Abstract
Objectives
Paraoxonase 1 (PON1) is a lactonase with important antioxidant and immunoprotective properties. We hypothesized that PON1 lactonase activity, PON1, and high-density lipoprotein (HDL) subclasses distribution are different in neonates than in adults.
Material and methods
We studied 83 healthy term neonates (34 males and 49 females) who were born by spontaneous, uncomplicated vaginal delivery. The study also included 17 paired maternal blood samples as well as 20 non-pregnant women collected for comparison. Total and free PON1 lactonase and arylesterase activity, HDL subclasses, PON1, and apolipoprotein distribution in the subclasses were assayed.
Results
PON1 arylesterase activity in the cord blood represented 37% ± 4 of the maternal activity, whereas the PON1 lactonase activity amounted to only 23% ± 5 of the maternal activity. The free arylesterase and lactonase activities were higher in the cord blood by 16 and 36%, respectively. There is a 65% lower HDL2b PON1 in the cord blood than in the maternal serum. When the Lipoprint HDL subclasses were assayed, the neonates showed a larger content (52% higher) of very large HDL as well as a characteristic peak in the middle-sized HDL5 which is unremarkable in the mothers.
Conclusion
The novel findings of this study are that the neonates have lower PON1 lactonase activity, higher free PON1, different distributions of PON1 in the HDL subclasses as compared with their mother and adults as well as a distinctive HDL subclass lipid profile. Our data also suggest that the neonate HDL is enriched with an intermediate-sized (and/or less charged HDL) that is also rich in active PON1.
Keywords: High-density lipoprotein subclasses, PON1, Arylesterase, Lactonase, Newborn HDL composition, Cardiovascular risk
Introduction
Neonates have a distinct qualitative and quantitative lipoprotein profile as compared with adults.1–16 High-density lipoprotein (HDL) cholesterol level in the cord blood is 50% of adult levels, and the HDL contains a proportionately higher level of apolipoprotein E.14,15 Previous studies in neonates have shown that the paraoxonase 1 (PON1) levels are lower than in adults, and that may be associated with a decreased defense against oxidative stress in the neonates, which is worse in premature infants.9,17–20 These studies have the setback that they did not explore the lactonase, physiological activity of PON1, which not only may be important to assess the different role of HDL cardioprotective activity at this period of life, but also may be of interest in innate immunity.21–23 The PON enzymes are capable of inactivating the quorum sensing lactones from the Gram-negative bacteria, thereby participating in natural immunity.21–23 Recent data show that PON1 dissociation from the HDL is enhanced in diabetes, which decreases its activity.24
The HDL subclasses are currently assessed by several approaches, such as selective precipitation ultracentrifugation, nuclear magnetic resonance, electronic microscopy, and two-dimensional electrophoresis, none of which explore the function or apolipoprotein distribution.25–29 We have recently developed a method to measure PON1 activity in the HDL subclasses, which has been validated in adults.30 We hypothesize that the PON1 lactonase activity, its free and HDL-bound form, as well as its distribution in the HDL subclasses are different in the neonates than the adults. The present study aims to fill the gap in the knowledge as to which is the lactonase activity and the PON1 distribution in the HDL subclasses in the neonates and compare them to their mothers and the non-pregnant women.
Two recent studies have shed new light on the lipoprotein subclasses distribution in the neonates.14–16 The authors employ the Liposearch approach that separates the lipoproteins by high-pressure liquid chromatography (HPLC) by virtue of their size and cholesterol/triglyceride (TG) contents. The authors reveal higher very large and very small HDL in the neonates as compared with the adults. This methodology has the limitation of reflecting only the lipid content of the particles of different sizes. It offers no information on the apolipoprotein content and the function of HDL. In a particle with such a complex biology as the HDL, knowledge of the interaction between the apolipoproteins, the lipids, and the enzymes is key for an increased understanding of the yet multiple unknown features of its function. No study so far has analyzed the neonatal lipoproteins using the Lipoprint system, which also separates the particles by size with the addition of some charge effect. For this reason and also to compare the lipid vs. the apolipoprotein and the PON1 distribution in the neonates, is another aim of our study.
Materials and methods
Materials
All of the chemicals were from Sigma-Aldrich (St Louis, MO, USA) unless otherwise indicated.
Study subjects and general laboratory assessments
From January 2013 to June 2013, 83 healthy, appropriate for gestational age neonates (34 males and 49 females) who were born by spontaneous, uncomplicated vaginal delivery in the maternity ward of the Department of Obstetrics and Gynecology, Dokkyo University School of Medicine, Tokyo, Japan were included in the study. All of the mothers were healthy and their pregnancies were without complications. The study included 17 paired maternal blood samples collected for comparison. Twenty age-matched healthy females were employed as the controls. All of the neonates had a gestational age between 37 and 41 weeks. No neonates had asphyxia at birth and all were found to be well on physical examination throughout the study period. Anthropometric measurements and serum lipoprotein analyses were performed at birth. The body weight of each neonate was determined to the nearest 1 g by the use of an electronic scale. The body length was measured to the nearest 0.1 cm in the supine position. Head and chest circumferences were measured with a tape measured to the nearest 0.1 cm. At birth, cord blood sampling was performed from the umbilical vein after double clamping of the umbilical cord. Maternal blood samples were obtained during labor from 17 mothers from the antecubital vein. The samples were drawn in dry tubes, centrifuged at 4°C, and the serum was separated and analyzed or frozen at −80°C until analysis. Plasma glucose and serum lipids, such as low-density lipoprotein (LDL) cholesterol (LDL-C), HDL cholesterol (HDL-C), and TGs, were measured by using enzymatic methods. This study was approved by the Ethics Committee of Showa University Northern Yokohama Hospital, and all of the subjects gave their informed consent.
Total and free PON1 activity
Serum PON1 arylesterase activity was kinetically measured by using phenylacetate as a substrate at 37°C, and the absorbance changes were recorded at 270 nm in a Versamax Microplate Reader (Molecular Diagnostics, CA, USA), as described previously.31 The PON1 lactonase activity was kinetically measured by using dihydrocoumarin (DHC) as a substrate at 37°C, and recorded at 270 nm in a Versamax Microplate Reader as described previously.32 DHC is also a substrate for PON3 which also circulates; however, its activity is 1/100–1/1000 that of PON1, therefore this method essentially measures the PON1 lactonase activity.
To assess free PON1, both of the activities were measured in lipoprotein depleted serum (LPDS) in all of the mothers, the controls, and a subset of 40 neonates. Briefly, 100 µl serum was diluted with 100 µl NaBr to achieve a d = 1.220 g/ml. A 10 µl aliquot was taken (for total enzyme activities), and the rest of the samples were centrifuged at 150 000 g in a Beckman 42.2 Ti-rotor, for 24 h at 10°C. This ensures that both the total and the free PON1 are salt activated.24,33 LPDS was taken from the non-floating fraction by using a Hamilton syringe. For the paraxonase activity, 5 µl was used for the total and 10 µl for the free fraction. After correction for dilution, the activities are expressed in U/l and in the ratio free/total.24,33
PON1 activity detection in situ
Detection of the PON1 activity in the lipoprotein subclasses was performed by employing our published zymogram method.30 For practical reasons, the PON1 distribution in the HDL subclasses was assessed in all of the mothers, the controls, and a subset of 40 neonates. The intra-assay coefficient of variation (CV) of the method was 8.9% as assessed after 32 repetitions of the same sample in four independent gels. The mean inter-assay CV was 12.8% as determined by the analysis of the same sample in 10 independent electrophoresis gels.
HDL subclasses
HDL subclasses were also analyzed for comparison and control purposes by using the Lipoprint HDL subfraction analysis system from Quantimetrix (Redondo Beach, CA, USA) according to the manufacturer's instructions. Briefly, 25 µl serum samples were added to polyacrylamide gel tubes along with 300 µl a loading gel solution containing Sudan Black. After 30 minutes of photopolymerization at 25°C, electrophoresis was performed for 50 minutes with a 3 mA/gel tube. Each electrophoresis run included a quality control (Liposure Serum Lipoprotein Control, Quantimetrix Corp.). For quantification, scanning was performed with the manufacturer's scanner and stained HDL subfractions were identified by their mobility after electrophoresis. The LDL/very low-density lipoprotein (VLDL) band was the starting reference point (Rf 0.0) and albumin was the ending reference point (Rf 1.0). AUC% was calculated with the Lipoware computer software (Quantimetrix Corp.). Up to 10 HDL subfractions, distributed between the LDL/VLDL and the albumin bands are detected and can also be grouped into three major classes: large (HDL1–HDL3), intermediate (HDL4–HDL7), and small (HDL8–HDL10) HDL subfractions. Lipid distribution in the HDL subclasses was assessed in all of the mothers, the controls, and a subset of 40 neonates.
Isolation of HDL2 and HDL3 to confirm the location of the subclasses in the gels
In order to confirm the cut-off points for HDL; HDL2 and HDL3 were separated by ultracentrifugation of the pooled serum in a Beckman L8-70M ultracentrifuge at 110 000 rpm in 8.4 ml polycarbonate tubes in a 50 Ti rotor as described previously. Briefly, the total apo B-containing lipoproteins (density <1.063 mg/dl) were separated by 20 hours centrifugation, then the total HDL (density 1.063–1.21 g/ml) was obtained after 24 hours centrifugation and repurified at density 1.21 g/ml for another 24 hours centrifugation. HDL2 and HDL3 were isolated by ultracentrifugation at density 1.12 and 1.21 g/ml, respectively. The HDL fractions were dialyzed against phosphate-buffered saline containing antiproteases and 1 mM ascorbic acid. To compare the PON1 activity and the apolipoprotein distribution in the HDL, after the PON1 activity detection, the gels were transferred to polyvinylidene difluoride. By using a Dry Transfer Bio-Rad, apo (lipoprotein) A-I, was detected on the immunoblots using horseradish peroxidase-conjugated antibodies.
Statistical analysis
Data are expressed as mean ± SD for the variables with normal distribution and as median and interquartile range for the variables with skewed distribution. The variables with skewed distribution were log-transformed in the analyses. The difference in the parameters was tested by the Student's t-test. The correlations were calculated according to the Pearson test. Statistical significance was set at P < 0.05. Statistical analysis was performed by using SPSS ver. 11 software (SPSS Inc., Chicago, IL, USA).
Results
The clinical data of the neonates studied are shown in Table 1. All of the newborns were term and born by vaginal delivery and show standard distribution of weight and length. Table 2 shows the demographics of the mothers and the non-pregnant women.
Table 1.
Characteristics of the studied neonate subjects
| Variables | Male | Female | P value |
|---|---|---|---|
| n = 34 | n = 49 | ||
| Gestational age (weeks) | 39.7 ± 0.1 | 39.2 ± 0.2 | NS |
| Birth weight (g) | 3,517.9 ± 92 | 3,209.2 ± 85 | 0.05 |
| Birth length (cm) | 50.1 ± 0.2 | 49.2 ± 0.2 | 0.0451 |
Table 2.
Clinical characteristics of the mothers and the non-pregnant women
| Variables | Mothers | Non-pregnant women | P |
|---|---|---|---|
| N = 17 | N = 20 | ||
| Age (years) | 30.1 ± 4.0 | 30.0 ± 5.0 | NS |
| BMI at 12 weeks (kg/m2) | 21.8 ± 1.0 | 22 ± 2 | NS |
| BMI at delivery (kg/m2) | 26.1 ± 1.5 | ||
| Fasting plasma glucose (mmol/l) | 4.5 ± 0.4 | 4.7 ± 0.4 | NS |
Data represent mean ± SD.
NS, non-significant.
Lipoprotein data and PON1 activity in the neonates, the mothers, and the non-pregnant women are shown in Table 3. The mothers display the characteristic pro-atherogenic lipid profile of the third trimester: high total and LDL cholesterol as well as elevated TGs. The results in the neonates are in agreement with the reported profile of the lipids in the cord blood: the total cholesterol is 23%, LDL-C 17%, HDL-C 64%, and TG 12% of the paired mother values. The predominant fetal lipoprotein is the HDL and there are very low TG levels in the neonates. The PON1 arylesterase activity in the cord blood represented 37% ± 4 of the maternal activity, whereas the PON1 lactonase activity amounted to only 23% ± 5 of the maternal activity. Arylesterase and lactonase activity in the maternal serum and the cord blood showed a strong correlation as expected: r = 0.75, P < 0.001 and r = 0.75, P < 0.001; respectively. Arylesterase activity in the maternal serum correlated with the same activity in the paired neonates: r = 0.57, P = 0.001, and a stronger correlation was found for the lactonase activity in the paired mother/newborn: r = 0.68. PON1 circulates in strong association with the HDL (and to a very limited extent in the VLDL); however, a small fraction can be found in free form. To further characterize the PON1 metabolism in the cord blood, we also analyzed free PON1. Interestingly, the free arylesterase and lactonase activities were higher in the cord blood than in the maternal serum by 16 and 36%, respectively. We did not find significant gender differences in the total or the free arylesterase or lactonase in the neonates nor between the mothers and the non-pregnant women free of the PON1 activities.
Table 3.
Lipids and PON1 activity in the mothers and the neonates
| Variables | Mother (n = 17) | Non-pregnant women (n = 23) | Neonate (n = 83) | P value |
|---|---|---|---|---|
| Total cholesterol (mmol/l) | 6.9 ± 0.5 | 5.1 ± 0.5 | 1.61 ± 0.3 | 0.0001*, 0.01** |
| Low-density lipoprotein cholesterol (mmol/l) | 3.3 ± 0.8 | 2.8 ± 0.6 | 0.58 ± 0.03 | 0.0001*, 0.01** |
| High-density lipoprotein cholesterol (mmol/l) | 1.6 ± 0.4 | 1.7 ± 0.5 | 1.03 ± 0.04 | 0.001*, NS |
| Triglyceride (mmol/l) | 2.3 (1.2–2.9) | 0.9 ± 0.3 | 0.28 (0.1–0.42) | 0.0001*, 0.001** |
| Total PON-1 arylesterase (U/l) | 185.6 ± 24.8 | 180 ± 20.1 | 68.4 ± 17.1 | 1.5 × 10−9*, NS** |
| Total PON-1 lactonase (U/l) | 57.1 ± 18.6 | 60.3 ± 20.2 | 13.4 ± 8.0 | 1.5 × 10−8*, NS** |
| Free PON-1 arylesterase (%) | 16.5 ± 3.0 | 14.5 ± 3.0 | 19.1 ± 3.2a | 0.001*, NS** |
| Free PON-1 lactonase (%) | 14.5 ± 3.0 | 13.5 ± 3.0 | 19.1 ± 2.9a | 0.0001*, NS** |
Data represent mean ± SD or mean and the interquartile range for TG.
NS, non-significant.
*Neonate vs. mother.
**Mother vs. non-pregnant women.
an = 40.
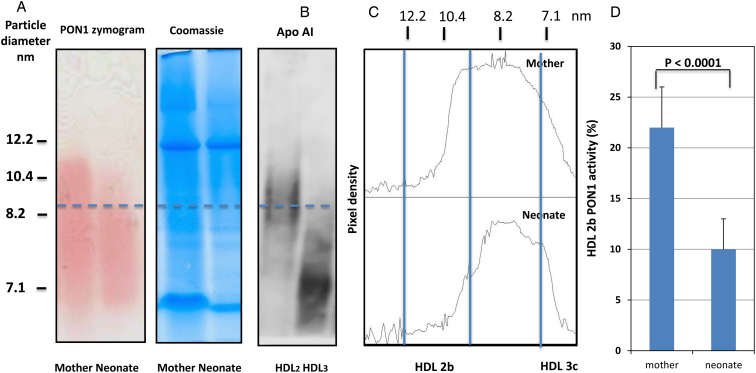
PON1 distribution in the HDL subclasses is shown in Fig. 1. Native HDL is separated by a 4–12% gradient non-denaturing polyacrylamide gel electrophoresis (PAGE) and its activity detected in situ as described in methods coupling the generation of the product and the phenol with a colorimetric reaction. PON1 in the HDL subclasses was not significantly different in the pregnant vs. the non-pregnant women (data not shown); therefore, we compared 17 of the neonates with their own mothers to reduce the impact of the genetics on the findings. We thereby ascertained that the neonate PON1 lactonase and the HDL distribution are different from the adult females and the males regardless of the changes in the pregnancy. In Fig 1A, a typical pair mother neonate PON1 activity across the HDL range is shown, together with the respective protein staining of the same gel for load and migration control. Fig 1B shows a western blot that localizes apoA-I in the purified HDL2 and HDL3. Fig 1C depicts a typical densitometry profile of the HDL-PON1 activity. A striking difference is apparent both visually and by densitometry: the distribution of the PON1 activity is narrower in the neonates. When AUC was quantified, PON1 in the area corresponding to HDL2b (9.7–12.9 nm) was much lower in the neonates than their mothers, as shown in Fig 1C. There is a 65% lower HDL2b PON1 in the cord blood than in the adult serum. The location of HDL2 in our gels is corroborated by the western blot in Fig 1B. No significant differences were apparent between the mother PON1 zymogram profiles and those of the age-matched non-pregnant women (data not shown).
Figure 1.
PON1 activity in the HDL subclasses from the neonates and their mothers separated by the native gradient electrophoresis gels. (A) The PON1 zymogram of a typical and representative pair of the mother and her newborn and Coomassie staining of the same gel for load and migration control. The HDLs were separated by their hydrodynamic diameter in an 8 × 10 × 0.15 cm3 non-denaturing 4–12% gradient PAGE (Novex® 4–12% Tris-Glycine gel, Invitrogen, Carlsbad, CA, USA) as described in the Materials and methods section. PON1 activity in the HDL subclasses was determined by the enzymatic detection of the PON1 hydrolysis of phenylacetate in situ (by means of a coupled reaction with aminoantipyrine and potassium ferricyanide, which quantitatively measures phenol, the product, at 405 nm, as described in the Materials and methods section. (B) A western blot that localizes the apoAI in the purified HDL2 and HDL3 which allows us to better determine the cut-off points for quantitation. (C) The densitometry profiles as indicated (same runs as in A). The relative proportions of the PON1 activity in each HDL subclass were estimated by optical densitometry analysis employing the Image J software (NIH, Bethesda, MA, USA), using as reference the globular proteins (thyroglobulin, 17 nm; ferritin, 12.2 nm; lactate dehydrogenase, 8.2 nm; and albumin, 7.1 nm: high-molecular weight calibration kit, Amersham Pharmacia Biotech, Buckimghamshire, UK) that were run in each gel. (D) The relative activity of PON1 in HDL2b measured as area under the curve for the PON1 activity in HDL2b/total PON1 activity.
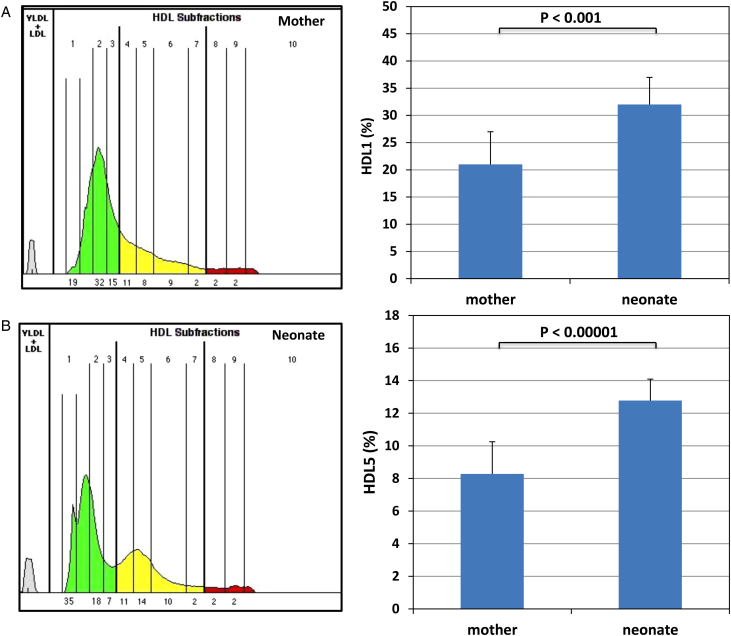
To further characterize the PON1 distribution in the neonates, we conducted HDL subclasses analysis by employing the FDA approved Lipoprint method in all of the mothers and a subset of 40 neonates. This procedure allows for the Sudan Black pre-stained lipoproteins to separate in the tube gels by their size and charge in a similar way as the separation achieved in the slab gels for our study. Table 4 shows the percentage distribution of the HDL subclasses in the two populations. Fig. 2A depicts a typical representative pair of the mother and the neonate. The two most striking differences (shown in graph C) are: the neonates have a larger content (52% higher) of very large HDL as well as a characteristic peak in the middle-sized HDL5 which is unremarkable in the mothers as well as in the non-pregnant women. Note the congruence of this latter finding that only accounts for the lipid content of these fractions, with the apolipoprotein findings in Fig. 2. These data suggest that the neonate HDL is enriched with an intermediate-sized or less charged HDL. As shown in Table 3, very small HDL are also lower in the neonates as compared to their mothers.
Table 4.
HDL subclasses by Lipoprint in the mothers and the neonates
| (% of total HDL) | Mother (n = 17) | Neonate (n = 40) | P value |
|---|---|---|---|
| HDL large | 50.08 ± 7.91 | 53.53 ± 6.46 | NS |
| HDL intermediate | 37.55 ± 7.21 | 38.67 ± 4.54 | NS |
| HDL small | 14.29 ± 6.31 | 7.49 ± 3.02 | 0.006552 |
| HDL1 | 20.57 ± 6.17 | 31.52 ± 4.52 | 0.00026 |
| HDL2 | 18.51 ± 5.37 | 15.01 ± 4.40 | NS |
| HDL3 | 11.01 ± 1.90 | 6.99 ± 0.89 | NS |
| HDL4 | 11.62 ± 2.15 | 10.96 ± 1.15 | NS |
| HDL5 | 8.28 ± 1.97 | 12.78 ± 1.31 | 1.09 × 10−5 |
| HDL6 | 11.61 ± 2.11 | 11.69 ± 2.26 | NS |
| HDL7 | 4.03 ± 0.98 | 3.27 ± 1.05 | NS |
| HDL8 | 4.17 ± 1.37 | 3.2 ± 1.06 | NS |
| HDL9 | 4.25 ± 1.63 | 3.2 ± 0.95 | 0.095258 |
| HDL10 | 5.84 ± 3.51 | 1.1 ± 1.30 | 0.000835 |
Data represent mean ± SD or mean and the interquartile range for TG.
NS, non-significant.
Figure 2.
Lipid distribution in the HDL subclasses from the neonates and their mothers separated by Lipoprint. A typical and representative pair of the mother (A) and her newborn (B) is depicted. Sera were analyzed by using the Lipoprint HDL subfraction analysis system from Quantimetrix according to the manufacturer's instructions. For quantification, scanning was performed with the manufacturer's scanner and stained HDL subclasses were identified by their mobility after electrophoresis. The LDL/VLDL band was the starting reference point (Rf 0.0) and albumin was the ending reference point (Rf 1.0). AUC% were calculated with the Lipoware computer software (Quantimetrix Corp.). Up to 10 HDL subfractions, distributed between the LDL/VLDL and the albumin bands are detected. The most striking differences are an increase in HDL1 and a prominent HDL5 in the neonates. See also Table 3 for a complete quantitative analysis.
Discussion
The novel findings of this study are that the neonates have lower PON1 lactonase activity, higher free PON1, different distribution of PON1in the HDL subclasses as compared to their mother and the adults as well as a distinctive HDL subclass lipid profile.
A key functional component of the HDL is PON1. During the past decade, PON1 has been proven to be an important contributor to the antioxidant activity of HDL. PON1 is versatile and plays a role in several pathways, many of which are protective against atherothrombosis: (1) it effectively hydrolyzes peroxides and lactones in LDL and HDL as well as protects the macrophages from oxidation; (2) it participates in endothelial homeostasis; (3) it is an effective xenobiotic metabolizer; (4) it is a homocysteine-thiolactonase. PON1 evolved from the bi-functional enzymes that act as homoserine-lactonases, which are the quorum sensing molecules employed by many Gram-negative bacteria, and is in part a component of innate immunity. In recent years, a low PON1 activity has been consistently linked with an increased risk of major cardiovascular events in the setting of secondary prevention of coronary artery disease.
Previous work by several authors has consistently shown lower PON1 activity in the neonates as compared to their mothers and the adults, which is proportionally lower than the HDL-C content, suggesting either less protein expression or activation.9,17–20 A lower PON1 activity in the neonates makes them more susceptible to oxidative stress and organophosphate toxicity. On the other hand, this lower content, in the light of the typical lipoprotein profile of the neonates, is consistent with the PON1 role as an antioxidant: the neonates contain very little VLDL and LDL, the latter being protected by the HDL PON1 by hydrolysis of the oxidized phospholipids. PON1 is the most active when bound to the HDL and the appropriate interaction with apoA-I or apoE occurs; and it is more active when present in small HDL3 particles.28 The previous studies on neonatal PON1 have the limitation of measuring its activity by using substrates that do not account for its lactonase, physiological activity. Would a lower lactonase activity compromise neonate natural immunity? Moreover, although proteomic studies have heralded significant progress in our understanding of the HDL composition in the cord blood, no study so far had assessed the distribution of PON1 across the HDL subclasses and plasma (free PON1) in the neonate or its association with specific lipid distribution across those HDL subspecies.
To address the aforementioned gaps in our knowledge, we conducted the present study for measuring total and free lactonase in neonate blood, PON1 distribution across the HDL subclasses employing our novel method, its association with apolipoprotein distribution, and its correlation with the HDL subclasses measured by current, established clinical laboratory methods. We compared the profile with the mothers at labor with the rationale of reducing the covariate of genetic variability, after determining that no significant differences are found between pregnant and non-pregnant women with regard to PON1 distribution in the HDL subclasses.
All of the newborns studied were term and born by vaginal delivery and show standard distribution of weight and length. The mothers show the well-described pro-atherogenic lipid profile of the third trimester and the neonates displayed lipid levels congruent with the previously reported profile of the lipids in the cord blood. The main fetal lipoprotein is the HDL and there are very low TG levels. The PON1 arylesterase activity in the cord blood represented 37% of the maternal activity, which confirms the previous reports. A first novel finding is that the PON1 lactonase activity amounted to only 23% of THE maternal activity. We chose to measure the PON1 arylesterase activity to provide comparison with the published studies and because arylesterase is considered a surrogate marker of the PON1 mass and is not significantly modified by common PON1 polymorphisms. Our findings confirm and expand the published data. Even when neonatal HDL-C was 64% of maternal HDL-C, the physiologically relevant lactonase activity is disproportionately lower. This may result from the differential activation of one vs. the other catalytic activity due to the environment of the PON1 in different HDL subclasses, and is another element showing that HDL-C poorly reflects the HDL function.
Even lower relative concentrations of the PON1 protein have been recently shown in proteomic studies. However, these studies used the HDL purified in high salt by ultracentrifugation, the HDL consistently loses peripheral proteins, as it is widely accepted, and it loses most of HDL3b and HDL3c-PON1 as previously shown in our laboratory.30 As expected, arylesterase and lactonase activity in the maternal serum and the cord blood showed a strong correlation. Arylesterase and lactonase activity in the maternal serum correlated with the same activity in the paired neonates as expected.
PON1 can also dissociate from the HDL in physiological conditions and increased free PON1 has been associated with diseases with high oxidative stress.24 Which is the condition in the neonates? Another novel finding of our study is that the neonates have higher free arylesterase and lactonase activities than their mothers or female controls as previously reported by us.33 Free lactonase was disproportionally higher which is also consistent with the changes in total activity as compared with THE HDL-C. Lower total lactonase activity and less functional free lactonase in the neonates could be linked to a deficient innate immunity vis-à-vis the Gram-negative infections. Although, in its evolution from a bi-functional enzyme, PON1 activity vis-à-vis the quorum sensing lactones decreased while functional gains in other activities were acquired, the possibility deserves further exploration.34,35 Plausible explanations for these findings are looser associations of the enzyme to the HDL subclasses that are different in the neonates as well as the differential activation and the conformation requirements of the promiscuous vs. the physiological activity of the enzyme. As recently shown, the pre-term HDL and the other lipoprotein profiles are different in the term infants, and the profiles change dramatically after birth, the changes in the PON1 lactonase activities also deserve further exploration.14,15
To shed further light on whether our finding of lower PON1 activity is associated with a different distribution in the HDL subclasses, we analyzed the HDL subclasses by using current clinical laboratory procedures as well as our recently developed PON1 zymogram. PON1 distribution in the HDL subclasses is shown in Fig. 1. The distribution of the PON1 activity is narrower in the neonates than in their mothers shown here or the female controls shown previously.30 A striking differential feature is that the PON1 in the particle sizes corresponding to HDL2b (9.7–12.9 nm) is much lower in the neonates than their mothers. It has been recently shown that the neonates have increased large HDL; our data suggest that these fractions are therefore very poor in PON1 as compared to the adults.30,37 It must be noted that the HPLC method employed by these authors provides separation by size and only accounts for the lipids. Large, buoyant, lipid-loaded HDL particles may then appear abundant whereas their relative molar concentration is not necessarily higher, and their protein content (including PON1) is proportionally lower. Our data thus show for the first time that the neonatal PON1 is differently distributed among the HDL subclasses. As previously indicated, the most thorough and recent analysis of the neonatal lipoprotein subclasses was performed by using HPLC and the lipid content of the fractions separated as a function of their mass.15 Among the several approaches to analyze the HDL, one of the most currently available in the clinical laboratories is the Lipoprint method.36 To date, no comparative data of the maternal and the neonatal serum HDL using this approach exists. To further dissect the associations of the PON1 and the lipid distribution in the HDL subclasses, we proceeded to analyze our samples with this method. One advantage is that both of the methods separate the native lipoproteins by their mass, with contribution, to a certain extent by their respective charges, allowing for better comparison of our PON1 and apolipoprotein distribution with the lipid content of each fraction. Indeed, in the previous reports, isolated neonatal HDL protein distribution has been shown to be shifted toward more cationic or larger sized fractions.8,16
When we compared the maternal and the cord blood serum HDL subclasses distribution, the neonates showed a 52% higher content of very large HDL as well as a characteristic peak in the middle-sized HDL5, which is inconspicuous in the mothers. Taken together, our data suggest that the neonate HDL is enriched with an intermediate-sized (and/or less charged HDL). This fraction is also rich in active PON1. Our data show that very small HDL is also lower in the neonates as compared to their mothers. Since HDL3 is known to carry most of the active PON1, this finding can also partly explain the lower activity of the enzyme in the neonates found by others and us. Our findings are in part consistent and in part do not support the previous findings using HPLC, which show a lower proportion of middle-sized HDL and a higher proportion of very small HDL in the neonates.14,15 A reasonable explanation is that the neonates may have subclasses of small HDL that are less charged and thereby migrate as the intermediate fractions in the gels.8,16
A limitation of our study is the relatively modest sample size, albeit larger than recent proteomic studies and that the subjects are from only one ethnic group. However, this is also an advantage since it allowed us to compare our results with recent studies of similar sample sizes in Japanese subjects.
Conclusion
The neonates have lower PON1 lactonase activity, increased free PON1 than the adults. PON1 distribution across the HDL subclasses differs between the neonates and the adults. The neonates have a very low activity of PON1 in large HDL, whereas they have increased lipid content in large HDL. Taken together, our results on the PON1 zymogram, and Lipoprint suggest that the neonates have distinctive middle-sized HDL particles, carrying active PON1. The very low lactonase activity in the neonates we report may explain in part the well-known relative immunodeficient state and the oxidative stress susceptibility of the newborns or reflect the relative low need of PON1 in a metabolism whereby the VLDL and the LDL concentrations are very low. Further studies are needed to assess the functional implications of this finding. Since the neonate lipoprotein pattern has been recently shown to be different in the preterm infants and to change very quickly after birth, our approach could be useful for further studies assessing the predictive value of some of these parameters in the prognosis of the preterm newborns.
Acknowledgments
We are grateful to Ms Mallory Davis for expert editorial and technical help. This work was funded in part by Touro University California and by JSPS KAKENHI Grant Number 25460701 to S.K.
References
- 1.Dyerberg J, Hjorne N, Nymand G, Olsen JS. Reference values for cord blood lipid and lipoprotein concentrations. Acta Paediatr Scand 1974;63:431–6. [DOI] [PubMed] [Google Scholar]
- 2.Stozicky F, Slaby P, Volenikova L. The pattern of major serum apolipoproteins during the early neonatal period. Acta Paediatr Scand 1982;71:239–41. [DOI] [PubMed] [Google Scholar]
- 3.Toth P, Klujber L, Baranyai Z, Molnar E. Serum lipid and lipoprotein-cholesterol values in cord blood and on the sixth postnatal day in newborns of varying maturity. Acta Paediatr Hung 1984;25:275–81. [PubMed] [Google Scholar]
- 4.Genzel-Boroviczeny O, Forte TM, Austin MA. High-density lipoprotein subclass distribution and human cord blood lipid levels. Pediatr Res 1986;20:487–91. [DOI] [PubMed] [Google Scholar]
- 5.Genzel-Boroviczeny O, D'Harlingue AE, Kao LC, Scott C, Forte TM. High-density lipoprotein subclass distribution in premature newborns before and after the onset of enteral feeding. Pediatr Res 1988;23:543–7. [DOI] [PubMed] [Google Scholar]
- 6.Kherkeulidze P, Johansson J, Carlson LA. High density lipoprotein particle size distribution in cord blood. Acta Paediatr Scand 1991;80:770–9. [DOI] [PubMed] [Google Scholar]
- 7.Bellu R, Ortisi MT, Agostoni C, Incerti P, Besana R, Riva E,. et al. Familial history of cardiovascular disease and blood lipid pattern in newborn infants. Acta Paediatr Scand 1992;81:21–4. [DOI] [PubMed] [Google Scholar]
- 8.Nagasaka H, Chiba H, Kikuta H, Akita H, Takahashi Y, Yanai H,. et al. Unique character and metabolism of high density lipoprotein (HDL) in fetus. Atherosclerosis 2002;161:215–23. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Kumar M, Chan W, Berkowitz G, Wetmur JG. Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect 2003;111:1403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Descamps OS, Bruniaux M, Guilmot PF, Tonglet R, Heller FR. Lipoprotein concentrations in newborns are associated with allelic variations in their mothers. Atherosclerosis 2004;172:287–98. [DOI] [PubMed] [Google Scholar]
- 11.Ophir E, Oettinger M, Nisimov J, Hirsch Y, Fait V, Dourleshter G,. et al. Cord blood lipids concentrations and their relation to body size at birth: possible link between intrauterine life and adult diseases. Am J Perinatol 2004;21:35–40. [DOI] [PubMed] [Google Scholar]
- 12.Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol 2007;21:518–24. [DOI] [PubMed] [Google Scholar]
- 13.Pac-Kozuchowska E. Evaluation of lipids, lipoproteins and apolipoproteins concentrations in cord blood serum of newborns from rural and urban environments. Ann Agric Environ Med 2007;14:25–9. [PubMed] [Google Scholar]
- 14.Fujita H, Okada T, Inami I, Makimoto M, Hosono S, Minato M,. et al. Heterogeneity of high-density lipoprotein in cord blood and its postnatal change. Clin Chim Acta 2008;389:93–7. [DOI] [PubMed] [Google Scholar]
- 15.Nagano N, Okada T, Yonezawa R, Yoshikawa K, Fujita H, Usukura Y,. et al. Early postnatal changes of lipoprotein subclass profile in late preterm infants. Clin Chim Acta 2012;413:109–12. [DOI] [PubMed] [Google Scholar]
- 16.Sreckovic I, Birner-Gruenberger R, Obrist B, Stojakovic T, Scharnagl H, Holzer M,. et al. Distinct composition of human fetal HDL attenuates its anti-oxidative capacity. Biochim Biophys Acta 2013;1831:737–46. [DOI] [PubMed] [Google Scholar]
- 17.Altunhan H, Annagur A, Kurban S, Ertugrul S, Konak M, Ors R. Total oxidant, antioxidant, and paraoxonase levels in babies born to pre-eclamptic mothers. J Obstet Gynaecol Res 2013;39:898–904. [DOI] [PubMed] [Google Scholar]
- 18.Schulpis KH, Barzeliotou A, Papadakis M, Rodolakis A, Antsaklis A, Papassotiriou I,. et al. Maternal chronic hepatitis B virus is implicated with low neonatal paraoxonase/arylesterase activities. Clin Biochem 2008;41:282–7. [DOI] [PubMed] [Google Scholar]
- 19.Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr 2008;167:81–5. [DOI] [PubMed] [Google Scholar]
- 20.Cole TB, Jampsa RL, Walter BJ, Arndt TL, Richter RJ, Shih DM,. et al. Expression of human paraoxonase (PON1) during development. Pharmacogenetics 2003;13:357–64. [DOI] [PubMed] [Google Scholar]
- 21.Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol 2004;369:78–88. [DOI] [PubMed] [Google Scholar]
- 22.Jakubowski H. Protein N-homocysteinylation: implications for atherosclerosis. Biomed Pharmacother 2001;55:443–7. [DOI] [PubMed] [Google Scholar]
- 23.Draganov DI, Stetson PL, Watson CE, Billecke SS, La Du BN. Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. J Biol Chem 2000;275:33435–42. [DOI] [PubMed] [Google Scholar]
- 24.Fuhrman B, Volkova N, Aviram M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: studies in vitro and in diabetic patients. Nutrition 2010;26:359–66. [DOI] [PubMed] [Google Scholar]
- 25.Asztalos BF, Tani M, Schaefer EJ. Metabolic and functional relevance of HDL subspecies. Curr Opin Lipidol 2011;22:176–85. [DOI] [PubMed] [Google Scholar]
- 26.Krauss RM, Nichols AV. Metabolic interrelationships of HDL subclasses. Adv Exp Med Biol 1986;201:17–27. [DOI] [PubMed] [Google Scholar]
- 27.Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- 28.Kontush A, Chapman MJ. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr Opin Lipidol 2010;21:312–8. [DOI] [PubMed] [Google Scholar]
- 29.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev 2006;58:342–74. [DOI] [PubMed] [Google Scholar]
- 30.Gugliucci A, Caccavello R, Kotani K, Sakane N, Kimura S. Enzymatic assessment of paraoxonase 1 activity on HDL subclasses: a practical zymogram method to assess HDL function. Clin Chim Acta 2013;415:162–8. [DOI] [PubMed] [Google Scholar]
- 31.Li WF, Costa LG, Furlong CE. Serum paraoxonase status: a major factor in determining resistance to organophosphates. J Toxicol Environ Health 1993;40:337–46. [DOI] [PubMed] [Google Scholar]
- 32.Rosenblat M, Gaidukov L, Khersonsky O, Vaya J, Oren R, Tawfik DS,. et al. The catalytic histidine dyad of high density lipoprotein-associated serum paraoxonase-1 (PON1) is essential for PON1-mediated inhibition of low density lipoprotein oxidation and stimulation of macrophage cholesterol efflux. The J Biol Chem 2006;281:7657–65. [DOI] [PubMed] [Google Scholar]
- 33.Gugliucci A, Schulze J, Kinugasa E, Ogata H, Kimura S. The free fraction of paraoxonase 1 is not increased in patients with end stage renal disease undergoing hemodialysis. Clin Chim Acta 2009;402:209–10. [DOI] [PubMed] [Google Scholar]
- 34.Bar-Rogovsky H, Hugenmatter A, Tawfik DS. The evolutionary origins of detoxifying enzymes: the mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases. J Biol Chem 2013;288:23914–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias M, Tawfik DS. Divergence and convergence in enzyme evolution: parallel evolution of paraoxonases from quorum-quenching lactonases. J Biol Chem 2012;287:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harangi M, Seres I, Harangi J, Paragh G. Benefits and difficulties in measuring HDL subfractions and human paraoxonase-1 activity during statin treatment. Cardiovasc Drugs Ther 2009;23:501–10. [DOI] [PubMed] [Google Scholar]
- 37.Yonezawa R, Okada T, Kitamura T, Fujita H, Inami I, Makimoto M,. et al. Very low-density lipoprotein in the cord blood of preterm neonates. Metabolism 2009;58:704–7. [DOI] [PubMed] [Google Scholar]