Abstract
Objective
Acetaminophen (APAP) is a substance that harms human health by stimulating free radical production. This study investigated the ability of Trifolium alexandrinum root (TAR) extract to reduce the hepatotoxicity induced by APAP in rats.
Methods
Animals were classified into four groups and treated for 6 weeks. Group 1: normal control-treated (saline); Group 2: TAR extract-treated (100 mg/kg); Group 3: APAP-treated; Group 4: APAP plus TAR extract.
Results
APAP significantly elevated AST (aspartate amino transferase), ALT (amino alanine transferase), ALP (alkaline phosphatase), GGTP (gamma glutamyl transpeptidase), bilirubin, and malondialdehyde with a significant decrease in glutathione, superoxide dismutase, glutathione peroxidase, catalase, and glutathione S-transferase compared with the control group. Administration of TAR extract combined with APAP improved the liver damage induced by APAP. Histopathological evidence, together with observed DNA fragmentation, supported the detrimental effect of APAP and the ameliorating effect of TAR extract on liver toxicity.
Conclusion
TAR extract has beneficial properties and can reduce the liver damage and toxicity induced by APAP.
Discussion
Free radical mediated processes have been implicated in the pathogenesis of many diseases. The protective effect of TAR root extract on APAP-induced hepatotoxicity in rats appears to be related to inhibition of lipid peroxidation and enhancement of antioxidant enzyme levels, in addition to a free radical scavenging action.
Keywords: Acetaminophen, Trifolium alexandrinum, Hepatotoxicity, DNA fragmentation
Introduction
Drug-induced hepatotoxicity has been attributed to be the cause of a major percentage of patient morbidity and mortality. It is well established that the liver plays an important role in drug metabolism and is, thus, highly susceptible to drug toxicity. Acetaminophen (APAP) is a widely used analgesic and antipyretic agent. It is metabolized by the liver via three main pathways; sulfonation, glucuronidation, and oxidation.1 Oxidation of APAP occurs in the hepatic microsomes and is primarily catalyzed by cytochrome P450.2 The process produces a highly reactive arylating compound called N-acetyl-p-benzoquinoneimine (NAPQI).3 In human liver microsomes, P450 enzymes were shown to be the principal catalysts of APAP activation.4
NAPQI is normally rapidly conjugated with glutathione (GSH) and is excreted eventually as the cysteinyl conjugate or the corresponding mercapturic acid. As long as the rate of formation of this toxin is not greater than the maximal rate of synthesis of GSH, there will be no damage to the cell or the organ.5 Hepatic synthesis of GSH, which is directly suppressed within the first few hours following ingestion of hepatotoxic dose of APAP, is overwhelmed and manifestations of toxicity appear when the GSH level falls below 30% of normal.6 When more NAPQI if formed than can be conjugated to GSH, the unbound NAPQI becomes toxic by binding to the macromolecules, including cellular proteins;7 some investigators have suggested that hepatic macrophages may have a pathogenic role, as inhibition of their function status attenuates the degree of APAP-induced hepatic injury by a mechanism that is independent of NAPQI production.8
During the last decades, considerable attention has been focused on the involvement of oxygen free radicals in various diseases. Active oxygen molecules such as superoxide and hydroxyl radicals have been demonstrated to play an important role in the inflammation process. Reactive oxygen species (ROS) are formed during increased metabolic states due to partial reduction of O2. ROS are part and parcel of life. However, during chronic or recurrent stress, there is increased utilization of energy and more ROS are produced. If the concentration of these ROS exceeds the body's capacity to neutralize them, they begin to harm the cells and, in cases of chronic stress, tissues and organs. ROS also damage mitochondria, the cellular components that produce energy, thereby reducing the capacity to maintain cellular energy levels.9
Many plant extracts and plant products have been shown to have significant antioxidant activity,10–11 which may be an important property of medicinal plants associated with the treatment of several ill-fated diseases including liver toxicity. Thus, herbal plants are considered a useful means to prevent and/or ameliorate certain disorders, such as diabetes, atherosclerosis, hepatotoxicity, and other complications.10,12 The plant Trifolium alexandrinum Linn. (family: Fabaceae) (common name: Egyptian clover, berseem clover) is a fodder plant and is distributed all over Asia, Europe, and the USA. Earlier phytochemical investigations of T. alexandrinum have revealed the presence of terpenoid glycosides, amino acids and their derivatives, proteins, flavonoids and their glycosides, isoflavonoids, and fatty acids in different parts of the same plant.13,14 Daily intake of (water, hexane, and ethanolic) extracts of the flower head of T. alexandrinum in drinking water for 4 weeks immediately after diabetes induction in male rats has been reported to cause significant decreases in glucose and glycated hemoglobin levels and an increase in insulin level. It also greatly improved the levels of serum lipid parameters and significantly decreased lipid peroxidation in addition to increasing the hepatic GSH content significantly.15 It contains a good yield of antioxidant enzyme and a number of flavonoids and phenolic components, which have been suggested to be responsible for such actions.16 However, there is no literature about its hepatoprotective activity in reference to APAP intoxication. In the present investigation, studies were conducted on an extract of T. alexandrinum for its effects on APAP-induced hepatotoxicity in animals.
Materials and methods
Chemicals
APAP, 5,5-dithiobis-2-nitrobenzoic acid (DTNB), dinitrophenylhydrazine, 1-chloro 2,4-dinitro benzoic acid (CDNB), and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical Co., (St Louis, MO, USA) and absolute ethanol was from Fisher Scientific (Hudson, NH, USA). Thiobarbituric acid (TBA) and reduced GSH were obtained from Buchs (Switzerland). Trichloroacetic acid was obtained from Merck K.G. (Darmstadt, Germany). All of the other chemicals used were of analytical grade.
Animals
Adult albino rats weighing around 180–200 g were purchased from the Faculty of Veterinary Medicine, Cairo University. The animal use protocol had been approved by the Institutional Animals Ethics Committee of Tanta University. The animals were housed in cages maintained under suitable conditions of temperature (25 ± 1°C), humidity (60 ± 10%), ventilation (continuous circulation of fresh air), and illumination (a 12 hours dark and 12 hours light cycle).
Extract preparation
The extraction procedure for the hydroalcoholic extract was carried out according to Charles et al.;17 100 g of T. alexandrinum dry roots were macerated in 1 l of methanol overnight at room temperature, filtered, and crude extract was collected. Another portion of 1 l of methanol was added to each sample residue and boiled for 2 hours under refluxing in a water bath and then filtered. The filtrate was collected in the previous crude extract. In the same way, 1 l of distilled water was added to each residue of the samples and left at room temperature overnight, and then filtered. The filtrate was added to the previous crude extract. Another 1 l volume of distilled water was added to the residue, boiled for 2 hours under the reflux condenser, and filtered. The hot water filtrate was added to the previous crude extract to form the hydroalcoholic crude extract. The final hydroalcoholic extract was evaporated to dryness under reduced pressure at 60°C, and then kept in dark bottles and stored in a deep freezer until usage.
Animal treatments
The rats were fasted overnight (16–18 hours) prior to administration of an intraperitoneal (i.p.) dose (100 mg/kg) of APAP, twice a day for 6 weeks dissolved in sterile normal saline warmed to 40°C. The rats were randomly assigned into four experimental groups; each group contained 10 rats, including the saline group as control (1 ml/kg for 6 weeks), the APAP group, Trifolium alexandrinum root (TAR) extract (100 mg/kg for 6 weeks), and APAP plus TAR extract. For co-administration of APAP and TAR extract, TAR was gavaged at a dose of 100 mg/kg immediately after i.p. injection of APAP for 6 weeks. We chose a minimal effective dose of 100 mg/kg of TAR root extract and observed no signs of mortality and toxicity up to 2000 mg/kg (data not shown).
Sample preparation
The animals were fasted overnight and clinical chemistry blood samples were obtained from the orbital sinus18 using capillary tubes (with and without heparin as per requirement) under mild ether anesthesia. Blood for hematology studies was collected into tubes containing EDTA as an anti-coagulant. After the animals were sacrificed, the liver was immediately removed, washed three times in ice cold saline, and blotted on ash-free filter paper, then used for preparation of tissue homogenates for estimation of tissue malondialdehyde (MDA) and GSH levels and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione S-transferase (GST), and catalase (CAT) enzymes.
Biochemical assays
Serum ALT, AST,19 alkaline phosphatase,20 bilirubin,21 GGTP,22 and total serum protein23 were estimated.
Preparation of tissue homogenates
Specimens were separated into two parts. Each piece was weighed and homogenized separately with a Potter Elvehjem tissue homogenizer. One part was homogenized in phosphate buffer saline (50 mM, pH 7.4) for estimation of protein content, SOD, CAT enzymes activities, and GSH level; the second was homogenized in potassium phosphate buffer 10 mM pH 7.4 for estimation of MDA, GST, and GPx activity. The crude tissue homogenate was centrifuged at 11,739 g, for 15 minutes in a cold centrifuge, and the resultant supernatant was used for different estimations. Protein content in tissue homogenate was measured according to the method of Lowry et al.23
Enzymatic and non-enzymatic antioxidant assays
Superoxide dismutase
The SOD activity was estimated in liver tissue homogenates by the method of Marklund and Marklund.24 Enzyme kinetic activity was recorded at 540 nm for 3 minutes and change in optical density (OD) per minute (ΔOD) was used to calculate % auto-oxidation inhibition to derive SOD units (U). One unit of SOD was defined as 50% inhibition of the auto-oxidation caused by a certain value of enzyme. The results of SOD activity have been expressed as U/mg protein.
Catalase
The CAT activity was measured in liver tissue homogenates by the method of Aebi25 The reaction mixture consisted of 2.9 ml buffer substrate (containing 0.1% H2O2 in 50 mM sodium potassium phosphate buffer, pH 7.0) and 0.1 ml sample in the final 3.0 ml assay volume. Change in absorbance was recorded for 150 seconds (every 15 seconds) at 240 nm. CAT activity was calculated using an extinction coefficient of 0.041 cm2/mmol and expressed as mmol H2O2 consumed per minute per mg of protein (mmol/minute/(mg protein)).
Glutathione peroxidase
GPx was determined in homogenate according to the method of Lawrence and Burk.26 This method is based on measuring the oxidation of NADPH by using hydrogen peroxide as the substrate. Absorbance was measured at 340 nm for 5 minutes, and an extinction coefficient of 6.22 × 103 was used for the calculation. The results were expressed as μmol/min/g tissue. The changes in the absorbance at 340 nm were recorded at 1 minute intervals for 5 minutes.
Glutathione S-transferase
The GST activity in tissue homogenates was performed as reported by Saggu and Kumer.27 The reaction mixture consisted of 1.0 ml potassium phosphate buffer (0.3 M, pH 6.5), 0.05 ml CDNB (7.5 mM in ethanol), 0.4 ml reduced GSH (7.5 mM), 0.2 ml of sample, and 1.35 ml of water in a total assay volume of 3.0 ml. The changes in absorbance were recorded at 340 nm. The enzymatic activity was calculated using a molar extinction coefficient of 9.6 × 103/M/cm and expressed as nmol CDNB conjugate formed/min/mg protein.
Reduced GSH
GSH content was determined with dithionitrobenzoic acid using the method described by Beutler et al.28 and was expressed in μmol GSH/mg protein. The method is based on the reduction of DTNB to produce a yellow compound. The reduced chromogen is directly proportional to GSH concentration and its absorbance can be measured at 412 nm.
MDA assay
MDA, a marker for lipid peroxidation, was measured according to Dousset et al.29 as modified by Saggu and Kumar.27 The MDA results were expressed as the nmol/mg protein.
Histopathological examination
For light microscopic investigations, samples from the liver were placed in 15% neutral buffered formalin and processed routinely for embedding in paraffin. Tissue sections (4 µm) were stained with hematoxylin and eosin (H&E) and examined under a photomicroscope.
Evaluation of DNA fragmentation
Liver samples were mechanically dissociated in hypotonic lysis buffer and centrifuged at 13 800 g for 15 minutes. The supernatant containing small DNA fragments was separated immediately and the pellet containing large DNA pieces was used for colorimetric determination by diphenylamine assay.30 The developed blue color was colorimetrically quantified spectrophotometrically at 578 nm. Percentage of DNA fragmentation in each sample was expressed by the formula: % DNA fragmentation = (OD supernatant/OD supernatant + OD pellet) × 100 (OD is the optical density).
Statistical analysis
Statistical analysis was performed using one-way analysis of variance followed by Student–Newman–Keuls test to assess significant differences among different groups. The results are considered to be significant when P < 0.05. All of the statistical analyses were performed using SigmaPlot, Systat Software program version 11.
Results
Effect of TAR extract on liver function
The leakage of ALT, AST, ALP, total bilirubin, and GGTP activities in the blood reflects indirectly the failure of liver function due to APAP-induced hepatotoxicity. Administration of APAP to the overnight fasted animals resulted in a 10-fold increase in serum ALT level and a 13-fold increase in AST levels compared with the control group (Table 1). The levels of ALP, total bilirubin, and GGTP were also markedly elevated in APAP-treated animals, indicating liver damage. Prophylactic treatment with APAP and TAR extract (100 mg/kg) prevented APAP-induced elevations compared with the APAP control group (Table 1).
Table 1.
Effect of TAR root extracts on liver function markers and total bilirubin in APAP intoxicated rats. Bar represents values that are mean ± SE, n = 10 animals per group
| Group | ALT (U/l) | AST (U/l) | ALP (U/l) | GGTP (U/l) | Total bilirubin (mg/dl) |
|---|---|---|---|---|---|
| Control | 28 ± 1.2 | 45 ± 2.1 | 168.5 ± 2.8 | 55 ± 1.2 | 0.17 ± 0.02 |
| TAR extract | 33 ± 2.8 | 48.5 ± 2.2 | 162.8 ± 3.6 | 53 ± 1.3 | 0.15 ± 0.03 |
| APAP | 285 ± 1.8a,b | 589 ± 3.1a,b | 259.5 ± 2.3a,b | 117 ± 1.8a,b | 1.32 ± 0.05a,b |
| APAP + TAR extract | 45 ± 1.1a,b,c | 106 ± 2.5a,b,c | 175.8 ± 2.9a,b,c | 72 ± 1.5a,b,c | 0.22 ± 0.02a,b,c |
Statistical significances: (aP < 0.005, compared with the normal control group; bP < 0.05, compared with the TAR extract group; and cP < 0.005, compared with the APAP group, where P < 0.001 in all of the compared groups).
Effect of TAR root extract on oxidative stress markers in APAP-induced toxicity
MDA, a toxic product of lipid peroxidation, was detected by TBA reactive substances analysis. Administration of APAP to experimental animals caused an increase in MDA production (4.06-fold) compared with the group administered with normal control. However, APAP-administered rats treated with 100 mg/kg of hydroalcholic root extract of T. alexandrium significantly reduced MDA amount by 68.4% in the liver homogenate, respectively, as compared with the APAP-intoxicated group (P < 0.001; F3,36 = 12.01) (Fig. 1A).
Figure 1.
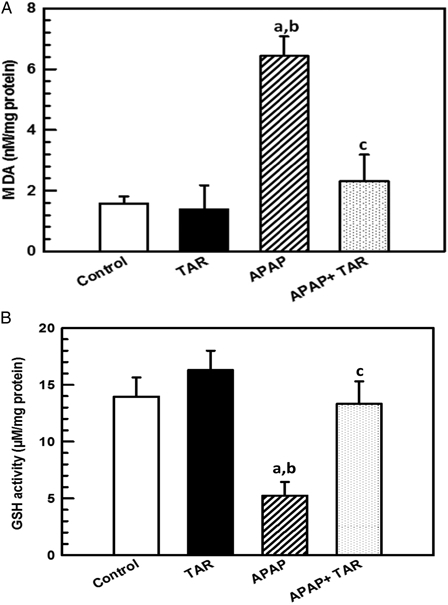
Effect of TAR root extracts administration on hepatic MDA (A) and GSH (B) levels in APAP-induced hepatotoxicity in experimental rats. Bar represents mean ± SE, n = 10 animals per group. Statistical significances: (aP < 0.05, compared with the normal control group; bP < 0.01, compared with the TAR extract group; and cP < 0.01, compared with the APAP group).
APAP causes marked depletion of hepatic GSH stores by 37.6% compared with the control group (Fig. 1B). When APAP-administered rats were treated with TAR root extract at a dosage of 100 mg/kg for 6 weeks, this significantly (P = 0.002) increased the level of GSH to 13.32 ± 2.01 µmol/mg protein (F3,36 = 8.23). These results show that TAR root extracts were able to preserve the amount of GSH, which is crucial to counteract the effects of APAP toxicity.
SOD is known to be the primary defense system against oxidative stress. APAP intoxication produces significant adverse effects on the redox status of the liver, which was evidenced by biochemical tests. The activity of SOD was significantly decreased in APAP intoxicated rats as compared with the control group (P = 0.001) (Fig. 2A).
Figure 2.
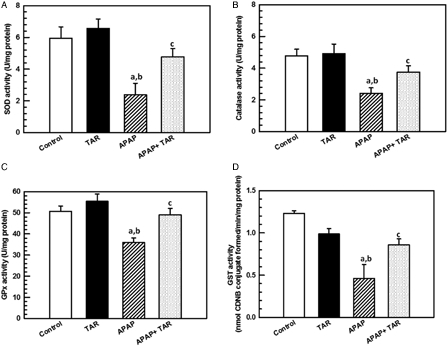
Effect of TAR root extract treatment on hepatic enzymatic antioxidants SOD (A), CAT (B), GPx (C), and GST (D) activity in APAP-induced hepatotoxicity in experimental rats. Bar represents mean ± SE, N = 10 animals per group. Statistical significances: (aP < 0.05, compared with the normal control group; bP < 0.01, compared with the TAR extract group; and cP < 0.05, compared with the APAP group).
CAT is one of the most important intracellular enzymes in the detoxification of the oxidant hydrogen peroxide. The activity of this enzyme was inhibited due to a high level of toxic metabolites. After administration of the TAR root extract, the activity of these enzymes was increased significantly (P < 0.05) (Fig. 2B).
APAP treatment caused a significant decrease in the levels of GPx and GST (P < 0.001) in liver tissue when compared with the control group. The treatment with TAR root extract at the dose of 100 mg/kg resulted in a significant increase of GPx and GST when compared with the drug-treated rats (Fig. 2C and D).
Effect of TAR root extracts on histopathology and DNA fragmentation
Normal morphology was observed in the control and TAR root extract groups following light microscopic examination of H&E-stained hepatic tissues (Fig. 3A and B). Examination of the control group indicated that 10 out of 10 rats had no evidence of hepatic necrosis. Examination of the TAR root extract groups showed normal hepatic parenchyma with clearing of the cytoplasm. Moderate degenerative changes and zonal centrilobular necrosis were evident in the APAP-treated group (Fig. 3C). In the APAP plus TAR extract group, there were mild changes and the absence of necrosis (Fig. 3D) compared with the APAP-treated mice.
Figure 3.
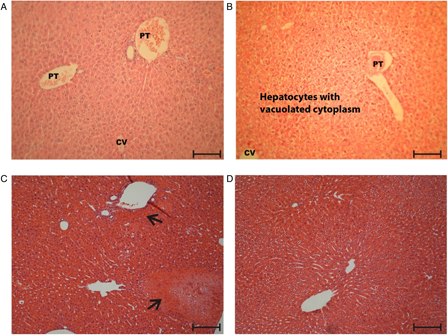
Hepatoprotective effect of TAR root extract on APAP-induced liver damage (H&E stain 100×). (A) Normal control group, (B) TAR extract group (100 mg/kg), (C) APAP group (100 mg APAP/kg, twice daily for 6 weeks), and (D) APAP + TAR extract. Arrows identify the necrotic areas. The bar is 100 µm. PT, portal triad; CV, central vein.
In the present study, we found that administration of APAP to rats resulted in a significant increase in hepatic DNA fragmentation (P < 0.001 vs. control group), indicating that APAP is capable of inducing hepatocyte apoptosis, which is a form of cell death that differs from necrosis. Treatment with 100 mg/kg of TAR root extracts inhibited the cellular damage significantly (P < 0.001, F3,36 = 5304.84) (Fig. 4).
Figure 4.
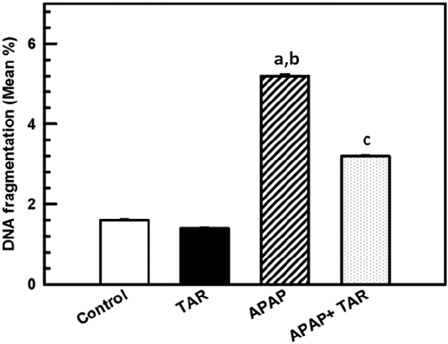
TAR extract treatment reduces APAP-induced hepatocyte apoptosis in the rat as represented by the accumulation of DNA fragmentation. DNA fragments in liver homogenates were detected at the end of the experimental period as described in the Materials and Methods Section. The animals were treated with control; 100 mg/kg of APAP (i.p.), twice a day for 6 weeks and TAR extract (100 mg/kg by gavage) for 6 weeks. For co-administration of APAP and TAR extract, the extract was gavaged immediately after i.p. injection of APAP. Data are means ± SE (n = 10). Statistical significances: (aP < 0.05, compared with the normal control group; bP < 0.001, compared with the TAR extract group; and cP < 0.01, compared with the APAP group).
Discussion
APAP is a known antipyretic and an analgesic, which produces hepatic necrosis in high doses. It is established that a fraction of APAP is converted via the cytochrome P450 pathway to a highly toxic metabolite, NAPQI,4 which is normally conjugated with GSH and excreted in urine. APAP overdose depletes the GSH stores, leading to the accumulation of NAPQI, mitochondrial dysfunction,31 and the development of acute hepatic necrosis. Several P450 enzymes are known to play an important role in APAP bioactivation to NAPQI. P4502E1 has been suggested to be the primary enzyme for APAP bioactivation in liver microsomes.5 Studies demonstrated that APAP-induced hepatotoxicity can be modulated by substances that influence P450 activity.25
In the assessment of liver injury by APAP, the analysis of enzyme levels such as AST and ALT is largely used. Necrosis or membrane damage releases the enzyme into circulation and hence it can be measured in the serum. High levels of AST indicate liver damage. It catalyzes the conversion of alanine to pyruvate and glutamate and is released in a similar manner. ALT is more specific to the liver, and is thus a better parameter for detecting liver injury. Elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membranes in the liver.32 Serum ALP and bilirubin levels, on the other hand, are related to the function of hepatic cells. Administration of APAP caused a significant (P < 0.001) elevation of enzyme levels such as AST, ALT, ALP, GGTP, as well as total bilirubin, when compared with the control. There was a significant (P < 0.001) restoration of these enzyme levels on administration of the TAR extract (Table 1). The reversal of increased serum enzymes in APAP-induced liver damage by the extract may be due to the prevention of the leakage of intracellular enzymes by its membrane stabilizing activity. This is in concurrence with the frequently accepted view that serum levels of transaminases return to normal with the healing of hepatic parenchyma and the regeneration of hepatocytes.33
We observed that the lipid peroxidation levels in the APAP group were increased, which clearly indicates that there is significant hepatic damage due to APAP and this is further evident from the fact that there was elevation in the levels of various markers of hepatic damage like ALT, AST, ALP, GGTP, and bilirubin. Treatment with 100 mg/kg TAR root extract decreased the levels of lipid peroxidation.
Decrease in the enzyme activity of SOD is a sensitive index of hepatocellular damage and is the most sensitive enzymatic index in liver injury. SOD is one of the most vital enzymes in the enzymatic antioxidant defense system. It scavenges the superoxide anion to form hydrogen peroxide, thus diminishes the toxic effect caused by this radical. TAR root extract at the dose of 100 mg/kg protected the liver against APAP-induced hepatotoxicity and restored the levels of SOD (P = 0.013, F3,36 = 8.175). CAT is an enzymatic antioxidant widely distributed in all animal tissues, and the highest activity is found in the red cells and the liver. CAT decomposes hydrogen peroxide and protects the tissues from highly reactive hydroxyl radicals.34 Therefore, reduction in the activity of CAT may result in a number of lethal effects due to the assimilation of superoxide radical and hydrogen peroxide. A dose of 100 mg/kg of TAR root extract increases the level of CAT as compared with APAP. GSH is one of the most abundant tripeptide, non-enzymatic biological antioxidants present in the liver. It removes free radical species such as hydrogen peroxide, superoxide radicals, and maintains membrane protein thiols. Also, it is the substrate for GPx.35 Administration of TAR root extract significantly (P < 0.001) increased the levels of GPx and GST.
Similar studies by Yen et al.36 showed that treatment with hydroethanolic extract of Cuscuta chinensis prevented the progression of liver damage due to hepatotoxins in an APAP-induced experimental system. It enhanced the activity of antioxidant enzymes and diminished the amount of toxic lipid peroxides.
The hepatoprotective effect of the TAR root extract was further revealed by histopathological analysis. Histopathological findings of liver samples and DNA fragmentation analysis (Figs. 3 and 4) were in agreement with the results obtained in biochemical studies, indicating that the extract is able to inhibit APAP-induced hepatotoxicity. It is clear from the current study that the repeated dosage of APAP (i.p. injection of 100 mg/kg, twice per day for 6 weeks), reliably induced hepatotoxicities in the treated rats. These were evidenced by the significant ameliorating effects of the TAR extract on both the biochemical alterations and histological lesions caused by the APAP hepatotoxicities. Similar results were obtained in the studies of Adeneye et al.37 and Aouacheri et al.,38. Hence, the hepatoprotective effect of T. alexandrinum is probably due to the prevention of depletion of the antioxidants and preventing lipid peroxidation, thereby preventing DNA damage. The drug is remarkably safe with an excellent safety record in terms of unwanted side effects. However, accidental or intentional APAP overdosage could cause life-threatening liver damage and associated diseases.
Conclusion
T. alexandrinum can be considered as a potential source of natural antioxidant with hepatoprotective activity. Furthermore, detailed investigations on these plants are needed in order to identify and isolate the hepatoprotective components in the extract and to justify its use in polyherbal formulations prescribed in the treatment of liver disorders. Finally, the education of the public and medical profession is needed to increase awareness of the potential toxic effects of APAP overdose.
References
- 1.Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keisser H. Acetaminophen induced hepatic injury. V. Protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther 1974;16:676–84. [DOI] [PubMed] [Google Scholar]
- 2.Potter WZ, Davis DC, Mitchell JR, Jollow GJ, Gillette JR, Brodie BB. Aetaminphen-induced hepatic necrosis. III. Cytochrome p450-mediated covalent binding in vitro. J Pharmacol Exp Ther 1973;187:203–10. [PubMed] [Google Scholar]
- 3.Dahlin DC, Miwa GT, Lu YA, Nelson DS. N-acetyl-p-benzoquinoneimine: a cytochrome p-450 mediated oxidation product of acetaminophen. Proc Natl Acad Sci 1984;81:1327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raucy JL, Lasker HM, Lieber CS, Black M. Acetaminophen activation by human liver cytochromes p-450 II EI and p-450 IA2. Arch Biochem Biophys 1989;27:270–83. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen induced hepatic injury. V. Protective role of glutathione. J Pharmacol Exp Ther 1973;187:211–7. [PubMed] [Google Scholar]
- 6.Makin AJ, Williams R. Acetaminophen induced hepatotoxicity: predisposing factors and treatments. Adv Int Med 1997;42:453–83. [PubMed] [Google Scholar]
- 7.Vermeulen NPE, Bassems JCM, Van de Straat R. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism based prevention. Drug Metab Rev 1992;24:367–407. [DOI] [PubMed] [Google Scholar]
- 8.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology 1995;21:1045–50. [PubMed] [Google Scholar]
- 9.Halliwell B. Antioxidants sense or speculation. Nutr Today 1994;29(6):15–9. [Google Scholar]
- 10.AL-Howiriny TA, Adnan J, AL-Rehailyb A, Joanna R, Polsc J, Porter S,. et al. Three new diterpenes and the biological activity of different extracts of Jasonia montana. Nat Prod Res 2005;19:253–65. [DOI] [PubMed] [Google Scholar]
- 11.Hussein MA. Protective effect of green tea extract against paraquat-induced toxicity in rat hepatocytes. Bull Fac Pharm Cairo Univ 2009;47:185–93. [Google Scholar]
- 12.Hussein MA. Antidiabetic and antioxidant activity of Jasonia montana extract in streptozotocin-induced diabetic rats. Saudi Pharm J 2008;16:214–21. [Google Scholar]
- 13.Temine S, Guler N. Trifolium L. – a review on its phytochemical and pharmacological profile. Phytother Res 2009;23:439–46. [DOI] [PubMed] [Google Scholar]
- 14.Sharaf M. Chemical constituents from the seeds of Trifolium alexandrinum. Nat Prod Res 2008;22:1620–3. [DOI] [PubMed] [Google Scholar]
- 15.Amer M, El-Sayed EH, Ahkam EG. Effects of Trifolium alexandrinum extracts on streptozotocin-induced diabetes in male rats. Ann Nutr Metab 2004;48:343–47. [DOI] [PubMed] [Google Scholar]
- 16.Rouhi HR, Aboutalebian MA, Moosavi SA, Karimi FA, Karimi F, Saman M,. et al. Change in several antioxidant enzymes activity of Berseem clover (Trifolium alexandrinum L.) by priming. Int J Agric Sci 2012;2(3):237–43. [Google Scholar]
- 17.Charles DJ, Morales R, Simon E. Essential oil content and chemical composition of hydroalcoholic extract of fennel. New crops 1993;570–3. [Google Scholar]
- 18.Riley V. Adaptation of orbital bleeding technique to rapid serial blood studies. Proc Soc Exp Biol Med 1960;104:751–4. [DOI] [PubMed] [Google Scholar]
- 19.Reitman S, Frankel S. Colorimeteric determination of GOT and GPT activity. Am J Clin Path 1957;28:56–63. [DOI] [PubMed] [Google Scholar]
- 20.Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J Biol Chem 1946;164:321–9. [PubMed] [Google Scholar]
- 21.Pearlman FC, Lee RT. Detection and measurement of total bilirubin in serum, with use of surfactants as solubilizing agents. Clin Chem 1974;20:447–53. [PubMed] [Google Scholar]
- 22.Rosalki SB. Gamma-glutamyltranspeptidase. In: Bodanski O, Latner AL, (eds.) Advances in clinical chemistry. vol. 17 New York, London: Academic Press; 1975. p. 53–107. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–72. [PubMed] [Google Scholar]
- 24.Marklund A, Marklund G. Involvement of the superoxide anion radical in the auto oxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem 1974;47:469–74. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. Catalase. In: Bergmeyer HU, (ed.) Methods of enzymatic analysis. New York: Academic Press; 1984. p. 673–84. [Google Scholar]
- 26.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976;71:952–8. [DOI] [PubMed] [Google Scholar]
- 27.Saggu S, Kumar R. Effect of seabuckthorn leaf extracts on circulating energy fuels, lipid peroxidation and antioxidant parameters in rats during exposure to cold, hypoxia and restraint (C–H–R) stress and post stress recovery. Phytomedicine 2008;15:437–46. [DOI] [PubMed] [Google Scholar]
- 28.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8. [PubMed] [Google Scholar]
- 29.Dousset JC, Trouilh M, Fogliettis MJ. Plasma malonaldehyde levels during myocardial infarction. Clin Chem Acta 1983;53:153–61. [DOI] [PubMed] [Google Scholar]
- 30.Perandones CE, lllera AV, Peckham D, Stunzl LL, Ashman RF. Regulation of apoptosis in vitro in mature murine spleen T-cell. J Immunol 1993;151:3521–29. [PubMed] [Google Scholar]
- 31.Parmar D, Kandakar M. Mitochondrial ATPase: a target for paracetamol-induced hepatotoxicity. Eur J Pharmacol 1995;293:225–29. [DOI] [PubMed] [Google Scholar]
- 32.Drotman R, Lawhan G. Serum enzymes are indications of chemical induced liver damage. Drug Chem Toxicol 1978;1:163–171. [DOI] [PubMed] [Google Scholar]
- 33.Thabrew M, Joice P. A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med 1987;53:239–41. [DOI] [PubMed] [Google Scholar]
- 34.Chance B, Greenstein DS. The mechanism of catalase actions-steady state analysis. Arch Biochem Biophys 1992;37:301–39. [DOI] [PubMed] [Google Scholar]
- 35.Prakash J, Gupta SK, Singh N. Chemopreventive activity of Withania somnifera in experimentally induced fibro sarcoma tumors in Swiss albino rats. Phytother Res 2001;15:200–4. [DOI] [PubMed] [Google Scholar]
- 36.Yen FL, Wu TH, Lin LT, Lin CC. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol 2007;111:123–8. [DOI] [PubMed] [Google Scholar]
- 37.Adeneye AA, Olagunju JA, Elias SO, Olatunbosun DO, Mustafa AO, Adeshile OI,. et al. Protective activities of the aqueous root extract of Harungana madagascariensis in acute and repeated acetaminophen hepatotoxic rats. Int J Appl Res Nat Prod 2008;3:29–42. [Google Scholar]
- 38.Aouacheri W, Saka S, Djafer R, Lefranc G. Protective effect of diclofenac towards the oxidative stress induced by paracetamol toxicity in rats. Ann Biol Clin (Paris) 2009;67(6):619–27. [DOI] [PubMed] [Google Scholar]


