Abstract
Objectives
Endothelia, intima, and connective tissues comprise the heart valves, but the relationship between heart valve damage, the pathogenesis of valve degeneration, and vitamin D, oxidative stress remains unclear. Here, we assessed serum 25(OH) vitamin D (calcidiol), parathormone (PTH), and redox balance in patients with mitral valve regurgitation (MR) and aortic valve regurgitation (AR).
Methods
This study includes 56 chronic heart valve disease (HVD) patients. Patients were diagnosed with MR or AR depending on the echocardiographic findings. Also, 40 sex-matched healthy control participants were enrolled for comparison. Serum calcidiol, PTH, total oxidative status (TOS), and total antioxidative capacity were measured, and the oxidative stress index (OSI) was calculated.
Results
Patients with HVD demonstrated significantly higher PTH, increased TOS and OSI, and a higher frequency of calcidiol deficiency than the control participants. Calcidiol and TOS were negatively correlated (r = −0.29; P <0.005), as were calcidiol and OSI (r = −0.413; P = 0.001). PTH and OSI were positively correlated (r = 0.22; P = 0.02).
Discussion
We demonstrate that vitamin D deficiency and secondary increases in PTH are highly prevalent. Heart valve regurgitation (AR and MR) is correlated to oxidative stress and hypovitaminosis D.
Keywords: Heart valve disease, Oxidative stress, Parathormone, Vitamin D
Introduction
Heart valve disease (HVD), a major public health problem, can only be effectively treated using surgery. Its pathophysiology is not fully understood. The estimated overall age-adjusted prevalence of valvular heart disease is 2.5%, and mitral valve regurgitation (MR) is the most common valvular heart condition reported worldwide. MR, aortic regurgitation, aortic stenosis, and mitral stenosis demonstrate prevalences of 1.7, 0.5, 0.4, and 0.1%, respectively.1
HVD develops and progresses with age. Aging results in reduced outdoor activity, food intake, and vitamin D synthesis in the skin. The nutritional intake of vitamin D is often too low to compensate for reduced dermal vitamin D synthesis. Vitamin D synthesis in the skin usually contributes 80–90% of the vitamin D required by a healthy person.2,3
The cellular actions of vitamin D are mediated by the membrane-bound and cytosolic vitamin D receptors (VDR). VDR is nearly universally expressed, and nearly all cells react to vitamin D exposure. About 3% of the human genome is organized, directly or indirectly, by the vitamin D endocrine system.3–5
Furthermore, it has been previously reported that cardiac myocytes and fibroblasts express the 1α-OHlase and 24-OHlase enzymes. The conversion of 25-hydroxycholecalciferol to calcitriol (1,25-dihydroxyvitamin D3), the active form of vitamin D, by 1α-OHlase mainly depends on substrate availability in extrarenal tissues; this finding suggests that circulating calcidiol concentrations might be a significant determinant of vitamin D effects in the myocardium.6–9 So, the available accumulating evidence suggests that calcitriol exerts important physiological effects on cardiomyocytes, smooth muscle cells, and the vascular endothelium. Several large, prospective, observational, and cohort studies have reported that high vitamin D status is associated with approximately 50% lower cardiovascular morbidity and mortality risk compared with low vitamin D status.9–14
Patients with secondary hyperparathyroidism demonstrate a high incidence of cardiac hypertrophy, calcium deposits in the myocardium, and aortic and mitral valve calcification; thus, these patients may be at higher risk of death from cardiovascular system diseases.5,15–19 Calcium deposits in the myocardium are found in 74% of patients.5
A growing body of evidence demonstrates that oxidative stress is a key player in the progression of HVD.1,20–22 Most especially, high oxidative stress is involved in hypovitaminosis D and the progression of cardiovascular damage.2,7 Circulating calcidiol is an indicator of vitamin D status. As needed, calcidiol is converted to its active hormonal form, calcitriol (1,25-dihydroxyvitamin D3), in the kidneys, a process that is usually tightly controlled by parathormone (PTH).2,23,24 PTH levels start rising at ≤ 20 calcidiol ng/ml.
We hypothesized that plasma calcidiol levels would be lower in patients with HVD. The objectives of this study are to estimate the prevalence of calcidiol deficiency in HVD patients with MR and aortic valve regurgitation (AR) and evaluate the relationship between calcidiol deficiency and redox balance in these patients.
Materials and methods
The study population consists of 56 patients who received clinical examinations at the cardiology outpatient clinic between November 2010 and April 2011. Patients were diagnosed with MR or AR depending on their echocardiographic findings. In addition, 40 sex-matched healthy control participants (hospital staff) were enrolled for comparison. All patients were evaluated and diagnosed by the same cardiologist based on their echocardiographic findings. Patients with any secondary primary hyperparathyroidism, chronic renal failure, other cardiac diseases, acute or chronic inflammatory diseases, malignancy, cerebrovascular disease, pregnancy, and patients using any medications that could influence the results (e.g. vitamin use, lipid-lowering agents, antioxidant drugs) were excluded. All participants belonged to the same ethnic group and socioeconomic status. All participants received a full physical examination, were asked to complete a general questionnaire, and provided informed consent before the onset of the study. The following data were recorded for each patient: age, sex, chronic treatments, alcohol consumption, smoking habits, and family history of cardiovascular disease. Blood pressure was manually measured using a sphygmomanometer. This study was performed in accordance with the ethical standards set by the Declaration of Helsinki and approved by the local ethics committee.
Blood sample collection
Blood samples were obtained from the patients and control participants after overnight fasting. Sera were separated from the cells by centrifugation at 448 rcf for 10 minutes. Lipid parameters and serum creatinine were immediately measured. The remaining serum samples were stored at −80°C and used to analyze calcidiol, PTH, total oxidative status (TOS), and total antioxidant status (TAS).
Analytical methods
Measurement of serum calcidiol
25 OH-D (calcidiol) assays (DiaSorin, Stillwater,MN) were performed using a and the direct competitive chemiluminescence immunoassay method. The LIAISON assay is linear up to 125 ng/ml total 25 OH-D in unaltered samples. The limit of detection is 3.5 ng/ml and the coefficient of variation ranges between 4.8–11.1% 25 OH-D for this assay.
Measurement of serum PTH
Serum intact PTH levels were determined using commercially available assay kits (Beckman Coulter) and an autoanalyzer (Access DxI800; Beckman Coulter Diagnostics, Germany). The intact PTH assay is a two-site immunoenzymatic (‘sandwich’) assay.
Determination of serum total oxidant status
Serum TOS levels were analyzed using the novel automated colorimetric measurement method developed by Erel.25 Using this method, the oxidants in the sample oxidize the ferrous ion-chelator complex to ferric ion, which makes a colored complex with the chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, correlates to the total amount of oxidant molecules present in the sample. The results are expressed in micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./l).
Determination of serum total antioxidant status
Serum TAS levels were analyzed by using the novel automated colorimetric measurement method developed by Erel.26 Using this method, the antioxidants in the sample reduce the dark blue-green 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical to the colorless reduced ABTS form. The change in absorbance at 660 nm is correlated with the total antioxidant level of the sample. This method can be used to determine the antioxidative effects of the sample against the potent free radical reactions initiated by the produced hydroxyl radical. The results are expressed as micromolar trolox equivalent per liter (μmol trolox Equiv./l).
Oxidative stress index
The percentage ratio of TOS:TAS has been suggested as an oxidative stress index (OSI).27 To calculate OSI, the resulting micromolar unit of TAS was converted to millimoles per liter using the formula: OSI = TOS (μmol H2O2 Equiv./l)/TAS (μmol trolox Equiv./l).
Routine parameters
Triglycerides, total cholesterol, high-density lipoprotein (HDL)-cholesterol, and serum creatinine levels were determined using commercially available assay kits (Abbott) and an autoanalyzer (Architect c16000; Abbott Diagnostics, Texas, USA).
Statistical analysis
Statistical analyses were carried out using statistical software. For normally distributed data, the results are presented as the mean and standard deviation; the median and interquartile range are used for non-normally distributed data. Significant differences between groups were determined using the Student unpaired t-test for normal distributions and the Mann–Whitney U test for abnormal distributions. Categorical variables were evaluated using the Fisher exact test. Pearson and Spearman correlation coefficients were used to assess the strength of any associations between different variables. In this study, P < 0.05 indicates statistical significance.
Results
Mean body mass index was significantly higher, and mean age was significantly lower, in the control participants in comparison with the HVD patients. HVD patients demonstrated higher rates of smoking, hypertension, and family history of cardiovascular disease in comparison with the control participants, though these differences were not statistically significant. When laboratory findings were compared, TC, low-density lipoprotein (LDL), and HDL levels were significantly higher in the control participants (P < 0.01, P = 0.02, and P < 0.01, respectively). The demographic and laboratory data are summarized in Table 1.
Table 1.
Demographic and laboratory data of HVD patients and control participants
| Parameter | Patients (n = 56) | Control (n = 40) | P |
|---|---|---|---|
| Age, years | 64.4 ± 13.3 | 58 ± 10.4 | 0.06 |
| Men, n (%) | 28 (50%) | 20 (50%) | 0.99 |
| HT, n (%) | 30 (53.5%) | 22 (55%) | 0.99 |
| DM, n (%) | 21 (37.5%) | 5 (12.5%) | <0.01 |
| Smoker, n (%) | 12 (21.4%) | 9 (22.5%) | 0.99 |
| BMI (kg/m2), mean ± SD | 26.9 ± 4.1 | 29.3 ± 3.7 | <0.01 |
| CVD history, n (%) | 21 (37.5%) | 8 (20%) | 0.07 |
| Creatinine (mg/dl) | 0.8 (0.7–1.1) | 0.7 (0.7–0.9) | 0.06 |
| TC, mg/dl | 171 ± 39 | 207 ± 50 | <0.01 |
| TG, mg/dl | 126 ± 65 | 147 ± 55 | 0.09 |
| HDL-C, mg/dl | 35 ± 12 | 48 ± 12 | <0.01 |
| LDL-C, mg/dl | 110 ± 31 | 126 ± 37 | 0.02 |
HT, hypertension; DM, diabetes mellitus; BMI, body mass index; CVD, cardiovascular disease; TC, total cholesterol; TG, triglyceride; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol.
In this study, 14 and 42 patients were diagnosed with AR and MR, respectively. The echocardiographic findings are summarized in Table 2.
Table 2.
Echocardiographic findings
| Parameter | Mean ± SD/median (interquartile range) | Reference interval |
|---|---|---|
| Ejection fraction (%) | 50.7 (15.5) | ≥ 55 |
| LV end-diastolic diameter (mm) | 54.4 ± 7.9 | 50.8 ± 3.6 |
| LV end-systolic diameter (mm) | 39.2 ± 10.1 | 32.9 ± 3.4 |
| Aortic velocity (m/second) | 1.5 (1.3–2.0) | 0.7–1.6 |
| Aort diameter (cm) | 3.19 ± 0.46 | 2.5–2.9 |
| Left atrium diameter (mm) | 46.7 ± 9.1 | 37.5 ± 3.6 |
| Interventricular septum (cm) | 1.18 ± 0.23 | 0.6–0.9 |
LV, left ventricle.
PTH, TOS, and OSI levels were significantly higher in HVD patients (P < 0.001, P = 0.002, and P < 0.001, respectively), but calcidiol levels were significantly lower compared with the control participants (P < 0.001; Table 3).
Table 3.
Calcidiol, PTH, and oxidative stress in HVD patients and control participants
| Parameter | Patients (n = 56) | Control (n = 40) | P |
|---|---|---|---|
| Calcidiol, ng/ml | 10 ± 8 | 22 ± 7 | <0.001 |
| PTH, pmol/l | 59 (46–66) | 39 (30–47) | <0.001 |
| TAS (nmol Trolox/l) | 1.34 ± 0.4 | 1.37 ± 0.2 | 0.7 |
| TOS (μmol H2O2 Equiv./l) | 8 (5.6–19.6) | 6.3 (4.4–7.3) | 0.002 |
| OSI | 6.2 (5.2–9.3) | 4.3 (4–4.9) | 0.001 |
Results are either shown as the mean ± SD (normal distributions) or median (interquartile range) (abnormal distributions). PTH normal range: 10–76 pmol/L.
When analyzing the echocardiographic findings, there was only a negative correlation between left ventricular ejection fraction (%) and TOS (r = −0.34; P = 0.01). There was no statistically significant correlation between calcidiol, PTH, TAS, OSI, and the other echocardiographic findings. Correlation analysis was used to assess calcidiol, PTH, and oxidative stress in the entire study population (n = 96), demonstrating negative correlations between calcidiol and TOS (r = −0.29; P = 0.005) and calcidiol and OSI (r = −0.413; P = 0.001). There was also a positive correlation between PTH and OSI (r = 0.22; P = 0.02). The negative correlation between calcidiol and PTH observed in the patient group (r = −0.334; P = 0.01) was not observed in the control group (Fig. 1).
Figure 1.
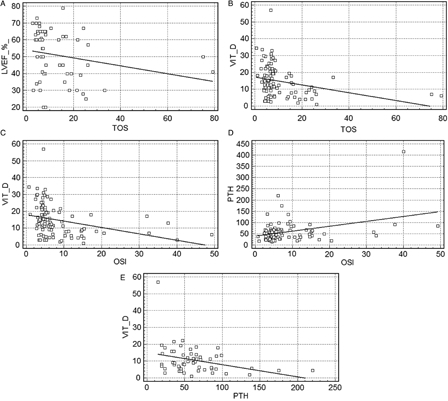
Graphics of significant correlations: (A) left ventricular ejection fraction (%) and TOS (r = −0.34, P = 0.01); (B) calcidiol and TOS (r = −0.29, P = 0.005); (C) calcidiol and OSI (r = −0.413, P = 0.001); (D) PTH and OSI (r = 0.22, P = 0.02); (E) calcidiol and PTH (in the patient group) (r = −0.334, P = 0.01)
Discussion
In this study, oxidative stress markers, particularly PTH, were significantly higher in calcidiol-deficient patients with MR and AR. These data suggest the direct oxidative effects of high parathyroid levels on the heart valves, as measured by TOS enhancement, and that OSI is most likely mediated by VDR. To the best of our knowledge, this is the first study to demonstrate an association between serum calcidiol and cardiac valve regurgitation (MR and AR) in patients with chronic HVD. We previously reported that high oxidative stress induced by the downregulation of the paraoxonase (HDL-associated antioxidant enzyme) affects the progression of mitral and aortic insufficiency. These results suggest that oxidative stress is associated with the pathophysiology of HVD.1 Indeed, HVD is a multifactorial process that results from the interactions of several risk factors, including genetic risks, immuno-inflammatory responses, infectious agents, and oxidative stress, but its pathophysiology is not fully understood.
Heart valves are composed primarily of an extracellular matrix, smooth muscle cells, fibroblasts, and endothelial cells. However, the biochemical mechanisms of calcium deposition in the heart valve and its turnover are poorly understood, but they do depend on the different cell layers and mechanical, developmental, and metabolic features of the diseased valve. Many fundamental questions still need to be addressed. In a community-based population of older adults with no known history of CVD, high serum PTH (yet still within the normal range) was associated with aortic valve sclerosis and mitral annular calcification.28 The observed associations were dependent on calcidiol and PTH, both of which play biological roles in redox balance and oxidative stress. Recent data on secondary hyperparathyroidism due to chronic renal failure suggest that the progression of aortic valve stenosis is related to PTH.29 In order to restore extracellular Ca2+ and Mg2+ homeostasis, increasing plasma PTH paradoxically leads to intracellular Ca2+ overload in diverse tissues, including heart valves.30,31 This study did not include patients with secondary hyperparathyroidism due to chronic renal failure. There was a negative correlation between calcidiol and PTH in the patient group, but not in the control group. Serum PTH also increased in response to hypovitaminosis D, similar to secondary hyperparathyroidism. The average PTH level of our cases was not only higher than controls but was also within the normal range below the threshold. However, average PTH levels of HVD patients was 59 pmol/l (46–66), the high end of the normal reference range.
Recent studies on the pathophysiological mechanisms of valvular disease tend to focus on impaired oxidative mechanisms. Located in the heart valve, endothelial cells may also be affected by oxidative stress. It is believed that the oxidative modification of LDL by free oxygen radicals plays a pivotal role in the onset and progression of the heart valve lesions.1,32 Recently, it was reported that calcitriol protects endothelial cells against H2O2 oxidative stress, counteracting the generation of superoxide anions, the onset of apoptosis, and blocking the extrinsic caspase cascade by positively controlling the phosphorylation of ERK. Thus, calcitriol significantly reduces endothelial malfunction and damage due to oxidative stress via the activation of the MEK/ERK/SirT-1 axis.33 Ahmed et al.34 reported that marked myocyte myofibrillar degeneration can occur as a result of increased oxidative stress and might be responsible for the progression of MR.
In this study, we demonstrate that vitamin D deficiency and secondary increases in PTH are highly prevalent. Heart valve regurgitation (AR and MR) is also correlated with oxidative stress and more common in hypovitaminosis D patients than control participants. Impaired oxidative balance, calcidiol deficiency, and the characteristics associated with HVD patients are less understood. To the best of our knowledge, this study is the first to report the high prevalence of calcidiol deficiency and elevated PTH levels in different types of heart valve pathologies. Adequate calcidiol levels may promote cardiovascular health by improving endothelial function and downregulating inflammation. In addition, calcitriol modulates endothelial and nitric oxide functions in the heart valves.35 Oxidative stress is also associated with CVD. In this study, serum TOS and OSI, which reflect the redox balance between oxidation and antioxidation, demonstrated differences between HVD patients and control participants. Oxidative modification of LDL by free oxygen radicals reportedly influences the initiation and progression of valve lesions.1
Heart valves are primarily composed of the extracellular matrix and surrounded by an endothelial cell monolayer and a few scattered cells that demonstrate characteristics that are intermediary between fibroblasts and smooth muscle cells.36 Prolonged oxidative damage inevitably leads to the endothelial dysfunction that ultimately allows lipids and toxins to penetrate the endothelial layer and accumulate, which in turn elicits an oxidative and inflammatory cascade that is mainly mediated by cytokins.37 This effect might contribute to the high risk of developing HVD conditions caused by the chronic reduction in bioavailable calcitriol.
Although the older age of the study patients is a major limitation, in addition to the fact that we did not obtain follow-up data, this is the first study to report that calcidiol deficiency is highly prevalent in patients with AR and MR. Low calcidiol is inversely correlated with oxidative markers and PTH-related differences in HVD outcomes. Future studies could help develop therapies for low vitamin D levels and improve clinical outcomes in chronic MR and AR patients.
References
- 1.Yilmaz N, Simsek N, Aydin O, Yardan E, Aslan S, Eren E,. et al. Decreased paraoxonase 1, arylesterase enzyme activity, and enhanced oxidative stress in patients with mitral and aortic valve insufficiency. Clin Lab 2013;59(5–6):597–604. [DOI] [PubMed] [Google Scholar]
- 2.Zittermann A, Gummert JF. Nonclassical vitamin D action. Nutrients 2010;2(4):408–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF,. et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29(6):726–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zittermann A, Koerfer R. Vitamin D in the prevention and treatment of coronary heart disease. Curr Opin Clin Nutr Metab Care 2008;11(6):752–7. [DOI] [PubMed] [Google Scholar]
- 5.Stefenelli T, Abela C, Frank H, Koller-Strametz J, Globits S, Bergler-Klein J,. et al. Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up. J Clin Endocrinol Metab 1997;82(1):106–12. [DOI] [PubMed] [Google Scholar]
- 6.Contreras S, Garcia LA, Mehrotra R, Gibbons G, Shohet R, Martins D,. et al. Vitamin D and cardiovascular disease: potential role in health disparities. J Health Care Poor Underserved 2011;22(4):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yavuz B, Sen O, Deveci OS, Akin KO, Dal K, Ata N,. et al. Serum 25-hydroxyvitamin D levels are correlated with mitral valve calcification score in patients with rheumatic mitral stenosis. J Heart Valve Dis 2012;21(5):570–5. [PubMed] [Google Scholar]
- 8.Camici M, Galetta F, Franzoni F, Carpi A, Zangeneh F. Vitamin D and heart. Intern Emerg Med 2013;8(1):5–9. [DOI] [PubMed] [Google Scholar]
- 9.Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int 2013;24(8):2167–80. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Ma J, Zhang X, Fan Y, Wang L. Protective role of the vitamin D receptor. Cell Immunol 2012;279(2):160–6. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Zhu Y, Wang X, Yang Y, Cheng S. Cardioprotective effect of calcitriol on myocardial injury induced by isoproterenol in rats. J Cardiovasc Pharmacol Ther 2013;18(4):386–91. [DOI] [PubMed] [Google Scholar]
- 12.Bae S, Singh SS, Yu H, Lee JY, Cho BR, Kang PM. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol 2013;114(8):979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Ballegooijen AJ, Visser M, Cotch MF, Arai AE, Garcia M, Harris TB,. et al. Serum vitamin D and parathyroid hormone in relation to cardiac structure and function: the ICELAND-MI substudy of AGES-Reykjavik. J Clin Endocrinol Metab 2013;12. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welsh P, Doolin O, McConnachie A, Boulton E, McNeil G, Macdonald H,. et al. Circulating 25OHD, dietary vitamin D, PTH, and calcium associations with incident cardiovascular disease and mortality: the MIDSPAN Family Study. J Clin Endocrinol Metab 2012;97(12):4578–87. [DOI] [PubMed] [Google Scholar]
- 15.Khouzam RN, Dishmon DA, Farah V, Flax SD, Carbone LD, Weber KT. Secondary hyperparathyroidism in patients with untreated and treated congestive heart failure. Am J Med Sci 2006;331(1):30–4. [DOI] [PubMed] [Google Scholar]
- 16.Yan H, Sharma J, Weber CJ, Guyton RA, Perez S, Thourani VH. Elevated parathyroid hormone predicts mortality in dialysis patients undergoing valve surgery. Surgery 2011;150(6):1095–101. [DOI] [PubMed] [Google Scholar]
- 17.Linefsky JP, O'Brien KD, Katz R, de Boer IH, Barasch E, Jenny NS,. et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol 2011;58(3):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celik A, Davutoglu V, Sarica K, Erturhan S, Ozer O, Sari I,. et al. Relationship between renal stone formation, mitral annular calcification and bone resorption markers. Ann Saudi Med 2010;30(4):301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozic B, Loncar G, Prodanovic N, Lepic T, Radojicic Z, Cvorovic V,. et al. Parathyroid hormone response to vitamin D insufficiency in elderly males with chronic heart failure. Physiol Res 2011;60(1):S155–63. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A,. et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes 2012;5(6):819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagler MA, Hadley TM, Zhang H, Mehra K, Roos CM, Schaff HV,. et al. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res 2013;99(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE,. et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol 2013;33(2):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argacha JF, Egrise D, Pochet S, Fontaine D, Lefort A, Libert F,. et al. Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol 2011;58(1):65–71. [DOI] [PubMed] [Google Scholar]
- 24.Brøndum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol 2012;32(11):2794–2802. [DOI] [PubMed] [Google Scholar]
- 25.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–11. [DOI] [PubMed] [Google Scholar]
- 26.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation more stable ABTS radical cation. Clin Biochem 2004;37:277–85. [DOI] [PubMed] [Google Scholar]
- 27.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly 2003;133:563–6. [DOI] [PubMed] [Google Scholar]
- 28.Fujise K, Amerling R, Sherman W. Rapid progression of mitral and aortic stenosis in a patient with secondary hyperparathyroidism. Br Heart J 1993;70(3):282–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarrass F, Benjelloun M, Zamd M, Medkouri G, Hachim K, Benghanem MG,. et al. Heart valve calcifications in patients with end-stage renal disease: analysis for risk factors. Nephrology (Carlton) 2006;11(6):494–6. [DOI] [PubMed] [Google Scholar]
- 30.Rutledge MR, Farah V, Adeboye AA, Seawell MR, Bhattacharya SK, Weber KT. Parathyroid hormone, a crucial mediator of pathologic cardiac remodeling in aldosteronism. Cardiovasc Drugs Ther 2013;27(2):161–70. [DOI] [PubMed] [Google Scholar]
- 31.Boucher BJ. The problems of vitamin D insufficiency in older people. Aging Dis 2012;3(4):313–29. [PMC free article] [PubMed] [Google Scholar]
- 32.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 1999;19:1218–22. [DOI] [PubMed] [Google Scholar]
- 33.Polidoro L, Properzi G, Marampon F, Gravina GL, Festuccia C, Di Cesare E,. et al. Vitamin D protects human endothelial cells from H2O2 oxidant injury through the Mek/Erk-Sirt1 axis activation. J Cardiovasc Transl Res 2013;6(2):221–31. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S,. et al. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction > 60%. Am Coll Cardiol 2010;55:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valiña-Tóth AL, Lai Z, Zhang S, Flack JM. Vitamin D and parathyroid hormone relationships with urinary nitric oxide metabolites and plasma isoprostanes in African-Americans. Cardiorenal Med 2012;2(3):234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MC, Chang JP, Liu WH, Yang CH, Chen CJ, Fang CY,. et al. Increased serum oxidative stress in patients with severe mitral regurgitation: a new finding and potential mechanism for atrial enlargement. Clin Biochem 2009;42:943–8. [DOI] [PubMed] [Google Scholar]
- 37.Davutoglu V, Celik A, Aksoy M. Contribution of selected serum inflammatory mediators to the progression of chronic rheumatic valve disease, subsequent valve calcification and NYHA functional class. J Heart Valve Dis 2005;14:251–56. [PubMed] [Google Scholar]


