Abstract
Various stressors activate the hypothalamo-pituitary-adrenal axis (HPA-axis) that stimulates adrenal secretion of glucocorticoids, thereby playing critical roles in the modulation of immune responses. Transcriptional regulation of nuclear genes has been well documented to underlie the mechanism of glucocorticoid-dependent modulation of cytokine production and immune reactions. Glucocorticoids also regulate inflammatory responses via non-genomic pathways in cytoplasm and mitochondria. Recent studies have revealed that glucocorticoids modulate mitochondrial calcium homeostasis and generation of reactive oxygen species (ROS). Although redox status and ROS generation in inflammatory cells have been well documented to play important roles in defense against pathogens, the roles of glucocorticoids and mitochondria in the modulation of immunological responses remain obscure. This review describes the role of stress-induced activation of the HPA-axis and glucocorticoid secretion by the adrenal gland in mitochondria-dependent signaling pathways that modulate endotoxin-induced inflammatory reactions and innate immunity.
Keywords: Sepsis, Lipopolysaccharide, Inflammation, Glucocorticoid, Cytokine, Hypothalamo-pituitary-adrenal axis, Mitochondrial oxidative stress
Introduction
Stress is defined as a response to various stressors that include hazardous chemicals, pathogens, and psychological events. Various stressors activate the hypothalamo-pituitary-adrenal axis (HPA-axis) to maintain a wide variety of homeostatic processes including immune responses. When exposed to stressors, HPA-axis-stimulated adrenal secretion of glucocorticoid modulates immunological reactions by regulating the transcription of nuclear genes encoding various cytokines and inflammation-related proteins. It has been well documented that strong stress suppresses innate immune reactions against a variety of pathogens, though its precise mechanism remains obscure. Hyper-activation of the HPA-axis and dysregulation of the neuro-endocrine network have been reported to underlie the pathogenesis of stress-induced emotional disorders, such as anxiety, anorexia nervosa and depression, and down-regulation of the host defense system against bacterial infection.1–4
Mitochondria are multi-functional organelles that synthesize ATP and regulate cell proliferation, differentiation, and oxidative signaling pathways leading to cell death. Thus, oxidative stress in and around mitochondria and their dysfunction have been postulated to underlie the pathogenesis of various diseases including mental disorders with abnormal immune reactions.5–7 Recent studies have revealed that glucocorticoids also modulate mitochondrial functions8,9 to regulate reactive oxygen species (ROS) generation and immune responses.10,11
The present article describes the role of mitochondria and HPA-axis-stimulated glucocorticoid release in the modulation of endotoxin-induced immunological reactions and the mechanism of immunosuppression.
Inflammatory response in sepsis
Sepsis is a syndrome induced by severe infection that causes uncontrolled acute systemic inflammation and organ dysfunction, and is a major cause of death in many countries.12,13 The acute phase reaction against bacterial and/or viral infection is first initiated locally, followed by systemic reactions including fever, leukocytosis, tachycardia, tachypnea, and other symptoms of inflammation.14 The current understanding of the immune response to sepsis is still controversial. Classically, the acute inflammatory response that elicits sepsis has been characterized by an over-reaction of innate immunity followed by perturbation of systemic immune reactions. Recent studies with patients and animal models have revealed that immunosuppression plays critical roles in the pathogenesis of sepsis.14–16 Both pro-inflammatory and anti-inflammatory responses occur in an early phase of sepsis that causes immunosuppression. Hotchkiss et al.17 suggested that immunosuppression associated with T-cell exhaustion is the major abnormality in sepsis. Thus, the balance between pro-inflammatory and anti-inflammatory responses and subsequent modulation of antigen-specific adaptive immune responses might determine the fate of patients with sepsis.
In an early phase of sepsis, ROS generation by damaged mitochondria, diminished cellular ATP level, and dysfunction of antioxidant systems might contribute to induction of multiple organ failure and systemic inflammatory response syndrome (SIRS).18,19 An uncontrolled innate immune reaction activates signaling pathways to generate pro-inflammatory cytokines that enhance ROS production and oxidative stress (Fig. 1). Interferon-γ, a pro-inflammatory cytokine, plays critical roles in the formation of ROS in macrophages to protect hosts against a wide variety of pathogens.20 Furthermore, pro-inflammatory cytokines activate inflammation-related enzymes, such as GTP cyclohydrolase-I, indoleamine 2,3-dioxygenase, and inducible nitric oxide synthase.20 Activation of these enzymes alters cellular redox balance to modulate the activity of NF-κB, a redox-sensitive transcription factor (TF), and immune responses.21,22 Plasma levels of the products of these enzymes have been shown to be useful markers of the inflammatory response in patients with sepsis.20,23,24
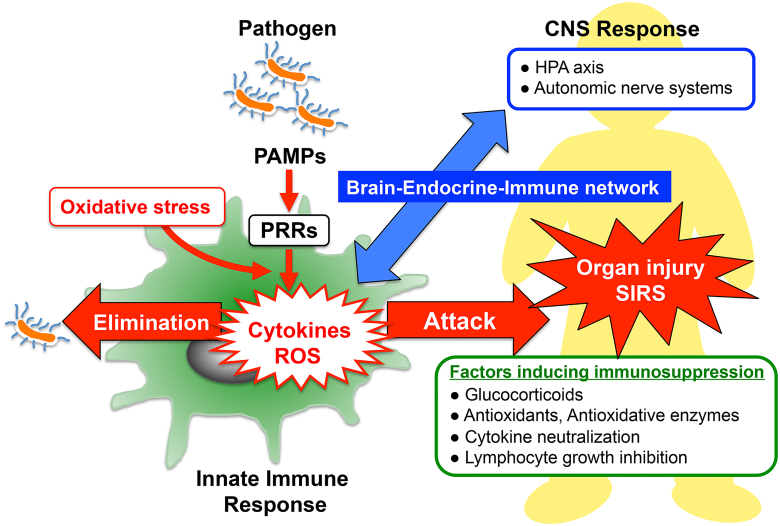
Figure 1.
Innate immune response and host defense systems: The recognition of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) located on the surface of host immune cells is a primitive part of the innate immune systems for protecting the host against invading pathogen. In the early phase of sepsis, an uncontrolled innate immune system enhances release of cytokines and generation of reactive oxygen species (ROS), which contribute to induce multiple organ failure and systemic inflammatory response syndrome (SIRS). Enhanced protective activity of the antioxidant network, cytokine neutralization or inhibition of lymphocyte growth might be of therapeutic potential in SIRS. In addition, optimal glucocorticoid secretion via HPA-axis stimulation in response to immune stress shows anti-inflammatory effects and enhances resistance against septic shock. Thus, the brain–endocrine–immune network might be important for the modulation of immunological balance during sepsis.
Macrophages play pivotal roles in innate immune responses in patients with sepsis by producing ROS, nitric oxide, and cytokines. Stimulation of macrophages by pathogens is crucial for triggering the subsequent innate immune reactions.25 The perception and recognition of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) localized on the surface of host immune cells is a primitive reaction of the innate immune system (see Fig. 1). Discovery of toll-like receptors (TLRs) and transmembranous PRR as primary sensors for microbial infection led to significant advances in understanding the pathogenesis of sepsis.26 TLRs are expressed in various types of cells including neutrophils, T-cells, macrophages, and dendritic cells. Lipopolysaccharide (LPS) of gram-negative bacteria activates the TLR4 signaling pathway that stimulates ROS generation and cytokine production in immune cells.27,28
Besides stimulation by invading pathogens, extracellular and/or intracellular oxidative stress also enhances immune responses to LPS via a TLR4-dependent signaling pathway.27,29–31 Oxidative stress enhances the recruitment of TLR4 to lipid rafts in plasma membranes of macrophages, thereby stimulating their response to LPS.31 Oxidative stress-enhanced innate immune reactions might induce priming of inflammatory cells. Thus, modulation of the TLR4 signaling pathway and suppression of oxidative stress and/or oxidant-induced TLR4 recruitment to membranous lipid rafts of macrophages might be of therapeutic potential for the prevention of LPS-induced inflammation leading to septic shock.
Danger signals, including PAMPs and host-derived molecules, such as uric acid crystals, DNA and RNA, enhance the generation of inflammatory cytokines and elicit sepsis through the activation of inflammasomes. Recent studies have revealed the presence of the signaling pathway for innate immune responses involving mitochondria-dependent activation of inflammasomes.32 Inflammasomes are molecular platforms for inflammatory signaling pathways that control the activity of caspase-1 to catalyze the maturation of pro-inflammatory cytokines, such as IL-1β and IL-18. Accumulating evidence suggests that modulation of mitochondrial functions, such as ROS generation, membrane potential, and mitochondrial DNA release into cytosol, is associated with inflammasome-dependent innate immune reactions. Enhanced activation of inflammasomes by inhibiting autophagy causes mitochondrial dysfunction and increases the susceptibility of macrophages to release mature forms of IL-1β and IL-18 after challenge with LPS and ATP.32,33 Furthermore, mice lacking autophagy-related proteins in hematopoietic cells showed enhancement of ROS generation in macrophages and the inflammatory immune response to LPS.34 These reports suggest that mitochondrial dysfunction and/or ROS generation in immune cells enhance the LPS-induced inflammatory response through the activation of inflammasome.
Factors that modulate inflammatory response
The redox buffer capacity plays a pivotal role in regulating immune responses. The roles of endogenous antioxidants and systemic redox balance have been investigated in patients and animals with sepsis.35,36 Endogenous antioxidants, such as vitamin E, reduced glutathione (GSH), and ascorbic acid, play important roles in the metabolism of ROS. As ascorbic acid and GSH are water-soluble major antioxidants, various types of cells possess these antioxidants in high concentrations.37,38 Perturbation of GSH metabolism by infection enhances cellular susceptibility to oxidative stress, thereby causing apoptosis and tissue injury in animals during sepsis. Since oxidative stress underlies the pathogenesis of endotoxin-induced septic shock,21,35,39 the effects of various antioxidants and related enzymes have been evaluated.19,40–42 Administration of the glutathione precursor N-acetylcysteine (NAC) decreased bacterial colonies, and improved survival in a mouse with polymicrobial sepsis induced by cecal ligation and puncture.40 Wang et al.43 reported that repeated administration of NAC during the early stage of severe sepsis effectively inhibited the activation of lung dendritic cells and their apoptosis, thereby preserving cellular functions in animals with zymosan-induced, generalized inflammation. Selenium-dependent antioxidant enzymes including glutathione peroxidase and thioredoxin reductase also play important roles in the regulation of cellular redox balance. The activity of these enzymes is affected by the availability of selenium. Patients with septic shock exhibited low levels of selenium and glutathione peroxidases in plasma and these showed negative correlations with the severity of manifestation of patients with sepsis.44 Angstwurm et al.45 reported that administration of a high dose of sodium selenite reduced the mortality of patients with severe sepsis and septic shock. Nrf2 is a major sensor of oxidative stress and a master regulator of antioxidant defense mechanisms.46 Oxidative stress induces the dissociation of Nrf2 from Keap1, a negative regulator, and its translocation into the nucleus where it binds to the antioxidant response element in the promoter region of several antioxidant genes.47 Since Nrf2 plays essential roles in the regulation of NADPH oxidase-dependent ROS generation, its disruption significantly increases the mortality of animals with polymicrobial sepsis and endotoxin shock.29 As mentioned above, oxidative damage of mitochondria is also involved in the development of organ dysfunction associated with sepsis.18,19 Treatment of septic animals with mitochondria-targeted antioxidants inhibited mitochondrial damage and inflammatory responses, thereby ameliorating organ failure.41,42 Thus, increases in the protective activity of the antioxidant network and mitochondrial integrity might be of therapeutic potential in septic patients with elevated oxidative stress.
Various stresses modulate signaling pathways leading to cytokine production, thereby altering immune responses.48–50 Stress can either enhance or reduce immune responses.51,52 Strong exercise stress exacerbated skin inflammation while mild exercise stress suppressed inflammatory responses in atopic dermatitis.53 The mechanism for the strength-dependent pivotal modulation of immune responses by stress remains unknown. Dhabhar and McEwen54 have reported that mild and short-acting stress enhances delayed-type hypersensitivity (DTH) reactions by adrenal stress hormones glucocorticoid and epinephrine, whereas high-dose corticosterone, chronic corticosterone administration significantly suppressed skin DTH. Yin et al.55 have reported that strong and chronic stress induces immunosuppression by decreasing immune cells through a mechanism involving Fas-mediated apoptosis dependent on endogenous opioids. Inhibition of lymphocyte growth and changes in the balance of Th1/Th2 cytokines also affect inflammatory responses during sepsis. Chronic restraint stress increased Th2-related cytokines but decreased Th1-type cytokines by a TLR4-dependent mechanism,55,56 and stress-induced up-regulation of TLR4 expression in lymphocytes and activation of the TLR4-PI3 K/AKT pathway play critical roles in lymphocyte apoptosis and immune suppression.56 These results showed the role of stress-related hormones in immune response, and the divergent effects of stress on immune function.
When challenged with endotoxin, the HPA-axis stimulates adrenal secretion of glucocorticoids that have potent immunomodulatory and anti-inflammatory activities. Stress-induced glucocorticoids have been shown to induce enhanced resistance against septic shock.57 Beneficial effects of glucocorticoids in sepsis-associated organ injury have been reported in animal experiments.58,59 Thus, glucocorticoids have been used for treating patients with septic shock.60–62 However, the effectiveness of glucocorticoids for improving the mortality of patients with sepsis is still under debate. Although a high dose of corticosteroid showed no beneficial effect on the prognosis of septic patients, physiologically low doses provided promising results.60,62 Thus, optimal levels and duration of HPA-axis-stimulated glucocorticoid secretion might be important for the modulation of immunological balance in patients with sepsis.
Role of the HPA-axis in the modulation of immune reactions
Responses to stressors play critical roles in the maintenance of homeostasis in animals, aiding their survival by adapting to their environments. Stimulation of the HPA-axis and sympathetic adrenal–medullary axis through neuronal networks enhances the secretion of glucocorticoids and catecholamine, an adrenergic neurotransmitter. The neuro-endocrine system plays important roles in the regulation of stress-induced biological reactions including digestion of food, energy metabolism, immune systems, and control of emotion. Neuro-endocrinological regulation of immune responses is essential for the survival of a host suffering from infection and inflammatory diseases and glucocorticoids released by HPA-axis-dependent mechanisms plays critical roles in the regulation of immune systems.63 In response to various stressors, corticotropin-releasing factor (CRF) is released from the hypothalamus into the hypophysial portal vein to stimulate the anterior pituitary gland, thereby releasing adrenocorticotropic hormone (ACTH) into the systemic circulation (Fig. 2). ACTH in the circulation binds to the type-2 melanocortin receptor (MC2R) in the adrenal cortex, where it stimulates the synthesis and release of glucocorticoids into the circulation.64 Glucocorticoids secreted into the circulation inhibit further activation of the HPA-axis through the glucocorticoid receptor (GR)-mediated feedback mechanism in the brain, to regulate homeostasis of stress-related hormones.
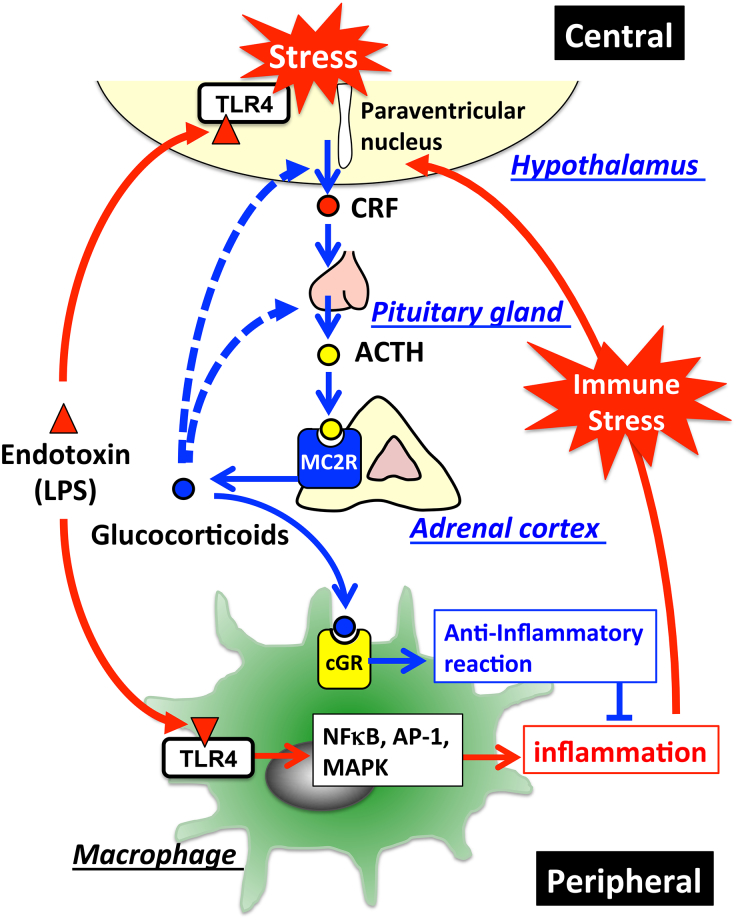
Figure 2.
HPA-axis in response to LPS and cross-talk between the brain and immune system: The major part of the HPA-axis system is constituted by the hypothalamus, the pituitary gland, and the adrenal glands. When exposed to stressors, secretion of corticotropin-releasing factor (CRF) into hypophysial portal vessels stimulates the anterior pituitary gland thereby releasing adrenocorticotropic hormone (ACTH) into the circulation. Circulating ACTH binds to the type-2 melanocortin receptor (MC2R) in the adrenal cortex, where it stimulates glucocorticoid release. Elevated glucocorticoids in the circulation inhibit further activation of the HPA-axis through glucocorticoid receptor-mediated mechanisms in the brain so as to maintain homeostasis. Invading pathogens enhance glucocorticoid release that modulates the systemic immune response. TLR4-stimulated immune cells in response to LPS release excessive amounts of cytokines into the circulation, thereby stimulating the HPA-axis. In addition, LPS directly affects activation of the HPA-axis via the TLR4 signaling pathway exhibited in both the resident immune cells and neurons in the brain. Blue lines represent the HPA-axis to maintain homeostasis; blue dotted lines represent suppression of glucocorticoid release by negative feedback loops; red lines represent stimulation of the HPA-axis in response to LPS.
Dysregulation of the HPA-axis also exacerbates the manifestation of sepsis syndrome and SIRS. Lipinska-Gediga et al.65 reported that the median level of pro-atrial natriuretic peptide (pro-ANP), an ACTH-inhibiting factor, was significantly higher in non-survivors than in survivors with septic shock. They suggested that the plasma level of pro-ANP in these patients could be a valuable prognostic marker. Chida et al.64 have established mice having an inactive MC2R gene in order to study its roles in vivo. MC2R-deficient mice lack the ability to produce glucocorticoids. In response to LPS, they showed increased release of inflammatory cytokines to exacerbate endotoxin-induced septic shock suggesting the important role of adrenal glucocorticoid release for immunosuppression.66
In contrast, the central nervous system (CNS) is also modulated by excessive release of pro-inflammatory cytokines, reactive oxygen and nitrogen species in the circulation and hypotension, associated with inflammation.67,68 Macrophages at the site of infection are important for the initiation of the acute phase reaction of the innate immune system69 that activates the HPA-axis in patients with sepsis. Circulating pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, stimulate the secretion of CRF and/or arginine vasopressin to release glucocorticoids. Thus, the release of glucocorticoids in response to peripheral inflammatory stress might be attributed to the ability of immune cells to produce various cytokines. The cross-talk between the peripheral immune system and the CNS via cytokines has important implications for modulation of host defense systems in stress condition.49,70
Direct effects of pathogens on the immunological activity of the brain also have been investigated. It has been reported that blood–brain barrier breakdown presents an early key step of pathogenesis in sepsis-associated encephalopathy.71 TLR4, widely expressed in mouse brain, functions independently from the circulating cytokines to modulate brain-specific inflammatory reactions and HPA-axis-induced adrenal secretion of corticosterone during endotoxemia.72 Restraint stress significantly increased TLR4 transcripts in the hypothalamus compared to those in control mice, suggesting that preconditioning with restraint stress primes responsiveness of the hypothalamus to LPS through TLR4 signaling.66 Neurons also function as key sensors for the modulation of inflammation through the TLR4 signaling pathway in the brain.73 Thus, LPS-mediated direct activation of CNS may result in enhanced activation of the HPA-axis, leading to elevated levels of ACTH and glucocorticoids, which in turn suppress the release of pro-inflammatory cytokines from peripheral tissues. It appears that LPS may exert the bimodal effects in case of septic inflammation; that is, LPS may initiate and accelerate the inflammatory responses in the systemic organs, and, at the same time, LPS may activate the HPA-axis directly for suppressing the systemic inflammatory responses (Fig. 2).
Role of adrenal glucocorticoids in inflammatory response and immunological signaling pathways
Although therapeutic effects of corticosteroids in patients with septic shock remain controversial, it is certain that HPA-axis-stimulated adrenal secretion of glucocorticoids shows various beneficial effects against infectious diseases and disruption of the immune systems in patients with sepsis. Recent studies showed that stimulation of the HPA-axis followed by glucocorticoid release, increased expression of GRs, and up-regulation of glucocorticoid-induced leucine zipper (GILZ) contribute to enhanced resistance to endotoxin-induced septic shock.57,74,75 Furthermore, antagonism of GR by RU486 enhanced the generation of LPS-induced pro-inflammatory cytokines and decreased the survival rate of septic mice75 without altering the stimulation of HPA-axis-induced glucocorticoid secretion.66 These findings suggest that receptor-dependent glucocorticoid signaling plays critical roles in the modulation of the LPS-induced immune response.
Glucocorticoids regulate various cellular events, such as energy metabolism, mitochondrial activity, apoptosis, and immune responses.76 Among various functions of glucocorticoids, anti-inflammatory and immunosuppressive effects have been well documented.77 The cellular response to glucocorticoids is initiated by binding to an inactive form of GRs in the cytosol (Fig. 3). Then, the GR complex exhibits transrepression to inhibit the transcriptional promoting activity in cytosol by interacting with TFs and transactivation in nucleus by binding to its responsive elements on DNA. Transcriptional activation by homodimeric GRs increases the synthesis of anti-inflammatory proteins including annexin A1, GILZ, and mitogen-activated protein kinase phosphatase-1. On the other hand, glucocorticoids bound to monomeric receptors form a complex with TFs, such as NFκB and AP-1, to inhibit their binding to the pro-inflammatory target genes (TFRE) including pro-inflammatory genes such as IL-1B, TNFA, and chemokines (transrepression).
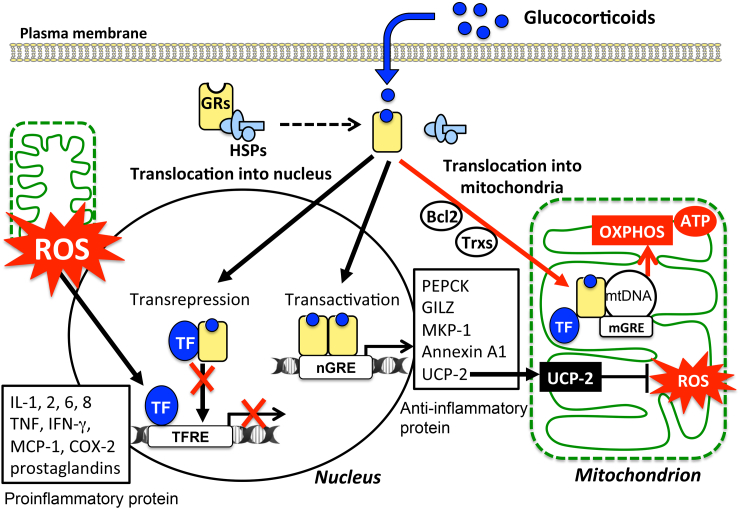
Figure 3.
Variety of cellular responses to glucocorticoids and modulation of mitochondrial functions: Cellular responses to glucocorticoids are initiated by its binding to the cytosolic glucocorticoid receptors (GRs) in their inactive form. Homodimers of the GR complex bind to nuclear glucocorticoid response elements (nGREs) and activate the gene transcriptions driving the expression of anti-inflammatory genes including those encoding annexin A1, glucocorticoid-induced leucine zipper protein (GILZ), and mitogen-activated protein kinase phosphatase-1 (MKP-1) (transactivation). In addition, GR complex up-regulate the expression of uncoupling protein-2 (UCP2) thereby suppressing mitochondrial ROS generation. On the other hand, GRs may complex with transcription factors (TFs) such as NFκB and AP-1 thereby preventing them from binding to their target genes, including pro-inflammatory genes such as IL-1B, TNFA, and chemokines (transrepression). Mitochondrial generation of ROS enhances the activity of these TFs. Activated GRs also modulate mitochondrial functions in various cells including neurons, hepatocytes, and immune cells. The mitochondrial genomes of human and rat contain sequences similar to that of the nGREs. GREs in the mitochondrial genome (mGRE) modulate the biosynthesis of the proteins involved in oxidative phosphorylation (OXPHOS) by regulating mitochondrial gene expression. Furthermore, interactions of GRs with B-cell lymphoma-2 (Bcl-2), and mitochondrial thioredoxin (Trx2) regulate mitochondrial function including oxidation, energy production, and cellular survival.
Besides transrepression of pro-inflammatory cytokines and transactivation of anti-inflammatory proteins, a non-genomic glucocorticoid signaling pathway has been reported recently. Several lines of evidence suggest the presence of a novel pathway of glucocorticoid action that modulates mitochondrial respiration, calcium homeostasis, and apoptosis.8–10,78,79 The nucleus and mitochondria coordinately regulate cellular expression of various genes encoding gluconeogenesis-related enzyme (PEPCK), fatty acid synthase (FASN), and mitochondrial respiratory proteins in order to maintain cellular homeostasis. Recent studies demonstrated the presence of activated GRs in mitochondria.78 Furthermore, the genomes of human and rat mitochondria contain sequences similar to those of the glucocorticoid responsive elements (GREs) in the nucleus.80,81 Glucocorticoids modulate the biosynthesis of proteins involved in oxidative phosphorylation (OXPHOS) by regulating mitochondrial gene expression.78,82 Besides a direct action of the GR complex on mitochondrial gene expression, GRs in mitochondria interact with TFs and proteins to modulate mitochondrial functions. GRs have been shown to interact with thioredoxin (Trx2) and regulate gene expression in mitochondria.83 In response to corticosterone, the glucocorticoid–GR complex also interacts with Bcl-2 and translocates into mitochondria to regulate mitochondrial oxidation, membrane potential, and calcium homeostasis, thereby affecting neuronal death and plasticity.8 Thus, exposure to glucocorticoids might directly modulate mitochondrial function through the GR signaling pathway.
The activities of the glucocorticoid–GR complex often exhibit conflicting and biphasic effects on cell survival and immune responses. Elucidation of non-genomic mechanisms of glucocorticoids that modulate mitochondrial functions and immune responses might contribute to evaluating the efficacy of glucocorticoid therapy in patients with sepsis.
Role of mitochondrial ROS and glucocorticoids in the mechanism of immunomodulation
Mammalian tissues have a high concentration of ATP generated by OXPHOS. In a human being, fairly large amounts of oxygen (∼500 l/day) are required to regenerate this amount of ATP. Most molecular oxygen used in mitochondria is converted to H2O by a four-electron reduction mechanism. However, under physiological conditions, significant fractions (about ∼2%) of the inspired oxygen are converted to the superoxide radical and related ROS using electrons released from the electron transport system. Therefore, mitochondria play critical roles in intracellular generation of ROS. Most but not all ROS rapidly react with a variety of molecules, thereby modulating cell functions. Because of the high reactivity of ROS, they should be effectively metabolized at or near the site of generation by enzymatic and/or non-enzymatic mechanisms.
Mitochondria in macrophages play critical roles in the regulation of innate immunity.84 We previously reported that mitochondrial density and their respiratory state contribute to LPS-induced immune responses of macrophages via an ROS-dependent mitogen-activated protein kinase (MAPK) pathway, without activating NFκB.85 Uncoupling protein-2 (UCP2) regulates the extent of uncoupling of the electrochemical gradient of mitochondria and reduces ROS generation in mitochondria. Suppression of mitochondrial ROS generation by UCP2 in macrophages has been shown to modulate the innate immune response.86 Several reports have suggested the physiological importance of UCP2 for suppressing the generation of ROS in cells and tissues.87–89 Expression of UCP2 is fairly high in the spleen, lung, and macrophages,90 suggesting its role in innate immunity and/or inflammatory reactions. UCP2−/− mice are more resistant to infection with Toxoplasma gondii and Listeria monocytogenes by generating a higher level of ROS than wild-type mice.90 They showed high levels of cytokines that mediate macrophage-dependent innate immunity. In contrast, transgenic mice overexpressing UCP2 showed reduced oxidative damage and inflammatory response in ischemic brain injury.91 These reports suggested that regulation of mitochondrial ROS by UCP2 plays critical roles in the signaling pathways that control immune responses.
Recent studies have suggested the involvement of glucocorticoids in the anti-inflammation and immunosuppression through inhibition of mitochondrial ROS generation.66,92,93 Recent studies in this laboratory using restraint stress mice suggested that glucocorticoid-induced up-regulation of UCP2 in macrophages reduced mitochondrial ROS generation and production of pro-inflammatory cytokines in LPS-induced sepsis.66 The promoter region of UCP2 gene contains GC-rich domains and the regulatory elements for Sp1, AP-2, AP-1, CREB, and the GREs.94 These reports suggest the possible novel glucocorticoid action for suppressing immune response through UCP2 up-regulation. In immune cells, LPS-stimulated signals itself suppress UCP2 expression and up-regulate mitochondrial ROS generation, thereby increase production of pro-inflammatory mediators in macrophages.87,95 In contrast, it has been reported that oxidative stress induce UCP2 expression thereby down-regulating ROS generation in immune cells.87,96 Although expression of UCP2 in immune cells is down-regulated in mice during the early stage of the LPS response, it increased at a later stage and protected cells from oxidative stress.87,95,97
The glucocorticoid-dependent immunosuppression might be caused, in part, by alteration of mitochondrial functions and ROS generation. Thus, stress-induced glucocorticoid release by the HPA-axis might contribute to the suppression of inflammatory immune response of patients with sepsis through both nuclear and mitochondrial signaling pathways (Fig. 4).
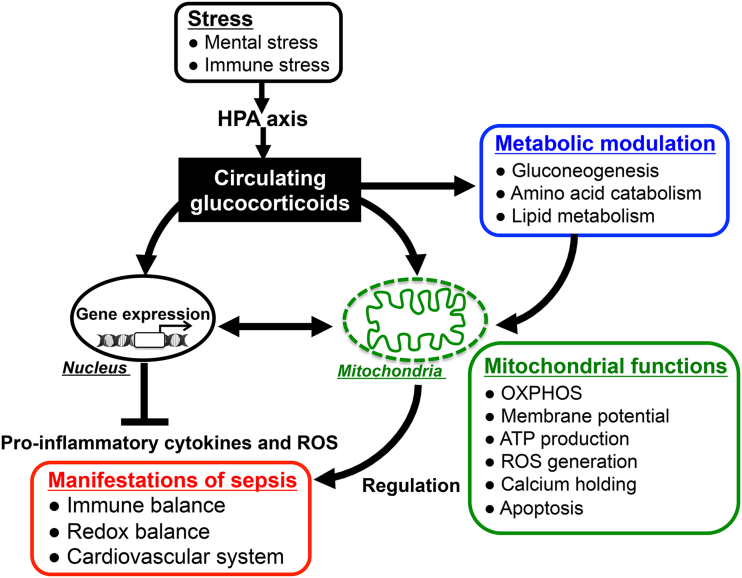
Figure 4.
Glucocorticoid actions for immunomodulation via nucleus- and mitochondria-dependent signaling pathways: Glucocorticoids have pleiotropic effects on energy metabolism (gluconeogenesis, amino acid catabolism, fatty acid metabolism), mitochondrial activity, and immune responses. The alteration of mitochondrial functions such as oxidative activity, ATP production, and apoptotic signaling in response to glucocorticoids plays important roles in modulating host immune responses and redox systems. Thus, appropriately stimulated HPA-axis followed by glucocorticoid release in response to endotoxin-induced immune stress might reduce the parameters of inflammatory response and improve the mortality in sepsis through both nucleus- and mitochondria-dependent signaling pathways.
Conclusion
The neuro-endocrine-immune network driven by the cross-talk of the HPA-axis, glucocorticoids, and mitochondria plays critical roles in the modulation of host defense systems against invasive pathogens that cause septic shock. Modulation of this cross-talk might have therapeutic potential for treating patients with severe infection and septic shock.
Disclaimer statements
Contributors Kasahara conducted this work. Inoue contributed to this study with his critical discussion and suggestion. Kasahara and Inoue interpreted this study and wrote the paper.
Funding This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japanese Government (21700698 and 24501353) and The Sakamoto Research Foundation of Psychiatric Diseases.
Conflict of interest None.
Ethics approval This work was approved by the ethical committee of Osaka City University, Graduate School of Medicine.
Acknowledgment
The authors thank Professor Atsuo Sekiyama, Department of Preemptive Medical Pharmacology for Mind and Body, Graduate School of Pharmaceutical Sciences, Osaka University for his useful comments and suggestions.
References
- 1.Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation 2006;13:268–76. [DOI] [PubMed] [Google Scholar]
- 2.Schatzberg AF, Rothschild AJ, Langlais PJ, Bird ED, Cole JO. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res 1985;19:57–64. [DOI] [PubMed] [Google Scholar]
- 3.Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G,. et al. Salivary cortisol measurement in normal-weight, obese and anorexic women: comparison with plasma cortisol. Eur J Endocrinol 2001;145:165–71. [DOI] [PubMed] [Google Scholar]
- 4.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012;1261:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmieri L, Persico AM. Mitochondrial dysfunction in autism spectrum disorders: cause or effect? Biochim Biophys Acta 2010;1797:1130–7. [DOI] [PubMed] [Google Scholar]
- 6.Konradi C, Sillivan SE, Clay HB. Mitochondria, oligodendrocytes and inflammation in bipolar disorder: evidence from transcriptome studies points to intriguing parallels with multiple sclerosis. Neurobiol Dis 2012;45:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012;36:764–85. [DOI] [PubMed] [Google Scholar]
- 8.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C,. et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 2009;106:3543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E. Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. J Exp Med 2006;203:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids 2002;67:529–34. [DOI] [PubMed] [Google Scholar]
- 11.Hall CJ, Boyle RH, Astin JW, Flores MV, Oehlers SH, Sanderson LE,. et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ROS production. Cell Metab 2013;18:265–78. [DOI] [PubMed] [Google Scholar]
- 12.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet 2013;381:774–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM,. et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580–637. [DOI] [PubMed] [Google Scholar]
- 14.Russell JA. Management of sepsis. N Engl J Med 2006;355:1699–713. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50. [DOI] [PubMed] [Google Scholar]
- 16.Hoetzenecker W, Echtenacher B, Guenova E, Hoetzenecker K, Woelbing F, Bruck J,. et al. ROS-induced ATF3 causes susceptibility to secondary infections during sepsis-associated immunosuppression. Nat Med 2012;18:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013;13:862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth 2011;107:57–64. [DOI] [PubMed] [Google Scholar]
- 19.Rocha M, Herance R, Rovira S, Hernandez-Mijares A, Victor VM. Mitochondrial dysfunction and antioxidant therapy in sepsis. Infect Disord Drug Targets 2012;12:161–78. [DOI] [PubMed] [Google Scholar]
- 20.Gostner JM, Becker K, Fuchs D, Sucher R. Redox regulation of the immune response. Redox Rep 2013;18:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth 2003;90:221–32. [DOI] [PubMed] [Google Scholar]
- 22.Victor VM, Rocha M, De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol 2004;4:327–47. [DOI] [PubMed] [Google Scholar]
- 23.Changsirivathanathamrong D, Wang Y, Rajbhandari D, Maghzal GJ, Mak WM, Woolfe C,. et al. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med 2011;39:2678–83. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M,. et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 2010;16:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–84. [DOI] [PubMed] [Google Scholar]
- 26.Uematsu S, Akira S. Toll-like receptors and innate immunity. J Mol Med 2006;84:712–25. [DOI] [PubMed] [Google Scholar]
- 27.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P,. et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011;472:476–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D,. et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci USA 2009;106:2348–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong X, Thimmulappa R, Kombairaju P, Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol 2010;185:569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N,. et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med 2006;203:2377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powers KA, Szaszi K, Khadaroo RG, Tawadros PS, Marshall JC, Kapus A,. et al. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J Exp Med 2006;203:1951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–5. [DOI] [PubMed] [Google Scholar]
- 33.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC,. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011;12:222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T,. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 2008;456:264–8. [DOI] [PubMed] [Google Scholar]
- 35.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal 2014;20:1000–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan J, Ralston MM, Meng X, Bongiovanni KD, Jones AL, Benndorf R,. et al. Glutathione reductase is essential for host defense against bacterial infection. Free Radic Biol Med 2013;61C:320–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Kashiba M, Kasahara E, Tsuchiya M, Sato EF, Utsumi K,. et al. Metabolic cooperation of ascorbic acid and glutathione in normal and vitamin C-deficient ODS rats. Physiol Chem Phys Med NMR 2001;33:29–39. [PubMed] [Google Scholar]
- 38.Kasahara E, Kashiba M, Jikumaru M, Kuratsune D, Orita K, Yamate Y,. et al. Dynamic aspects of ascorbic acid metabolism in the circulation: analysis by ascorbate oxidase with a prolonged in vivo half-life. Biochem J 2009;421:293–9. [DOI] [PubMed] [Google Scholar]
- 39.Kolls JK. Oxidative stress in sepsis: a redox redux. J Clin Invest 2006;116:860–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villa P, Saccani A, Sica A, Ghezzi P. Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J Infect Dis 2002;185:1115–20. [DOI] [PubMed] [Google Scholar]
- 41.Lowes DA, Thottakam BM, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med 2008;45:1559–65. [DOI] [PubMed] [Google Scholar]
- 42.Lowes DA, Webster NR, Murphy MP, Galley HF. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 2013;110:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HW, Yang W, Lu JY, Li F, Sun JZ, Zhang W,. et al. N-acetylcysteine administration is associated with reduced activation of NF-kB and preserves lung dendritic cells function in a zymosan-induced generalized inflammation model. J Clin Immunol 2013;33:649–60. [DOI] [PubMed] [Google Scholar]
- 44.Forceville X, Aouizerate P, Guizard M. [Septic shock and selenium administration]. Therapie 2001;56:653–61. [PubMed] [Google Scholar]
- 45.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P,. et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med 2007;35:118–26. [DOI] [PubMed] [Google Scholar]
- 46.Marzec JM, Christie JD, Reddy SP, Jedlicka AE, Vuong H, Lanken PN,. et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J 2007;21:2237–46. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C,. et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 2010;38:5718–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol 2003;24:444–8. [DOI] [PubMed] [Google Scholar]
- 49.Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci 2002;966:20–7. [DOI] [PubMed] [Google Scholar]
- 50.Sekiyama A, Ueda H, Kashiwamura S, Sekiyama R, Takeda M, Rokutan K,. et al. A stress-induced, superoxide-mediated caspase-1 activation pathway causes plasma IL-18 upregulation. Immunity 2005;22:669–77. [DOI] [PubMed] [Google Scholar]
- 51.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun 1997;11:286–306. [DOI] [PubMed] [Google Scholar]
- 52.Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol 1993;44:53–85. [DOI] [PubMed] [Google Scholar]
- 53.Orita K, Hiramoto K, Inoue R, Sato EF, Kobayashi H, Ishii M,. et al. Strong exercise stress exacerbates dermatitis in atopic model mice, NC/Nga mice, while proper exercise reduces it. Exp Dermatol 2010;19:1067–72. [DOI] [PubMed] [Google Scholar]
- 54.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA 1999;96:1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin D, Tuthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med 2000;191:1423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Zhang Y, Miao J, Hanley G, Stuart C, Sun X,. et al. Chronic restraint stress promotes immune suppression through toll-like receptor 4-mediated phosphoinositide 3-kinase signaling. J Neuroimmunol 2008;204:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Wang Y, Lu Y, Yu D, Wang Y, Chen F, Yang H,. et al. Enhanced resistance of restraint-stressed mice to sepsis. J Immunol 2008;181:3441–8. [DOI] [PubMed] [Google Scholar]
- 58.Choi HM, Jo SK, Kim SH, Lee JW, Cho E, Hyun YY,. et al. Glucocorticoids attenuate septic acute kidney injury. Biochem Biophys Res Commun 2013;435:678–84. [DOI] [PubMed] [Google Scholar]
- 59.Johannes T, Mik EG, Klingel K, Dieterich HJ, Unertl KE, Ince C. Low-dose dexamethasone-supplemented fluid resuscitation reverses endotoxin-induced acute renal failure and prevents cortical microvascular hypoxia. Shock 2009;31:521–8. [DOI] [PubMed] [Google Scholar]
- 60.Annane D, Cavaillon JM. Corticosteroids in sepsis: from bench to bedside? Shock 2003;20:197–207. [DOI] [PubMed] [Google Scholar]
- 61.Vincent JL, Marshall JC. Surviving sepsis: a guide to the guidelines. Crit Care 2008;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med 2012;185:133–9. [DOI] [PubMed] [Google Scholar]
- 63.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol 2004;181:207–21. [DOI] [PubMed] [Google Scholar]
- 64.Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T,. et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci USA 2007;104:18205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lipinska-Gediga M, Mierzchala M, Durek G. Pro-atrial natriuretic peptide (pro-ANP) level in patients with severe sepsis and septic shock: prognostic and diagnostic significance. Infection 2012;40:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasahara E, Sekiyama A, Hori M, Kuratsune D, Fujisawa N, Chida D,. et al. Stress-induced glucocorticoid release up-regulates UCP2 expression and enhances resistance to endotoxin-induced lethality. Neuroimmunomodulation 2014 [Ahead of print]. DOI: 10.1159/000368802. [DOI] [PubMed] [Google Scholar]
- 67.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1Beta, TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One 2013;8:e69123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laflamme N, Rivest S. Effects of systemic immunogenic insults and circulating proinflammatory cytokines on the transcription of the inhibitory factor kappaB alpha within specific cellular populations of the rat brain. J Neurochem 1999;73:309–21. [DOI] [PubMed] [Google Scholar]
- 69.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev 2003;16:379–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005;5:243–51. [DOI] [PubMed] [Google Scholar]
- 71.Frister A, Schmidt C, Schneble N, Brodhun M, Gonnert FA, Bauer M,. et al. Phosphoinositide 3-Kinase gamma Affects LPS-Induced Disturbance of Blood-Brain Barrier Via Lipid Kinase-Independent Control of cAMP in Microglial Cells. Neuromolecular Med 2014 [Ahead of print]. DOI: 10.1007/s12017-014-8320-z. [DOI] [PubMed] [Google Scholar]
- 72.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci 2005;25:1788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leow-Dyke S, Allen C, Denes A, Nilsson O, Maysami S, Bowie AG,. et al. Neuronal Toll-like receptor 4 signaling induces brain endothelial activation and neutrophil transmigration in vitro. J Neuroinflammation 2012;9:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilibert S, Galle-Treger L, Moreau M, Saint-Charles F, Costa S, Ballaire R,. et al. Adrenocortical scavenger receptor class B type I deficiency exacerbates endotoxic shock and precipitates sepsis-induced mortality in mice. J Immunol 2014;193:817–26. [DOI] [PubMed] [Google Scholar]
- 75.Dejager L, Pinheiro I, Puimege L, Fan YD, Gremeaux L, Vankelecom H,. et al. Increased glucocorticoid receptor expression and activity mediate the LPS resistance of SPRET/EI mice. J Biol Chem 2010;285:31073–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol 2008;4:525–33. [DOI] [PubMed] [Google Scholar]
- 77.Ayroldi E, Cannarile L, Migliorati G, Nocentini G, Delfino DV, Riccardi C. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB J 2012;26:4805–20. [DOI] [PubMed] [Google Scholar]
- 78.Psarra AM, Sekeris CE. Glucocorticoid receptors and other nuclear transcription factors in mitochondria and possible functions. Biochim Biophys Acta 2009;1787:431–6. [DOI] [PubMed] [Google Scholar]
- 79.Kino T, Charmandari E, Chrousos GP. Glucocorticoid receptor: implications for rheumatic diseases. Clin Exp Rheumatol 2011;29:S32–41. [PMC free article] [PubMed] [Google Scholar]
- 80.Demonacos C, Djordjevic-Markovic R, Tsawdaroglou N, Sekeris CE. The mitochondrion as a primary site of action of glucocorticoids: the interaction of the glucocorticoid receptor with mitochondrial DNA sequences showing partial similarity to the nuclear glucocorticoid responsive elements. J Steroid Biochem Mol Biol 1995;55:43–55. [DOI] [PubMed] [Google Scholar]
- 81.Lee SR, Kim HK, Song IS, Youm J, Dizon LA, Jeong SH,. et al. Glucocorticoids and their receptors: insights into specific roles in mitochondria. Prog Biophys Mol Biol 2013;112:44–54. [DOI] [PubMed] [Google Scholar]
- 82.Psarra AM, Sekeris CE. Glucocorticoids induce mitochondrial gene transcription in HepG2 cells: role of the mitochondrial glucocorticoid receptor. Biochim Biophys Acta 2011;1813:1814–21. [DOI] [PubMed] [Google Scholar]
- 83.Psarra AM, Hermann S, Panayotou G, Spyrou G. Interaction of mitochondrial thioredoxin with glucocorticoid receptor and NF-kappaB modulates glucocorticoid receptor and NF-kappaB signalling in HEK-293 cells. Biochem J 2009;422:521–31. [DOI] [PubMed] [Google Scholar]
- 84.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol 2011;11:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasahara E, Sekiyama A, Hori M, Hara K, Takahashi N, Konishi M,. et al. Mitochondrial density contributes to the immune response of macrophages to lipopolysaccharide via the MAPK pathway. FEBS Lett 2011;585:2263–8. [DOI] [PubMed] [Google Scholar]
- 86.Emre Y, Nubel T. Uncoupling protein UCP2: when mitochondrial activity meets immunity. FEBS Lett 2010;584:1437–42. [DOI] [PubMed] [Google Scholar]
- 87.Alves-Guerra MC, Rousset S, Pecqueur C, Mallat Z, Blanc J, Tedgui A,. et al. Bone marrow transplantation reveals the in vivo expression of the mitochondrial uncoupling protein 2 in immune and nonimmune cells during inflammation. J Biol Chem 2003;278:42307–12. [DOI] [PubMed] [Google Scholar]
- 88.Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB,. et al. Persistent nuclear factor-kappa B activation in Ucp2-/- mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem 2005;280:19062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J 2007;402:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B,. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 2000;26:435–9. [DOI] [PubMed] [Google Scholar]
- 91.Haines B, Li PA. Overexpression of mitochondrial uncoupling protein 2 inhibits inflammatory cytokines and activates cell survival factors after cerebral ischemia. PLoS One 2012;7:e31739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long F, Wang YX, Liu L, Zhou J, Cui RY, Jiang CL. Rapid nongenomic inhibitory effects of glucocorticoids on phagocytosis and superoxide anion production by macrophages. Steroids 2005;70:55–61. [DOI] [PubMed] [Google Scholar]
- 93.Wang JF, Jerrells TR, Spitzer JJ. Decreased production of reactive oxygen intermediates is an early event during in vitro apoptosis of rat thymocytes. Free Radic Biol Med 1996;20:533–42. [DOI] [PubMed] [Google Scholar]
- 94.Yamada M, Hashida T, Shibusawa N, Iwasaki T, Murakami M, Monden T,. et al. Genomic organization and promoter function of the mouse uncoupling protein 2 (UCP2) gene. FEBS Lett 1998;432:65–9. [DOI] [PubMed] [Google Scholar]
- 95.Kizaki T, Suzuki K, Hitomi Y, Taniguchi N, Saitoh D, Watanabe K,. et al. Uncoupling protein 2 plays an important role in nitric oxide production of lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA 2002;99:9392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giardina TM, Steer JH, Lo SZ, Joyce DA. Uncoupling protein-2 accumulates rapidly in the inner mitochondrial membrane during mitochondrial reactive oxygen stress in macrophages. Biochim Biophys Acta 2008;1777:118–29. [DOI] [PubMed] [Google Scholar]
- 97.Tang SE, Wu CP, Wu SY, Peng CK, Perng WC, Kang BH,. et al. Stanniocalcin-1 ameliorates lipopolysaccharide-induced pulmonary oxidative stress, inflammation, and apoptosis in mice. Free Radic Biol Med 2014;71C:321–31. [DOI] [PubMed] [Google Scholar]