Abstract
Objectives
This study was focused on the role of indole acetic acid (IAA) in the defense against oxidative stress damage caused by drought in soybean plants and to elucidate whether heme oxygenase-1 (HO-1) and nitric oxide (NO) are involved in this mechanism. IAA is an auxin that participates in many plant processes including oxidative stress defense, but to the best of our knowledge no information is yet available about its possible action in drought stress.
Methods
To this end, soybean plants were treated with 8% polyethylene glycol (PEG) or 100 µM IAA. To evaluate the behavior of IAA, plants were pretreated with this compound previous to PEG addition. Lipid peroxidation levels (thiobarbituric acid reactive substances (TBARS)), glutathione (GSH) and ascorbate (AS) contents, catalase (CAT), superoxide dismutase (SOD), and guaiacol peroxidase (POD) activities were determined to evaluate oxidative damage.
Results
Drought treatment (8% PEG) caused a significant increase in TBARS levels as well as a marked decrease in the non-enzymatic (GSH and AS) and enzymatic (CAT, SOD, and POD) antioxidant defense systems. Pre-treatment with IAA prevented the alterations of stress parameters caused by drought, while treatment with IAA alone did not produce changes in TBARS levels, or GSH and AS contents. Moreover, the activities of the classical enzymes involved in the enzymatic defense system (SOD, CAT, and POD) remained similar to control values. Furthermore, this hormone could enhance HO-1 activity (75% with respect to controls), and this increase was positively correlated with protein content as well as gene expression. The direct participation of HO-1 as an antioxidant enzyme was established by performing experiments in the presence of Zn-protoporphyrin IX, a well-known irreversible inhibitor of this enzyme. It was also demonstrated that HO-1 is modulated by NO, as shown by experiments performed in the presence of an NO donor (sodium nitroprusside), an NO scavenger (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide), or an NO synthesis inhibitor (N-nitro-l-arginine methyl ester, NAME).
Discussion
It is concluded that IAA is responsible, at least in part, for the protection against oxidative stress caused by drought in soybean plants through the modulation of NO levels which, in turn, enhances HO-1 synthesis and activity.
Keywords: Drought stress, Glycine max. L, Heme oxygenase, Indole acetic acid, Nitric oxide
Introduction
Soybean (Glycine max. L) is an important crop in the world because it is used as a source of high-quality protein. Different environmental conditions may affect its yield. In this sense, drought is one of the major abiotic stresses that impair plant productivity.1 Water deficit is characterized by a disturbance between the generation and quenching of reactive oxygen species (ROS).2 In the absence of an effective protective system, ROS provokes lipid peroxidation, protein degradation, DNA breakage, and cell death.3 Plant cells can overcome ROS deleterious effects by means of endogenous protective mechanisms involving non-enzymatic as well as enzymatic systems.4
Indole acetic acid (IAA) is one of the naturally occurring growth hormones that enhances cell division and elongation. The behavior of IAA in drought resistance is still rather contradictory. For a long time, it was generally assumed that water deficiency results in a decrease of IAA content.5 However, it has become more evident that adaptation to drought is accompanied by an increase in the IAA levels.6
Several reports have demonstrated that heme oxygenase-1 (HO-1) participates in the response to different stress stimuli in plants,7–10 but to the best of our knowledge no information is still available about its behavior against the oxidative stress damage caused by drought stress in soybean plants.
Heme oxygenase (HO; EC 1.14.99.3) catalyzes the degradation of heme into biliverdin IXa (BV), Fe2+, and carbon monoxide (CO). This reaction requires molecular oxygen and electrons from NADPH.11–13 The mechanism of heme degradation (Fig. 1) by HO is a common feature of all organisms and has been intensively investigated.14 In plants, HO-1 is also associated with the biosynthetic pathway leading to phytochrome chromophore formation,15–17 protection against20 oxidative damage,18,19 and root development.20,21 BV generated by the HO-1 catalyzed reaction is an efficient scavenger of ROS.22 In the soybean leaf, HO has been localized in chloroplast and mitochondria23 and in the peribacteroid membrane of root nodules.24 Moreover, expression of HO-1 in plants is regulated by ultraviolet-B (UV-B), H2O2, nitric oxide (NO), cytokinin, and heavy metals.18,20,25–29 Heme catabolism also gives rise to CO production, and this molecule can participate in many physiological reactions,30 including cell protection against oxidative stress.31,32
Figure 1.
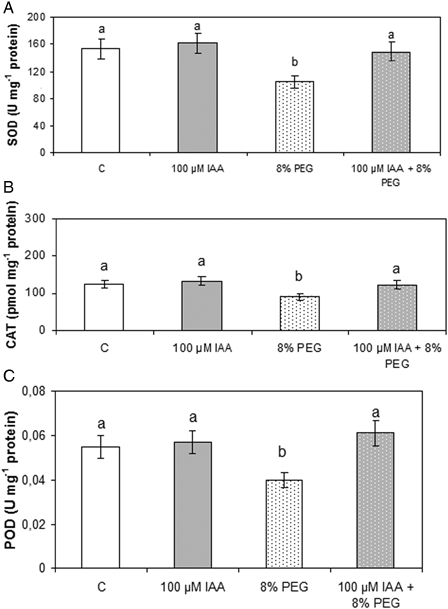
Effect of PEG and IAA pre-treatment on soybean roots SOD (A), CAT (B), and POD (C) activities. Experiments were performed according to the Material and methods section. Values are the mean of three independent experiments and bars indicate SE. Different letters indicate significant differences (P < 0.05) according to Tukey's multiple range test.
Recently, it has been demonstrated that HO-1 also performs a crucial role in NaHS-induced lateral root (LR) formation in tomato (Solanum lycopersicum L.) seedlings33 and that HO-1 may be a central repeater for crosstalk among NO, CO, and hydrogen peroxide in plants.34
NO production is associated with many physiological situations in plants, and NO is a key signaling molecule throughout the lifespan of a plant. In fact, it plays many roles in biotic and abiotic stresses3,35–37 and, depending on its concentration, it produces either protective or toxic effects. It has been established that low doses modulate superoxide anion formation and inhibit lipid peroxidation, acting in antioxidant defense during stress. Furthermore, microarray studies have shown that NO induces a large number of genes at the transcriptional level, among them those of antioxidant enzymes.38 It also has been reported that NO gives rise to signaling pathways mediating responses of specific genes to UV-B radiation, such as chalcone synthase and phenylalanine ammonia lyase39 and that NO is involved in auxin-induced LR formation.40
However, knowledge about the role that NO plays in regulating antioxidant enzymes to counteract drought-induced oxidative stress is rather scarce. Recently, a few studies have suggested that NO could act in protecting plants from oxidative damage;40,41 therefore, NO-donor treatment would prevent plants from damage by increasing the activity of antioxidant enzymes.
These facts prompted us to investigate the role of IAA in the defense against oxidative stress damage caused by drought in soybean plants and to elucidate whether HO-1 and NO could be involved in this mechanism.
Materials and methods
Plant material and growing conditions
Seeds of soybean (Glycine max L., A6445RG) were surface sterilized with 5% v/v sodium hypochlorite for 10 minutes and washed with distilled water four times, and then they were pretreated with 100 µM IAA solution. Controls were performed in distilled water in the absence of the hormone. Control and treated seeds were maintained in the darkness for 48 hours. Afterwards, seeds were planted in vermiculite for 5 days in the presence of different IAA concentrations ranging from 0 to 100 µM. After 5 days of germination, plants were removed from pots; roots were gently washed and transferred to separated flasks containing PEG (MW20000) solutions ranging from 0 to 8% (w/v) for 72 hours in a controlled climate room at 24 ± 2°C and 50% relative humidity, with a photoperiod of 16 hours and a light intensity of 175 µmol/m2/s. The hydroponics medium was Hoagland's nutrient solution. The medium was continuously aerated and replaced every 3 days. Roots were excised and used for determinations. To evaluate the effect of NO, seeds were pretreated with sodium nitroprusside (SNP), an NO donor, the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), or N-nitro-l-arginine methyl ester (l-NAME), an NO synthase (NOS) inhibitor, at 100, 100, and 200 µM final concentration, respectively, for 48 hours. When the effect of Zn-protoporphyrin IX (ZnPPIX, 20 µM) was investigated, it was added to the solutions in the presence or absence of IAA for 48 hours. Determinations were performed in triplicate in three different experiments employing six plants for each treatment.
Thiobarbituric acid reactive substances level
Lipid peroxidation was measured as the amount of thiobarbituric acid reactive substances (TBARS) determined by the thiobarbituric acid (TBA) reaction as described.42 Fresh control and treated roots (0.3 g) were homogenized in 3 ml of 20% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 3500 g for 20 minutes. To 1 ml of the aliquot of the supernatant, 1 ml of 20% TCA containing 0.5% (w/v) TBA and 100 µl 4% butylated hydroxytoluene in ethanol were added. The mixture was heated at 95°C for 30 minutes and then quickly cooled on ice. The contents were centrifuged at 10 000 g for 15 minutes and the absorbance was measured at 532 nm. Value for non-specific absorption at 600 nm was subtracted from the value at 532 nm. The concentration of TBARS was calculated using malondialdehyde extinction coefficient of 155 mM−1 cm−1.
Glutathione determination
Non-protein thiols were extracted by homogenizing 0.3 g of roots in 3 ml of 0.1 N HCl (pH 2), 1 g polyvinylpyrrolidone (PVP).43 After centrifugation at 10 000 g for 10 minutes at 4°C, the supernatants were used for analysis. Total glutathione content (GSH) was determined in the homogenates by spectrophotometry at 412 nm, using yeast-GR, 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), and Nicotinamide adenine dinucleotide phosphate (NADPH). A calibration curve was performed using GSH (Sigma, Buenos Aires, Argentina) as a standard.
Ascorbate determination
Ascorbate (AS) was determined as described.43 One gram of tissue was homogenized in 10% (w/v) TCA, and the supernatant was used for the assay. Sodium hydroxide (10 µl, 5 M) was added to 400 µl of extract, mixed, and the mixture was centrifuged for 2 minutes at 3500 g. To a 200 µl sample of the supernatant were added 200 µl of 150 mM NaH2PO4 buffer, pH 7.4, and 200 µl of water. To another 200 µl of supernatant, 200 µl of buffer and 100 µl of 10 mM dithiothreitol were added and, after thorough mixing and being left at room temperature for 15 minutes, 100 µl of 0.5% (w/v) N-ethylmaleimide was added. Samples were vortex-mixed and incubated at room temperature for 30 seconds. To each was then added 400 µl of 10% (w/v) TCA, 400 µl of 44% (v/v) H3PO4, 400 µl of 4% (w/v) bipyridyl in 70% (v/v) ethanol, and 200 µl of 3% (w/v) FeCl3. After vortex mixing, samples were incubated at 37°C for 60 minutes and the absorbance at 525 was recorded. A standard curve of AS was used for calibration.
Antioxidant enzyme assay
Extracts for determination of superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), and guaiacol peroxidase (POD; EC 1.11.1.7) activities were prepared from 0.3 g of roots homogenized under ice-cold conditions in three of extraction buffers, containing 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP, and 0.5% (v/v) Triton X-100 at 4°C. The homogenates were centrifuged at 10 000 g for 20 minutes and the supernatant fraction was used for the assays. CAT activity was determined in the homogenates by measuring the decrease in absorption at 240 nm in a reaction medium containing 50 mM potassium phosphate buffer (pH 7.2) and 2 mM H2O2. The pseudo-first-order reaction constant (k′ = k[CAT]) of the decrease in H2O2 absorption was determined and the CAT activity in H2O2 pmol/mg protein was calculated using k = 4.7 × 107 M−1 s−1.44 Total SOD activity was assayed by the inhibition of the photochemical reduction of Nitrotetrazolium Blue chloride (NBT), as described by Becana et al.45 The reaction mixture consisted of 50–150 µl of enzyme extract and 3.5 ml O2− generating solution which contained 14.3 mM methionine, 82.5 µM NBT, and 2.2 µM riboflavin. Protein extracts were brought to a final volume of 0.3 ml with 50 mM potassium phosphate (pH 7.8) and 0.1 mM Na2EDTA. Test tubes were shaken and placed 30 cm from light bank consisting of six 15 W fluorescent lamps. The reaction was allowed to run for 10 minutes and stopped by switching the light off. The reduction in NBT was followed by reading absorbance at 560 nm. Blanks and controls were run the same way but without illumination and enzyme, respectively. One unit of SOD was defined as the amount of enzyme which produced a 50% inhibition of NBT reduction under the assay conditions. POD activity was determined in the homogenates by measuring the increase in absorption at 470 nm due to the formation of tetraguaiacol (ɛ: 26.6 mM−1 cm−1) in a reaction contained extract, 50 mM potassium phosphate buffer pH 7, 0.1 mM EDTA, 10 mM guaiacol, and 10 mM H2O2. One unit is defined as the amount of enzyme that catalyzes the formation of 1 µM of guaiacol per minute under the assay conditions.
HO preparation and assay
Roots (0.3 g) were homogenized in a Potter-Elvehjem homogenizer using four volumes of ice-cold 0.25 M sucrose solution containing 1 nM phenylmethyl sulfonyl fluoride, 0.2 nM EDTA, and 50 mM potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 20 000 g for 20 minutes and supernatant fractions were used for activity determination. HO activity was determined as previously described with minor modifications. The standard incubation mixture in a final volume of 500 ml contained 10 mM potassium phosphate buffer (pH 7.4), 60 nmol NADPH, 250 µl HO (0.5 mg protein), and 200 mM hemin. Incubations were carried out at 37°C during 60 minutes. The concentration of BV was estimated using a molar absorption coefficient at 650 nm of 6.25 mM−1 cm−1 in 0.1 M HEPES-NaOH buffer (pH 7.2). One unit is defined as the amount of enzyme catalyzing the formation of 1 nmol of BV per 30 minutes under stander conditions.
DNA protein gel blot analysis for HO-1
Homogenates obtained for HO activity assay were also analyzed by DNA protein gel immunoblot technique. Forty milligrams of protein from root homogenates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis using a 12% acrylamide resolving gel (Mini Protean II System, BioRad, Hertz, UK), according to Laemmli (1970).45 Separated proteins were then transferred to nitrocellulose membranes and non-specific binding of antibodies was blocked with 3% non-fat dried milk in phosphate-buffered saline, pH 7.4 for 1 hour at room temperature. Membranes were then incubated overnight at 4°C in primary antibodies raised against Arabidopsis thaliana HY-146 diluted 1:2000 in Tris-NaCl buffer plus 1% non-fat milk. Immune complexes were detected using alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G. The phosphatase-labeled antigens were visualized with the colorigenic substrate 5-bromo-4-chloro-3-indoleyl phosphate and nitroblue tetrazolium.
Real-time quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated using Trizol reagent (Invitrogen, Buenos Aires, Argentina), treated with RNase-free DNase I (Promega, Buenos Aires, Argentina), and reverse-transcribed into cDNA using random hexamers and M-MLV Superscript II RT (Invitrogen). Quantitative reverse transcription-polymerase chain reaction (RT-PCR) reactions were carried out using G. max HO-1 specific primers 5′-GATCCCCAAGCATTCATTTG-3′ and 5′-TTTCTTCCAGACAGTGGTCC-3′, they were designed to amplify the 476–701 bp region of the G. max HO-1 cDNA (GenBank accession no. AF320024) and Power SYBR Green master mix (Applied Biosystems). Samples were assayed in triplicate on a 7900HT real-time PCR system (Applied Biosystems) with the following conditions: one cycle for 10 minutes at 95°C, 40 cycles with 95°C for 15 seconds, 58°C for 20 seconds, and 60°C for 40 seconds, followed by a melting curve analysis. Results were analyzed using the relative quantification (ΔΔCt) method. The threshold cycle (Ct) values were normalized against the reference gene 18S, which has shown to be stable under several UV-B conditions.48 The data were calculated using the formula 2−ΔΔCt and are presented as the fold change in the relative steady-state level of specific mRNA normalized and relative to the untreated control.
Protein determination
Protein concentration was evaluated by the method of Bradford,49 using bovine serum albumin as a standard.
Statistics
Values in the text, figures, and tables indicate mean values ± standard error (SE). Differences among treatments were analyzed by one-way analysis of variance, taking P < 0.05 as significant according to Tukey's multiple range test.
Table 1.
Effect of PEG and IAA pre-treatments on GSH and AS content in soybean roots
| Treatment | GSH (nmol/mg FW) | AS (µmol/mg FW) |
|---|---|---|
| Control | 245 ± 20a | 0.21 ± 0.03a |
| 100 µM IAA | 249 ± 18a | 0.23 ± 0.02a |
| 8% PEG | 167 ± 12b | 0.15 ± 0.02b |
| 100 µM IAA + 8% PEG | 242 ± 19a | 0.24 ±0.04a |
Determinations were performed as described in the Experimental section.
Different letters within columns indicate significant differences (P < 0.05) according to Tukey's multiple range test.
Results
TBARS level
Taking into account the fact that TBARS content is a good indicator of cell membrane damage, it was determined in plants subjected to different polyethylene glycol (PEG) concentrations ranging from 0 to 8% (w/v).
On one hand, PEG enhanced this parameter in a dose-depending manner. Fig. 2A shows that 2 and 4% PEG provoked an increase by about 40% while 8% PEG enhanced TBARS levels by 87%, with respect to controls. Considering these results, 8% PEG was chosen to perform further experiments. On the other hand, different IAA concentrations ranging from 0 to 100 µM did not modify TBARS levels with respect to controls (data not shown). Taking into account this fact, pre-treatments with different IAA concentrations were performed before 8% PEG application. Fig. 2B indicates that only 100 µM IAA was capable of preventing TBARS enhancement caused by 8% PEG. Based on these results, 8% PEG and 100 µM IAA were used to perform further experiments.
Figure 2.
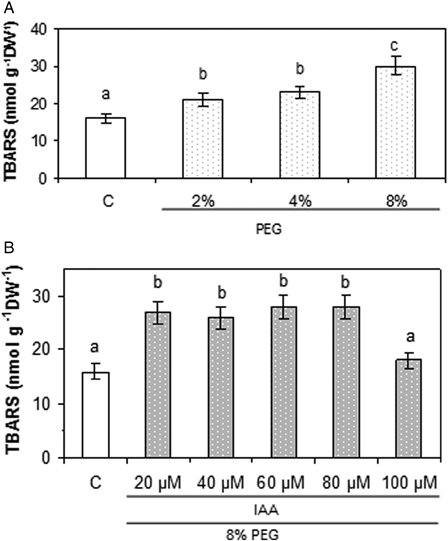
Lipid peroxidation evaluated as TBARS formation in soybean roots exposed to (A) different PEG concentrations and (B) pre-treatments with different IAA concentrations and addition of 8% PEG. Experiments were performed as described in the Materials and methods section. Values are the mean of three independent experiments and bars indicate SE. Different letters indicate significant differences (P < 0.05) according to Tukey's multiple range test.
AS and GSH levels
It could therefore be expected that if drought induces ROS formation, it would also modify GSH levels. The data in Table 1 indicate that GSH concentration was not affected by IAA application. Plants subjected to drought showed a 32% decrease in GSH levels with respect to controls. Pre-treatment with IAA prevented the GSH decrease produced by 8% PEG. Similar results were obtained when AS content was assayed.
Antioxidant enzyme system
As shown in Fig. 1A, IAA did not affect SOD activity while drought brought about a 33% inhibition with respect to controls. Pre-treatment with IAA totally prevented the SOD activity inhibition caused by PEG treatment.
Fig. 1B shows that on one hand, IAA did not affect CAT activity and, on the other hand, PEG caused a 26% inhibition with respect to controls. Moreover, pre-treatment with IAA before drought avoided the decrease previously observed in CAT activity. This behavior is similar to that obtained with SOD activity.
As can been see in Fig. 1C, IAA did not affect POD activity and PEG treatment provoked a 27% decrease in the enzyme activity. Once again, IAA pre-treatment counteracted this effect and the enzyme activity approached control levels.
HO activity, protein content, and RNA expression
The data indicate that drought did not affect HO-1 activity (Fig. 3A), nor the protein levels or relative steady-state level of specific mRNA (Fig. 3B and C). IAA treatment enhanced HO-1 activity with respect to controls (75%). Plants pretreated with IAA and then subjected to drought showed an enhancement of HO-1 activity (43%) with respect to plants treated with IAA alone. These results prompted us to investigate the behavior of HO-1 as a consequence of IAA treatment.
Figure 3.
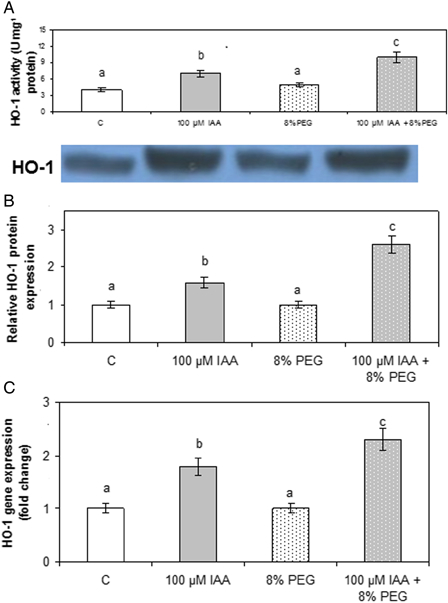
Effect of PEG and IAA pre-treatments on soybean roots HO activity (A), protein content (B), and gene expression (C). (A) Enzyme activity was assayed as described in the Materials and methods section. (B) HO-1 protein expression was analyzed by DNA protein gel blot as described in the Material and methods section. Densitometry was done by Gel-Pro® analyzer to quantify HO-1 protein expression. (C) HO-1 mRNA expression was analyzed by real-time RT-PCR as described in the Material and methods section. The 18S amplification band is shown to confirm equal loading of RNA and RT efficiency. Relative HO-1 transcript expression taking control as 1 U. Data are means of three independent experiments and bars indicate SE. Different letters indicate significant differences (P < 0.05) according to Tukey's multiple range test.
As already stated, HO-1 has been described as a feature of plant responses to stress conditions. To asses whether HO-1 is involved in the protection exerted by 100 µM IAA against drought, experiments were carried out in plants treated with ZnPPIX, a well-known irreversible HO-1 inhibitor, in the presence of 100 µM IAA alone and in plants pretreated with 100 µM IAA before 8% PEG addition. When HO-1 was inhibited, enhancement of TBARS levels (142%, with respect to controls) as well as a diminution of GSH (50%, with respect to controls) was observed (Table 2). In the presence of ZnPPIX, the above-mentioned protective role of IAA was not observed (Fig. 2, Tables 1 and 2). Similar results were obtained when the effects of HO-1 inhibition on SOD and CAT activities were assayed (Fig. 1, Table 3). Therefore, we can assume that protection exerted by 100 µM IAA may also be due to the increase of HO activity.
Table 2.
Effect of ZnPPIX on GSH and TBARS content in soybean roots
| Treatment | GSH (nmol/mg FW) | TBARS (nmol/mg DW) | ||
|---|---|---|---|---|
| Without ZnPPIX | With ZnPPIX | Without ZnPPIX | With ZnPPIX | |
| 100 µM IAA + 0% PEG | 245 ± 20a | 241 ± 12a | 16.6 ± 0.9a | 18.6 ± 0.3a |
| 100 µM IAA + 8% PEG | 242 ± 18a | 121 ± 11b | 17.5 ± 1.1a | 45.1 ± 14.3b |
Determinations were performed as described in the Experimental section.
Different letters within columns indicate significant differences (P < 0.05) according to Tukey's multiple range test.
Table 3.
Effect of ZnPPIX on SOD and CAT activities in soybean roots
| Treatment | SOD (U/mg protein) | CAT (pmol/mg protein) | ||
|---|---|---|---|---|
| Without ZnPPIX | With ZnPPIX | Without ZnPPIX | With ZnPPIX | |
| 100 µM IAA + 0% PEG | 162 ± 20a | 170 ± 12a | 132.6 ± 10.8a | 130.7 ± 11.3a |
| 100 µM IAA + 8% PEG | 160 ± 18a | 95 ± 11b | 122.5 ± 10.1a | 105.1 ± 10.2b |
Determinations were performed as described in the Experimental section.
Different letters within columns indicate significant differences (P < 0.05) according to Tukey's multiple range test.
On the other hand, DNA protein gel blot analysis for HO-1 as well as RT-PCR analysis (Fig. 3B and C) revealed a positive relationship between enzyme activity, protein content, and transcript levels. These results indicated that the enhancement of HO-1 activity was due to an increase in gene transcription.
Effect of NO on HO-1 activity
Plants were treated with water (C), IAA, or the NO-donor SNP either in the presence or the absence of cPTIO (100 µM) or l-NAME (200 µM), a specific NO scavenger and inhibitor of NO synthesis, respectively. Treatments with IAA in the presence of cPTIO or l-NAME reduced by 50% the HO-1 activity, indicating that NO is involved in the enhancement of HO-1 activity induced by IAA (Fig. 4). This result is confirmed by the fact that SNP alone also produced a 75% increase in HO-1 activity. In addition, plants subjected to SNP in the presence of cPTIO or l-NAME exhibited a significant reduction in HO-1 activity with respect to plants treated with SNP alone. On the other hand, cPTIO or l-NAME significantly decreased HO-1 activity (50%).
Figure 4.
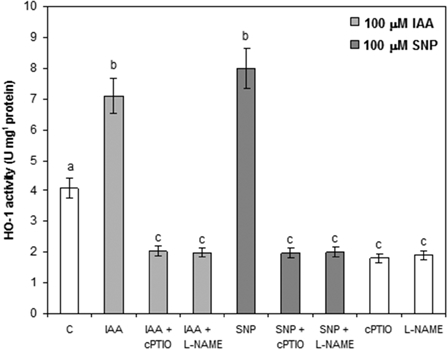
Effect of NO on HO-1 activity. Experiments were performed according to the Materials and methods section. Seeds were pretreated with IAA, SNP, NO donor, the NO scavenger cPTIO, or l-NAME, a NOS inhibitor, at 100, 100, or 200 µM final concentrations, respectively. Values are the mean of three independent experiments and bars indicate SE. Different letters indicate significant differences (P < 0.05) according to Tukey's multiple range test.
Discussion
It has been previously demonstrated that IAA action as well as HO-1 activity and gene expression are mediated by NO,50,51 but to the best of our knowledge no information is available with respect to the role that this hormone could have in the defense system against drought in soybean plants. In this paper we present, for the first time, evidence that IAA contributes to the defense against oxidative stress generation in soybean plants subjected to a simulated drought, and that this effect is mediated by HO-1 activity, which in turn is modulated by NO.
Roots are very sensitive to IAA fluctuations and their response to increasing amounts of exogenous IAA has been described.52 This is why this tissue has been chosen for this study. In this drought model, the non-enzymatic as well as the enzymatic defense systems are altered by drought and pre-treatment with IAA prevents these alterations.
Plants develop non-enzymatic and enzymatic antioxidants to reduce ROS-induced oxidative stress, which is indirectly induced by drought.53 Taking into account the fact that the AS–GSH cycle plays an important role in the defense against ROS production, AS as well as GSH content were analyzed. GSH is a leading substrate for enzymatic antioxidant functions and it is also, together with AS, a known radical scavenger. In this work, drought treatment provokes a significant increase in TBARS levels (Fig. 2) and important decreases of soluble antioxidant compounds such as GSH and AS (Table 1). These alterations indicate that oxidative stress occurred.
Under unstressed conditions, the production as well as the removal of O2− are in balance. However, under stress ROS formation can be overwhelming. Within a cell, the SODs constitute the first line of defense against O2−. Hydrogen peroxide is an important signal molecule involved in plant development and environmental responses. Changes in H2O2 availability can result from increased production or decreased metabolism. While plants contain several types of H2O2-metabolizing proteins, CATs are highly active enzymes that do not require cellular reductants as they directly catalyze a dismutase reaction. On the other hand, as an adaptive enzyme in the antioxidant system, POD has an important role in maintaining the redox state in the cells. Drought conditions diminished CAT, SOD, and POD activities with respect to non-stressed plants. These results strongly indicate that drought induced ROS production and oxidative stress. Nevertheless, pre-treatment with 100 µM IAA completely prevented these effects and kept the activities of SOD, CAT, and POD at control levels (Figs. 1 and 2, Table 1). These antioxidant enzymes might work cooperatively to eliminate the excess of ROS and to counteract drought-induced oxidative damage.
The protective role that HO-1 plays against oxidative stress caused by different stimuli is well documented.18,19,53 When HO-1 was studied, it was observed that drought did not affect its activity, protein content, or relative steady-state level of specific mRNA. Moreover, 100 µM IAA increased HO-1 activity by 75% with respect to controls, and a significant increase (150%, with respect to controls) was observed in plants pretreated with 100 µM IAA and then subjected to drought stress. This enhancement was positively correlated with protein amount and gene expression (Fig. 3). Organisms tend to up-regulate the synthesis of heat shock proteins and to activate antioxidant enzymes under adverse environmental stresses. HO-1 induction enhances protection of normal cell functioning by yielding the potent antioxidant BV.18 Therefore, it is assumed that as consequence of IAA treatment, an enhancement of HO-1 occurred to protect the plant against the oxidative damage. Experiments carried out in the presence of ZnPPIX clearly revealed the involvement of HO-1 in the protective action exerted by IAA (Tables 2 and 3).
It was demonstrated that NO is engaged in the signaling pathway of many phytohormones, for instance, the auxin response during adventitious root formation in cucumber50 and soon after it was shown that a NO-mediated cGMP-dependent pathway is operating in that process.51 Furthermore, accumulating evidence has shown that NO plays an important role in signaling plant responses to biotic and abiotic stresses41,54 and it is also involved in the induction of HO-1 in soybean plants treated with UV-B.50 However, it is worth pointing out that relevant information on the role of NO in dehydration/drought tolerance in soybean plants is relatively insufficient. In this study, we wanted to elucidate whether IAA action on HO-1 could be mediated by NO. Experiments performed in the presence of SNP or NO inhibitors, such as cPTIO or l-NAME, indicated that NO acts as a messenger in the cell response against drought. The enhancement of HO-1 activity observed in the presence of IAA, diminished when plants were pretreated with NO inhibitors. Moreover, treatment with SNP alone considerably increased HO-1 activity (Fig. 4). These data provide compelling evidence that IAA serves as an important molecule for signaling the dehydration/drought response in soybean, as has been found in other plants.55
Despite the fact that HO-1 is not affected by drought, an enhancement in its activity, protein amount, and gene expression was observed in IAA-treated plants. Surprisingly, an even more pronounced increase was obtained in plants pretreated with IAA prior to drought with respect to plants treated with IAA alone. The results obtained in the experiments performed with NO donors or inhibitors clearly indicate that, in this case, the enhancement of HO-1 activity is mediated by NO.
Summing up, a possible mechanism can be proposed to explain these effects (Fig. 5). IAA modules NO levels which, in turn, enhances HO-1 synthesis and activity. This increase in HO-1 activity raised BV and CO production. On one hand, BV is a potent antioxidant16 that could be, at least in part, responsible for the observed protective role against drought. On the other hand, it also has been demonstrated that CO provides potent cytoprotective effects in plants.56,57 Taken together, these results indicate that application of IAA to soybean seeds, previous to sowing, could be an interesting and novel strategy to improve soybean crop yield.
Figure 5.
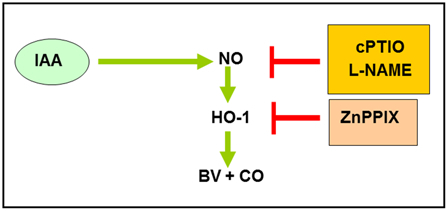
Possible mechanism of IAA action on HO-1. NO mediates HO-1 induction caused by IAA. IAA, indole acetic acid; l-NAME, NO synthesis inhibitor; cPTIO, NO scavenger; ZnPPIX, Zn-protoporphyrin IX; BV, biliverdin; NO, nitric oxide; CO, carbon monoxide.
Disclaimer statements
Contributors None.
Funding This work was supported by Universidad de Buenos Aires – CONICET.
Conflicts of interest None.
Ethics approval Ethical approval was not required.
Acknowledgements
This work was supported by grants from the Universidad de Buenos Aires (Argentina) and from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (Argentina). K.B.B., M.L.T., and A.B. are career investigators from CONICET; G.O.N. holds the post of Principal research assistant at CONICET.
References
- 1.Bajaj S, Targolli J, Liu L, Ho T, Wu R. Transgenic approaches to increase dehydration-stress tolerance in plants. Mol Breed 1999;5:493–503. [Google Scholar]
- 2.Smirnoff N. Plant resistance to environmental stress. Curr Opin Biotech 1998;9(2):214–9. [DOI] [PubMed] [Google Scholar]
- 3.Beligni M, Lamattina L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 1999;208(3):337–44. [Google Scholar]
- 4.Asada K. Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. In: Baker NR, Bowyer JR, (eds.) Photoinhibition of photosynthesis. From molecular mechanisms to the field. Oxford: Bios Scientific Publishers; 1994. pp. 129–42. [Google Scholar]
- 5.Pustovoitova T, Zholkevich V. Basic trends in the investigation of drought effects on physiological processes in plants. Fiziol Biokhim Kul't Rast 1992;24:14–27. [Google Scholar]
- 6.Zholkevich V, Pustovoitova T. Growth and phytohormone content in Cucumis sativus L. leaves under water deficiency. Russ Plant Physiol 1993;40:595–9. [Google Scholar]
- 7.Zhang X, Ervin EH, Evanylo GK, Haering KC. Impact of biosolids on hormone metabolism in drought-stressed tall fescue. Crop Sci 2009;49(5):1893–901. [Google Scholar]
- 8.Muramoto T, Tsurui N, Terry M, Yokota A, Kohchi T. Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol 2002;130:1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terry M, Linley P, Kohchi O. Making light of it: the role of plant haem oxygenases in phytochrome chromophore synthesis. Biochem Soc Trans 2002;30:604–9. [DOI] [PubMed] [Google Scholar]
- 10.Gohya T, Zhang X, Yoshida T, Migita C. Spectroscopic characterization of a higher plant heme oxygenase isoform-1 from Glycine max (soybean): coordination structure of the heme complex and catabolism of heme. FEBS J 2006;273:5384–99. [DOI] [PubMed] [Google Scholar]
- 11.Synder X, Baranano D. Heme oxygenase: a font of multiple messengers. Neuropsychopharmacology 2001;25:294–8. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Kurepa J, Vierstra R. The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA 1999;96:6541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis S, Bhoo S, Durski A, Walter J, Vierstra R. The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol 2001;126:656–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emborg T, Walker J, Noh B, Vierstra R. Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol 2006;140:856–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisk B, Yasui Y, Kohchi T, Frankenbeg-Dinkel N. Characterization of the haem oxygenase protein family in Arabidopsis. Biochem J. 2010;425(2):425–34. [DOI] [PubMed] [Google Scholar]
- 16.Noriega G, Balestrasse K, Batlle A, Tomaro M. Heme oxygenase exerts a protective role against oxidative stress in soybean leaves. Biochem Biophys Res Commun 2004;323:1003–8. [DOI] [PubMed] [Google Scholar]
- 17.Shen Q, Jiang M, Li H, Che L, Yang Z. Expression of a Brasssica napus heme oxygenase confers plant tolerance to mercury toxicity. Plant Cell Environ 2011;34:752–63. [DOI] [PubMed] [Google Scholar]
- 18.Xuan W, Zhu F, Xu S, Huang B, Ling T, Qi J,. et al. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious root process. Plant Physiol 2008;148:881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo K, Kong W, Yang Z. Carbon monoxide promotes root hair development in tomato. Plant Cell Environ 2009;32:1033–45. [DOI] [PubMed] [Google Scholar]
- 20.Noriega G, Yannarelli G, Balestrasse K, Batlle A, Tomaro M. The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta 2007;226:1155–63. [DOI] [PubMed] [Google Scholar]
- 21.Stocker R. Induction of haemoxygenase as a defence against oxidative stress. Free Radic Res Commun 1990;9:101–12. [DOI] [PubMed] [Google Scholar]
- 22.Dixit S, Verma K, Shekhawat G. In vitro evaluation of mitochondrial–chloroplast subcellular localization of heme oxygenase1 (HO1) in Glycine max. Protoplasma 2014;251(3):671–5. [DOI] [PubMed] [Google Scholar]
- 23.Zilli C, Santa-Cruz D, Polizio A, Tomaro M, Balestrasse K. Symbiotic association between soybean plants and Bradyrhizobium japonicum develops oxidative stress and heme oxygenase-1 induction at early stages. Redox Rep 2011;16(2):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena I, Shekhawat G. Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 2013;32:13–20. [DOI] [PubMed] [Google Scholar]
- 25.Santa-Cruz D, Pacienza N, Polizio A, Balestrasse K, Tomaro M, Yannarelli G. Nitric oxide synthase-like dependent NO production enhances heme oxygenase up-regulation in ultraviolet-B-irradiated soybean plants. Phytochemistry 2010;71(14–15):1000–7. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Han B, Xu S, Zhou M, Shen W. Heme oxygenase-1 is involved in the cytokinin-induced alleviation of senescence in detached wheat leaves during dark incubation. J Plant Physiol 2011;168:768–75. [DOI] [PubMed] [Google Scholar]
- 27.Wei Y, Zheng Q, Liu Z, Yang Z. Regulation of tolerance of Chlamydomonas reinhardtii to heavy metal toxicity by heme oxgenase-1 and carbon monoxide. Plant Cell Physiol 2011;52:1665–75. [DOI] [PubMed] [Google Scholar]
- 28.Xu S, Zhang B, Cao Z, Ling T, Shen W. Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals 2011;24(2):181–91. [DOI] [PubMed] [Google Scholar]
- 29.Otterbein L, Bach F, Alam J, Soares M, Tao H, Wysk M,. et al. Carbon monoxide mediates anti-inflammatory effects via the mitogen activated protein kinase pathway. Nat Med 2000;6:422. [DOI] [PubMed] [Google Scholar]
- 30.Xuan W, Zhu F, Xu S, Huang B, Ling T, Qi J,. et al. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol 2008;148(2):881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekhawat G, Verma K. Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot 2010;61:2255–70. [DOI] [PubMed] [Google Scholar]
- 32.Fang T, Li J, Cao Z, Chen M, Shen W, Huang L. Heme oxygenase-1 is involved in sodium hydrosulfide-induced lateral root formation in tomato seedlings. Plant Cell Rep 2014;33:969–78. [DOI] [PubMed] [Google Scholar]
- 33.He H, He L. Heme-oxygenase-1 and abiotic stresses in plants. Acta Physiol Plant 2014;36(3):581–8. [Google Scholar]
- 34.Romero-Puertas M, Perazzolli M, Zago E, Delledonne M. Nitric oxide signaling functions in plant-pathogen interactions. Cell Microbiol 2004;6(9):795–808. [DOI] [PubMed] [Google Scholar]
- 35.Corpas F, Palma J, Del Río L, Barroso J. Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol 2009;184:9–14. [DOI] [PubMed] [Google Scholar]
- 36.Leitner M, Vandelle E, Gaupels F, Bellin D, Delledonne M. NO signals in the haze: nitric oxide signalling in plant defence. Curr Opin Plant Biol 2009;12(4):451–64. [DOI] [PubMed] [Google Scholar]
- 37.Parani M, Rudrabhatla S, Myers R, Weirich H, Smith B, Leaman D,. et al. Microarray analysis of nitric oxide responsive transcripts in Arabidopsis. Plant Biotechnol J 2004;2(4):359–68. [DOI] [PubMed] [Google Scholar]
- 38.Mackerness S, John C, Jordan B, Thomas B. Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett 2001;489:237–42. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Kao C. Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma 2012;249:187–95. [DOI] [PubMed] [Google Scholar]
- 40.Lamattina L, García-Mata C, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 2003;54:109–36. [DOI] [PubMed] [Google Scholar]
- 41.Shantel A, Fowler R, Virgen A, Gossett D, Banks S, Rodriguez S. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ Exp Bot 2008;62:60–8. [Google Scholar]
- 42.Heath R, Packer L. Photoperoxidation in isolated chloroplasts. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 1968;125:189–98. [DOI] [PubMed] [Google Scholar]
- 43.Law M, Charles S, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J 1983;210:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 1979;72:527–605. [DOI] [PubMed] [Google Scholar]
- 45.Becana M, Aparicio-Tejo P, Irigoyen J, Sánchez-Díaz M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol 1986;82:1169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM. The Arabidopsis photomorphogenic mutant HY1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid haeme oxygenase. Plant Cell 1999;11:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5. [DOI] [PubMed] [Google Scholar]
- 48.Yannarelli G, Noriega G, Batlle A, Tomaro M. Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 2006;224:1154–62. [DOI] [PubMed] [Google Scholar]
- 49.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 50.Pagnussat G, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol 2002;129:954–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagnussat G, Lanteri M, Lamattina L. Nitric oxide and cyclic GMP are messengers in the IAA-induced adventitious rooting process. Plant Physiol 2003;132:1241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finnie J, Van Staden J. Effect of seed weeds concentrate and applied hormones on in vitro cultured tomato roots. J Plant Physiol 1985;120:215–22. [Google Scholar]
- 53.Cruz de Carvalho M. Drought stress and reactive oxygen species. Plant Signal Behav 2008;3:156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siddiqui M, Al-Whaibi M, Basalah M. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2011;248(3):447–55. [DOI] [PubMed] [Google Scholar]
- 55.Hao Z, Li X, Su Z, Xie C, Li M, Liang X,. et al. A proposed selection criterion for drought resistance across multiple environments in maize. Breed Sci 2011;61:101–8. [Google Scholar]
- 56.Zhi-Sheng S, Li-Qin H, Guo-Lin W, Jin-Peng D, Xiao-Yue C, Tian Y,. et al. Carbon monoxide: a novel antioxidant against oxidative stress in wheat seedling leaves. J Integr Plant Biol 2007;49:638–45. [Google Scholar]
- 57.Bai XG, Chen JH, Kong XX, Todd CD, Yang YP, Hu XY,. et al. Carbon monoxide enhances the chilling tolerance of recalcitrant Baccaurea ramiflora seeds via nitric oxide-mediated glutathione homeostasis. Free Rad Biol Med 2012;53:710–20. [DOI] [PubMed] [Google Scholar]


