Abstract
Objective
This study was undertaken to investigate the antihypertensive and antihyperlipedimic potential of morin against deoxycorticosterone acetate (DOCA)-salt hypertensive rats.
Methods
Hypertension was induced in uninephrectomized rats (UNX) by weekly twice subcutaneous injection of DOCA (25 mg/kg) and 1% NaCl in the drinking water for six consecutive weeks. Morin (50 mg/kg) was administered to DOCA-salt rats orally using an intragastric tube daily for a period of 6 weeks.
Results
The DOCA-salt hypertensive rats showed significant elevation in mean arterial pressure (MAP), heart rate (HR) and reduction in body weight. A significant increase in the concentrations of plasma and tissue (liver, kidney, heart, and aorta) lipids such as total cholesterol, triglycerides, free fatty acids, phospholipids, plasma low-density and very low-density lipoproteins cholesterol, and a decrease in the concentration of high-density lipoprotein cholesterol were noticed in DOCA-salt hypertensive rats. Also, the levels of urinary protein and the activity of 3-hydroxy 3-methylglutaryl coenzyme A reductase in the plasma and tissues were increased, and lecithin cholesterol acyl transferase activity in the plasma was decreased in DOCA-salt rats. Morin supplementation (50 mg/kg) throughout the experimental period restored all the above parameters significantly.
Conclusion
Morin has a potential role in attenuating severe hypertension and hyperlipedimia.
Keywords: Uninephrectomy, Dyslipidemia, Reactive oxygen species, Collagen
Introduction
The latest statistics from the World Health Organization indicate that more people die annually from cardiovascular disease (CVD) than any other ailment. In 2004, an estimated 17.1 million people died from CVD, representing nearly 29% of all global mortalities. Of these, an estimated 7.2 million were due to coronary heart disease and 5.7 million were due to stroke.1 Hypertension, a prevalent risk factor for CVD, frequently occurs in conjunction with metabolic disturbances and in particular with dyslipidemia.2 Dyslipidemia is a broad term which implies imbalance between elevated circulating levels of cholesterol and it occurs concomitantly in over one-third of patients with hypertension.3
In humans, hypertension and hyperlipidemia are frequent causes of CVD and major risk factors for atherosclerosis; the presence of both conditions accelerates atherosclerosis.4 Epidemiological studies provide a large body of evidence for the independent relationship between lipid profile and cardiovascular risk.5 Moreover, lipoproteins can stimulate the release and/or expression of endothelial mediators which may, in turn, contribute to the development of endothelial dysfunction and hypertension.6 Recent evidence indicates that oxidative stress as the main mechanism is responsible for cardiovascular complications such as alteration in lipid metabolism.7
Many modern drugs are effective in preventing CVD, but their use is often limited because of their adverse effects. Therefore, the screening and development of drugs for CVD is still in progress. This can be achieved by plants and natural products that may offer better effect than currently used drugs. Flavonoids are one of the largest (>6000 identified) and most widely distributed groups of secondary plant metabolites8 and are found in all photosynthesizing plants. Flavonols vary from one another based on their number and position of hydroxyl groups and pharmacological actions. Each flavonol has its own specific biological function and activity.9 Several cohort studies suggest that generous consumption of fruits and vegetables has been associated with reduced risk of CVD including hypertension.10
Morin (3,5,7,2′,4′-pentahydroxyflavone) is a flavonoid that belongs to the class of flavonols, which is widely distributed in plants and foods of plant origin;9 it is especially abundant in guava leaves,11 onion, and apple.12 Morin has been reported to possess a variety of biological properties including antioxidant,13 antiallergic,14 anti-inflammatory, and anticarcinogenic effects.15,16 In our previous study, we established the effects of morin on systolic and diastolic blood pressure, hepatic and renal function markers, nitric oxide metabolites, lipid peroxidation products, and antioxidant status in DOCA-salt hypertensive rats.17,18 In continuation of our previous work, in this study we have concentrated on the effects of morin on mean arterial pressure (MAP), heart rate (HR), lipids, lipoproteins, lipid marker enzymes, urinary protein, and histopathology of liver against DOCA-salt hypertensive rats. Thus, this is a novel study with different set of animals and entirely different from the previous study.
Materials and methods
Animal care and diet
Male albino Wistar rats (Rattus norvegicus; 11–13-week-old) were obtained from the Central Animal House, Department of Experimental Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University, Tamil Nadu, India. They were housed (3 rats/cage) in polypropylene cages (47 × 34 × 20 cm) lined with husk, renewed every 24 hours under a 12:12 hours light/dark cycle at around 22°C and had free access to tap water and food. The rats were fed on a standard pellet diet (Kamadhenu Agencies, Bangalore, India). The whole experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi, India and approved by the Animal Ethical Committee of Annamalai University (Reg no: 160/1999/CPCSEA, Approval no: 622).
Source of chemicals
Morin (95% of purity), DOCA, and dimethyl formamide (DMF) were purchased from Sigma-Aldrich Chemical Company, St Louis, Missouri, USA. All other chemicals used in this study were of highest analytical grade obtained from Himedia, Mumbai, India.
Experimental induction of DOCA-salt hypertension rats
Left uninephrectomy was performed on all rats. Rats were anaesthetized with intraperitonial injection of ketamine (75 mg/kg), kidney was visualized by a left lateral abdominal incision, and the left renal artery and ureter were ligated by silk thread, and then the left kidney was removed and weighed. The muscle and skin layer (incision site) were sutured with sterile suture needles. After uninephrectomy, rats were allowed to drink pure water ad libitum, with no further treatment (UNX-rats), and uninephrectomy given 1% NaCl in the drinking water with weekly twice subcutaneous injection of DOCA (25 mg/kg in 0.4 ml of DMF19,20) for six consecutive weeks (DOCA-salt hypertensive rats).
Suspension of the test compound and mode of administration
Morin was freshly solubilized in water21 and was administered to rats orally using an intragastric tube daily for a period of six consecutive weeks.
Experimental time line
The rats were randomly divided into four groups of 10 rats each. Two rats in each group were taken for histopathological analysis. Group I: rats served as UNX-control; group II: UNX-control treated with morin alone (50 mg/kg); group III: served as hypertensive control; and group IV: hypertensive rats which received morin (50 mg/kg).
Group I: UNX
Group II: UNX + morin (50 mg/kg)
Group III: DOCA-salt
Group IV: DOCA-salt + morin (50 mg/kg)
Water intake and body weights were measured daily for all rats. At the end of the experimental period, rats were placed in metabolic cages, and 24 hours urine samples were collected in sealed beakers. Protein concentration in the urine determined using the method of Bradford,22 using bovine serum albumin as standard.
Measurement of blood pressure by non-invasive method
MAP and HR were recorded every week during the entire period of the study by tail-cuff method (IITC, model 31, Woodland Hills, CA, USA).
Tissue sampling and extraction of lipids
Total lipids were extracted from plasma, kidney, heart, aorta, and liver tissues according to the method of Folch et al.23 using chloroform: methanol mixture (2:1, v/v). For the lipid extraction, the tissues were rinsed in cold physiological saline thoroughly and dried by pressing between folds of filter paper. The samples were homogenized in cold chloroform–methanol (2:1, v/v) and the contents were extracted after 24 hours. The extraction was repeated four times. The combined filtrate was washed with 0.7% of potassium chloride (0.1 N) and the aqueous layer was discarded. The organic layer was made up to a known volume with chloroform and used for the analysis of lipids.
Estimation of TC
The levels of total cholesterol (TC) were estimated by the method of Zlatkis et al.24 Lipid extract of 0.5 ml was evaporated to dryness. To this, 5.0 ml of ferric chloride-acetic acid reagent was added. The tubes were mixed well and 3.0 ml of concentrated sulfuric acid (H2SO4) was added. A series of standards containing cholesterol in the range 3–15 µg were made up to 5.0 ml with the reagent and a blank containing 5.0 ml of the reagent were prepared. The absorbance was read after 20 minutes at 560 nm.
Estimation of TG
The content of triglycerides (TG) was estimated by the method of Fossati and Lorenzo.25 Lipid extract of 0.5 ml was evaporated to dryness. To this, 0.1 ml of methanol was added followed by 4.0 ml of isopropanol. About 0.4 g of alumina was added to all the tubes and shaken well for 15 minutes. It was centrifuged and then accurately 2.0 ml of the supernatant was transferred to appropriately labeled tubes. The tubes were placed in a water bath at 65°C for 60 minutes for saponification after adding 0.6 ml of the saponification reagent followed by 0.1 ml of sodium metaperiodate and 0.5 ml of acetyl acetone reagent. After mixing, the tubes were kept in a water bath at 65°C for an hour. A series of standards of concentration 8–40 µg triolein were treated similarly along with a blank containing only the reagents. All the tubes were cooled and read at 405 nm.
Estimation of FFA
Free fatty acid (FFA) level was estimated by the method of Falholt et al.26 An aliquot (0.5 ml) of the lipid extract was evaporated to dryness. To this, 1.0 ml of phosphate buffer, 6.0 ml of extraction solvent, and 2.5 ml of copper (Cu-TEA) reagent were added. All the tubes were shaken vigorously for 90 seconds and were kept aside for 15 minutes. Then the tubes were centrifuged and 3.0 ml of the upper layer was transferred to another tube containing 0.5 ml of diphenylcarbazide solution and mixed carefully. The absorbance was read at 550 nm after 15 minutes. A reagent blank containing (1.0 ml) of phosphate buffer was processed as blank.
Estimation of PL
Phospholipid (PL) levels were estimated by the method of Zilversmit and Davis.27 An aliquot of 0.5 ml of the lipid extract was pipetted out into a Kjeldahl flask and evaporated to dryness. To the extract/0.2 ml of plasma, 1 ml of 5 NH2SO4 was added and digested in a digestion rack till the appearance of light brown color. Two to three drops of concentrated nitric acid was added and the digestion continued till it became colorless. The Kjeldahl flask was cooled and 1.0 ml of distilled water was added and heated in a boiling water bath for about 5 minutes. Then, 1.0 ml of 2.5% ammonium molybdate and 0.1 ml of 1-amino-2-napthol-4-sulfonic acid were added. The volume was then made upto 5.0 ml with distilled water and the absorbance was measured at 660 nm within 10 minutes.
Estimation of lipoprotein fractions
The high-density lipoprotein cholesterol (HDL-C) content in plasma was estimated by a standard commercial kit (Agappe Diagnostics, Pattimattom, Kerala, India). Low-density lipoprotein cholesterol (LDL-C) and very low-density lipoproteins cholesterol (VLDL-C) fractions were calculated as follows: VLDL-C = TGs/5 and LDL-C = total cholesterol – (HDL-C + VLDL-C), respectively.
Estimation of lipid marker enzymes
HMG-CoA reductase
The activity of 3-hydroxy 3-methylglutaryl coenzyme A (HMG-CoA) reductase in the plasma and tissues were assayed by the method of Philipp and Shapiro.28 In brief, equal volumes of 10% fresh plasma/tissue homogenate and diluted perchloric acid were mixed, kept for 5 minutes and centrifuged at 2000 × g for 10 minutes. To 1.0 ml of filtrate, 0.5 ml of freshly prepared hydroxylamine reagent (alkaline hydroxylamine in the case of HMG-CoA) was added and mixed. After 5 minutes, 1.5 ml of ferric chloride was added and shaken well. Readings were taken after 10 minutes at 540 nm against similarly treated saline-arsenate blank. The ratio of HMG-CoA to mevalonate was calculated. Lower ratio indicates higher enzyme activity.
LCAT activity
Plasma lecithin cholesterol acyl transferase (LCAT) activity was assayed by the method of Hitz et al.29 To 1.0 ml of plasma, 0.5 ml of 0.2% dextran sulfate was added. The mixture was incubated at 4°C for 15 minutes and centrifuged at 1750 × g for 15 minutes. The supernatant was separated and used as an enzyme source. The substrate of 0.6 ml was mixed with 0.6 ml of the enzyme. A 0.2 ml of this mixture was mixed with 1.0 ml of isopropanol whereas the remaining mixture was incubated at 27°C for 90 minutes. The precipitate was removed by centrifugation and the supernatant was taken for the estimation of free cholesterol.24 This represented the amount of free cholesterol present in the test sample at zero time. After 90 minutes, 0.2 ml of the incubated mixture was mixed with 1.0 ml of isopropanol and the remaining mixture was incubated at 27°C for a further period of 90 minutes. At the end of 180 minutes, 0.2 ml of the incubated mixture was treated with 1.0 ml of isopropanol to arrest the reaction. Control tubes containing only the substrate were treated similarly to check for complete inactivation of plasma during substrate preparations. LCAT activity was expressed as a function of the disappearance of free cholesterol during the incubation period.
Histopathological examination of liver
Liver tissues obtained from all experimental groups were washed immediately with 0.9% saline and then fixed in 10% buffered formalin. After fixation, the liver tissues were processed by embedding in paraffin wax. Then, the tissues were sectioned (5–6 µm thickness) using a microtome and stained with hematoxylin and eosin (H&E) dye or Masson's trichrome stain (MT). Sections were examined under a high power microscope (Nikon ECLIPSE TS 100; Tokyo, Japan) and photomicrographs were taken.
Statistical analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test (DMRT) using Statistical Package for the Social Science (SPSS Inc, Chicago, IL, USA) software version 12.00. Results were expressed as mean ± standard deviation (SD) for six rats in each group. A value of P ≤ 0.05 was considered to be statistically significant.
Results
Fig. 1A–1C depicts the effect of morin on MAP, HR and body weight. The DOCA-salt hypertensive rats exhibited significant elevation in MAP and HR and diminished body weight as compared with UNX-control. Morin supplementation daily for a period of six consecutive weeks significantly reduced the MAP and HR and improved body weight in DOCA-salt rats.
Figure 1.
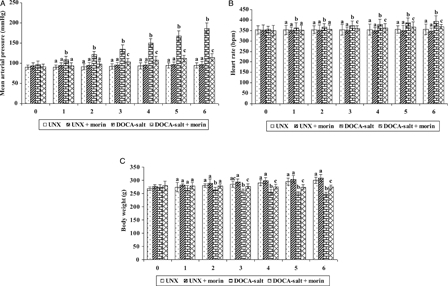
Effect of morin on MAP (A), heart rate (B), and body weight (C) in UNX and DOCA-salt hypertensive rats. Values are expressed as means ± standard deviation (SD) for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Tables 1 and 2 portray the levels of lipids (TC, TG, FFA, and PL) in plasma and tissues (kidney, heart, aorta, and liver) of UNX and DOCA-salt hypertensive rats. The concentrations of plasma and tissue lipids (TC, TG, FFA, and PL) were significantly increased in hypertensive rats as compared with UNX-control. Morin supplementation significantly declined the levels of plasma and tissue lipids in DOCA-salt rats toward normal.
Table 1.
Effect of morin on TC and TG in plasma and tissues of UNX and DOCA-salt hypertensive rats
| Parameter | UNX | UNX + morin | DOCA-salt | DOCA-salt + morin | |
|---|---|---|---|---|---|
| TC (mg/g tissue) | Plasma (mg/dl) | 81.13 ± 6.41a | 81.76 ± 5.8 3a | 163.06 ± 11.24b | 94.13 ± 5.43c |
| Kidney | 4.16 ± 0.28a | 4.03 ± 0.19ac | 6.93 ± 0.46b | 4.52 ± 0.39c | |
| Heart | 2.49 ± 0.15a | 2.38 ± 0.17a | 4.53 ± 0.24b | 2.86 ± 0.21c | |
| Aorta | 3.03 ± 0.23a | 2.98 ± 0.16a | 6.82 ± 0.43b | 4.12 ± 0.27c | |
| Liver | 4.51 ± 0.33a | 4.48 ± 0.31a | 7.36 ± 0.53b | 5.03 ± 0.41c | |
| TG (mg/g tissue) | Plasma (mg/dl) | 56.17 ± 3.87a | 55.11 ± 3.97a | 158.16 ± 9.33b | 69.66 ± 5.65c |
| Kidney | 4.31 ± 0.29a | 4.17 ± 0.23a | 7.01 ± 0.59b | 4.86 ± 0.26c | |
| Heart | 3.96 ± 0.21a | 3.99 ± 0.26a | 5.83 ± 0.32b | 4.07 ± 0.29c | |
| Aorta | 2.89 ± 0.19a | 2.83 ± 0.22a | 6.24 ± 0.46b | 4.02 ± 0.26c | |
| Liver | 3.72 ± 0.26a | 3.53 ± 0.28a | 7.61 ± 0.43b | 3.94 ± 0.32c | |
Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Table 2.
Effect of morin on FFA and PL in plasma and tissues of UNX and DOCA-salt hypertensive rats
| Parameter | UNX | UNX + morin | DOCA-salt | DOCA-salt + morin | |
|---|---|---|---|---|---|
| FFA (mg/g tissue) | Plasma (mg/dl) | 53.91 ± 3.66a | 54.03 ± 4.26a | 121.66 ± 9.55b | 66.21 ± 5.34c |
| Kidney | 4.23 ± 0.26a | 4.18 ± 0.32a | 7.96 ± 0.45b | 5.03 ± 0.36c | |
| Heart | 4.96 ± 0.27a | 4.77 ± 0.29a | 9.13 ± 0.74b | 6.12 ± 0.42c | |
| Aorta | 3.92 ± 0.31a | 3.92 ± 0.26a | 7.26 ± 0.52b | 5.02 ± 0.39c | |
| Liver | 7.73 ± 0.36a | 7.56 ± 0.61a | 16.86 ± 1.16b | 9.14 ± 0.63c | |
| PL (mg/g tissue) | Plasma (mg/dl) | 96.43 ± 6.62a | 94.16 ± 7.61ac | 156.13 ± 8.11b | 104.11 ± 7.38c |
| Kidney | 15.76 ± 0.99a | 14.63 ± 1.02a | 28.66 ± 1.98b | 19.03 ± 1.31c | |
| Heart | 13.18 ± 0.76a | 12.97 ± 0.98a | 23.14 ± 1.58b | 15.43 ± 1.23c | |
| Aorta | 4.73 ± 0.25a | 4.52 ± 0.19a | 10.66 ± 0.82b | 6.26 ± 0.57c | |
| Liver | 21.16 ± 1.51a | 21.32 ± 1.72a | 53.23 ± 3.76b | 28.31 ± 1.82c | |
Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Fig. 2 reveals the effect of morin on plasma lipoproteins (LDL-C, VLDL-C, and HDL-C) in UNX and DOCA-salt hypertensive rats. The elevation in the levels of LDL-C and VLDL-C and diminished level of HDL-C were observed in DOCA-salt hypertensive rats. Morin supplementation significantly decreased the levels of plasma LDL-C and VLDL-C and increased the level of HDL-C in DOCA-salt rats.
Figure 2.
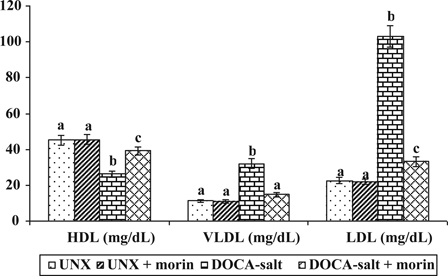
Effect of morin on LDL-C, VLDL-C, and HDL-C in plasma of UNX and DOCA-salt hypertensive rats. Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
The activity of HMG-CoA reductase in UNX and DOCA-salt hypertensive rats were shown in Fig. 3. Increased activity of HMG-CoA reductase in plasma and tissues (kidney, heart, aorta, and liver) were observed in DOCA-salt hypertensive rats as compared with UNX-control. Treatment with morin significantly diminished the activity of HMG-CoA reductase in DOCA-salt rats. Lower ratio of HMG-CoA indicates higher enzyme activity.
Figure 3.
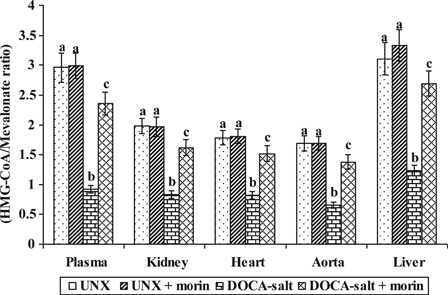
Effect of morin on the activity of HMG-CoA reductase in plasma and tissues of UNX and DOCA-salt hypertensive rats. Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT). *Lower ratio indicates higher enzyme activity.
DOCA-salt hypertensive rats exhibited significantly decreased plasma LCAT activity when compared with UNX-control. Morin supplementation significantly improved the activity of LCAT toward normal level in DOCA-salt rats (Fig. 4).
Figure 4.
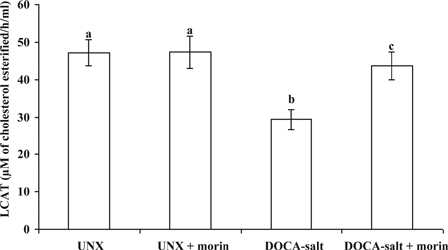
Effect of morin on the activity of LCAT in plasma of UNX and DOCA-salt hypertensive rats. Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Compared with UNX-controls, urinary protein levels was markedly elevated in rats that received DOCA-salt. Morin supplementation significantly prevented and reversed these levels in DOCA-salt rats (Fig. 5).
Figure 5.
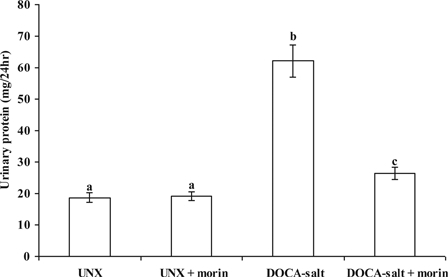
Effect of morin on the levels of urinary protein in UNX and DOCA-salt hypertensive rats. Values are expressed as means ± SD for six rats in each group. Values not sharing a common superscript differ significantly at P < 0.05 (DMRT).
Table 3 and Fig. 6 illustrate the histological analysis of liver by H & E and MT stain, respectively. UNX-control and UNX-control treated with morin alone revealed normal liver architecture without any pathological changes (A) (see Figs. 6A and 6B). DOCA-salt hypertensive rats exhibited severe liver damage characterized by sinusoidal dilatation (+++), kuffer cell hyperplasia (+++), congested blood vessels (+++), and necrosis with fatty deposits (+++) (see Figs. 6C–6E). Morin supplementation in DOCA-salt rats showed near normal architecture with mild sinusoidal dilatation (+) and without kuffer cell hyperplasia (A), congested blood vessels (A), and necrosis (A) (see Fig. 6F).
Table 3.
Effect of morin on the degree of histological changes of liver in UNX and DOCA-salt hypertensive rats
| Groups | UNX | UNX + morin | DOCA-salt | DOCA-salt + morin |
|---|---|---|---|---|
| H&E staining | ||||
| Sinusoidal dilatation | A | A | ++ + | + |
| Kuffer cell hyperplasia | A | A | ++ + | A |
| Congested blood vessels | A | A | ++ + | A |
| Necrosis with fatty deposits | A | A | ++ + | A |
| MT staining | ||||
| Collagen deposition | A | A | ++ + | A |
Photomicrographs were used to evaluate the degree of damages in the liver tissues. (A) no changes; (+) mild changes; (++ ) moderate changes; (+++) marked changes.
Figure 6.
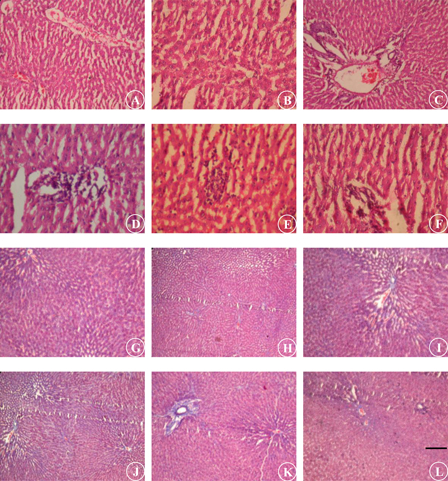
Photomicrograph of liver section staining with H & E (A–F; 10×) and MT (G–L; 10×). UNX-control (A and G), UNX-control treated with morin alone (50 mg/kg) (B and H), DOCA-salt hypertensive rats (C–E and I–K) and DOCA-salt rats treated with morin (50 mg/kg) (F and L). Scale bar: 5 µm.
MT staining of liver section from DOCA-salt rats revealed intense collagen deposition (+++) (see Figs. 6I–6K). Intense collagen accumulation was remarkably reduced in liver of rats treated with morin (A) (see Fig. 6L). Morin administered UNX-control showed normal collagen distribution in liver as that of control (A) (see Figs. 6G and 6H).
Discussion
The major findings of the current study are the following (i) Morin supplementation significantly diminished the MAP and HR and improves the body weight and (ii) Morin exhibited beneficial effects on lipid profiles, urinary protein and ameliorated liver injury, and collagen accumulation imposed by DOCA-salt.
In our study, we observed that subcutaneous injection of DOCA with 1% NaCl loading via drinking water produced significant raise in MAP and HR on treatment with morin resulted in a remarkable reduction in MAP and HR in hypertensive rats, effects that may be attributed to its antioxidant properties. Several epidemiological studies have supported the hypothesis that the antioxidant actions of flavonoids may reduce the risk of developing CVD.30
In agreement with previous report31 we also found that significantly diminished body weight in DOCA-salt hypertensive rats. This observation may be because of an increase in urinary protein excretion. This was evidenced in our current study. When hypertensive rats were treated with morin, the weight loss significantly controlled which might be as a result of its ability to reduce the protein loss in urine.
The presence of high blood pressure (BP) and hyperlipidemia is so common in hypertension that many have argued that the high BP itself may play a role in altering lipid metabolism, resulting in abnormalities.32 In our study, we observed increased levels of TC in plasma and tissues of hypertensive rats. High levels of circulating cholesterol and its accumulation in tissues are well associated with cardiovascular damage.33 Morin supplementation decreased the levels of TC in hypertensive rats which may be due to the suppression of endogenous cholesterol biosynthesis by inhibiting the activity of HMG-CoA reductase.
Hypertriglyceridemia is independent risk factors that can accelerate the development of coronary artery disease and progression of atherosclerotic lesions.34 In this study, we observed a higher concentration of TG in DOCA-salt hypertensive rats. This may be due to increased secretion of VLDL by the liver, disturbed catabolism of VLDL and decreased removal of triglyceride due to diminished lipoprotein lipase activity.35 Morin supplementation lowered the levels of TG in plasma and tissues of hypertensive rats. This beneficial action might be due to the effective quenching of free radicals by morin.
The oxidative tissue damage can release the membrane lipids such as FFA and PL into blood.36 Elevated levels of FFA and PL in plasma and tissues of DOCA-salt hypertensive rats may be due to membrane damage caused by increased lipid peroxidation. We previously reported that DOCA-salt hypertension is associated with increased lipid peroxidation.18 Morin supplementation rescues the tissues from lipid peroxidation by mopping up free radicals and diminished the levels of FFA and PL in hypertensive rats. This effect revealed the antilipid peroxidation property of morin.
Presently, the level of LDL-C and HDL-C is considered to be superior index of CVD risk than total cholesterol. Increased LDL concentration in plasma of DOCA-salt hypertensive rats might be due to defect in LDL-C receptor either through failure in its production or function. In addition, elevated LDL concentration, which in turn leads to the build-up of harmful deposits in the arteries, thus favoring coronary heart disease.37
HDL is considered to be a beneficial lipoprotein.38 The lowered HDL levels in hypertensive rats may be due to diminished plasma LCAT activity and also contribute to the increased cholesterol levels. Usually, a high level of LDL-C and a low level of HDL-C indicate an imbalance between cholesterol transport from the liver to extrahepatic tissues and back to the liver. A greater increase of VLDL-C may also cause a greater decrease of HDL-C as there is a reciprocal relation between the concentration of VLDL-C and HDL-C. Morin supplementation considerably increased HDL-C and lowered LDL-C and VLDL-C in hypertensive rats. These results indicate that morin could provide an antihyperlipidemic activity.
HMG-CoA reductase plays a major role in the regulation of cholesterol metabolism and a rate-limiting enzyme in the pathway of cholesterol biosynthesis. In our study, we have observed enhanced activity of HMG-CoA reductase in plasma and tissues of DOCA-salt hypertensive rats might be due to increased lipid peroxidation. An increase in HMG-CoA reductase activity leads to excessive production and accumulation of cholesterol, resulting in the formation of foam cell, a prerequisite step in the development of atherosclerosis.39 Morin supplementation daily for a period of six consecutive weeks diminished the activity of HMG-CoA reductase in plasma and tissues of hypertensive rats.
LCAT is an enzyme responsible for the conversion of cholesterol to cholesterol esters on the surface of HDLs. Deficiency of LCAT in DOCA-salt hypertensive rats could be attributed to the augmented oxidative stress. Morin supplementation improved the activity of LCAT in hypertensive rats and it may be due to its potent antioxidant property by offering a possible role in reducing the oxidative stress by inducing cellular antioxidant enzymes.40
In this study, we used urinary protein excretion rate as a marker of overall renal injury. Morin supplementation significantly reduced urinary protein levels in DOCA-salt hypertensive rats. These results strongly indicate that morin treatment remarkably attenuated against DOCA-salt induced renal injury by diminished urinary protein.
Morin administration ameliorated the DOCA-salt induced sinusoidal dilatation, kuffer cell hyperplasia, congested blood vessels, necrosis with fatty deposits and intense collagen accumulation in liver. UNX-control treated with morin alone (50 mg/kg) exhibited normal liver architecture without any pathological changes. This histological observation indicates the beneficial effect of morin against hypertension.
Conclusion
Major findings from the present study demonstrated that morin supplementation could effectively prevent the development of hypertension and hyperlipedimia in DOCA-salt rats. Our findings also illustrate that the liver injury was ameliorated by morin supplementation.
Acknowledgements
This research was supported by the grants from the Department of Science and Technology (DST; SR/FT/LS-150/2008), New Delhi, India. The authors wish to thank Dr P. Viswanathan, Professor, Department of Pathology, Rajah Muthiah Medical College and Hospital, Annamalai University, Tamil Nadu, India for his help in histopathological studies.
References
- 1.World Health Organization Available from http://www.who.int/mediacentre/factsheets/fs317/en/ [accessed 31 January 2011].
- 2.Chapman MJ, Sposito AC. Hypertension and dyslipidemia in obesity and insulin resistance: pathophysiology, impact on atherosclerotic disease and pharmacotherapy. Pharmacol Ther 2008;117:354–73. [DOI] [PubMed] [Google Scholar]
- 3.Wong ND, Lopez V, Tang S, Williams GR. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the United States. Am J Cardiol 2006;98:204–8. [DOI] [PubMed] [Google Scholar]
- 4.Kwon HM, Sangiorgi G, Ritman EL. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clinic Invest 1998;101:1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, treatment of high blood pressure. Hypertens 2003;42:1206–52. [DOI] [PubMed]
- 6.Jagla A, Schrezenmeir J. Postprandial triglycerides and endothelial function. Exp Clin Endocrinol Diabetes 2001;109:533–47. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri VO, Grattagliano I, Portincasa P. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr 2006;136:3022–6. [DOI] [PubMed] [Google Scholar]
- 8.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481–504. [DOI] [PubMed] [Google Scholar]
- 9.Hou YC, Chao PDL, Ho HJ. Profound difference in pharmacokinetics between morin and its isomer quercetin in rats. J Pharm Pharmacol 2003;55:199–203. [DOI] [PubMed] [Google Scholar]
- 10.Retelny VS, Neuendorf A, Roth JL. Nutrition protocols for the prevention of cardiovascular disease. Nutr Clin Pract 2008;23:468–76. [DOI] [PubMed] [Google Scholar]
- 11.Rattanachaikunsopon P, Phumkhachorn P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium quajava. J Med Plants Res 2010;4:393–6. [Google Scholar]
- 12.Lotito SB, Frei B. Consumption of flavonoid rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon. Free Radic Biol Med 2006;41:1727–46. [DOI] [PubMed] [Google Scholar]
- 13.Subash S, Subramanian P. Morin a flavonoid exerts antioxidant potential in chronic hyperammonemic rats: a biochemical and histopathological study. Mol Cell Biochem 2009;32:153–61. [DOI] [PubMed] [Google Scholar]
- 14.Francis AR, Shetty TK, Bhattacharya RK. Modifying role of dietary factors on the mutagenicity of aflatoxin B1: in vitro effect of plant flavonoids. Mutat Res 1989;222:393–401. [DOI] [PubMed] [Google Scholar]
- 15.Sivaramakrishnan V, Shilpa P, Kumar V, Niranjali Devaraj S. Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel favonol-morin. Chem Biol Inter 2008;171:79–88. [DOI] [PubMed] [Google Scholar]
- 16.Sivaramakrishnan V, Devaraj SN. Morin regulates the expression of NF-kB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chem Biol Inter 2009;180:353–9. [DOI] [PubMed] [Google Scholar]
- 17.Prahalathan P, Kumar S, Raja B. Effect of morin, a flavonoid against DOCA-salt hypertensive rats: a dose dependent study. Asian Pac J Trop Biomed 2012;2:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prahalathan P, Kumar S, Raja B. Morin attenuates blood pressure and oxidative stress in deoxycorticosterone acetate -salt hypertensive rats: a biochemical and histopathological evaluation. Metabolism 2012; doi: 10.1016/j.metabol.2011.12.012 (In press). [DOI] [PubMed] [Google Scholar]
- 19.Abishek I, Andrew F, Junxian L, Giang T, Robert C, Maria A. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. Brit J Pharmacol 2010;159:1408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent C, Andrew F, Abishek I, Andrew H, Lindsay B. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharm Biotechnol 2011;12:429–36. [DOI] [PubMed] [Google Scholar]
- 21.Karthik Kumar V, Vennila S, Nalini N. Inhibitory effect of morin on DMH-induced biochemical changes and aberrant crypt foci formation in experimental colon carcinogenesis. Environ Toxicol Phar 2010;29:50–7. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane SGH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 24.Zlatkis A, Zak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J Lab Clin Med 1953;41:486–92. [PubMed] [Google Scholar]
- 25.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 1982;28:2077–80. [PubMed] [Google Scholar]
- 26.Falholt K, Lund B, Falholt W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin Chim Acta 1973;46:105–1. [DOI] [PubMed] [Google Scholar]
- 27.Zilversmit DB, Davis AK. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J Lab Clin Med 1950;35:155–60. [PubMed] [Google Scholar]
- 28.Philipp B, Shapiro DJ. Improved methods for the assay and activation of 3-hydroxy-3-methyl glutaryl coenzyme A reductase. J Lipid Res 1970;20:588–93. [PubMed] [Google Scholar]
- 29.Hitz J, Steinmetz G. Siest Plasma lecithin: cholesterol acyltransferasereference values and effects of xenobiotics. Clin Chim Acta 1983;133:85–96. [DOI] [PubMed] [Google Scholar]
- 30.Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr 2003;77:1400–8. [DOI] [PubMed] [Google Scholar]
- 31.Rosario J, Rocio Lopez S, Maria K. Polyphenols restore endothelial function in DOCA-salt hypertension: role of endothelin-1 and NADPH oxidase. Free Radic Biol Med 2007;43:462–73. [DOI] [PubMed] [Google Scholar]
- 32.Friedwald WT, Levy RJ, Fredricken DS. Estimation of the concentration of LDL-cholesterol in the plasma without the use of preparative ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 33.Salter AM, White DA. Effects of dietary fat on cholesterol metabolism: regulation of plasma LDL concentrations. Nutr Res 1996;9:241–57. [DOI] [PubMed] [Google Scholar]
- 34.McKenney JM. Pharmacotherapy of dyslipidemia. Cardiovasc Drugs Ther 2001;15:413–22. [DOI] [PubMed] [Google Scholar]
- 35.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol 1998;81:7–12. [DOI] [PubMed] [Google Scholar]
- 36.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993;91:2546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein JL, Brown MS. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res 1984;25:1450–61. [PubMed] [Google Scholar]
- 38.Miller NE, Thelle DS, Forde OH. The Tromso heart-study. HDL and coronary heart-disease: a prospective case control-study. Lancet 1977;1:965–8. [DOI] [PubMed] [Google Scholar]
- 39.Easterbauer H, Gebick J, Phal H. The role of lipidperoxidation and antioxidants in the oxidative modifications of LDL. Free Radic Biol Med 1992;13:341–90. [DOI] [PubMed] [Google Scholar]
- 40.Kok DL, Wong YP, Wu TW, Chan HC, Kwok TT, Fung KP. Morin hydrate a potential antioxidant in minimizing the free radicals-mediated damage to cardiovascular cells by anti-tumor drug. Life Sci 2000;67:91–9. [DOI] [PubMed] [Google Scholar]


