Abstract
We investigated the antihypertensive and antioxidant potential of vanillic acid (VA) in Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME) – treated adult male albino Wistar rats. Treatment of rats with l-NAME (40 mg/kg Bw for 30 days) caused a sustained increase in systolic- (SBP) and diastolic blood pressure (DBP) and significantly decreased the concentration of nitrite/nitrate (NOx) in plasma as compared with that in the control. Rats treated with VA restored SBP and DBP to normal level and preserve the plasma NO metabolites concentration. Moreover, VA reduced lipid peroxidation products (thiobarbituric acid reactive substances, lipid hydroperoxides, conjugated dienes) and significantly restored enzymatic antioxidants (superoxide dismutase, catalase, and glutathione peroxidase), non-enzymatic antioxidants (vitamin C, vitamin E, and reduced glutathione) in the plasma. To assess the toxicity if any of VA treatment, hepatic and renal function markers were measured. Our results showed that the effect at a dose of 50 mg/kg Bw of VA was more pronounced than that of the other two doses, 25 and 100 mg/kg Bw. These results were supported by histopathology studies. We conclude that VA possesses an antihypertensive and antioxidant activity in l-NAME-induced hypertensive rats.
Keywords: Hypertension, l-NAME, Antioxidant, Vanillic acid
Introduction
Cardiovascular diseases (CVD) are the leading cause of death worldwide, accounting for an estimated 14 million deaths in 1990 and projected to cause 25 million deaths in 2020.1 Hypertension is an important risk factor for CVD among the adult population and a significant contributor to poor health, resulting in an excess of both morbidity and mortality. Nitric oxide (NO) is a vital regulator of vascular endothelial function and blood pressure. The chronic administration of nitric oxide synthase inhibitors provides an animal experimental model of hypertension.2 A recent study focused on hypertension showed that an increase in arterial blood pressure leads to a decrease in NO in the circulation, which reflects the role of arginine-NO in the pathophysiology of hypertension.3
Oxidative stress constitutes a unifying mechanism of injury in many types of vascular diseases. It occurs when there is a serious imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defense systems in the body.4 The generated ROS induce lipid peroxidation, a type of oxidative deterioration in polyunsaturated fatty acids (PUFAs), which has been linked with altered membrane structure and enzyme inactivation. Increases in the levels of lipid peroxidation products such as thiobarbituric acid-reactive substances (TBARS), lipid hydroperoxides (LOOH), and conjugated dienes (CD) appear to be the initial stage in the tissue, making it more susceptible to oxidative damage. There is a dynamic relationship between ROS and antioxidants.5 Free-radical scavenging enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and non-enzymatic vitamin C, vitamin E, and reduced glutathione (GSH) are cellular defense agents against oxidative injury. The external antioxidants can scavenge oxidative stress-generated free radicals and contributes to improved antioxidant status in the body.
In recent years dietary agents such as increased consumption of fruits, vegetables, whole grains, and fish have been shown to be are important in the control of CVD including hypertension.6 The protective effects of plant ingestion can be due to the presence of phenolic compounds and flavionoids. Phenolic acids are hydroxylated derivatives of benzoic and cinnamic acids. Vanillic acid (VA, 4-hydroxy-3-methoxybenzoic acid) is a phenolic derivative of edible plants and fruits; its structure is given in Fig. 1. It is also an intermediate in the production of vanillin from ferulic acid.7 The largest amount of VA is found in the plant roots of Angelica sinensis.8 It has several medicinal properties including antifilarial,9 antibacterial,10 and antimicrobial activities,11 free-radical scavenging abilitity,12 and a chemopreventive effect.13 Earlier reports from our lab showed the hepatoprotective effect of VA on actaminophen (APAP)-induced toxicity in rats.14 Recently, there has been an upsurge of interest to explore the antihypertensive and antioxidant potential of natural products. Very few scientific reports are available on the antihypertensive and antioxidant effects of phenolic acids in hypertensive rats. Hence, in view of the above facts, we evaluated the antihypertensive effect of VA on Nitro-l-arginine methyl ester (l-NAME)-induced hypertension in rats.
Figure 1.
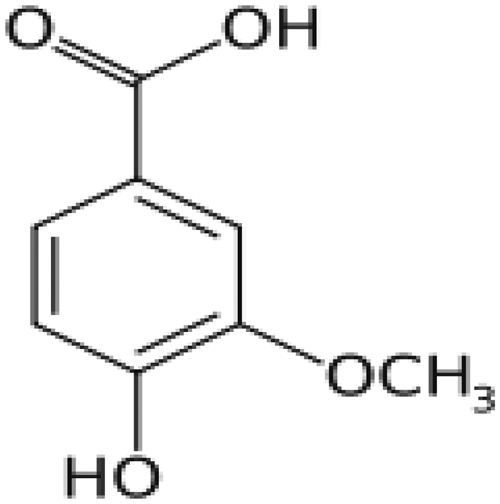
Chemical structure of VA (4-hydroxyl-3-methoxy benzoic acid C8H8O4).
Material and methods
Experimental animals
All the experiments were carried out with male albino Wistar rats weighing 180–220 g, obtained from The Central Animal House, Rajah Muthiah Institute of Health Sciences, Annamalai University, Tamilnadu, India. They were housed in polypropylene cages (47 cm × 34 cm × 20 cm) lined with husk, renewed every 24 hours under a 12:12 hours light/dark cycle at around 22°C. The rats had free access to tap water and food. The rats were fed on a standard pellet diet. The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and approved by the Animal Ethical Committee of Annamalai University. (Proposal No.736: 2010).
Drug and chemicals
VA and l-NAME were purchased from Sigma–Aldrich, Bangalore, India. All other chemicals and analytical grades were obtained from Merck and Himedia, Mumbai, India.
Model of l-NAME-induced arterial hypertension
l-NAME (40 mg/kg Bw) was dissolved in drinking water and given to rats at an interval of 24 hours for 30 days. Tail cuff systolic blood pressure (SBP) was measured using a tail cuff blood pressure measuring system. SBP measurement was performed of 1–4 weeks.15
Experimental design
The animals were randomly divided into six groups of six animals each as given below. VA was dissolved in 0.9% saline and administered at a dosage of 25, 50, and 100 mg/kg Bw post-orally by intragastric intubation, once each day at 9–10 am for 30 days.
| Group I | Control (0.9% saline) |
| Group II | Control + VA (100 mg/kg Bw) |
| Group III | l-NAME (40 mg/kg Bw) |
| Group IV | l-NAME + VA (25 mg/kg Bw) |
| Group V | l-NAME + VA (50 mg/kg Bw) |
| Group VI | l-NAME + VA (100 mg/kg Bw) |
After 30 days of the treatment period all the animals were anaesthetized between 8 and 9 am using intramuscular injection of ketamine (24 mg/kg Bw) and sacrificed by cervical decapitation. Blood was collected and serum and plasma were separated by centrifugation. Tissues (250 mg heart and kidney and 100 mg aorta) were sliced into pieces and homogenized in 5.0 ml of cold 0.1 M Tris-HCl buffer (pH 7.4). The homogenate was centrifuged at 56 × g for 10 minutes at 4°C in a cooling centrifuge. The supernatant was collected in separate test tubes and used for various biochemical estimations.
Blood pressure measurements
SBP and diastolic blood pressure (DBP) in unanesthetized rats was measured by non-invasive tail cuff plethysmography (IITC, model 31, Woodland Hills, CA, USA). Tail blood pressure was tested once a week till the end of the experiment. The tested rats were restrained and the ambient temperature was controlled at 32°C. After the blood pressure readings stabilized, three consecutive readings were averaged.
Biochemical estimations
Estimation of NOx
Nitrite/nitrate (stable NO metabolites) in the plasma samples were measured based on the Griess reaction,16 in which a chromophore with a strong absorbance at 550 nm is formed by reaction of nitrate with a mixture of naphthylethylenediamine and sulfanilamide. The nitrate was reduced to nitrite by 30 minutes incubation with nitrate reductase in the presence of NADPH. The amount of nitrite/nitrate present in the plasma sample was estimated from the standard curve obtained. Nitrite/nitrate levels were expressed as μmol/l.
Estimation of lipid peroxidation products and antioxidant status
The levels of TBARS in plasma and the tissues (aorta, kidney, and heart) were estimated by the methods of Niehaus.17 The levels of LOOH were estimated by the method of Jiang et al.18 and CD according to Rao and Recknagel.19 The activities of enzymatic antioxidants such as SOD, CAT, and GPx were assayed in erythrocytes and tissues (aorta, kidney, and heart) by the methods of Kakkar et al.,20 Sinha,21 and Rotruck et al.22 The levels of the non-enzymatic antioxidants GSH, vitamin C, and vitamin E were estimated in plasma and tissues (aorta, kidney, and heart) by the methods of Baker et al.23 and Ellman.24
Estimation of hepatic and renal function markers
The activities of serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, urea, uric acid, and creatinine were determined by the method proposed by Raja and Deepa Mol.14
Histopathological examination
The heart tissue obtained from all experimental animals was washed with 0.9% saline and then fixed in 10% buffered neutral formalin solution. After fixation, the heart tissue was processed and embedded in paraffin. Then, the tissue was sectioned and stained with hematoxylin and eosin dye and examined with a high-power microscope and photomicrographs were captured with a CCD cold camera.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance followed by Duncan's multiple range test (DMRT) using Software Package for the Social Science software package version 12.00. Results were expressed as mean ± SD for six rats in each group. P values <0.05 were considered significant.
Results
Table 1 shows the level of SBP and DBP measured by plethysmography in conscious rats. l-NAME-treated rats showed increases in SBP and DBP levels (P < 0.05) which were significantly attenuated by administration of VA (25, 50, and 100 mg/kg Bw) throughout the 30 days of the study. Oral treatment of VA in control rats did not significantly modify SBP and DBP. Among the three doses 50 mg/kg Bw improved the SBP and DBP to near-normal level.
Table 1.
Effect of VA on SBP and DBP in control and l-NAME-induced hypertensive rats
| Groups | SBP | DBP | ||||
|---|---|---|---|---|---|---|
| 0th week | 2nd week | 4th week | 0th week | 2nd week | 4th week | |
| Control | 105 ± 5.23 | 109 ± 9.51a | 116 ± 7.89a | 76 ± 7.32 | 78 ± 5.23a | 80 ± 7.26a |
| Control + VA (100 mg/kg Bw) | 104 ± 6.58 | 107 ± 8.54a | 114 ± 9.26a | 75 ± 2.28 | 76 ± 9.3a | 78 ± 7.21a |
| l-NAME (40 mg/kg Bw) | 107 ± 5.21 | 156 ± 8.21b | 185 ± 8.35b | 78 ± 4.64 | 110 ± 7.56b | 116 ± 9.26b |
| l-NAME + VA (25 mg/kg Bw) | 105 ± 6.24 | 132 ± 7.29c | 148 ± 9.28c | 76 ± 6.61 | 96 ± 6.21c | 108 ± 7.20c |
| l-NAME + VA (50 mg/kg Bw) | 108 ± 7.10 | 115 ± 8.24d | 126 ± 9.26d | 77 ± 7.42 | 88 ± 6.26d | 92 ± 6.21d |
| l-NAME + VA (100 mg/kg Bw) | 110 ± 9.26 | 128 ± 7.29e | 134 ± 8.2e | 72 ± 2.90 | 92 ± 8.29e | 99 ± 8.95e |
Values are mean ± SD of six rats from each group.
Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
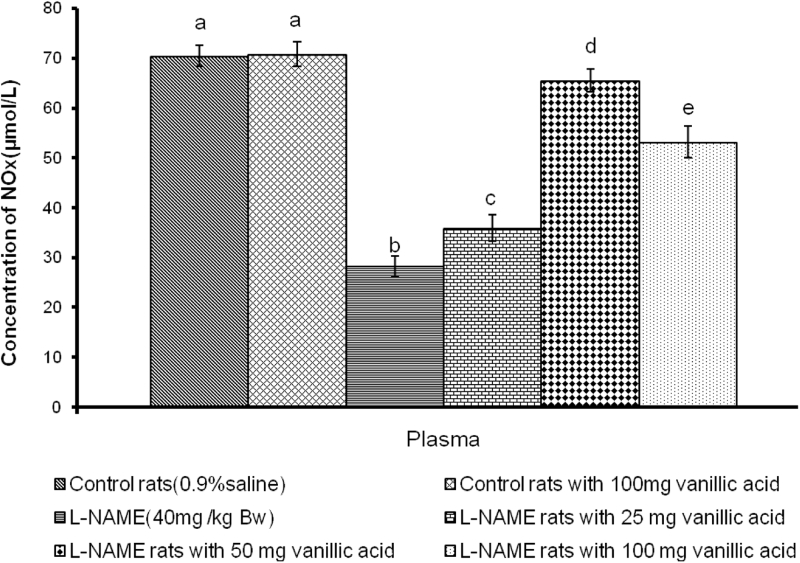
The plasma NO metabolites NOx (nitrite and nitrate) were significantly decreased (P < 0.05) in l-NAME-administered rats compared to control rats (Fig. 2). The administration of VA for 4 weeks significantly restored the level of plasma NO metabolites in the l-NAME-treated rats.
Figure 2.
Effect of VA on plasma concentration of NOx in control and l-NAME-induced hypertensive rats. Values are mean ± S.D. of six rats from each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
The levels of TBARS, LOOH, and CD in the plasma and tissues are summarized in Table 2. The levels of TBARS, LOOH, and CD were significantly (P < 0.05) elevated in the l-NAME-administered rats and treatment with VA significantly decreased the levels of TBARS, LOOH, and CD.
Table 2.
Effect of VA on TBARS, LOOH, and CD in plasma and tissues in control and l-NAME- induced hypertensive rats
| Parameters | Control | Control + VA (100 mg/ kg Bw) | l-NAME (40 mg/kg Bw) | l-NAME + VA (25 mg/kg Bw) | l-NAME + VA (50 mg/kg Bw) | l-NAME + VA 100 mg/ kg Bw) | |
|---|---|---|---|---|---|---|---|
| Thiobarbituric acid reactive substances (mmoles/100 g wet tissue) | Plasma (mmol/dl) | 0.15 ± 0.01a | 0.14 ± 0.02a | 0.45 ± 0.46b | 0.38 ± 0.15c | 0.17 ± 0.22a | 0.30 ± 0.02d |
| Aorta | 0.42 ± 0.04a | 0.40 ± 0.03a | 1.92 ± 0.12b | 1.36 ± 0.12c | 0.77 ± 0.55d | 1.11 ± 0.83e | |
| Kidney | 1.42 ± 0.12a | 1.35 ± 0.12a | 4.22 ± 0.11b | 3.42 ± 0.17c | 1.45 ± 0.15a | 2.72 ± 0.11d | |
| Heart | 0.52 ± 0.08a | 0.45 ± 0.13a | 3.17 ± 0.22b | 2.62 ± 0.18c | 0.65 ± 0.15a | 1.55 ± 0.15d | |
| Lipid hydroperoxides (mmoles/100 g wet tissue) | Plasma (mmol/dl) | 8.92 ± 0.31a | 8.75 ± 0.19a | 21.48 ± 1.32b | 18.57 ± 1.08c | 10.41 ± 0.52a | 15.53 ± 1.11d |
| Aorta | 75.79 ± 5.65a | 73.21 ± 4.37a | 117.85 ± 8.65b | 108.09 ± 6.76c | 86.90 ± 8.35d | 98.80 ± 8.35e | |
| Kidney | 63.69 ± 5.72a | 60.71 ± 6.79a | 157.14 ± 9.84b | 148.21 ± 6.68c | 77.19 ± 4.8d | 117.06 ± 6.55e | |
| Heart | 66.07 ± 5.14a | 64.28 ± 3.19a | 129.16 ± 8.23b | 120.80 ± 7.71c | 76.19 ± 4.8d | 105.95 ± 5.85e | |
| Conjugated dienes (mmoles/100 g wet tissue) | Plasma (mmol/dl) | 0.63 ± 0.03a | 0.62 ± 0.36a | 1.68 ± 0.04b | 0.99 ± 0.12c | 0.83 ± 0.13d | 0.96 ± 0.14e |
| Aorta | 39.87 ± 2.31a | 38.12 ± 2.62a | 63.96 ± 4.65b | 59.40 ± 3.77c | 46.27 ± 3.92d | 52.62 ± 4.39e | |
| Kidney | 20.3 ± 2.56a | 19.25 ± 1.82a | 42.41 ± 1.91b | 34.86 ± 3.50c | 25.87 ± 6.68d | 28.80 ± 1.18e | |
| Heart | 42.07 ± 2.72a | 41.77 ± 3.67a | 68.85 ± 4.83b | 60.65 ± 2.22c | 49.62 ± 4.44d | 54.77 ± 4.63e | |
Values are mean ± SD of six rats from each group.
Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
The activities of SOD, CAT, and GPx are given in Table 3. SOD, CAT, and GPx activities were significantly (P < 0.05) decreased in l-NAME-induced hypertensive rats and treatment with VA significantly restored the activities.
Table 3.
Effect of VA on enzymatic antioxidant activities in erythrocytes and tissues in control and l-NAME-induced hypertensive rats
| Parameters | Control | Control + VA (100 mg/kg Bw) | l-NAME (40 mg/ kg Bw) | l-NAME + VA (25 mg/kg Bw) | l-NAME + VA (50 mg/kg Bw) | l-NAME + VA (100 mg/kg Bw) | |
|---|---|---|---|---|---|---|---|
| Superoxide dismutase | Erythrocytes (U*/mg Hb) | 8.30 ± 0.76a | 8.08 ± 0.41a | 3.10 ± 1.60b | 3.94 ± 3.67c | 6.77 ± 0.64d | 4.52 ± 2.19e |
| Aorta (U*/mg protein) | 12.26 ± 1.85a | 11.46 ± 2.19a | 5.37 ± 0.40b | 6.75 ± 0.60c | 8.35 ± 0.55d | 7.32 ± 0.61e | |
| Kidney (U*/mg protein) | 15.77 ± 3.42a | 14.50 ± 3.10a | 8.09 ± 0.87b | 8.70 ± 4.21c | 12.73 ± 2.47d | 10.02 ± 4.30e | |
| Heart (U*/mg protein) | 6.72 ± 2.62a | 5.83 ± 1.85a | 2.30 ± 2.76b | 3.74 ± 0.95c | 5.01 ± 4.10a | 3.68 ± 3.74e | |
| Catalase | Erythrocytes (U#/mg Hb) | 180.79 ± 9.47a | 175.80 ± 7.30a | 96.39 ± 8.66b | 124.18 ± 6.62c | 156.14 ± 8.13d | 133.48 ± 7.65e |
| Aorta (U#/mg protein) | 54.46 ± 5.70a | 52.28 ± 5.40a | 28.49 ± 2.20b | 37.79 ± 4.26c | 48.66 ± 3.66a | 41.21 ± 5.62d | |
| Kidney (U#/mg protein) | 35.65 ± 2.09a | 34.94 ± 3.22a | 18.38 ± 2.87b | 21.76 ± 3.53c | 33.04 ± 4.63a | 26.20 ± 2.70d | |
| Heart (U#/mg protein) | 50.48 ± 6.86a | 48.43 ± 7.85a | 26.71 ± 2.20b | 36.20 ± 2.12c | 46.47 ± 4.56a | 40.71 ± 4.10d | |
| Glutathione peroxidase | Erythrocytes (U$/mg Hb) | 14.32 ± 2.38a | 13.35 ± 2.08a | 6.69 ± 0.65b | 8.27 ± 2.96c | 13.10 ± 1.82a | 10.52 ± 4.62d |
| Aorta (U$/mg protein) | 8.99 ± 2.40a | 9.79 ± 1.54a | 3.63 ± 1.20b | 4.14 ± 0.78c | 7.24 ± 2.40a | 5.24 ± 2.16d | |
| Kidney (U$/mg protein) | 12.42 ± 3.26a | 11.25 ± 2.26a | 3.73 ± 1.36b | 4.44 ± 2.30c | 10.91 ± 4.98d | 5.56 ± 2.20e | |
| Heart (U$/mg protein) | 8.84 ± 2.10a | 8.20 ± 2.43a | 3.65 ± 0.48b | 4.10 ± 0.75c | 7.10 ± 2.30a | 6.18 ± 1.58d | |
U*, enzyme concentration required to inhibit the NBT to 50% reduction in 1 minute.
U#, μmol of H2O2 consumed/minute U$, μg of GSH utilized/minute.
Values are mean ± SD of six rats from each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Changes in the levels of the non-enzymic antioxidants vitamin C, vitamin E, and GSH in the plasma and tissues are shown in Table 4. l-NAME hypertensive rats exhibited (P < 0.05) a decrease in the levels of vitamin C, vitamin E and GSH, and administration of VA significantly increased the levels.
Table 4.
Effect of VA on non-enzymatic antioxidant activities in plasma and tissues in control and l-NAME- induced hypertensive rats
| Parameters | Control | Control + VA (100 mg/ kg Bw) | l-NAME (40 mg/ kg Bw) | l-NAME + VA (25 mg/ kg Bw) | l-NAME + VA (50 mg/kg Bw) | l-NAME + VA (100 mg/ kg Bw) | |
|---|---|---|---|---|---|---|---|
| Vitamin C | Plasma (mg/dl) | 2.56 ± 0.22a | 2.51 ± 0.18a | 0.84 ± 0.03b | 1.24 ± 0.04c | 2.19 ± 0.19a | 1.42 ± 0.14e |
| Aorta (μg/mg protein) | 0.58 ± 0.11a | 0.55 ± 0.08a | 0.26 ± 0.05b | 0.35 ± 0.54c | 0.55 ± 0.083a | 0.38 ± 0.50e | |
| Kidney (μg/mg protein) | 0.75 ± 0.13a | 0.680 ± 0.11a | 0.31 ± 0.07b | 0.43 ± 0.05c | 0.66 ± 0.06a | 0.55 ± 0.05e | |
| Heart (μg/mg protein) | 0.616 ± 0.07a | 0.580 ± 0.11a | 0.250 ± 0.05b | 0.330 ± 0.05c | 0.530 ± 0.08d | 0.380 ± 0.07e | |
| Vitamin E | Plasma (mg/dl) | 1.88 ± 0.17a | 1.12 ± 0.188a | 0.61± 0.09b | 0.99 ± 0.10c | 1.74 ± 0.34d | 1.32 ± 0.16e |
| Aorta (μg/mg protein) | 4.15 ± 0.35a | 4.10 ± 0.24a | 1.34 ± 0.24b | 2.25 ± 0.20c | 3.52 ± 0.45d | 2.45 ± 0.31e | |
| Kidney (μg/mg protein) | 4.33 ± 0.39a | 4.29 ± 0.22a | 1.55 ± 0.31b | 2.03 ± 0.35c | 3.70 ± 0.36d | 2.60 ± 0.42e | |
| Heart (μg/mg protein) | 4.25 ± 0.45a | 4.32 ± 0.28a | 1.48 ± 0.43b | 2.14 ± 0.30c | 3.70 ± 0.36d | 2.48 ± 0.58e | |
| Reduced glutathione | Plasma (mg/dl) | 36.76 ± 2.75a | 34.91 ± 3.21a | 17.40 ± 4.36b | 24.76 ± 3.13c | 31.18 ± 4.07d | 27.18 ± 2.10e |
| Aorta (μg/mg protein) | 8.71 ± 1.41a | 8.35 ± 2.07a | 3.91 ± 1.10b | 4.26 ± 0.95c | 7.46 ± 0.95d | 4.82 ± 1.18e | |
| Kidney (μg/mg protein) | 12.26 ± 0.89a | 11.91 ± 1.41a | 4.62 ± 0.87 b | 5.33 ± 0.95c | 9.42 ± 0.80d | 6.75 ± 1.10e | |
| Heart (μg/mg protein) | 9.24 ± 1.45a | 8.88 ± 2.30a | 3.73 ± 1.18b | 4.26 ± 0.95c | 7.64 ± 1.24d | 5.15 ± 1.24e | |
Values are means ± SD for six rats.
Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
The rats treated with l-NAME showed a significant (P < 0.05) increase in the levels of serum hepatic and renal pathophysiological markers (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, urea, uric acid, and creatinine) when compared with control rats, as shown in Table 5. On treatment with VA, rats given l-NAME showed a significant (P < 0.05) decrease in serum hepatic and renal pathophysiological markers when compared with l-NAME hypertensive rats and the best effect was observed with the 50 mg/kg Bw dose.
Table 5.
Effect of VA on hepatic and renal function markers in serum of control and l-NAME-induced hypertensive rats
| Parameters | Hepatic function markers | Renal function markers | |||||
|---|---|---|---|---|---|---|---|
| Aspartate aminotransferase (IU@/l) | Alanine aminotransferase (IU@/l) | Alkaline phosphatase (IU*/l) | Gamma-glutamyl transferase (IU$/l) | Urea (mg/dl) | Uric acid (mg/dl) | Creatinine (mg/dl) | |
| Control | 67.21 ± 4.24a | 29.27 ± 1.56a | 76.74 ± 3.35a | 16.73 ± 1.28a | 22.51 ± 2.62a | 1.33 ± 0.10a | 0.86 ± 0.07a |
| Control + VA (100 mg/ kg Bw) | 66.55 ± 4.75a | 27.98 ± 1.55a | 75.46 ± 3.11a | 15.91 ± 1.33a | 20.35 ± 1.85a | 1.28 ± 0.15a | 0.85 ± 0.02a |
| l-NAME (40 mg/kg Bw) | 122.28 ± 6.97b | 65.89 ± 2.73b | 127.05 ± 7.44b | 35.02 ± 1.69b | 44.42 ± 1.19b | 3.70 ± 0.32b | 2.70 ± 0.16b |
| l-NAME + VA (25 mg/ kg Bw) | 110.44 ± 7.30c | 53.11 ± 5.46c | 118.17 ± 4.61c | 28.42 ± 1.99c | 38.16 ± 2.74c | 3.02 ± 0.33c | 2.22 ± 0.14c |
| l-NAME + VA (50 mg/ kg Bw) | 75.32 ± 4.56d | 36.94 ± 4.72d | 85.00 ± 5.43d | 21.18 ± 2.32d | 27.91 ± 1.76d | 1.85 ± 0.10d | 1.10 ± 0.14d |
| l-NAME + VA (100 mg/ kg Bw) | 87.71 ± 7.40e | 50.98 ± 5.81e | 106.99 ± 4.67e | 26.08 ± 1.14e | 34.83 ± 3.13e | 2.76 ± 0.19e | 1.66 ± 0.12e |
IU@/l – μmol of pyruvate liberated per hour.
U*/l – μmol of phenol liberated per minute.
IU$/l – μmol of p-nitroanilide liberated per minute.
Values are means ± SD for six rats.
Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
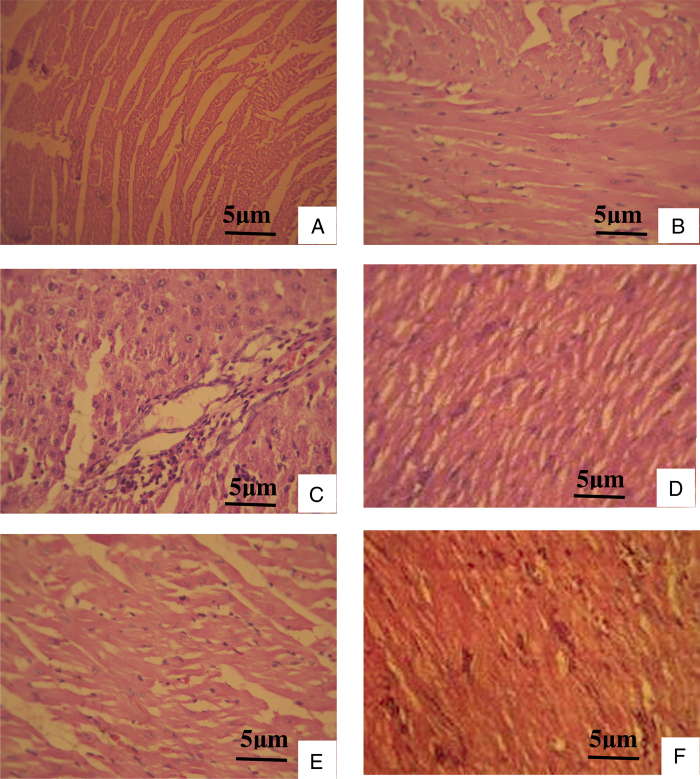
Histopathological changes of the heart in control and l-NAME-induced hypertensive rats were shown in Fig. 3. l-NAME-treated rats showed a splitting of cardiac muscle fibers and influx of inflammatory cells. Treatment with VA at 50 mg/kg Bw brought these changes toward near-normal morphology. Control rats treated with VA at higher doses showed normal muscle fibers without any pathological changes.
Figure 3.
Histopathological changes of cardiac muscle (hematoxylin and eosin stain). (A) Normal cardiac muscle bundles. (B) VA-treated control rat heart showing normal muscle fibers without any pathological changes. (C) l-NAME-treated rat heart showing splitting of cardiac muscle fibers and inflammatory cells. (D) l-NAME with 25 mg VA treatment reduced the rupture of muscle fibers, and mononuclear infiltration. (E) l-NAME with 50 mg VA treated rat heart showing muscle fibers with mild hyalinization. (F) l-NAME with 100 mg VA focal collection of mononuclear cells and fibrosis.
Treatment with VA (50 mg/kg Bw) in l-NAME-administered rats showed significant effects on blood pressure and all other biochemical changes. Histopathological examination also showed the protective effect of VA at 50 mg/kg Bw against heart tissue damage. There was no obvious change in normal control rats treated with VA at the higher concentration.
Discussion
Hypertension is considered one of the most important risk factors associated with the development of vascular diseases. NO synthesis and release by endothelial cells contribute to the modulation of vascular tone.25 In addition, NO is important in other cellular events, such as vascular smooth muscle cell proliferation.26 Besides, it is well established that chronic inhibition of NO biosynthesis by in vivo administration of l-NAME, an l-arginine analog, leads to arterial hypertension and renal vasoconstriction,27 characterized by cardiac remodeling,28 an impairment of endothelial-dependent relaxation,29 and renal function changes.30 Chronic treatment with l-NAME-induces hypertension and provides an experimental model to study hypertension.31 Earlier studies have shown that a decrease in plasma NO increases the blood pressure in l-NAME-treated rats.32 We have shown that treatment with VA (25, 50 mg, and 100 mg/kg) improved plasma NOx and decreased SBP and DBP.
Oxidative stress can damage many biological molecules; indeed, proteins and DNA are often more significant targets of oxidative injury than lipids, and lipid peroxidation often occurs late in the injury process.33 Large amounts of ROS such as superoxide, hydrogen peroxide, and hydroxyl radicals are produced during hypertension. Our results showed that the lipid peroxidation end products, measured as TBARS, LOOH, and CD, were increased in plasma and tissues of l-NAME-induced hypertensive rats. Lipid peroxidation is an important pathogenic event in hypertension and accumulation of LOOH reflects the various stages of this disease and its complications.34 ROS have been shown to be a critical determinant in hypertension. The increased levels of lipid peroxides in l-NAME-induced rats might be due to free-radical-mediated membrane damage. Oral treatment with VA (25, 50, and 100 mg/kg Bw) significantly decreased the levels of TBARS, LOOH, and CD in l-NAME-treated rats.
Crucial components of the antioxidant defense system in the body are cellular antioxidant enzymes, which play a primary role in the maintenance of a balanced redox status. A previous study has suggested that the antioxidant enzymes SOD, CAT, and GPx were significantly decreased in the erythrocytes and tissues of l-NAME-induced hypertensive rats.32 In the current study, VA administration significantly improved the activities of SOD, CAT, and GPx. The increased activities of these enzymes in VA-treated rats might be due to the free-radical scavenging ability of VA.12
The non-enzymatic antioxidants, namely vitamin C, vitamin E, and GSH, scavenge the residual free radicals escaping from decomposition enzymes.35 Ascorbic acid present in the aqueous environment has multiple antioxidant properties including the ability to reduce alpha-tocopheryl radicals present on the surface of the membrane.36 GSH is a powerful cellular antioxidant, which is directly involved in the removal of superoxide radicals, peroxyl radicals, and singlet oxygen.37 The lowered concentrations of vitamin C, vitamin E, and GSH observed in l-NAME-induced hypertensive rats might be due to neutralizing the production of free radicals. Treatment with VA enhanced the levels of non-enzymatic antioxidants in l-NAME-treated rats.
Hypertension is frequently associated with liver and renal damage, which depends on the degree to which the microcirculation is exposed to the elevated blood pressure. Oxidative stress and alteration of cellular redox status are linked to many types of acute and chronic liver and kidney injury.38 The liver and kidney actively detoxify and handle endogenous and exogenous chemicals, making them vulnerable to injury. Disruption of liver tissue architecture and vacuolation under hypertension and NO-deficiency are an indication of hepatic fatty infiltration and hepatocellular injury.39 AST, ALT, and ALP are relatively liver-specific enzymes. Elevation of AST, ALT, and ALP activities in the plasma is the result of leakage from damaged cells and therefore reflects hepatocyte damage.40 We found that the activities of AST, ALT, ALP, and GGT in serum were significantly decreased in VA treated rats when compared with rats treated with l-NAME alone. The kidney plays a central role in regulating of the balance of body salt and water, and disordered regulation of renal functions is responsible for the altered balance of salt and water in pathophysiological states including hypertension.41 Urea is the major nitrogen-containing metabolic end-product of protein metabolism; uric acid is the major product of purine nucleotides; creatinine is endogenously produced to release into the body fluids and its clearance is measured as an indicator of glomerular filtration rate.42 Our results clearly demonstrated that VA administration significantly decreased serum concentrations of renal markers such as urea, uric acid, and creatinine in the hypertensive rats. This shows that VA partially preserves the functional capacity of the kidney from the adverse effects of l-NAME. Histopathological findings in VA treated hypertensive rat heart were a near normal morphology with the absence of necrosis compared to the pattern with l-NAME treatment alone.
Conclusion
The present study overall demonstrated that VA at a dose of 50 mg/kg Bw exhibited a greater antihypertensive effect than the other two doses (25 mg and 100 mg/kg Bw), as evidenced by lowered blood pressure, lipid peroxidation products, hepatic and renal function markers and increased plasma NOx level, and antioxidant status in the l-NAME-induced hypertensive rats. Further investigations on the exact mechanism of action of VA may identify a new target and a novel therapy for hypertension.
References
- 1.Yusuf S, Ounpuu S, Anand A. Global burden of cardiovascular diseases: part 1 general considerations, the epidemiologic transition, risk factors and impact of urbanization. Circulation 2001;104:2746–53. [DOI] [PubMed] [Google Scholar]
- 2.Silva-Herdade A, Saldanha C. Hemorheological effects of valsartan in L-NAME induced hypertension in rats. Open Circul Vasc J 2011;4:1–5. [Google Scholar]
- 3.Gerald W, Dryden Ion D, Gavin A, Craig J. McClain clinical implications of oxidative stress and antioxidant therapy. Cur Gastroenterol Rep 2005;7:308–16. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs 1995;49:345–61. [DOI] [PubMed] [Google Scholar]
- 5.Getz GS, Reardon CA. Nutrition and cardiovascular disease. Arterioscler Thromb Vasc Biol 2007;27:2499–506. [DOI] [PubMed] [Google Scholar]
- 6.Retelny VS, Neuendorf A, Roth JL. Nutrition protocols for the prevention of cardiovascular disease. Nutr Clin Pract 2008;23:468–76. [DOI] [PubMed] [Google Scholar]
- 7.Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A. Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13. Appl Environ Microbiol 2000;66:2311–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duke JA. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton: CRC Press; 1992. [Google Scholar]
- 9.Varma RS, Shukla A, Chatterjee RK. Evaluation of vanillic acid analogues as a new class of antifilarial agents. Indian J Exp Biol 1993;31:819–21. [PubMed] [Google Scholar]
- 10.Rai RP, Maurya MS. Synthesis and evaluation of antibacterial activity of vanillin derivatives. J Sci Tech 1966;4:275–6. [Google Scholar]
- 11.Delaquis P, Stanich K, Toivonen P. Effect of pH on the inhibition of Listeria spp. by vanillin and vanillic acid. J Food Protec 2005;68:1472–6. [DOI] [PubMed] [Google Scholar]
- 12.Stanely Mainzen Prince P, Dhanasekar K, Rajakumar S. Preventive effects of Vanillic acid on lipids, Bax, Bcl-2 and myocardial infarct size on isoproterenol-induced myocardial infarcted rats: a biochemical and in vitro study. Cardiovasc Toxicol 2011;11:58–66. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda H, Uehara N, Iwahori Y, Asamoto M, Ligo M, Nagao M, et al.. Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo [4, 5] quinoline in the rat. Japanese J Cancer Res 1994;85:1214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja B, Deepa Mol S. The protective role of vanillic acid against acetaminophen induced hepatotoxicity in rats. J Pharm Res 2010;3:1480–4. [Google Scholar]
- 15.Afkir S, Benoit Nguelefack T, Aziz M, Zoheir J, Cuisinaud G, Bnouham M, et al.. Arbutus unedo prevents cardiovascular and morphological alterations in L-NAME-induced hypertensive rats Part I: cardiovascular and renal hemodynamic effects of Arbutus unedo in L-NAME-induced hypertensive rats. J Ethnopharmacol 2008;116:288–95. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrite and nitrate in biological fluids. Anal Biochem 1982;126:131–8. [DOI] [PubMed] [Google Scholar]
- 17.Niehaus WG, Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Pharmacol 1968;6:126–30. [DOI] [PubMed] [Google Scholar]
- 18.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem 1992;202:384–9. [DOI] [PubMed] [Google Scholar]
- 19.Rao KS, Recknagel RO. Early onset of lipid peroxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol 1968;9:271–8. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 1984;21:130–2. [PubMed] [Google Scholar]
- 21.Sinha KA. Colorimetric assay of catalase. Anal Biochem 1972;47:389–94. [DOI] [PubMed] [Google Scholar]
- 22.Rotruck JT, Pop AL, Ganther H, Hafeman BG, Hoeksira WG. Selenium: biochemical role as a component of glutathione peroxides. Science 1973;179:588–90. [DOI] [PubMed] [Google Scholar]
- 23.Baker H, Frank O, De Angelis B, Feingold S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Rep Int 1980;21:531–6. [Google Scholar]
- 24.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959;82:70–7. [DOI] [PubMed] [Google Scholar]
- 25.Katsumi H, Nishikawa M, Hashida H. Development of nitric oxide donors for the treatment of cardiovascular diseases. Cardiovasc Hematol Agents Med Chem 2007;5:204–8. [DOI] [PubMed] [Google Scholar]
- 26.Ribeiro MO, Antunes E, De Nucci G, Lovisolo SM, Zatz R. Chronic inhibition of nitric oxide synthase, a new model of arterial hypertension. Hypertension 1992;20:298–303. [DOI] [PubMed] [Google Scholar]
- 27.Jover B, Herizi A, Ventre F, Dupont M, Mimran A. Sodium and angiotension in hypertension induced by long-term nitric oxide blockade. Hypertension 1993;21:944–8. [DOI] [PubMed] [Google Scholar]
- 28.Baylis C, Mitruka B, Deng A. Chronic blockade of nitric oxide synthesis in the rat produces systemic hypertension and glumerular damage. J Clin Invest 1992;90:278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulker S, McKeown PP, Bayraktutan U. Vitamins Reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension 2003;41:534–9. [DOI] [PubMed] [Google Scholar]
- 30.Fridovich I. The biology of oxygen radicals. Science 1978;201:875–80. [DOI] [PubMed] [Google Scholar]
- 31.Frank L, Massaro D. Oxygen toxicity. Am J Med 1980;69:117–26. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Saravana Kumar M, Raja B. Efficacy of piperine, an alkaloidal constituent of pepper on nitric oxide, antioxidants and lipid peroxidation markers in L-NAME induced hypertensive rats. Int J Res Pharm Sci 2010;3:300–7. [Google Scholar]
- 33.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 2005;57:715–24. [DOI] [PubMed] [Google Scholar]
- 34.Hamberg M, Svensson J, Wakabayashi T, Samuelsson B. Isolation and structure of two prostaglandins endoperoxides that cause platelet aggregation. Proc Natl Acad Sci 1974;71:345–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roy AKD, Gordon MJ, Campbell FM, Duthie GG, James WPT. Vitamin-E requirement, transport and metabolism: role of α-tocopherol-binding protein. J Nutr Biochem 1994;5:562–70. [Google Scholar]
- 36.Karthikeyan J, Rani P. Enzymatic and non enzymatic antioxidants in selected piper speice. Ind J Exp Biol 2003;41:135–40. [PubMed] [Google Scholar]
- 37.Abidi P, Afaq F, Arif JM, Lohani M, Rahman Q. Chrysotilemediated imbalance in the glutathione redox system in the development of pulmonary injury. Toxicol Lett 1999;106:31–9. [DOI] [PubMed] [Google Scholar]
- 38.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology 2006;43:63–74. [DOI] [PubMed] [Google Scholar]
- 39.Hoetzel A, Welle A, Schmidt R, Loop T, Humar M, Ryter SW, et al.. Nitric oxide-deficiency regulates hepatic heme oxygenase-1. Nitric Oxide Biol Chem 2008;18:61–9. [DOI] [PubMed] [Google Scholar]
- 40.Loria P, Lonardo A, Carulli L, Verrone AM, Ricchi M, Lombardini S, et al.. The metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2005;22:31–6. [DOI] [PubMed] [Google Scholar]
- 41.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am Physiol 1998;274:914–23. [DOI] [PubMed] [Google Scholar]
- 42.Burtis CA, Ashwood ER. Enzymes, Teitz fundamentals of clinical chemistry. 4th ed Philadelphia, USA: NB Saunders Company; 1996. p. 312–35. [Google Scholar]