Abstract
Objective
The aim of the present study was to evaluate the protective effect of kaempferol against oxidative stress in streptozotocin (STZ)-induced diabetic rats.
Methods
Diabetes was induced in male, adult albino rats of the Wistar strain, by intraperitoneal administration of STZ (40 mg/kg body weight (BW)). Kaempferol (100 mg/kg BW) or glibenclamide (600 µg/kg BW) was administered orally once daily for 45 days to normal and STZ-induced diabetic rats.
Results
The STZ-induced diabetic rats showed significantly increased levels of plasma glucose, thiobarbituric acid reactive substances, lipid hydroperoxides, and conjugated dienes in plasma, liver, kidney, and heart whereas they showed significantly decreased level of plasma insulin. The levels of non-enzymic antioxidants (vitamin C, vitamin E, reduced glutathione) in plasma, liver, kidney, and heart and the activities of enzymatic antioxidants (superoxide dismutase, catalase, glutathione peroxidase, and glutathione-S-transferase) in liver, kidney, and heart were significantly decreased in diabetic rats. Administration of kaempferol to diabetic rats was showed brought back in plasma glucose, insulin, lipid peroxidation products, enzymatic, and non-enzymatic antioxidants to near normal.
Conclusion
The present study indicates that kaempferol has a good antioxidant property, as evidenced by its increase of antioxidant status and decrease of lipid peroxidation markers, thus providing protection from the risks of diabetic complications.
Keywords: Streptozotocin, Diabetes, Lipid peroxidation, Antioxidant, Kaempferol
Introduction
Diabetes mellitus is the world's largest endocrine disease. It is characterized by chronic hyperglycemia in association with abnormalities in carbohydrate, fat, and protein metabolism because of complete or relative insufficiency of insulin secretion and/or insulin action, and is usually accompanied by a variety of microvascular, macrovascular, neurological, or infectious complications. The World Health Organization (WHO) predicts that by the year 2025, 300 million people will have diabetes mellitus.1
Oxidative stress can be associated with an increase in the rate of reactive oxygen species (ROS) generation, a decrease in the level of antioxidant defense, or a combination of both. ROS-mediated alterations include damage to cells, tissues, or organs, and have been proposed to be major factors in causing several diseases.2 An increased production of oxygen-derived free radicals and a decrease in the activity of the free radical scavenger system have been reported in diabetes.3 It has also been proposed that an increase in oxidative stress could contribute to tissue damage in diabetes.4 Over the past decade, there has been increase in the level of oxidative stress and its role in the development of diabetic complications. Apart from traditional antidiabetic treatments, antioxidant therapy may be beneficial in diabetes. An earlier study has shown that treatment with antioxidants reduces diabetic complications.5 Efforts for discovering antioxidants, which can serve as useful drug for diabetic patients to combat diabetic complications, are being conducted relentlessly. The use of herbal medicines and plant-derived compounds for the treatment of diabetes mellitus has gained importance throughout the world. Since synthetic drugs have undesirable side effects or contraindications, the WHO has recommended the evaluation of traditional plant and plant-derived compound for treatment of diabetes.6
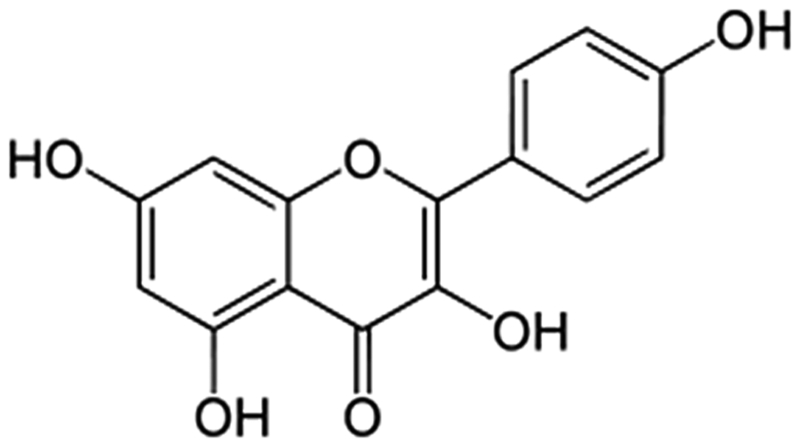
Flavonoids are ubiquitously present in common plant-based food items and beverages. Medicinal plants may serve as a good source for designing of drugs, which act as antidiabetic agents, and consequently, much attention has been paid to herbal medicine formulations.7 Potent antioxidant properties may be exhibited by flavonoids.8 Kaempferol, a flavonoid, naturally occurs in a variety of fruits, vegetables, wine, and tea. It can be isolated from tea, broccoli, witch-hazel, propslis, grapefruit, and other plant sources.9 Kaempferol usually exists in plant glycosides, which influence the plants’ pharmacokinetic properties.10 The pharmacological properties of kaempferol exhibit antioxidant,11 anti-inflammatory,12 and anticancer activity.13 Kaempferol also provides benefits for the treatment of atherosclerosis.14 Several studies have indicated that intake of kaempferol-containing foods is associated with curtailment of mortality and incidence of myocardial infarctions15 and cerebrovascular disease as well as a slight reduction in the risk of coronary heart disease.16 Previously, an in vitro study has shown that kaempferol ameliorates hyperglycemia by improving insulin-stimulated glucose uptake in adipocytes.17 Kaempferol also plays a beneficial role in diabetes by preventing oxidative damage in pancreatic beta cells.18 The structure of kaempferol is depicted below (Fig. 1). Glibenclamide are widely used to treat type 2 diabetes mellitus and also used for standard antidiabetic drug for the experimental animal model because they stimulate insulin secretion from pancreatic beta-cells. In our experimental study, diabetes was induced in adult male albino rats of the Wistar strain, by streptozotocin (STZ). STZ inhibits insulin secretion and causes a state of insulin-dependent diabetes mellitus. For that, in this study we are using glibenclamide as standard drug for the comparison of efficacy of kaempferol versus glibenclamide treated diabetic rats.
Figure 1.
Chemical structure of kaempferol.
No detailed investigation has addressed the efficacy of kaempferol in treating STZ-induced oxidative stress in rats. Thus, the present manuscript addresses the protective effects of kaempferol on lipid peroxidation in the antioxidant defense system of STZ-induced diabetic rats.
Materials and methods
Experimental animals
Male albino rats of Wistar strain of body weight (BW) ranging from 180 to 200 g were procured from Central Animal House, King Saud University, and they were maintained in an air-conditioned room (25±1°C) with a 12-hour light/12-hour dark cycle. The animals were fed ad libitum with normal laboratory pellet diet used in the study and procedures involving animals and their care were accordance with the Policy of Research Centre, King Saud University.
Drug and chemicals
STZ and kaempferol were purchased from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade.
Experimental induction of diabetes
The animals were made diabetic by an intraperitoneal injection of STZ (40 mg/kg BW, between 8:00 a.m. and 9:00 a.m.) in a freshly prepared citrate buffer (0.1M, pH 4.5) after an overnight fast. STZ-injected animals were given 20% glucose solution for 24 h to prevent initial drug-induced hypoglycemic mortality. Diabetes was confirmed by measuring the fasting plasma glucose concentration 96 hour after induction. Albino rats with a plasma glucose level above 220 mg/dL were considered diabetic and were used in this experiment.
Experimental design
Dose determination study
The animals were randomly separated into seven groups consisting of six animals each. Kaempferol (50, 100, and 200 mg/kg BW) or glibenclamide (600 µg/kg BW) was dissolved in 5% dimethyl sulfoxide (DMSO) and administered by intubation (p.o.) once a day, between 9 a.m. and 10 a.m., for 45 days.
Group I : normal rats (5% DMSO alone)
Group II : normal+kaempferol (200 mg/kg BW)
Group III : diabetic control
Group IV : diabetic+kaempferol (50 mg/kg BW)
Group V : diabetic+kaempferol (100 mg/kg BW)
Group VI : diabetic+kaempferol (200 mg/kg BW)
Group VII : diabetic+glibenclamide (600 µg/kg BW)
After 45 days, the animals were fasted for 12 hour, anesthetized between 8:00 a.m. and 9:00 a.m. using ketamine (24 mg/kg BW, intramuscular injection), and sacrificed by cervical dislocation. Blood was collected in tubes with a mixture of potassium oxalate and sodium fluoride (1:3) for the estimation of plasma glucose and insulin. Plasma glucose concentration was measured in each group at the various times during the morning 8:00 a.m.–9:00 a.m. The dose of 100 mg/kg BW showed the maximum glucose lowering effect compared to other two doses. So, the 100 mg/kg BW was fixed as an optimum and used for further study.
Experimental protocol for further study
The animals were randomly divided into five groups consisting of six animals each. Kaempferol or glibenclamide was dissolved in 5% DMSO and administered by intubation (p.o.) once a day, between 9 a.m. and 10 a.m., for 45 days.
Group I: normal rats (5% DMSO alone)
Group II: normal rats+kaempferol (100 mg/kg BW)
Group III: diabetic control
Group IV: diabetic rats+kaempferol (100 mg/kg BW)
Group V: diabetic rats+glibenclamide (600 µg/kg BW)
After 45 days administration of kaempferol and glibenclamide, the rats were fasted for 12 hour, anesthetized by ketamine (24 mg/kg BW via intramuscular injection) and sacrificed by decapitation. Blood sample was collected in tubes containing a mixture of ethylene diamine tetra acetic acid (EDTA) for the estimation of plasma lipid peroxidation and antioxidants. Tissue was sliced into pieces and homogenized in appropriate buffer in cold condition (pH 7.0) to give 20% homogenate. The homogenate were centrifuged at 1000 rpm for 10 minutes at 0°C in cold centrifuge. The supernatant was separated and used for various biochemical estimations.
Biochemical assays
Estimation of estimation of plasma glucose
Plasma glucose was estimated by the method of Trinder using reagent Kit.19 To 0.01 ml of plasma, standard and distilled water (blank) in to three separate tubes, 1.0 ml of the enzyme reagent was added, mixed, and kept at 37°C for 15 minutes. The color developed was read at 510 nm against reagent blank. The values were expressed as mg/dL of plasma.
Estimation of plasma insulin
Plasma insulin was assayed by the method of Burgi et al.20 Standards or samples containing insulin react with capture antibodies coated on a plastic well and with monoclonal antibodies labeled with horseradish peroxidase (HRP). Fifty microliter of each standard, control or samples was dispensed into the appropriate wells. Fifty microliter of antiserum HRP conjugate was dispended into all wells and incubated for 2 hour at room temperature on a horizontal shaker set at 700 rpm. The plates were washed after aspirating the liquid from the well. Then 0.4 ml of washing solution was dispensed into each well and the contents were aspirated. This was repeated twice for complete washing. Two hundred micrometer of the freshly prepared revelation solution was added into each well 15 minutes after washing. Then the plate was incubated for 15 minutes on a horizontal shaker set at 700 rpm at room temperature, avoiding direct sunlight and 50 μl of arresting reagent was added into each well. The absorbance was read within 1 hour at 450 nm. Insulin concentration was expressed as U/ml of plasma.
Estimation of thiobarbituric acid reactive substances
The concentration of thiobarbituric acid reactive substances (TBARS) in the plasma and tissues was estimated by the method of Niehaus and Samuelsson.21 Sample of 0.5 ml was diluted with 0.5 ml of double distilled water and mixed well, and then was treated with 2 ml of (1:1:1 ratio) TBA-tricarboxylic acid (TCA)-HCl reagent (0.37% thiobarbituric acid, 0.25 N HCl, and 15% TCA), placed in water bath for 15 minutes, cooled, and centrifuged at room temperature for 10 minutes. The absorbance of clear supernatant was measured against reference blank at 535. The values were expressed as mmol/dL of plasma or mmol/100 g of tissues.
Estimation of lipid hydroperoxides
The estimation of lipid hydroperoxides (LOOH) in the plasma and tissues was estimated by method of Jiang et al.22 Sample of 0.1 ml was treated with 0.9 ml of Fox reagent (88 mg of butylated hydroxytoluene, 7.6 mg of xylenol orange, and 9.8 mg of ammonium iron sulfate added to 90 ml of methanol and 10 ml of 250 mM sulfuric acid) and incubated at 37°C for 30 minutes. The color developed was read at 560 nm colorimetrically. Lipid hydroperoxides were expressed as mmol/dL of plasma or mmol/100 g of tissues.
Estimation of conjugated dienes
CD was assayed by the method of Rao and Recknagel.23 Sample of 1.0 ml was mixed well with 5.0 ml of chloroform-methanol reagent (2:1v/v) and centrifuged for 5 minutes. To this, 1.5 ml cyclohexane was added and the absorbance was read at 233 nm against a cyclohexane blank. The concentration of conjugated dienes (CD) was expressed as mmol/dL plasma or mmol/100 mg tissue.
Estimation of reduced glutathione
GSH in the plasma and tissues was estimated by method of Ellman.24 Sample of 0.5 ml was pipetted out and precipitated with 2.0 ml of 5% TCA. Supernatant of 2.0 ml was taken after centrifugation and 1.0 ml of Ellman's reagent and 4.0 ml of 0.3M disodium hydrogen phosphate were added. The yellow color developed was read in a Spectronic 20 at 412 nm. The amount of glutathione (GSH) was expressed as mg/dL of plasma or µg/mg of protein for tissues.
Estimation of ascorbic acid (vitamin C)
Vitamin C in the plasma and tissues was estimated by the method of Roe and Kuether.25 To 0.5 ml of sample, 1.5 ml of 6% TCA was added and allowed to stand for 5 minutes and centrifuged. To the supernatant, 0.3 g of acid washed norit was added, shaken vigorously, and filtered. This converts ascorbic acid to dehydroascorbic acid. Filtrate of 0.5 ml was taken and 0.5 ml of 2,4-dinitrophenylhydrazine (DNPH) was added, stoppered, and placed in a water bath at 37°C for exactly 3 hour. Removed, placed in ice-cold water, and added 2.5 ml of 85% sulfuric acid drop by drop. The contents of the tubes were mixed well and allowed to stand at room temperature for 30 minutes. The color developed was read at 540 nm. The values were expressed as mg/dL of plasma or µg/mg of protein for tissue.
Estimation of α-tocopherol (vitamin E)
Vitamin E in the plasma and tissues was estimated by the method of Baker et al.26 To 0.5 ml of sample, 1.5 ml of ethanol was added, mixed, and centrifuged. The supernatant was evaporated and to the precipitate, 3.0 ml of petroleum ether, 0.2 ml of 2, 2′dipyridyl solution, and 0.2 ml of ferric chloride solution were added, mixed well, and kept in dark for 5 minutes. An intense red color was developed. n-butanol of 4.0 ml was added to all the tubes and mixed well. The color in the n-butanol layer was read at 520 nm. The values were expressed as mg/dL for plasma or µg/mg protein for tissue.
Estimation of superoxide dismutase activity
Superoxide dismutase activity (SOD) in the tissues was assayed by the method of Kakkar et al.29 Sample of 0.5 ml was diluted with 1 ml of water. In this mixture, 2.5 ml of ethanol and 1.5 ml of chloroform (all reagents chilled) were added and shaken for 1 minutes at 4°C then centrifuged. The enzyme activity in the supernatant was determined. The assay mixture contained 1.2 ml of sodium pyrophosphate buffer (0.025 M, pH 8.3), 0.1 ml of 186 μM N-methyl dibenzopyrazine methyl sulfate, 0.3 ml of 30 μM nitro blue tetrazolium (NBT), and 0.2 ml of 780 μM reduced nicotinamide adenine dinucleotide (NADH), appropriately diluted enzyme preparation and water in a total volume of 3 ml. Reaction was started by the addition of NADH. After incubation at 30°C for 90 seconds the reaction was stopped by the addition of 1 ml glacial acetic acid. The reaction mixture was stirred vigorously and shaken with 4 ml of n-butanol. The intensity of the chromogen in the butanol layer was measured at 560 nm against butanol blank. A system devoid of enzyme served as control. One unit of the enzyme activity is defined as the enzyme reaction, which gave 50% inhibition of NBT reduction in 1 minutes under the assay conditions. The specific activity of the enzyme was expressed as Unit/min/mg of protein for tissues.
Estimation of catalase activity
The activity of catalase (CAT) in the tissues was measured by the method of Sinha.28 The reaction mixture (1.5 ml, vol) contained 1.0 ml of 0.01 M phosphate buffer (pH 7.0), 0.1 ml of samples, and 0.4 ml of 2 M H2O2. The reaction was stopped by the addition of 2.0 ml of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). Then the absorbance was read at 620 nm; CAT activity was expressed as µmol of H2O2 consumed/min/mg of protein for tissues.
Estimation of glutathione peroxides activity
The activity of glutathione peroxides (GPx) in the tissues was measured by the method of Rotruck et al.29 To 0.2 ml of Tris buffer, 0.2 ml of EDTA, 0.1 ml of sodium azide, and 0.5 ml of sample were added. To the mixture, 0.2 ml of GSH followed by 0.1 ml of H2O2 was added. The contents were mixed well and incubated at 37°C for 10 minutes. After 10 minutes, the reaction was arrested by the addition of 0.5 ml of 10% TCA. The tubes were centrifuged and the supernatant was assayed for GSH by the method of Ellman.24 The activity was expressed as µmol of GSH consumed/min/mg of protein for tissues.
Estimation of glutathione-S-transferase activity
Glutathione-S-transferase (GST) activity was determined spectrophotometrically by the method of Habig et al.30 The reaction mixture contained 1 ml of phosphate buffer, 0.1 ml of 1-chloro,2,4-dinitrobenzene (CDNB), 0.1 ml of sample, and 0.7 ml of distilled water. The mixture was incubated at 37°C for 5 minutes and then the reaction was started by the addition of 0.1 ml of 30 mM GSH. The change in absorbance was read at 340 nm for 5 minutes. Reaction mixture without the enzyme was used as the blank. The activity of GST was expressed as µg of CDNB conjugate formed/min/mg protein.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance followed by Duncan's multiple range test (DMRT) using SPSS software package 9.05. Results were expressed as mean ± SD from six rats in each group. P < 0.05 were considered as significant.
Results
Effects of kaempferol on plasma glucose and insulin
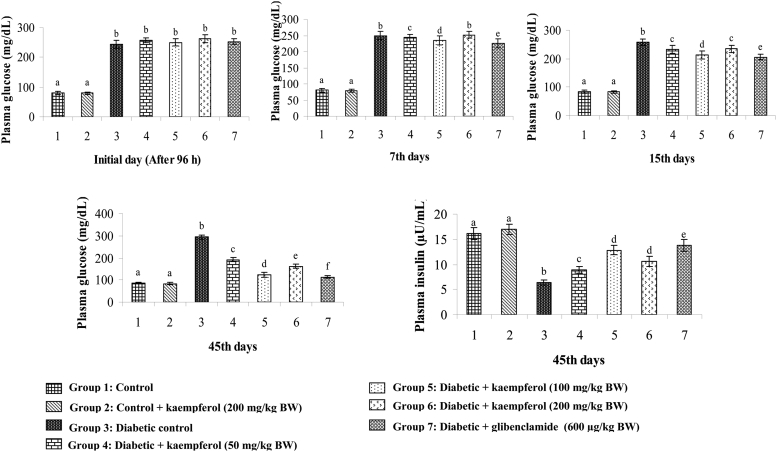
Figure 2 shows the effects of kaempferol at three different doses (50, 100, and 200 mg/kg BW) on plasma glucose (initial day, 7th days, 15th days, and 45th days) and insulin (45th days) levels in normal and STZ-diabetic rats. Diabetic rats showed a gradually elevated level of plasma glucose on initial, 7th, 15th, and 45th days, respectively, and a decreased level of insulin on 45th days when compared to these levels in normal rats. Oral administration of kaempferol or glibenclamide in diabetic rats showed a decrease level of plasma glucose and increase level of insulin when compared to these levels in the diabetic control rats. The dose of 100 mg/kg BW showed maximum glucose lowering effect in comparison to the lowering effects of the 50 and 200 mg/kg BW doses. So the 100 mg/kg BW dose was fixed as the optimum dose and used for further study.
Figure 2.
Effect of kaempferol on plasma glucose (Initial day, 7th days, 15th days, and 45th days)) and insulin (45th days) in STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Effects of kaempferol on TBARS, LOOH, and CD
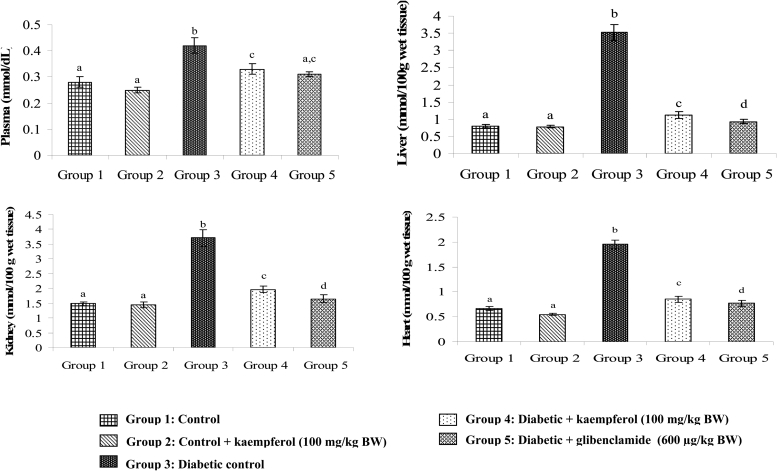
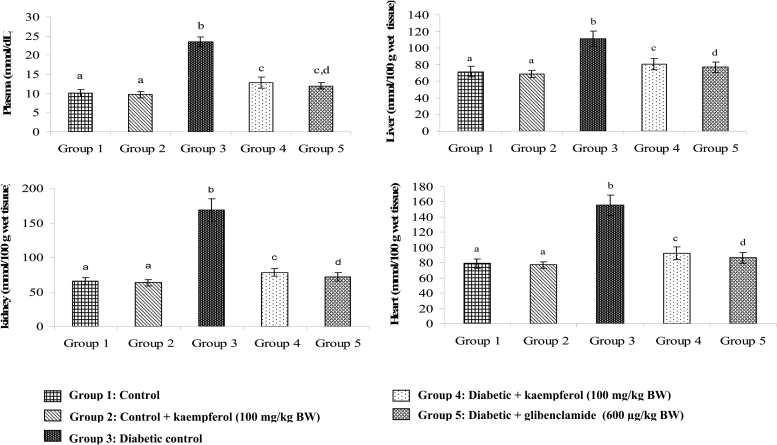
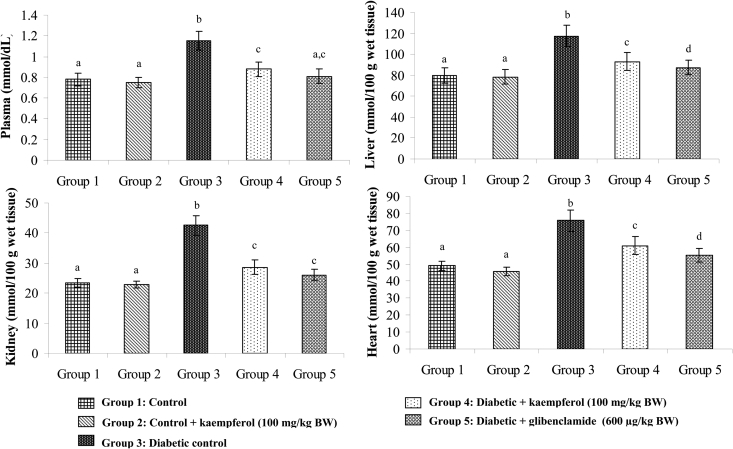
Figures 3Figure 4.5 show the levels of lipid peroxidative markers TBARS, LOOH, and CD in the plasma and tissues of normal and diabetic rats. These levels showed significant increase in diabetic rats, whereas, upon treatment with kaempferol or glibenclamide, these lipid peroxidative markers showed significant decline towards normal level.
Figure 3.
Effect of kaempferol on TBARS in the plasma and tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Figure 4.
Effect of kaempferol on LOOH in the plasma and tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Figure 5.
Effect of kaempferol on conjugated dienes (CD) in the plasma and tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Effects of kaempferol on GSH, vitamin C, and vitamin E
The levels of non-enzymatic antioxidants such as GSH, vitamin C, and vitamin E in the plasma and tissues of normal and diabetic rats are given in Tables 1Table 2.3, respectively. The GSH, vitamin C, and vitamin E levels underwent significant reduction in the plasma and tissues of the diabetic rats. However, treatment with kaempferol or glibenclamide prevented the above changes in diabetic rats, and the levels showed improvement towards normal.
Table 1.
Effect of kaempferol on reduced GSH in the plasma and tissues of STZ-diabetic rats
| Groups | Plasma GSH (mg/dL) | Tissue GSH (µg/mg protein) | ||
|---|---|---|---|---|
| Liver | Kidney | Heart | ||
| Control | 33.10 ± 2.58a | 16.88 ± 1.90a | 11.05 ± 0.85a | 12.13 ± 0.78a |
| Control+kaempferol (100 mg/kg BW) | 35.46 ± 3.05a | 17.27 ± 1.10a | 11.35 ± 1.41a | 12.58 ± 0.64a |
| Diabetic control | 14.20 ± 1.17b | 6.98 ± 0.46b | 5.65 ± 0.35b | 6.08 ± 0.55b |
| Diabetic+kaempferol (100 mg/kg BW) | 27.74 ± 1.85c | 14.30 ± 1.30a,c | 9.48 ± 0.71c | 9.15 ± 0.66c |
| Diabetic+glibenclamide (600 µg/kg BW) | 30.52 ± 2.13a,c | 15.24 ± 1.18a | 10.28 ± 0.97a,c | 10.20 ± 0.81c,d |
Values are given as means ± SD from six rats in each group.
a, b, c, d Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Table 2.
Effect of kaempferol on vitamin C in the plasma and tissues of STZ-diabetic rats
| Groups | Plasma vitamin C (mg/dL) | Tissue vitamin C (µg/mg protein) | ||
|---|---|---|---|---|
| Liver | Kidney | Heart | ||
| Control | 2.25 ± 0.25a | 0.90 ± 0.08a | 0.83 ± 0.07a | 0.55 ± 0.03a |
| Control+kaempferol (100 mg/kg BW) | 2.29 ± 0.14a | 0.91 ± 0.06a | 0.87 ± 0.05a | 0.59 ± 0.04a |
| Diabetic control | 0.86 ± 0.05b | 0.45 ± 0.03b | 0.54 ± 0.03b | 0.27 ± 0.01b |
| Diabetic+kaempferol (100 mg/kg BW) | 1.86 ± 0.10c | 0.83 ± 0.05c | 0.76 ± 0.06a | 0.48 ± 0.03a |
| Diabetic+glibenclamide (600 µg/kg BW) | 1.98 ± 0.06d | 0.87 ± 0.06a | 0.78 ± 0.05a | 0.51 ± 0.03a |
Values are given as means ± SD from six rats in each group.
a, b, c, d Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
Table 3.
Effect of kaempferol on vitamin E in the plasma and tissues of STZ-diabetic rats
| Groups | Plasma vitamin E (mg/dL) | Tissue vitamin E (µg/mg protein) | ||
|---|---|---|---|---|
| Liver | Kidney | Heart | ||
| Control | 1.78 ± 0.10a | 5.69 ± 0.34a | 3.65 ± 0.21a | 3.52 ± 0.16a |
| Control + kaempferol (100 g/kg BW) | 1.72 ± 0.08a | 5.80 ± 0.42a | 3.73 ± 0.17a | 3.63 ± .0.22a |
| Diabetic control | 0.82 ± 0.10b | 3.70 ± 0.20b | 1.46 ± 0.07b | 1.69 ± 0.06b |
| Diabetic + kaempferol (100 mg/kg BW) | 1.35 ± 0.14c | 4.52 ± 0.30c | 2.20 ± 0.18c | 2.40 ± 0.10c |
| Diabetic + glibenclamide (600 µg/kg BW) | 1.52 ± 0.10d | 4.95 ± 0.40d | 3.44 ± 0.20a | 2.78 ± 0.20d |
Values are given as means ± SD from six rats in each group.
a, b, c, d Values not sharing a common superscript differ significantly at P < 0.05. DMRT.
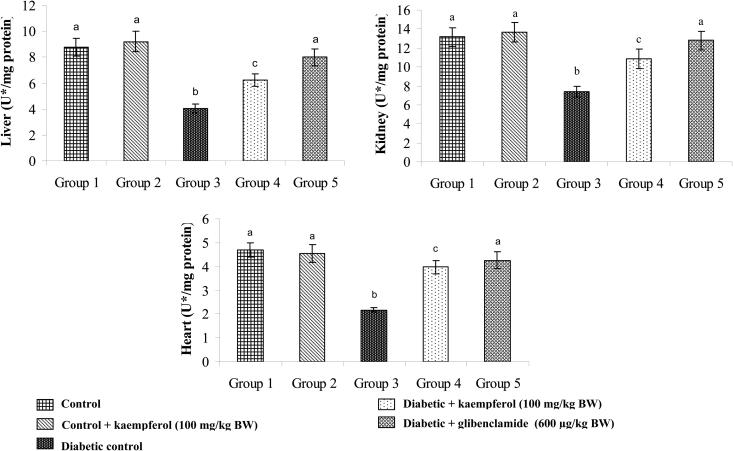
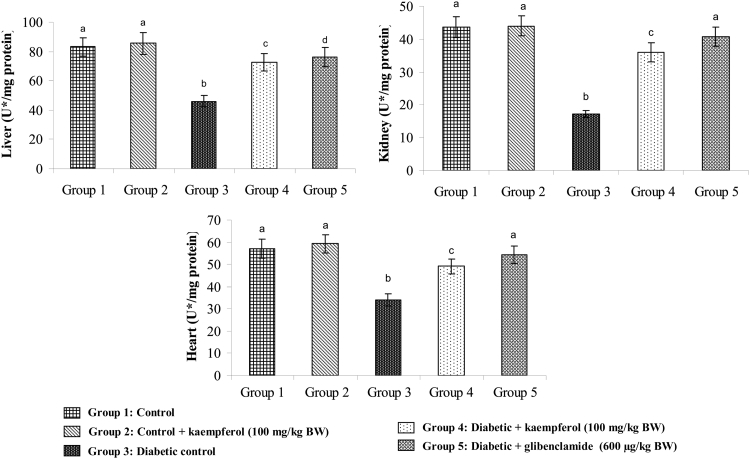
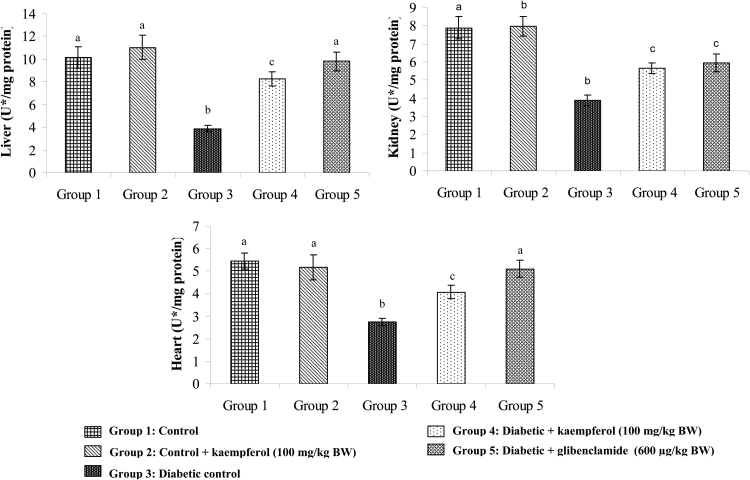
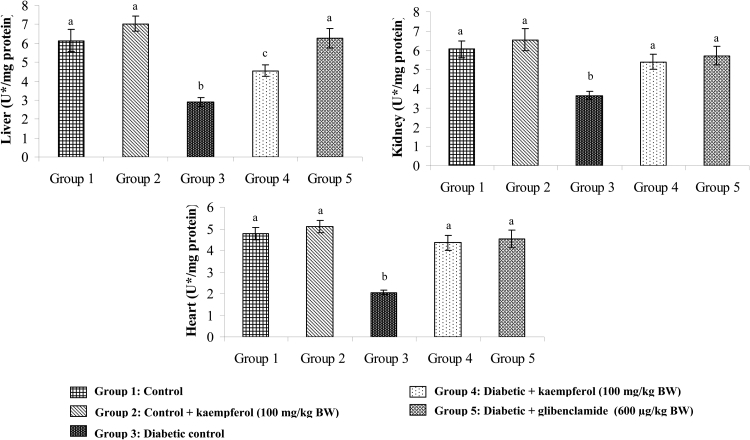
Effects of kaempferol on SOD, CAT, GPx, and GST
Figures 6Figure 7.Figure 8.9 show the activities of SOD, CAT, GPx, and GST in the tissues of normal and diabetic rats. The activities of SOD, CAT, GPx, and GST decreased in the tissues of diabetic rats, while with the administration of kaempferol or glibenclamide, the activities of these enzymatic antioxidants significantly increased.
Figure 6.
Effect of kaempferol on the activity of SOD in the tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT. U* = enzyme concentration required for 50% inhibition of NBT reduction/minute.
Figure 7.
Effect of kaempferol on the activity of CAT in the tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT. U* = µmol of hydrogen peroxide consumed/minute.
Figure 8.
Effect of kaempferol on the activity of glutathione peroxidase (GPx) in the tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT. U* = µmol of GSH utilized/minute.
Figure 9.
Effect of kaempferol on the activity of glutathione-S-transferase (GST) in the tissues of STZ-diabetic rats. Values are given as means ± SD from six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05. DMRT. U* = µg of CDNB conjugate formed/minute.
Discussion
Hyperglycemia in diabetes mellitus involves over-production (i.e., excessive hepatic glycogenolysis and gluconeogenesis) and reduction of glucose utilization by the tissues. Several studies have shown that the level of plasma glucose increases and the level of plasma insulin decreases in STZ-induced diabetic rats.31 Hence, in the present study, we have also observed increase in the level of plasma glucose and decrease in the level of plasma insulin in the STZ-induced diabetic rats. On the basis of the results obtained from the study, it is seen that oral administration of kaempferol has resulted in significant reduction in plasma glucose and increase in plasma insulin. The dose of 100 mg/kg BW has exhibited maximum reduction when compared to the reduction provided by the other two doses. Studies have demonstrated that flavonoid compounds act against diabetes mellitus either through their capacity of avoiding glucose absorption or by improving glucose tolerance.32 In vitro studies have shown that a soybean extract containing isoflavones genistein and daidzein inhibits glucose absorption into the intestinal brush border membrane vesicles of rabbits.33 Naringenin has also been found to reduce glucose uptake in the intestinal brush border membrane vesicles of diabetic rats to a level similar to that of normal rats.34 Kaempferol, a flavonoid, may avoid glucose absorption or improve glucose tolerance by stimulation of insulin secretion. Previously, an in vitro study has shown that kaempferol ameliorates hyperglycemia by improving insulin-stimulated glucose uptake in adipocytes.17
Enhanced oxidative stress and reduced antioxidant capacity are considered to play an important role in the pathogenesis of chronic diabetes mellitus.2 Oxygen-free radical generation is associated with auto-oxidation of glucose, impaired GSH metabolism, alterations in the antioxidant enzymes, and formation of lipid peroxides. Lipid peroxide-mediated damage has been observed in the development of diabetes mellitus.35 Increased endogenous peroxides may initiate uncontrolled lipid peroxidation, thus leading to cellular infiltration and islet cell damage.36 Increased lipid peroxidation impairs membrane functioning by decreasing membrane fluidity and causes free radical-induced membrane lipid peroxidation, including increased membrane rigidity and decreased cellular deformability, leading to disease.37 Lipid peroxidative markers such as TBARS, LOOH, and CD have shown significant increase as reported in the previous experimental models of STZ-induced diabetes.38 In the present study, the levels of TBARS, LOOH, and CD have also significantly increased in STZ-induced diabetic rats, and administration of kaempferol has significantly decreased the level of lipid peroxidative markers in their plasma and tissues. This result demonstrates the anti-peroxidative and antioxidant effects of kaempferol. In this respect, the previous studies have also supported that kaempferol has the ability to scavenge free radicals and inhibit lipid peroxidation.11 Drugs with antioxidant properties may supply endogenous defense systems and reduce both the initiation and propagation of ROS.39
Apart from the enzymic antioxidants, non-enzymic antioxidants such as GSH, vitamin C, and vitamin E play an excellent role in preventing the cells from sustaining oxidative stress. GSH is a major endogenous antioxidant which counterbalances free radical mediated damage. The earlier study has shown that the plasma and tissue GSH concentrations of STZ-induced diabetic rats were significantly lowered when compared to those of the control rats.40 It is well known that GSH is involved in protecting normal cell structure and function by maintaining redox homeostasis, quenching free radicals, and participating in detoxification reactions. It is a direct scavenger of free radicals as well as a co-substrate for peroxide detoxification by glutathione peroxidases.41 Furfaro et al.42 have suggested that the decrease in tissue GSH could be the result of decreased synthesis or increased degradation of GSH by oxidative stress in diabetes. In our study, GSH significantly decreased in the plasma and tissues of diabetic rats, which could be due to decreased synthesis or increased degradation of GSH. The elevation of GSH levels in plasma and tissues was observed in the kaempferol-treated diabetic rats. This indicates that the kaempferol may have either increased the biosynthesis of GSH or reduced the oxidative stress leading to less degradation of GSH, or both.
Vitamin E is a well-known physiological antioxidant and membrane stabilizer. It interrupts the chain reaction of LPO by reacting with lipid peroxy radicals, thus protecting cell structures against damage.43 The decreased level of vitamin E observed in the diabetic rats is compatible with the hypothesis that plasma vitamin E plays a protective role against increased peroxidation in diabetes. In our study, vitamin E significantly decreased in the plasma and tissues of diabetic rats, which could be due to increased membrane damage by ROS. Treatment with kaempferol brought vitamin E to near normal levels, which could be the result of decreased membrane damage as evidenced by decreased lipid peroxidation.
Vitamin C plays a central role in the antioxidant protective system, protecting all lipids undergoing oxidation and diminishing the number of apoptotic cells. Furthermore, vitamin C regenerates the oxidized vitamin E.44 In diabetic rats, the level of plasma and tissue vitamin C significantly decreased, which could be caused by increased utilization of vitamin C as an antioxidant defense against ROS or by a decrease in GSH, which is required for the recycling of vitamin C. Treatment with kaempferol brought the plasma and tissue levels of vitamin C to near normal levels, which could be the result of decreased membrane damage as evidenced by the antioxidant mechanism.
Antioxidative enzymes form the first line of defense against ROS in the organism. This defense includes the enzymes SOD, CAT, GPx, and GST. Disturbances of antioxidant defense systems in diabetes have been previously reported.45 Therefore, treatment with an antioxidant may contribute to the prevention or delay of diabetic complications.46 SOD protects tissues against oxygen-free radicals by catalyzing the removal of superoxide radicals, converting them into H2O2 and molecular oxygen, which damage the cell membrane and other biological structures. The observed decrease in SOD activity could result from inactivation by H2O2 or by glycation of enzymes.47 Oral treatment with kaempferol caused a significant increase in SOD activities in diabetic rats. This means that the kaempferol reduced the reactive oxygen-free radicals and improved the activity of the tissue antioxidant enzymes.
CAT is a hemeprotein which catalyzes the reduction of hydrogen peroxides and protects the tissues from highly reactive hydroxyl radicals. A decrease in CAT activity could result from inactivation by glycation of enzyme.48 CAT reduces hydrogen peroxide produced by a dismutation reaction. It also prevents the generation of hydroxyl radicals, thereby protecting the cellular constituents from oxidative damage in peroxisomes. The reduced activity of CAT in STZ-treated rats might result from the accumulation of H2O2, which produces deleterious effects. In the present study, it was observed that the kaempferol treatment caused a significant increase in the activity of CAT in diabetic rats. This action is predominantly due to the antioxidant nature of kaempferol and could involve a mechanism related to scavenging activity.
Glutathione peroxidase (GPx), a selenium-containing enzyme present in significant concentrations, detoxifies H2O2 to H2O through the oxidation of reduced GSH. Reduced activity of GPx may result from radical-induced inactivation and glycation of the enzyme.41 The low activity of GPx could be directly explained by the low content of GSH found in the diabetic state, since GSH is a substrate and cofactor of GPx. Reduced activity of GPx in the tissues was observed during diabetes, which may result in a number of deleterious effects due to the accumulation of toxic products. In this context, another study also reported a decrease in the activity of GPx in the tissues of diabetic rats.49 In the present study, kaempferol treatment significantly increased the activity of GPx in diabetic rats by inhibiting lipid peroxidation.
In addition to this enzyme, GST provides GSH and helps to neutralize toxic electrophiles. GSH-metabolizing enzyme GST works in concert with GSH in decomposing hydrogen peroxide and other organic hydroperoxides to nontoxic products at the expense of reduced GSH. The reduced activity of GST might be due to inactivation of this enzyme by ROS. In this study, the kaempferol-treated rats showed a significant increase in the activities of GST by inhibiting lipid peroxidation. Flavonoids are one of the most numerous and widespread groups of phenolic compounds in higher plants.50 Some of them, due to their phenolic structure, are known to be involved in the healing process of free radical mediated diseases including diabetes.51 Kaempferol is one of the flavonoids which are reported as preventing oxidative damage in pancreatic beta cells. This study, along with previous studies, confirms that kaempferol possesses antioxidant activity.11
In this study, we conclude that the administration of kaempferol and glibenclamide has significantly improved the antioxidant status as well as hyperglycemia on STZ-induced diabetic rats. Earlier studies have not reported the direct antioxidant action of glibenclamide. Thus, antioxidant properties of glibenclamide might be due to as a result of improved glycemic control. Previously, an in vitro study showed that kaempferol ameliorates hyperglycemia by improving insulin-stimulated glucose uptake in adipocytes.17 Kaempferol has been shown to possess direct antioxidant activity.52 Thus, antioxidant properties of kaempferol on STZ-induced diabetic rats might be due to the direct action of anti-free radical activities and also improved glycemic control.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RGP-VPP-249.
Disclaimer statements
Contributors KSA-N and MAA conceived and designed the study. CG supervised the experiments and critically revised the manuscript. CV conducted the study, analysed the data and wrote the manuscript.
Funding Research project No. RGP-VPP-249, Deanship of Scientific Research at King Saud University.
Conflict of interest The authors declare no conflict of interest.
Ethics approval Ethical was approved.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. [DOI] [PubMed] [Google Scholar]
- 2.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–25. [DOI] [PubMed] [Google Scholar]
- 3.Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonellafoenum graecum). Mol Cell Biochem 2002;236:7–12. [DOI] [PubMed] [Google Scholar]
- 4.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 2013;19:5695–703. [DOI] [PubMed] [Google Scholar]
- 5.Ali MM, Agha FG. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest 2009;69:371–9. [DOI] [PubMed] [Google Scholar]
- 6.Sellamuthu PS, Arulselvan P, Fakurazi S, Kandasamy M. Beneficial effects of mangiferin isolated from Salacia chinensis on biochemical and hematological parameters in rats with streptozotocin-induced diabetes. Pak J Pharm Sci 2014;27(1):161–7. [PubMed] [Google Scholar]
- 7.Vishwakarma SL, Sonawane RD, Rajani M, Goyal RK. Evaluation of effect of aqueous extract of Enicostemma littorale Blume in streptozotocin-induced type 1 diabetic rats. Indian J Exp Biol 2010;48:26–30. [PubMed] [Google Scholar]
- 8.Taha H, Arya A, Paydar M, Looi CY, Wong WF, Vasudeva MC,. et al. Upregulation of insulin secretion and downregulation of pro-inflammatory cytokines, oxidative stress and hyperglycemia in STZ-nicotinamide-induced type 2 diabetic rats by Pseuduvaria monticola bark extract. Food Chem Toxicol 2014;66:295–306. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Konishi M, Horinaka M, Yasuda T, Goda AE, Taniguchi H,. et al. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem Biophys Res Commun 2008;375:129–33. [DOI] [PubMed] [Google Scholar]
- 10.He XZ, Li WS, Blount JW, Dixon RA. Regioselective synthesis of plant (iso) flavone glycosides in Escherichia coli. Appl Microbiol Biotechnol 2008;80:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampkotter A, Gombitang NC, Zurawski RF, Timpel C, Chovolou Y, Watjen W,. et al. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch Toxicol 2007;81:849–58. [DOI] [PubMed] [Google Scholar]
- 12.Park MJ, Lee EK, Heo HS, Kim MS, Sung B, Kim MK,. et al. The anti-inflammatory effect of kaempferol in aged kidney tissues: the involvement of nuclear factor-kappa B via nuclear factor inducing kinase/Ikappa B kinase and mitogen-activated protein kinase pathways. J Med Food 2009;12:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen TT, Tran E, Ong CK, Lee SK, Do PT, Huynh TT,. et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J Cell Physiol 2003;197:110–21. [DOI] [PubMed] [Google Scholar]
- 14.Yu SF, Shun CT, Chen TM, Chen YH. 3-O-beta-Dglucosyl-(1→6)-beta-D-glucosyl-kaempferol isolated from Sauropus androgenus reduces body weight gain in Wistar rats. Biol Pharm Bull 2006;29:2510–3. [DOI] [PubMed] [Google Scholar]
- 15.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam study. Am J Clin Nutr 2002;75:880–6. [DOI] [PubMed] [Google Scholar]
- 16.Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A,. et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–8. [DOI] [PubMed] [Google Scholar]
- 17.Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci 2008;82:615–22. [DOI] [PubMed] [Google Scholar]
- 18.Lee YJ, Suh KS, Choi MC, Chon S, Oh S, Woo JT,. et al. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytother Res 2010;24:419–23. [DOI] [PubMed] [Google Scholar]
- 19.Trinder P. Determination of blood glucose using an oxidase peroxidase system with a non-carcinogenic chromogen. Ann Clin Biochem 1969;6:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgi W, Briner M, Franken N, Kessler AC. One step sandwich enzyme immunoassay for insulin using monoclonal antibodies. Clin Biochem 1988;21:311–4. [DOI] [PubMed] [Google Scholar]
- 21.Niehaus WG, Samuelson B. Formation of MDA from phospholipids arachidonate during microsomal lipid peroxidation. Eur J Biochem 1968;6:126–30. [DOI] [PubMed] [Google Scholar]
- 22.Jiang ZY, Hunt JV, Wolff SP. Detection of lipid hydroperoxides using the Fox method. Anal Biochem 1992;202:384–9. [DOI] [PubMed] [Google Scholar]
- 23.Rao KS, Recknagel RO. Early onset of lipid peroxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol 1968;9:271–8. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959;82:70–7. [DOI] [PubMed] [Google Scholar]
- 25.Roe JH, Kuether CA. Detection of ascorbic acid in whole blood and urine through 2, 4-DNPH derivative of dehydroascorbic acid. J Biol Chem 1943;147:399–407. [Google Scholar]
- 26.Baker H, Frank O, DeAngelis B, Feingold S. Plasma tocopherol in man at various times after ingesting free or acetylated tocopherol. Nutr Res 1980;21:531–6. [Google Scholar]
- 27.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophy 1978;21:130–2. [PubMed] [Google Scholar]
- 28.Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389–94. [DOI] [PubMed] [Google Scholar]
- 29.Rotruck JJ, Pope AL, Ganther HE, Swanson AB. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–90. [DOI] [PubMed] [Google Scholar]
- 30.Habig WH, Pabst MJ, Jakoby WBC. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 31.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi Sh, Farhangi A, Verdi AA,. et al. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem 2007;22(2):60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung UJ, Lee MK, Jeong KS, Choi MS. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J Nutr 2004;134(10):2499–2503. [DOI] [PubMed] [Google Scholar]
- 33.Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE). Phytother Res 1999;13:601–8. [DOI] [PubMed] [Google Scholar]
- 34.Anne WH, Yashomati MP. Naringenin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2006;345(1):516–22. [DOI] [PubMed] [Google Scholar]
- 35.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasupathi P, Chandrasekar V, Senthil Kumar U. Evaluation of oxidative stress, enzymatic and non-enzymatic antioxidants and metabolic thyroid hormone status in patients with diabetes mellitus. Diabetes Metab Syndr Clin Res Rev 2009;3:160–5. [Google Scholar]
- 37.Bandeira SM, Guedes GS, Fonseca LJS, Pires AS, Gelain DP, Moreira JC. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid Med Cell Longev 2012;2012:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 1995;151:113–9. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh B, Karuna R, Sreenivasa RS, Haritha K, Sai MD, Sasi BRB,. et al. Effect of Commiphora mukul gum resin on hepatic marker enzymes, lipid peroxidation and antioxidants status in pancreas and heart of streptozotocin induced diabetic rats. Asian Pac J Trop Biomed 2012;2(11):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ewis SA, Abdel-Rahman MS. Effect of metformin on glutathione and magnesium in normal and streptozotocin-induced diabetic rats. J Appl Toxicol 1995;15:387–90. [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn CC. Concerted antioxidant activity of glutathione and superoxide dismutase. In: Packer L, Fuchs J, eds. Biothiols in health and disease. New York: Marcel Dekker Inc; 1995. p. 117–34. [Google Scholar]
- 42.Furfaro AL, Nitti M, Marengo B, Domenicotti C, Cottalasso D, Marinari UM,. et al. Impaired synthesis contributes to diabetes-induced decrease in liver glutathione. Int J Mol Med 2012;29(5):899–905. [DOI] [PubMed] [Google Scholar]
- 43.Ingold KU, Webb AC, Witter D, Burton GW, Metcalf TA, Muller DP. Vitamin E remains the major lipid soluble, chain breaking antioxidant in human plasma even in individuals suffering from severe vitamin E deficiency. Arc Biochem Biophys 1987;259:224–5. [DOI] [PubMed] [Google Scholar]
- 44.Sadi G, Yılmaz O, Guray T. Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu-Zn SOD and catalase. Mol Cell Biochem 2008;309:109–16. [DOI] [PubMed] [Google Scholar]
- 45.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants. J Biochem Mol Toxicol 2003;17(1):24–38. [DOI] [PubMed] [Google Scholar]
- 46.Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci 2005;46(6):2092–9. [DOI] [PubMed] [Google Scholar]
- 47.Sozmen BY, Sozmen B, Delen Y, Onat T. Catalase/superoxidedismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Arch Med Res 2001;32:283–7. [DOI] [PubMed] [Google Scholar]
- 48.Yan H, Harding JJ. Glycation-induced inactivation and loss of antigenicity of catalase and superoxide dismutase. Biochem J 1997;328:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aliciguzel Y, Ozen I, Aslan M, Karayalcin U. Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J Lab Clin Med 2003;142(3):172–7. [DOI] [PubMed] [Google Scholar]
- 50.Carini M, Adlini G, Furlanetto S, Stefani R, Facino RM. LC coupled to ion-trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J Pharm Biomed 2001;24:517–26. [DOI] [PubMed] [Google Scholar]
- 51.Czinner E, Hagymasi K, Blazovies A, Kery A, Szoke E, Lemberkovics E. In vitro antioxidant properties of Helichrysum arenarium (L.). Moench J Ethnopharmacol 2000;73:437–43. [DOI] [PubMed] [Google Scholar]
- 52.Singh R, Singh B, Singh S, Kumar N, Kumar S, Arora S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Ex Del Toxicol In Vitro 2008;22(8):1965–70. [DOI] [PubMed] [Google Scholar]