Abstract
The status of lipid peroxidation, antioxidants, and detoxification enzymes were used as biochemical end points to assess the chemopreventive potential of geraniol, a monoterpene, in 7,12-dimethylbenz[a]anthracene (DMBA)-induced hamster buccal pouch carcinogenesis. Topical application of 0.5% DMBA in liquid paraffin, three times a week, for 14 weeks developed well-differentiated squamous cell carcinoma in the buccal pouch of golden Syrian hamsters. Although 100% tumor formation was noticed in hamsters treated with DMBA alone, intragastric administration of geraniol, at a dose of 250 mg/kg body weight (b.w.) to DMBA-treated hamster completely prevented the formation of oral tumors. Furthermore, geraniol significantly reduced lipid peroxidation by-products and improved the status of enzymatic and non-enzymatic antioxidants as well as modulated the status of phase I and phase II detoxification enzymes, favoring the excretion of carcinogenic metabolite, during DMBA-induced oral carcinogenesis. The present study concludes that the chemopreventive potential of geraniol relies on its anti-lipid peroxidative and antioxidant function as well as modulatory effects on phase I and II detoxification enzymes to excrete the carcinogenic metabolite, during DMBA-induced hamster buccal pouch carcinogenesis.
Keywords: Antioxidants, Chemoprevention, Detoxification, DMBA, Geraniol, Lipid peroxidation, Oral carcinogenesis
Introduction
Oral squamous cell carcinoma is the fifth most common malignancy worldwide and is the major cause of morbidity and mortality in Indian populations. Each year more than 500 000 new oral cancer cases are diagnosed worldwide. The incidence of oral cancer is rapidly increasing throughout the world, especially in developing countries including India, where this form of cancer accounts for 40–50% of all cancers.1 Increase in the incidence of oral cancer is largely associated with betel quid chewing with and without tobacco, smoking, and alcohol consumption. The overall 5-year survival rates for oral cancer patients have not changed significantly in the past 4–5 decades and still at 50% despite recent advancement in the treatment of oral cancer.2
Oral carcinogenesis is a multifocal disease and develops through a multistage process, comprised of initiation, promotion, and progression. 7,12-Dimethylbenz[a]anthracene (DMBA), a polycyclic aromatic hydrocarbon, is produced during the incomplete combustion of carbon-containing compounds, and mostly found in tobacco, smoke, and motor vehicle exhaust emissions. DMBA on metabolic activation generates a reactive metabolite, dihydrodiolepoxide, which subsequently binds to adenine and guanine residues of DNA, contributing to mutagenesis and carcinogenesis.3 DMBA-induced tumor in the buccal pouch of hamster closely resembles human oral tumor histologically, biochemically, and at molecular level.4 DMBA-induced oral carcinogenesis is therefore commonly employed to assess the cancer chemopreventive potential of natural products and synthetic agents.
Profound studies reported that the development of cancer is associated with oxidative stress, a condition leading to overproduction of reactive oxygen species (ROS) and compromise of enzymatic and non-enzymatic antioxidants.5 Lipid peroxidation (LPO), a free radical mediated chain reaction, causes oxidative deterioration of polyunsaturated fatty acids in the biomembrane. LPO by-products (thiobarbituric acid-reactive substances (TBARS), lipid hydroperoxides (LOOH), and conjugated dienes (CD)) causes extensive membrane damage and dysorganization of cell structure and function.6 Overproduction of ROS under normal conditions is, however, prevented by the host antioxidant defense system, which includes non-enzymatic (vitamin E and reduced glutathione (GSH)) and enzymatic (superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx)) antioxidants.7 Proper balance between oxidant and antioxidant status should be maintained in the cell because of their potential importance in the pathogenesis of various pathological disorders including cancer. Neoplastic cells sequester essential nutrients and antioxidants from circulation for their rapid growth as well as to meet the nutrient demand of growing tumor.8
The metabolic activation and detoxification of DMBA occurs mainly in the liver and the reactions are catalyzed by phase I (cytochrome P450 and cytochrome b5) and phase II detoxification enzymes (glutathione-S-transferase (GST), glutathione reductase (GR), oxidized glutathione (GSSG), and DT-diaphorase (DTD)) respectively.9 The activation of phase I and phase II detoxification enzymes were significantly altered during several cancerous conditions including oral carcinoma.10,11
A growing body of literature pointed out the importance of dietary antioxidants in the reduction of cellular oxidative damage in the human body. Antioxidants play a crucial role in the protection of cancer, inflammation, neurodegenerative, and cardiovascular diseases.12 Recently, researchers have focused on phytochemicals as antioxidants or free radical scavengers. Geraniol (3, 7-dimethylocta-trans-2, 6-dien-1-ol), an acyclic monoterpene alcohol, is found widely as a chief constituent of essential oil of ginger, lemon, lime, nutmeg, lavender, orange, rose, etc. It is also commonly used as fragrance components in cosmetics, soaps, and cleaning products and as food additives. Geraniol has been represented as a new class of agents for cancer chemoprevention. Geraniol has potent anti-lipid peroxidative, antioxidant, anti-microbial, and anti-inflammatory properties.13,14 It also exerted anticancer activity against various cancer cells both in vitro and in vivo and inducing the activities of the detoxifying enzyme such as GST.14,15 The chemical structure of geraniol is given in Fig. 1.
Figure 1.
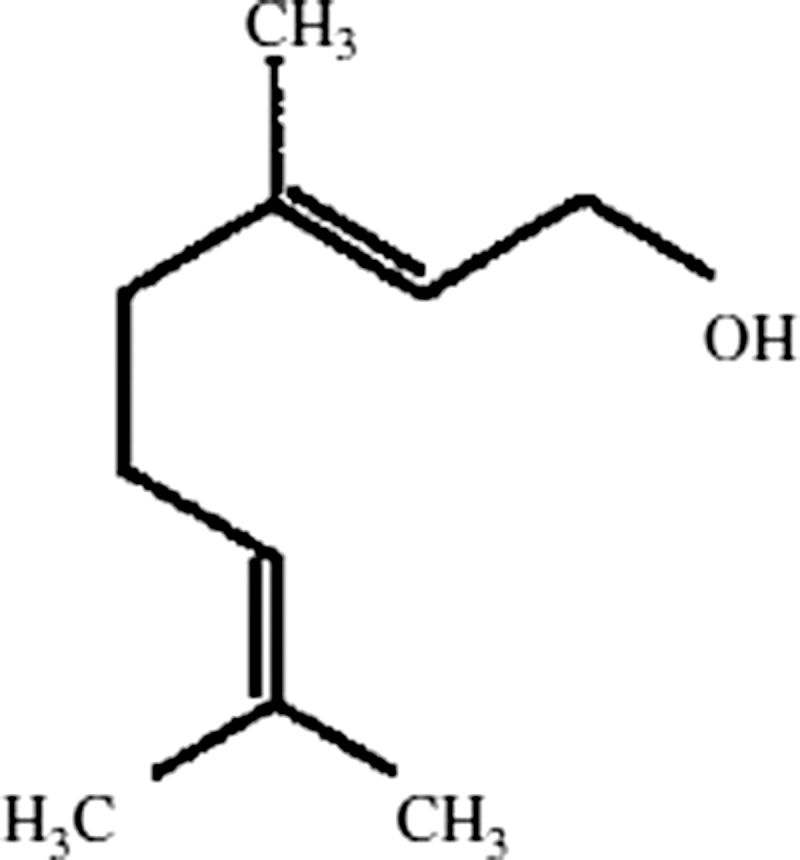
Chemical structure of geraniol.
To the best of our knowledge, there were no scientific studies on the chemopreventive potential of geraniol in the well-established DMBA-induced hamster buccal pouch carcinogenesis. Since the status of lipid peroxidation, antioxidants, and phase I and II detoxification enzymes are markedly altered during several cancerous conditions, the present study utilizes the same biochemical parameters as an end point to assess the chemopreventive potential of geraniol in DMBA-induced oral carcinogenesis.
Materials and methods
Chemicals
DMBA and geraniol (trans-3,7-dimethyl-2,6-octadien-1-ol;98%) were obtained from Sigma Aldrich Chemical Pvt. Ltd (Bangalore, India). All other chemicals used were of analytical grade.
Animals
Male golden Syrian hamsters, 8–10 weeks old, weighing 80–120 g, were purchased from National Institute of Nutrition, Hyderabad, India, and maintained in Central Animal House, Rajah Muthiah Medical College and Hospital, Annamalai University. The hamsters were housed four or five in a polypropylene cage and provided standard pellet diet and water ad libitum. The hamsters were maintained under controlled conditions of temperature and humidity with a 12 hours light/dark cycle. The local institutional animal ethics committee (Registration Number 160/1999/CPCSEA) of Annamalai University approved the experimental design.
DMBA-induced hamster buccal pouch carcinogenesis
Tumors were induced in hamster buccal pouches by topical application of 0.5% DMBA in liquid paraffin three times a week for 14 weeks. The number of tumors in each hamster buccal pouch was examined macroscopically when the hamsters were sacrificed.
Experimental design
Dose-dependent study
For dose-dependent study, a total number of 40 hamsters were randomized into four groups of 10 hamsters in each group. Groups 1–4 hamsters were painted with 0.5% DMBA in liquid paraffin three times a week for 14 weeks on their left buccal pouches with a number 4 brush. Geraniol was dissolved in corn oil (2.5 ml/kg b.w.) and administered orally to hamsters in groups 2, 3, and 4 starting a week before the exposure to the carcinogen and continued on days alternate to DMBA painting until the animals were sacrificed.
Group 1: DMBA (0.5% DMBA painted three times a week for 14 weeks).
Group 2: DMBA (0.5% DMBA painted three times a week for 14 weeks) + geraniol (125 mg/kg b.w., by orally for 14 weeks on days alternate to DMBA painting).
Group 3: DMBA (0.5% DMBA painted three times a week for 14 weeks) + geraniol (250 mg/kg b.w., by orally for 14 weeks on days alternate to DMBA painting).
Group 4: DMBA (0.5% DMBA painted three times a week for 14 weeks) + geraniol (500 mg/kg b.w., by orally for 14 weeks on days alternate to DMBA painting).
The experiment was terminated at the end of the 14th week, and all animals were sacrificed by cervical dislocation. The number of tumors in each hamster buccal pouch was examined macroscopically. The diameter of each tumor was measured with a Vernier caliper. The tumor volume was calculated by the formula, 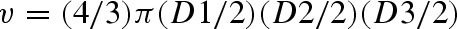 where D1, D2, and D3 are the three diameters (mm3) of the tumor. Tumor burden was calculated by multiplying tumor volume and the number of tumors/hamster. Of three doses, oral administration of geraniol at a dose of 250 and 500 mg/kg b.w. completely prevented the tumor formation in DMBA-treated hamsters. Therefore, we had chosen the low dose of 250 mg/kg b.w. for further chemoprevention study.
where D1, D2, and D3 are the three diameters (mm3) of the tumor. Tumor burden was calculated by multiplying tumor volume and the number of tumors/hamster. Of three doses, oral administration of geraniol at a dose of 250 and 500 mg/kg b.w. completely prevented the tumor formation in DMBA-treated hamsters. Therefore, we had chosen the low dose of 250 mg/kg b.w. for further chemoprevention study.
Chemoprevention study
For chemoprevention study, a total number of 50 hamsters were randomized into five groups of 10 hamsters in each. Geraniol dissolved in corn oil was administered orally to hamsters in groups 2 and 3 starting a week before the exposure to the carcinogen and continued on days alternate to DMBA painting until the animals were sacrificed.
Group 1: DMBA (0.5% DMBA painted three times a week for 14 weeks).
Group 2: DMBA (0.5% DMBA painted three times a week for 14 weeks) + geraniol (250 mg/kg b.w., by orally for 14 weeks on days alternate to DMBA painting).
Group 3: Geraniol alone (250 mg/kg b.w., by orally for 14 weeks).
Group 4: Corn oil (2.5 ml/kg b.w., by orally on days alternate to liquid paraffin painting) + liquid paraffin (painted three times a week for 14 weeks).
Group 5: Control (Untreated).
The experiment was terminated at the end of 14th week, and all animals were sacrificed by cervical dislocation. Biochemical studies were conducted on blood, buccal mucosa, and liver of control and experimental hamsters in each group.
Histopathology
Buccal mucosa were fixed in 10% formalin, routinely processed and embedded in paraffin; 2–3 µm sections were cut in a rotary microtome, fixed on glass slides, and stained with hematoxylin and eosin.
Biochemical estimations
Sample collection
Blood samples were collected into heparinized tubes. Plasma was separated by centrifugation at 1000 g for 15 minutes. Liver and buccal mucosa from hamsters were washed with ice-cold saline and homogenized using appropriate buffer in an All-glass homogenizer with Teflon pestle and used for biochemical estimations.
TBARS in plasma and buccal mucosa were assayed by the method described by Yagi16 and Ohkawa et al.17 The LOOH and CD were estimated with the method of Jiang et al.18, and Rao and Recknagel.19 The GSH levels were determined by the method described by Beutler and Kelley.20 The vitamin E level in the plasma and buccal mucosa were determined with the method of Desai21 and Palan et al.22 The activity of SOD, CAT, and GPx in plasma and buccal mucosa were assayed using the method of Kakkar et al.23 Sinha24, and Rotruck et al.25, respectively. The activities of GST and GR, and GSSG content were assayed using the method of Habig et al.26 Carlberg and Mannervik27, and Tietze28, respectively. The levels of cytochrome P450 and b5 were determined according to the method of Omura and Sato.29 The activity of DTD was estimated according to the method of Ernster.30 Tissue protein was assayed by the method of Lowry et al.31
Statistical analysis
The data are expressed as mean ± SD. Statistical comparisons were performed by one-way analysis of variance, followed by Duncan's multiple range test (DMRT). The results were considered statistically significant if the P values were 0.05 or less.
Results
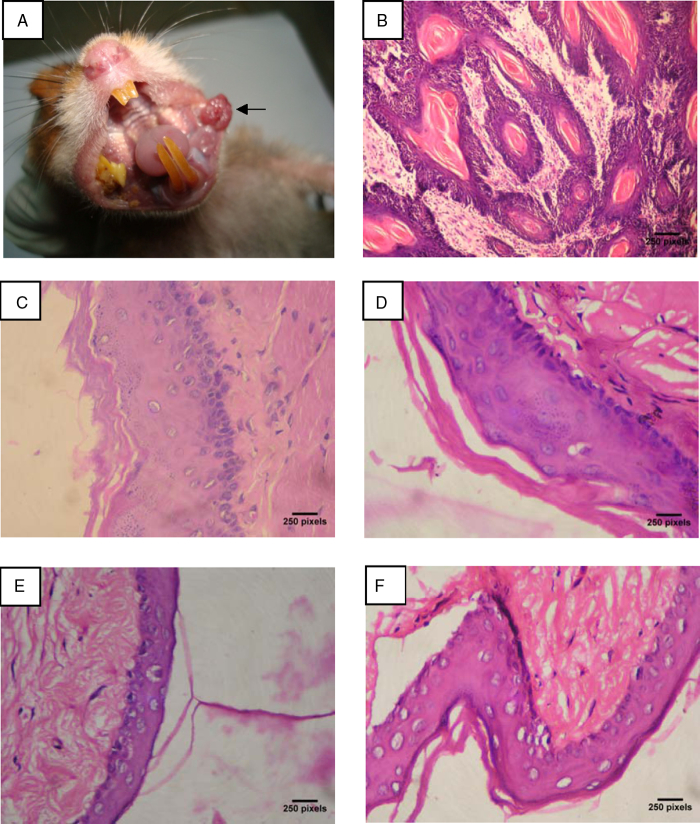
Table 1 shows the tumor incidence, tumor volume, and tumor burden of control and experimental hamsters in each group. We observed 100% tumor formation with mean tumor volume of 364.50 mm3 and tumor burden of 1020.60 mm3 in DMBA-alone-painted hamsters. Oral administration of geraniol (250 mg/kg b.w.) to DMBA-treated hamsters for 14 weeks completely prevented the formation of oral squamous cell carcinoma. The gross appearance of oral squamous cell carcinoma noticed in DMBA-alone-painted hamsters is shown in Fig. 2A.
Table 1.
Tumor incidence, tumor number, tumor volume, and tumor burden in control and experimental hamsters
| Groups | Treatment | Tumor incidence (oral squamous cell carcinoma) | Total number of tumors/hamsters | Tumor volume (mm3) | Tumor burden (mm3) |
|---|---|---|---|---|---|
| 1. | DMBA | 100% | 28/(10) | 364.50 ± 42.40 | 1020.60 ± 98.50 |
| 2. | DMBA + geraniol (125 mg/kg b.w.) | 50% | 12/(5) | 74.15 ± 5.60 | 177.96 ± 13.60 |
| 3. | DMBA + geraniol (250 mg/kg b.w.) | 0% | 0 | 0 | 0 |
| 4. | DMBA + geraniol (500 mg/kg b.w.) | 0% | 0 | 0 | 0 |
Values are expressed as mean ± SD for 10 hamsters in each group. Tumor volume was measured using the formula 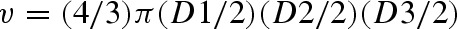 (where D1, D2, and D3 are the three diameters (mm3) of the tumor). Tumor burden was calculated by multiplying tumor volume and the number of tumors/animal.
(where D1, D2, and D3 are the three diameters (mm3) of the tumor). Tumor burden was calculated by multiplying tumor volume and the number of tumors/animal.
Figure 2.
(A) Gross appearance of oral squamous cell carcinoma (indicated by arrow) in DMBA-alone-painted hamsters. (B–F) Histopathological changes in the buccal mucosa of control and experimental hamsters in each group (hematoxylin and eosin, ×400). (B) Well-differentiated squamous cell carcinoma exhibiting epithelial and keratin pearls in DMBA-alone-treated group 1 hamsters. (C) Buccal pouch epithelium from group 2 hamsters (DMBA + geraniol) exhibiting moderate keratosis and hyperplasia. (D–F) Buccal pouch epithelium from group 3 (geraniol alone 250 mg/kg b.w.), group 4 (liquid paraffin + corn oil), and group 5 (untreated control) hamsters showed normal cellular architecture with no signs of cell proliferation.
Table 2 depicts the histopathological changes observed in control and experimental hamsters in each group. Hamsters treated with DMBA alone exhibited severe keratosis, hyperplasia, dysplasia, and well-differentiated squamous cell carcinoma (Fig. 2B). A mild-to-moderate preneoplastic lesion (hyperplasia, keratosis, and dysplasia (Fig. 2C)) were noticed in DMBA + geraniol-treated hamsters. Normal cellular architecture with no signs of cell proliferation were observed in geraniol-alone-treated (Fig. 2D), liquid paraffin + corn oil-treated (Fig. 2E), and control hamsters (Fig. 2F).
Table 2.
Histopathological changes in the buccal mucosa of control and experimental hamsters (n = 10/group)
| Group | Treatment | Keratosis | Hyperplasia | Dysplasia | Squamous cell carcinoma |
|---|---|---|---|---|---|
| 1. | DMBA | ++ + | ++ + | ++ + | ++ + |
| 2. | DMBA + geraniol (250 mg/kg b.w.) | ++ | ++ | + | − |
| 3. | Geraniol alone (250 mg/kg b.w.) | − | − | − | − |
| 4. | Corn oil + liquid paraffin | − | − | − | − |
| 5. | Control | − | − | − | − |
−, no change; +, mild; ++, moderate; ++ +, severe.
Table 3 shows the levels of LPO by-products in the plasma and buccal mucosa of control and experimental hamsters. The concentration of TBARS, LOOH, and CD were significantly (P < 0.05) elevated in the plasma whereas the levels of buccal mucosa TBARS, LOOH, and CD were significantly (P < 0.05) decreased in tumor-bearing hamsters as compared with control hamsters. Oral administration of geraniol to DMBA-painted hamsters significantly (P < 0.05) restored the status of LPO by-products to the near-normal range.
Table 3.
The levels of LPO by-products (TBARS, LOOH, and CD) in the plasma and buccal mucosa of control and experimental hamsters
| Plasma | Buccal mucosa | ||||||
|---|---|---|---|---|---|---|---|
| Groups | Treatment/parameters | TBARS (nmol/ml) | LOOH (mM/ml) | CD (μM/ml) | TBARS (nmol/100 mg protein) | LOOH (mmol/mg protein) | CD (mg/mg protein) |
| 1. | DMBA | 4.96 ± 0.49a | 0.92 ± 0.06a | 2.02 ± 0.16a | 49.72 ± 4.93a | 24.02 ± 2.12a | 21.62 ± 2.02a |
| 2. | DMBA + geraniol (250 mg/kg b.w.) | 3.07 ± 0.28b | 0.52 ± 0.06b | 1.12 ± 0.18b | 70.68 ± 5.26b | 29.02 ± 2.88b | 26.18 ± 2.20b |
| 3. | Geraniol alone (250 mg/kg b.w.) | 2.68 ± 0.20c | 0.34 ± 0.03c | 0.80 ± 0.07c | 79.86 ± 4.37c | 32.24 ± 3.02c | 29.02 ± 1.38c |
| 4. | Corn oil + liquid paraffin | 2.64 ± 0.21c | 0.36 ± 0.03c | 0.82 ± 0.08c | 79.75 ± 6.66c | 31.42 ± 2.98c | 28.76 ± 1.46c |
| 5. | Control | 2.74 ± 0.23c | 0.34 ± 0.02c | 0.78 ± 0.08c | 78.38 ± 5.69c | 32.12 ± 2.92c | 30.22 ± 1.56c |
Values are expressed as mean ± SD for 10 hamsters in each group.
a–cValues not sharing a common superscript letter (a, b, and c) differ significantly at P < 0.05 (DMRT).
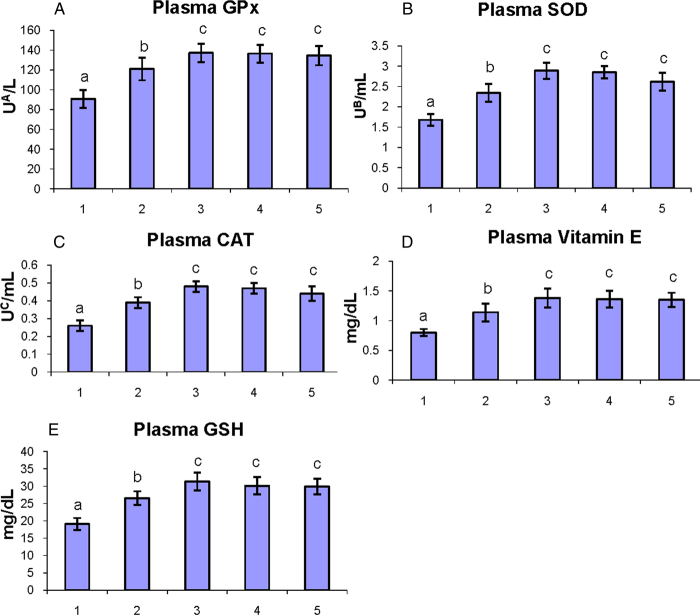
The status of enzymatic (GPx, SOD, and CAT) and non-enzymatic (vitamin E and GSH) antioxidants in the plasma of control and experimental hamsters in each group are shown in Fig. 3A–E. The levels of antioxidants were significantly (P < 0.05) decreased in tumor-bearing hamsters as compared to control hamsters. Oral administration of geraniol to DMBA-painted hamsters significantly (P < 0.05) restored the status of antioxidants to the near-normal range.
Figure 3.
The status of enzymatic antioxidants (GPx, SOD, and CAT) and non-enzymatic antioxidants (vitamin E and GSH) in the plasma of control and experimental hamsters. (1) DMBA, (2) DMBA + geraniol (250 mg/kg b.w.), (3) Geraniol alone (250 mg/kg b.w.), (4) Corn oil + liquid paraffin, (5) Control. Bars are mean ± SD for 10 hamsters in each group. a–cValues that do not share a common superscript letter (a, b, and c) in the same column differ significantly at P < 0.05 (DMRT). UA – micromoles of glutathione utilized/minute; UB – the amount of enzymes required to inhibit 50% nitroblue-tetrazolium (NBT) reduction; UC – micromoles of H2O2 utilized/second.
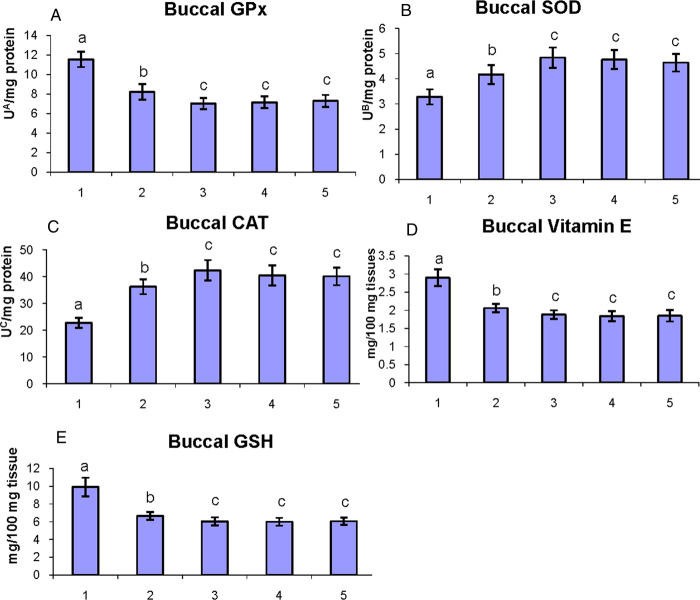
Fig. 4A–E shows the status of antioxidants in the buccal mucosa of control and experimental hamsters in each group. DMBA-alone-painted hamsters were shown disturbed antioxidants status (P < 0.05) (GPx, GSH, and vitamin E were increased; SOD and CAT were decreased) in the buccal mucosa as compared to control hamsters. Oral administration of geraniol to DMBA-painted hamsters significantly (P < 0.05) restored the status of antioxidants to the near-normal level.
Figure 4.
The status of enzymatic antioxidants (GPx, SOD, and CAT) and non-enzymatic antioxidants (vitamin E and GSH) in the buccal mucosa of control and experimental hamsters. (1) DMBA, (2) DMBA + geraniol (250 mg/kg b.w.), (3) Geraniol alone (250 mg/kg b.w.), (4) Corn oil + liquid paraffin, (5) Control. Bars are mean ± SD for 10 hamsters in each group. a–cValues that do not share a common superscript letter (a, b, and c) in the same column differ significantly at P < 0.05 (DMRT). UA – micromoles of glutathione utilized/minute; UB – the amount of enzymes required to inhibit 50% nitroblue-tetrazolium (NBT) reduction; UC – micromoles of H2O2 utilized/second.
Table 4 shows the status of phase I detoxification enzymes in the liver and buccal mucosa of control and experimental hamsters in each group. The status of phase I (cytochrome P450 and cytochrome b5) detoxification enzymes in the liver and buccal mucosa were significantly (P < 0.05) increased in tumor-bearing hamsters as compared to control hamsters. Oral administration of geraniol to DMBA-painted hamsters significantly (P < 0.05) decreased the levels of phase I detoxification enzymes to the near-normal range.
Table 4.
Activities of phase I detoxification enzymes in the liver of control and experimental hamsters
| Liver | Buccal mucosa | ||||
|---|---|---|---|---|---|
| Groups | Treatment/parameters | Cytochrome P450 (UX /mg protein) | Cytochrome b5 (UY/mg protein) | Cytochrome P450 (UX /mg protein) | Cytochrome b5 (UY/mg protein) |
| 1. | DMBA | 1.70 ± 0.12a | 1.98 ± 0.18a | 3.50 ± 0.28a | 0.76 ± 0.06a |
| 2. | DMBA + geraniol (250 mg/kg b.w.) | 0.76 ± 0.06b | 1.08 ± 0.16b | 2.00 ± 0.20b | 0.42 ± 0.04b |
| 3. | Geraniol alone (250 mg/kg b.w.) | 0.69 ± 0.04c | 0.98 ± 0.08c | 1.84 ± 0.16c | 0.40 ± 0.04c |
| 4. | Corn oil + liquid paraffin control | 0.66 ± 0.04c | 0.92 ± 0.06c | 1.80 ± 0.14c | 0.38 ± 0.03c |
| 5. | Control | 0.68 ± 0.04c | 0.94 ± 0.08c | 1.78 ± 0.14c | 0.34 ± 0.02c |
Values are expressed as mean ± SD for 10 hamsters in each group.
a–cValues not sharing a common superscript letter (a, b, and c) differ significantly at P < 0.05 (DMRT).
X – micromoles of cytochrome P450.
Y – micromoles of cytochrome b5.
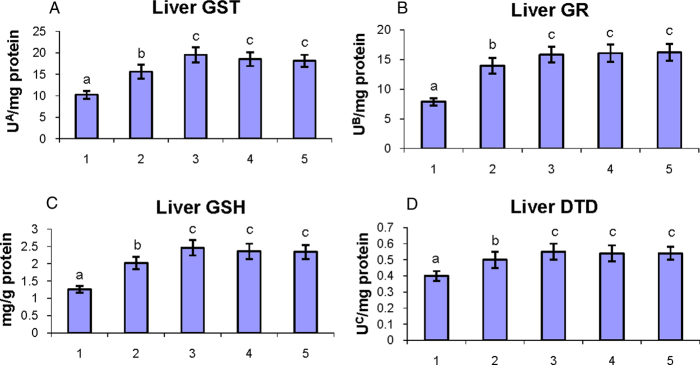
The status of phase II detoxification enzymes and GSH in the liver of control and experimental hamsters in each group are shown in Fig. 5A–D. The activities of phase II detoxification enzymes (GST, GR, and DTD) and GSH were significantly (P < 0.05) decreased in DMBA-alone-painted hamsters as compared to control hamsters. Oral administration of geraniol to DMBA-painted hamsters significantly (P < 0.05) restored the levels of phase II detoxification enzymes and GSH to the near-normal range.
Figure 5.
The activities of phase II detoxification enzymes (GST, GR, and DTD) and GSH in the liver of control and experimental hamsters. (1) DMBA, (2) DMBA + geraniol (250 mg/kg b.w.), (3) Geraniol alone (250 mg/kg b.w.), (4) Corn oil + liquid paraffin, (5) Control. Bars are mean ± SD for 10 hamsters in each group. a–cValues not sharing a common superscript letter (a, b, and c) in the same column differ significantly at P < 0.05 (DMRT). UA – micromoles of 1-chloro-2,4-dinitrobenzene-reduced glutathione conjugate formed/minute. UB – micromoles of NADPH oxidized/hour. UC – micromoles of 2,6-dichlorolindophenol reduced/minute.
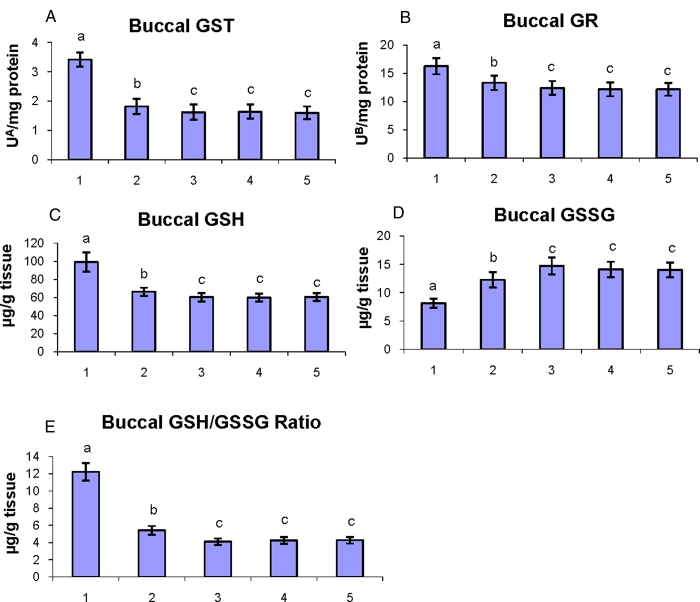
The status of phase II detoxification enzymes (GST and GR) and glutathione content (GSH, GSSG, and GSH/GSSG ratio) in the buccal mucosa of control and experimental hamsters in each group are shown in Fig. 6A–E. The concentrations of phase II detoxification enzymes and glutathione content in the buccal mucosa were disturbed (P < 0.05) (GST, GR, GSH, and GSH/GSSG ratio were increased; GSSG was decreased) in tumor-bearing hamsters as compared to control hamsters. Oral administration of geraniol to DMBA-treated hamsters significantly (P < 0.05) restored the status of phase II detoxification enzymes and glutathione content toward normal.
Figure 6.
The status of phase II detoxification enzymes (GST and GR) and glutathione content (GSH, GSSG, and GSH/GSSG ratio) in the buccal mucosa of control and experimental hamsters. (1) DMBA, (2) DMBA + geraniol (250 mg/kg b.w.), (3) Geraniol alone (250 mg/kg b.w.), (4) Corn oil + liquid paraffin, (5) Control. Bars are mean ± SD for 10 hamsters in each group. a–cValues not sharing a common superscript letter (a, b, and c) in the same column differ significantly at P < 0.05 (DMRT). UA – micromoles of 1-chloro-2,4-dinitrobenzene-reduced glutathione conjugate formed/minute. UB – micromoles of NADPH oxidized/hour.
Discussion
In recent years, researches on chemoprevention have enormously increased in parallel with an increased understanding of carcinogenesis. Although several chemopreventive and chemotherapeutic agents have shown promising results, they also produce side effects at desired doses. Studies are therefore warranted to investigate a non-toxic and safe chemopreventive agent against carcinogenesis. The purpose of the present study is to explore the chemopreventive potential of geraniol in DMBA-induced oral carcinogenesis.
Human as well as experimental oral carcinogenesis proceeds through sequential pathological changes from hyperplasia through dysplasia to malignant neoplasm. In the present study, all the hamsters treated with DMBA alone for 14 weeks developed tumors in their buccal pouches and thus the incidence of tumor formation was 100%. Also, severe hyperplasia, keratosis, and dysplasia were noticed in hamsters treated with DMBA alone. The tumors were histopathologically confirmed as well-differentiated squamous cell carcinoma. The tumor cell contains hyperchromatic nuclei with keratin pearl formation. Control hamsters and hamsters treated with geraniol alone showed well-defined intact and well-organized epithelial layers. Burke et al.32 demonstrated that Syrian golden hamsters fed geraniol or farnesol at 20 g/kg diet exhibited complete inhibition of PC-1pancreatic tumor growth. Burke et al.33 also reported that human BxPC3 pancreatic cancer cells treated with farnesol, geraniol, or perillyl alcohol exhibited a 3–10-fold increase in apoptosis and higher Bak protein expression than the controls. Cardozo et al.34 reported that geraniol showed promising chemopreventive effects during promotion of hepatocarcinogenesis by inducing apoptosis. He et al.35 demonstrated that isoprenoids suppressed the growth of murine B16 melanomas in vitro and in vivo. Yu et al.36 reported that geraniol suppressed the growth of hepatomas and melanomas transplanted to rats and mice. Carnesecchi et al.37 suggested that the use of geraniol as an adjuvant drug in combination with 5-fluorouracil may potentially represent an interesting approach for the treatment of colorectal cancer. Carnesecchi et al.38 suggested that polyamine metabolism is presumably a target in the anti proliferative properties of geraniol. In the present study, though oral administration of geraniol at a dose of 250 mg/kg b.w. to DMBA-treated hamsters completely prevented the tumor formation, moderate hyperplasia and mild dysplasia were noticed.
Tumor cells express biochemical variables in a manner different from their normal counterparts. DMBA generally manifests oral carcinogenesis in the buccal mucosa of hamsters through the formation of DNA adducts, chronic inflammation, and overproduction of ROS.39 Overproduction of ROS in the system causes oxidative modification of nucleic acids, which can lead to transformation of normal cell into malignant one.40 In the present study, decreased levels of tumor tissue LPO by-products were accompanied by increased levels of plasma LPO by-products in hamsters painted with DMBA alone. Several studies documented that rapidly proliferating tumor cells showed lower LPO by-products, as there is an inverse relationship between LPO and cell proliferation.6–8 Also, low availability of lipid peroxidative substrates such as polyunsaturated fatty acid, due to decreased levels of phospholipids, in oral tumors has been reported.41,42 Decreased levels of tumor tissue LPO by-products could therefore be due to rapid cell proliferation occurring in the hamster buccal pouch carcinogenesis or due to low availability of LPO substrates.
Changes in the antioxidant status in the cell play a key role in carcinogenesis. Overproduction of H2O2 has been shown in several tumor tissues.43 Decreased activity of SOD and CAT has been well documented in buccal mucosa tumor tissues.6,40 Our results corroborate with these previous findings. GPx and its co-substrate reduced glutathione have regulatory role in cellular proliferation. Increased activity of GPx and reduced glutathione content has been reported in tumor tissues of experimental animals.44 Increased content of vitamin E and reduced glutathione in tumor tissues indicate that tumor tissues sequester these antioxidants/nutrients to meet their nutritional demand as well as for their rapid growth.45,46 Oral administration of geraniol at a dose of 250 mg/kg b.w. to DMBA-treated hamsters improved the antioxidant defense mechanism to maintain the status of LPO by-products to a normal range in the buccal mucosa. Present results indicate that geraniol might have inhibited abnormal cellular proliferation by interfering with metabolic activation of DMBA in the buccal mucosa.
In the present study, the levels of plasma TBARS, LOOH, and CD were significantly increased, whereas the activities of enzymatic antioxidants and non-enzymatic antioxidants levels were decreased in hamsters painted with DMBA alone. Profound studies reported that ROS are excessively generated in the system, which subsequently enters into the plasma during pathological conditions including cancer.7,47 Moreover, ROS are excessively generated during the metabolic activation of DMBA into its active metabolite dihydrodiol epoxide.48 LPO by-products that are generated at primary sites could enter into circulation to provoke damage at other tissues.6 Increased levels of LPO by-products in plasma confirmed oxidative stress in tumor-bearing animals. Decreased activities of plasma enzymatic antioxidants are probably due to exhaustion of these enzymes to scavenge excessively generated LPO by-products or accumulated LPO by-products in the system.8 Oral administration of geraniol restored the status of plasma LPO by-products and antioxidants to the near-normal range, which indicates that geraniol has potent free radical scavenging (antioxidant) property during DMBA-induced oral carcinogenesis. Geraniol showed significant cyto-protective and antioxidant properties against t-BHP-induced oxidative stress.13 The antioxidant potential of geraniol is probably due to its lipophilic nature of structure that may have facilitated quick uptake and protective role against potent free radicals.
Liver phase I and phase II detoxification enzymes play crucial role in the detoxification of carcinogenic substances and thus measurement of these enzymes could help to assess the chemopreventive potential of geraniol during DMBA-induced oral carcinogenesis. In the present study, the activities of detoxification enzymes were altered significantly in both liver and buccal mucosa in hamsters treated with DMBA alone, which suggest that both liver and buccal mucosa were affected by toxic carcinogenic metabolites.8,11,49 Oral administration of geraniol significantly restored the status of phase I and phase II detoxification enzymes in the liver and buccal mucosa, which indicates that geraniol might have inhibited the metabolic activation or stimulated the excretion of carcinogenic metabolites during DMBA-induced oral carcinogenesis. The present study thus demonstrated the chemopreventive potential of geraniol in DMBA-induced hamster buccal pouch carcinogenesis.
Conclusion
The present study concludes that the chemopreventive potential of geraniol relies on its anti-lipid peroxidative and antioxidant function as well as modulatory effects on phase I and II detoxification enzymes to excrete the carcinogenic metabolites, during DMBA-induced hamster buccal pouch carcinogenesis. The major goal of cancer treatment is to increase or prolong the survival time of cancer patients. Although oral administration of geraniol completely prevented the tumor formation in the buccal pouch of DMBA-treated hamsters, we observed moderate hyperplasia and mild dysplasia. The present study suggests that geraniol has potent ability to prolong the survival time of tumor-bearing hamsters. Further studies are warranted to confirm the chemopreventive efficacy of geraniol by following DMBA + geraniol-treated experimental animals longer than 14 weeks.
References
- 1.Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of mouth cancer: a review of global incidence. Oral Dis 2000;6:65–74. [DOI] [PubMed] [Google Scholar]
- 2.Muwonge R, Ramadas K, Sankila R, Thomas G, Vinoda J. Role of tobacco smoking, chewing and alcohol drinking in the risk of oral cancer in Trivandrum, India; a nested case-control design using incident cancer cases. Oral Oncol 2008;44:446–54. [DOI] [PubMed] [Google Scholar]
- 3.Dipple A, Piggot MA, Moschel RC, Costantino N. Evidence that binding of 7,12-dimethylbenz[a]anthracene to DNA in mouse embryo cell cultures results in extensive substitution of both adenine and guanine residues. Cancer Res 1983;43:4132–5. [PubMed] [Google Scholar]
- 4.Lin LM, Chen YK. Creatine kinase iso-enzymes activity in serum and buccal pouch tissue of hamsters during DMBA-induced squamous cell carcinogenesis. J Oral Pathol Med 1991;20:479–85. [DOI] [PubMed] [Google Scholar]
- 5.Magon de la Villehuchet A, Brack M, Dreyfus G, Oussar Y, Bonnefont-Rousselot D, Chapman MJ, et al.. A machine-learning approach to the prediction of oxidative stress in chronic inflammatory disease. Redox Rep 2009;14:23–33. [DOI] [PubMed] [Google Scholar]
- 6.Manoharan S, Panjamurthy K, Menon VP, Balakrishnan S, mary Alias Linsa. Protective effect of withaferin-A on tumor formation in 7,12 dimethylbenz(a)anthracene induced oral carcinogenesis in hamsters. Ind J of Exp Biol 2009;47:16–23. [PubMed] [Google Scholar]
- 7.Manoharan S, Balakrishnan S, Menon VP, Alias LM, Reena AR. Chemopreventive efficacy of curcumin and piperine during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Singapore Med J 2009;50:139–46. [PubMed] [Google Scholar]
- 8.Manoharan S, VasanthaSelvan M, Silvan S, Baskaran N, Singh AK, Kumar VV. Carnosic acid: a potent chemopreventive agent against oral carcinogenesis. Chemico-Biol Int 2010;188:616–22. [DOI] [PubMed] [Google Scholar]
- 9.Shimada T. Xenobiotic-metabolising enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab Pharmacokinet 2006;21:257–76. [DOI] [PubMed] [Google Scholar]
- 10.McLellan LI, Wolf CR. Glutathione and glutathione dependent enzymes in cancer drug resistance. Drug Resist Updat 1999;2:153–64. [DOI] [PubMed] [Google Scholar]
- 11.Manikandan P, Vidjaya Letchoumy P, Gopalakrishnan M, Nagini S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and in vivo modulation of biomarkers of chemoprevention in the hamster buccal pouch carcinogenesis model. Food & Chem Toxicol 2008;46:2332–43. [DOI] [PubMed] [Google Scholar]
- 12.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53:4290–302. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari M, Kakkar P. Plant derived antioxidants-geraniol and camphene protects rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol In Vitro 2009;23:295–301. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Viljoen AM. Geraniol-A review of a commercially important fragrance material. S Afr J Bot 2010;76:643–51. [Google Scholar]
- 15.Ong TP, Heidor R, De Conti A, Dagli MLZ, Moreno FS. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis 2006;27:1194–203. [DOI] [PubMed] [Google Scholar]
- 16.Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids 1987;45:337–51. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351–8. [DOI] [PubMed] [Google Scholar]
- 18.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxides in low density lipoprotein. Anal Biochem 1992;202:384–9. [DOI] [PubMed] [Google Scholar]
- 19.Rao KS, Recknagel RO. Early onset of lipid peroxidation in rat liver after carbon tetrachloride administration. Exp Mol Pathol 1968;9:271–8. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Kelly BM. The effect of sodium nitrite on red cell GSH. Experientia 1963;19:96–7. [DOI] [PubMed] [Google Scholar]
- 21.Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol 1984;105:138–47. [DOI] [PubMed] [Google Scholar]
- 22.Palan PR, Mikhail MS, Basu J, Romney SL. Plasma levels of antioxidant beta-carotene and alpha-tocopherol in uterine cervix dysplasias and cancer. Nutr Cancer 1991;15:13–20. [DOI] [PubMed] [Google Scholar]
- 23.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 1984;21:130–2. [PubMed] [Google Scholar]
- 24.Sinha AK. Colorimetric assay of catalase. Anal Biochem 1972;47:389–94. [DOI] [PubMed] [Google Scholar]
- 25.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science 1973;179:588–90. [DOI] [PubMed] [Google Scholar]
- 26.Habig WH, Pabst MJ, Jakoby WBC. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–9. [PubMed] [Google Scholar]
- 27.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol 1985;113:484–90. [DOI] [PubMed] [Google Scholar]
- 28.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502–22. [DOI] [PubMed] [Google Scholar]
- 29.Omura T, Sato R. The carbon monoxide binding pigment of liver microsomes. J Biol Chem 1964;239:2370–8. [PubMed] [Google Scholar]
- 30.Ernster L. DT-Diaphorase. In: , Estabrook RW, Pullman ME (eds.) Methods in enzymology. New York: Academic Press; 1967;10 pp. 309–17. [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 32.Burke YD, Ayoubi AS, Werner SR, McFarland BC, Heilman DK, Ruggeri BA, et al.. Effects of the isoprenoids perillyl alcohol and farnesol on apoptosis biomarkers in pancreatic cancer chemoprevention. Anticancer Res 2002;22:3127–34. [PubMed] [Google Scholar]
- 33.Burke YD, Stark MJ, Roach SL, Sen SE, Crowell PL. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 1997;32:151–6. [DOI] [PubMed] [Google Scholar]
- 34.Cardozo MT, de Conti A, Ong TP, Scolastici C, Purgatto E, Horst MA, et al.. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J Nutr Biochem 2011;22:130–5. [DOI] [PubMed] [Google Scholar]
- 35.He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 1997;127:668–74. [DOI] [PubMed] [Google Scholar]
- 36.Yu SG, Hildebrandt LA, Elson CE. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J Nutr 1995;125:2763–7. [DOI] [PubMed] [Google Scholar]
- 37.Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, et al.. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett 2004;215:53–9. [DOI] [PubMed] [Google Scholar]
- 38.Carnesecchi S, Schneider Y, Ceraline J, Duranton B, Gosse F, Seiler N, et al.. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Ther 2001;298:197–200. [PubMed] [Google Scholar]
- 39.Balakrishnan S, Menon VP, Manoharan S. Chemopreventive efficacy of ferulic acid in 7,12-dimehylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis. J Med Food 2008;11:693–700. [DOI] [PubMed] [Google Scholar]
- 40.Mohan KV, Letchoumy PV, Hara Y, Nagini S. Combination chemoprevention of hamster buccal pouch carcinogenesis by bovine milk lactoferrin and black tea polyphenols. Cancer Invest 2008;26:193–201. [DOI] [PubMed] [Google Scholar]
- 41.Krishnakumar N, Manoharan S, Palaniappan PR, Venkatachalam P, Manohar MG. Chemopreventive efficacy of piperine in 7,12-dimethylbenz(a)anthracene (DMBA)- induced hamster buccal pouch carcinogenesis: an FT-IR study. Food Chem Toxicol 2009;47:2813–20. [DOI] [PubMed] [Google Scholar]
- 42.Diplock AT, Rice-Evans CA, Burdon RH. Is there a significant role for lipid peroxidation in the causation of malignancy and for antioxidants in cancer prevention? Cancer Res 1994;54:1952–6. [PubMed] [Google Scholar]
- 43.Pugalendhi P, Manoharan S. Chemopreventive potential of genistein and daidzein in combination during 7,12-dimethylbenz[a]anthracene (DMBA) induced mammary carcinogenesis in Sprague-Dawley rats. Pak J Biol Sci 2010;13:279–86. [DOI] [PubMed] [Google Scholar]
- 44.Balasenthil S, Saroja M, Ramachandran CR, Nagini S. Of humans and hamsters: comparative analysis of lipid peroxidation, glutathione and glutathione dependent enzymes during oral carcinogenesis. Br J Oral Maxillo Fac Surg 2000;38:267–70. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, et al.. Inhibition of 7,12-dimethylbenz(a)anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 2002;23:1307–13. [DOI] [PubMed] [Google Scholar]
- 46.Kavitha K, Manoharan S. Anticarcinogenic and antilipidperoxidative effects of Tephrosia purpurea (Linn) Pers in 7,12-dimethylbenz(a)anthracene (DMBA) induced hamster buccal pouch carcinoma. Indian J Pharmacol 2006;38:185–9. [Google Scholar]
- 47.Kolanjiappan K, Ramachandran CR, Manoharan S. Biochemical changes in tumor tissues of oral cancer patients. Clin Biochem 2003;36:61–5. [DOI] [PubMed] [Google Scholar]
- 48.Lim V, Korourian S, Todorova VK, Kaufmann Y, Klimberg VS. Glutamine prevents DMBA-induced squamous cell cancer. Oral Oncol 2009;45:148–55. [DOI] [PubMed] [Google Scholar]
- 49.Subapriya R, Velmurugan B, Nagini S. Modulation of xenobiotic-metabolizing enzymes by ethanolic neem leaf extract during hamster buccal pouch carcinogenesis. J Exp Clin Cancer Res 2005;24:223–30. [PubMed] [Google Scholar]