 8.Deakin S, Leviev I, Gomaraschi M, Calabresi L, Franceschini G, James RW. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J Biol Chem 2002;277(6):4301–8. [DOI] [PubMed] [Google Scholar]
- 9.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med 2004;37(9):1304–16. [DOI] [PubMed] [Google Scholar]
- 10.Precourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E,. et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 2011;214(1):20–36. [DOI] [PubMed] [Google Scholar]
- 11.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA,. et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39(8):1423–34. [DOI] [PubMed] [Google Scholar]
- 12.Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 2001;31(15):1033–62. [DOI] [PubMed] [Google Scholar]
- 13.Grandjean PW, Crouse SF, Rohack JJ. Influence of cholesterol status on blood lipid and lipoprotein enzyme responses to aerobic exercise. J Appl Physiol 2000;89(2):472–80. [DOI] [PubMed] [Google Scholar]
- 14.Crouse SF, O'Brien BC, Rohack JJ, Lowe RC, Green JS, Tolson H,. et al. Changes in serum lipids and apolipoproteins after exercise in men with high cholesterol: influence of intensity. J Appl Physiol 1995;79(1):279–86. [DOI] [PubMed] [Google Scholar]
- 15.Tomas M, Elosua R, Senti M, Molina L, Vila J, Anglada R,. et al. Paraoxonase1-192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J Lipid Res 2002;43(5):713–20. [PubMed] [Google Scholar]
- 16.Otocka-Kmiecik A, Lewandowski M, Stolarek R, Szkudlarek U, Nowak D, Orlowska-Majdak M. Effect of single bout of maximal exercise on plasma antioxidant status and paraoxonase activity in young sportsmen. Redox Rep 2010;15(6):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollaard NB, Shearman JP, Cooper CE. Exercise-induced oxidative stress: myths, realities and physiological relevance. Sports Med 2005;35(12):1045–62. [DOI] [PubMed] [Google Scholar]
- 18.Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A,. et al. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation 2000;101(21):2510–7. [DOI] [PubMed] [Google Scholar]
- 19.Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL,. et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med 1999;26(7–8):892–904. [DOI] [PubMed] [Google Scholar]
- 20.Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA,. et al. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol 1998;82(12A):74U–81U; discussion 5U–6U. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg A, Alagona P Jr, Capuzzi DM, Guyton J, Morgan JM, Rodgers J,. et al. Multiple-dose efficacy and safety of an extended-release form of niacin in the management of hyperlipidemia. Am J Cardiol 2000;85(9):1100–5. [DOI] [PubMed] [Google Scholar]
- 22.Deakin SP, James RW. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin Sci (Lond) 2004;107(5):435–47. [DOI] [PubMed] [Google Scholar]
- 23.Lamon-Fava S, Diffenderfer MR, Barrett PH, Buchsbaum A, Nyaku M, Horvath KV,. et al. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol 2008;28(9):1672–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH,. et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010;121(1):110–22. [DOI] [PubMed] [Google Scholar]
- 25.Plaisance EP, Mestek ML, Mahurin AJ, Taylor JK, Moncada-Jimenez J, Grandjean PW. Postprandial triglyceride responses to aerobic exercise and extended-release niacin. Am J Clin Nutr 2008;88(1):30–7. [DOI] [PubMed] [Google Scholar]
- 26.Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem 1991;37(3):377–86. [PubMed] [Google Scholar]
- 27.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem 1992;38(9):1632–8. [PubMed] [Google Scholar]
- 28.Benitez S, Sanchez-Quesada JL, Lucero L, Arcelus R, Ribas V, Jorba O,. et al. Changes in low-density lipoprotein electronegativity and oxidizability after aerobic exercise are related to the increase in associated non-esterified fatty acids. Atherosclerosis 2002;160(1):223–32. [DOI] [PubMed] [Google Scholar]
- 29.Iborra RT, Ribeiro IC, Neves MQ, Charf AM, Lottenberg SA, Negrao CE,. et al. Aerobic exercise training improves the role of high-density lipoprotein antioxidant and reduces plasma lipid peroxidation in type 2 diabetes mellitus. Scand J Med Sci Sports 2008;18(6):742–50. [DOI] [PubMed] [Google Scholar]
- 30.Cakmak A, Zeyrek D, Atas A, Erel O. Paraoxonase activity in athletic adolescents. Pediatr Exerc Sci 2010;22(1):93–104. [DOI] [PubMed] [Google Scholar]
- 31.Hamurcu Z, Saritas N, Baskol G, Akpinar N. Effect of wrestling exercise on oxidative DNA damage, nitric oxide level and paraoxonase activity in adolescent boys. Pediatr Exerc Sci 2010;22(1):60–8. [DOI] [PubMed] [Google Scholar]
- 32.Bergmeier C, Siekmeier R, Gross W. Distribution spectrum of paraoxonase activity in HDL fractions. Clin Chem 2004;50(12):2309–15. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D,. et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 2002;162(14):1568–76. [DOI] [PubMed] [Google Scholar]
- 34.Kuvin JT, Dave DM, Sliney KA, Mooney P, Patel AR, Kimmelstiel CD,. et al. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am J Cardiol 2006;98(6):743–5. [DOI] [PubMed] [Google Scholar]
- 35.Jafri H, Alsheikh-Ali AA, Mooney P, Kimmelstiel CD, Karas RH, Kuvin JT. Extended-release niacin reduces LDL particle number without changing total LDL cholesterol in patients with stable CAD. J Clin Lipidol 2009;3(1):45–50. [DOI] [PubMed] [Google Scholar]
- 36.Shearer GC, Pottala JV, Hansen SN, Brandenburg V, Harris WS. Effects of prescription niacin and omega-3 fatty acids on lipids and vascular function in metabolic syndrome: a randomized controlled trial. J Lipid Res 2012;53(11):2429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamanna VS, Kashyap ML. Mechanism of action of niacin on lipoprotein metabolism. Curr Atheroscler Rep 2000;2(1):36–46. [DOI] [PubMed] [Google Scholar]