Abstract
Objective: The aim of this study was to investigate the effects of lycopene (Lyc) on methotrexate (Mtx)-induced intestinal damage in rats.
Method: Twenty-eight male Sprague Dawley rats were divided into four equal groups: control, Mtx, Lyc, and Mtx-L.
Control group: Rats were given only the vehicle.
Lyc group: Rats were given Lyc (10 mg/kg) with corn oil by oral gavage for 10 days.
Mtx group: Rats were injected intraperitoneally with a single dose of 20 mg/kg of Mtx and given corn oil by oral gavage.
Mtx-L group: Rats were treated with Lyc (10 mg/kg) for 10 days after a single dose of Mtx (20 mg/kg). All of the rats were euthanized using terminal anesthesia, and the intestinal tissues were removed for histological examination and for pro-inflammatory cytokine measurement (tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β)), total oxidative status (TOS), total antioxidant capacity (TAC), and oxidative stress index (OSI).
Results: Mtx administration increased histopathological damage and increased TNF-α, IL-1β, TOS, TAC, and OSI levels in the small intestine tissues. Lyc therapy applied to the Mtx-L group provided significant improvement in all parameters of histopathological damage to the small intestine and significantly reduced the levels of IL-1β, TOS, and OSI in the intestinal tissues.
Conclusions: The results of this study indicate that Lyc might be useful for protecting intestinal damage induced by Mtx in rats by reducing the increased oxidative stress and pro-inflammatory cytokine (IL-1β) levels.
Keywords: Lycopene, Intestinal injury, Methotrexate, TNF-α, IL-1β, Oxidative stress
Introduction
Methotrexate (Mtx), an antineoplastic and immunosuppressive agent commonly used in the treatment of malignancies and various inflammatory diseases, is toxic not only to cancer cells but also to normal cells. Its cytotoxic structure causes life-threatening side effects, such as intestinal injury, and as a result of this, use of this agent is often limited.1–3 While the mechanism of Mtx-induced intestinal injury has not yet been fully elucidated, many studies have shown that reactive oxygen species play an important role in its pathogenesis. This mechanism is probably related to the increase in oxidative stress.4–6 Mtx administration induces oxidative stress and significantly reduces antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase in the intestinal mucosa of rats.7 Antioxidant agents that have been shown to have beneficial effects on Mtx-induced intestinal damage are ascorbic acid, melatonin, N-acetylcysteine, and vitamin A and these findings support the theory of oxidative stress.4,8–10 It is also currently believed that pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6, may also play a role in Mtx-induced intestinal damage. Human and animal experiments have shown that TNF-α inhibitors repair mucositis. It has been suggested that inflammation might have a role in the pathogenesis of Mtx-induced nephrotoxicity, intestinal damage, and pneumonitis.1,11–14 These cytokines have all been demonstrated in the mucosa and peripheral blood of patients undergoing cancer treatment.11,12
Lycopene (Lyc) is a carotenoid pigment produced by vegetables and fruits, such as tomatoes. It is one of the most powerful antioxidant, anticancer, and anti-inflammatory agents among all the dietary carotenoids,15–18 and it has been shown to be effective in reducing organ toxicity, e.g., pancreas, testis, and kidneys.19–22 Recent studies have indicated that Lyc plays an important role in the prevention of the gastrointestinal tract cancer. It protects mammalian cells from lipid peroxidation and oxidative DNA damage and inhibits the proliferation of cancer cells while accelerating the intestinal mucosal cell proliferation.23,24
Fruits and vegetables including Lyc have a very long history of use in the diet of humans without any problems. Additionally, the studies investigating the safety of Lyc support this. The deposition of Lyc in plasma, liver, and other tissues had no adverse effects and no teratogenic effects were observed in rat studies.25–29 The antioxidant, anticancer, and anti-inflammatory properties of Lyc are thought to be primarily responsible for its beneficial effects.24,30–32
Several antioxidant agents have been shown to be effective experimentally, by reducing the increased oxidative stress and pro-inflammatory cytokine levels in intestinal injury caused by Mtx. However, no study has been performed yet to evaluate the effects of Lyc on intestinal injury by Mtx in rats. Therefore, the aim of this study was to investigate the effects of Lyc on intestinal injury caused by Mtx in rats.
Materials and methods
Chemicals
Mtx was purchased from a local pharmacy (Koçak Farma, Turkey). Lyc (Redivivo® 10% CWS/S-TG, DSM, Basel, Switzerland) was provided by DSM Nutritional Products, Istanbul, Turkey. TNF-α and IL-1β enzyme-linked immunosorbent assay (ELISA) kits and oxidative stress kits used for biochemical analysis were obtained from RayBiotech, Diaclone, and Rel Assay Diagnostics, respectively.
Animals
Twenty-eight healthy, adult male Sprague Dawley rats weighing 200–275 g were used in this study. The animals were obtained from Dollvet Animal Laboratory (Sanliurfa, Turkey), where the experimental protocol was performed. The animals were allowed to acclimate under standard laboratory conditions (12 hours light, 12 hours dark) in a room with controlled temperature (24 ± 3°C) for 1 week prior to the experimental study. The animals had free access to water and were fed a standard commercial pellet diet ad libitum. The Dollvet Animal Care and Use Committee approved the study (approval number: 2014/35). All experimental procedures were conducted in accordance with the Guide to the Care and Use of Laboratory Animals.
Experimental protocol
After 7 days of acclimatization, the rats were divided into four groups of seven rats each, as follows (Table 1):
Table 1. Experimental study protocol.
| Groups | 1* | 2* | 3* | 4* | 5* | 6* | 7* | 8* | 9* | 10* | 11* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | a,b | b | b | b | b | b | b | b | b | b | e |
| Lyc | b,c | b,c | b,c | b,c | b,c | b,c | b,c | b,c | b,c | b,c | e |
| Mtx | d | e | |||||||||
| Mtx-L | b,c,d | b,c | b,c | b,c | b,c | b,c | b,c | b,c | b,c | b,c | e |
*Days, a:0.9% NaCl(i.p.), b:Corn Oil (o.g.), c:Lycopene (10 mg/kg)(o.g.), d:Mtx (20 mg/kg) (i.p.), e:Euthanized.
Control Group: The rats in this group served as the controls and were administered the vehicle only.
Lyc Group: The rats were given Lyc (10 mg/kg) with corn oil by oral gavage for 10 days.
Mtx Group: The rats were injected intraperitoneally (i.p.) with a single dose of Mtx (20 mg/kg) (1, 2) and given corn oil by oral gavage.
Mtx-L Group: The rats were treated with Lyc (10 mg/kg) for 10 days after being administered a single dose of Mtx (20 mg/kg).
All of the rats were euthanized by terminal anesthesia (ketamine – 75 mg/kg i.p. and xylazine – 8 mg/kg i.p.), and the small intestines were removed immediately and divided into two equivalent sections. One of the sections was fixed with 10% buffered formalin solution at room temperature for histopathological evaluation and the other part was stored at −80°C for biochemical analysis.
Histopathological examination
The small intestine tissues were removed aseptically from all of the rats, cut into small sample pieces, and fixed in 10% formaldehyde solution. The tissue sections (5 µm) were mounted on glass slides and stained with hematoxylin–eosin to evaluate the intestinal structure. Each stained section was semiquantitatively evaluated under a light microscope (Olympus BX51 microscope with a magnification of ×200) by a histologist who did not know which treatment group each sample was from. The histologist used the scoring system devised by Duman et al. Scores were assigned for each criterion 0 (none), 1 (mild), 2 (moderate), and 3 (severe) using the following semiquantitative scale: (1) degeneration of surface and crypt epithelium, (2) degeneration of villus structure, and (3) inflammatory cell infiltration. Five pathological sections were prepared from each rat. The microscopic score of the ileum was calculated as the sum of the scores given to each criterion, and at least five microscopic areas were examined to score each specimen. A maximum score of nine indicated the most severe damage of the small intestine.2
Biochemical analysis
Tissue preparation and homogenization
Before the biochemical assays, small intestine tissues were weighed, broken down into very small pieces, and placed in empty glass tubes. A portion (1 ml) of 140 mM potassium chloride (KCl) solution per gram of tissue was added to each tube, and then all the tissues were homogenized in a motorized homogenizer. The homogenate was centrifuged at 2800 × g for 10 minutes at 4°C.33 The resulting supernatant was used to obtain the levels of total antioxidant capacity (TAC), total oxidative status (TOS), TNF-α, and IL-1β.
Determination of oxidative stress biomarkers
Measurement of TAC
The TAC of the fractions of the supernatant was determined using a novel automated measurement method developed by Erel.34,35 Using this method, the antioxidative effect of the sample against the potent free radical reactions initiated by the hydroxyl radical produced was measured. The results are expressed as mmol Trolox equivalent/g protein.
Measurement of TOS
The TOS of the supernatant fractions was determined using a novel automated measurement method developed by Erel.34,35 The assay results are expressed as µmol hydrogen peroxide (H2O2) equivalent/g protein.
Oxidative stress index
The percentage ratio of TOS level to TAC level was accepted as the oxidative stress index (OSI). The OSI value was calculated according to the following formula:
OSI (arbitrary unit) = TOS (µmol H2O2 equivalent/g protein)/TAC (mmol Trolox equivalent/g protein).
Determination of pro-inflammatory cytokines
TNF-α and IL-1β levels were measured using ELISA kits according to the manufacturer's instructions. TNF-α and IL-1β levels are expressed as pg/g protein.
Statistical analysis
All data are expressed as mean ± standard deviation. The use of the Kruskal–Wallis analysis of variance method was followed by the application of the non-parametric Mann–Whitney U test, with a Bonferroni correction for binary comparisons to evaluate the differences between the experimental groups. Probability values less than 0.05 were considered to be statistically significant. All the data were processed using the SPSS 16.0 for Windows Statistical Software Package (SPSS, Inc., Chicago, IL, USA).
Results
Histopathological results
In the Mtx group, Mtx caused damage to the bowel tissue across all histopathological parameters (degeneration of surface and crypt epithelium, degeneration of villus structure, and inflammatory cell infiltration) (Fig. 1-IIa–IIc). The differences in intestinal tissue damage between the Mtx group and the control group were statistically significant in all histopathological parameters. The group with the most tissue damage was the Mtx group, and the group with the least tissue damage was the control group (Table 2). There were no statistically significant histopathological differences between the Lyc group and the control group (Table 2). There was significant improvement in all histological parameters in the Mtx-L group compared with the Mtx group (Fig. 1-III; Table 2).
Figure 1.
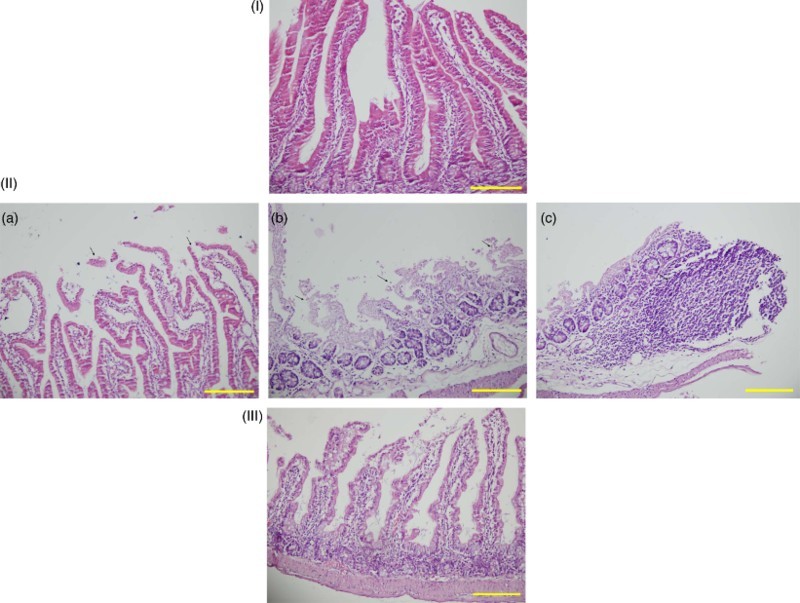
Sections of the small intestine were stained with hematoxylin–eosin, scale bar = 200 μm. (I) Normal histological appearance of the small intestine tissues in the control rats. (II) Histopathological effects of high dose methotrexate on the small intestine structure. (a) Degeneration of surface and crypt epithelium. (b) Degeneration of villus structure. (c) Inflammatory cell infiltration. (III) Section indicating that lycopene treatment in the Mtx-L group is providing significant histological improvement.
Table 2. Histopathologic examination results in rat small intestine tissues.
| Histopatalogic parameters | Groups | |||
|---|---|---|---|---|
| Controla | Lyca | Mtxa | Mtx-La | |
| Degeneration of surface and crypt epithelium | 0.00 (0.0–1.0) | 0.00 (0.0–1.0) | 1.00 (1.0–2.0)b | 0.50 (0.0–1.0)c |
| Degeneration of villus structure | 0.00 (0.0–1.0) | 0.00 (0.0–1.0) | 1.00 (1.0–2.0)b | 1.00 (0.0–1.0)c |
| İnflammatory cell infiltration | 0.00 (0.0–0.0) | 0.00 (0.0–0.0) | 1.00 (1.0–2.0)b | 0.00 (0.0–1.0)c |
aData were expressed as median (min–max).
bSignificance of Mtx compared with control.
cSignificance of Mtx-L compared with Mtx.
P < 0.01 is significant.
Biochemical results
Biochemical examination of the small bowel tissue samples showed improvements in all parameters in the Mtx group compared with the control group. However, only the increases in TNF-α and TOS were significant (Table 3). The Mtx-L group exhibited significant improvement in IL-1β, TOS, and OSI values (Table 3). There were no statistically significant differences in biochemical parameters between the Lyc and control groups.
Table 3. Biochemical assessment results in rat small intestine tissues.
| Groups | ||||
|---|---|---|---|---|
| Controla (n = 7) | Lyca (n = 7) | Mtxa (n = 7) | Mtx-La (n = 7) | |
| TNF-α (pg/g protein) | 1030 ± 176 | 1091 ± 100 | 1253 ± 114b | 1123 ± 120 |
| IL-1 β (pg/g protein) | 11.91 ± 3.09 | 11.92 ± 3.15 | 14.18 ± 2.73 | 10.64 ± 2.78c |
| TAC (mmol Trolox Eq/g protein) | 0.43 ± 0.06 | 0.46 ± 0.07 | 0.45 ± 0.08 | 0.46 ± 0.07 |
| TOS (μmol H2O2 Eq/g protein) | 6.93 ± 058 | 7.57 ± 2.25 | 8.84 ± 1.54b | 7.20 ± 1.33c |
| OSI (arbitrary units) | 1.63 ± 0.24 | 1.66 ± 0.59 | 2.18 ± 0.82 | 1.62 ± 0.65c |
TNF-α, tumor necrosis factor-alpha; IL-1β, interlekuin-1 beta; TAC, total antioxidant capacity; TOS, total oxidative status; OSI, oxidative stress index.
aData were expressed as mean ± standard deviation.
bSignificance Mtx compared with control
cSignificance Mtx-L compared with Mtx.
P < 0.01.
Discussion
The results of this experimental study revealed that Lyc reduced pathological damage, oxidative stress, and pro-inflammatory cytokine levels in rat small intestine tissues. Lyc therapy applied to the Mtx-L group provided significant improvement in all histopathological parameters and in IL-1β, TOS, and OSI levels.
Several studies have indicated that increased oxidative stress and pro-inflammatory cytokine levels might be effective in Mtx-induced intestinal damage.14,36–38 In the present pathological examination of intestinal tissue damage and tissue biochemical parameters, the rats in the Mtx group had the highest levels. However, only the TOS and TNF-α levels of the Mtx group were significantly higher than those of the control group, suggesting that both oxidative stress and increased levels of pro-inflammatory cytokines might be involved in the intestinal damage caused by Mtx.
The microscopic examination of tissue damage showed that in the Mtx-L group there was significant improvement in all of the histopathological parameters. This result can be interpreted to mean that the dose and length of time of Lyc administration were effective in treating the small bowel damage caused by Mtx.
Antioxidants inhibit damage to cells by binding the free oxygen radicals. Antioxidants such as amifostine, ascorbic acid, melatonin, N-acetylcysteine, and resveratrol have been used in Mtx-induced hepatotoxicity, and beneficial effects on oxidative stress have been demonstrated. Lyc, a strong antioxidant, anti-inflammatory, and anticancer agent, has been used in nephrotoxicity caused by cisplatin and has been found to reduce oxidative stress.10,18,20,39 It has also been shown to be effective in reducing organ toxicity in the pancreas, testes, and kidneys.3–6 Because of this, the effects of Lyc on Mtx-induced small bowel injury were investigated, and the results of this research showed that OSI and TOS levels decreased significantly after Lyc treatment. This result was interpreted to mean that Lyc might be effective by reducing oxidative stress in the damage of the small intestine caused by Mtx.
Cytokines are molecules secreted by inflammatory cells against antigens that regulate immune and inflammatory responses and two of these cytokines, TNF-α and IL-1β, play active roles in inflammation. Mtx is an agent that causes an increase in inflammatory cytokines.39 Several studies have shown that Lyc treatment decreases elevated TNF-α levels.36–38,40 However, to the best of our knowledge, no previous studies have shown the effect of Lyc on IL-1β levels in intestinal injury caused by Mtx in rats. This study is the first to demonstrate that Lyc might be effective by reducing IL-1β levels. In this study, IL-1β levels decreased significantly in the Mtx-L group compared to the Mtx group.
In our study, all biochemical parameters in the intestinal tissues of the rats in the Mtx group were high when compared with those of the control group, but the only significant differences were in TNF-α and TOS. These results suggest that Mtx created an effective bowel injury. IL-1β only improved significantly in the Mtx-L group. This result was interpreted as an indication that the effectiveness of Lyc in treating the Mtx-induced intestinal damage might be because of the reduced IL-1β levels.
Finally, Lyc might be useful for protecting intestinal damage from Mtx in rats by reducing the increased IL-1β and oxidative stress levels. It is thought that new studies with Lyc should be conducted to reveal the role of Lyc in Mtx-induced intestinal injury.
Disclaimer statements
Contributors All authors contributed equally.
Funding None.
Conflict of interest All the authors have no conflict of interest to declare. This work was carried out at Harran University School of Medicine, Department of General Surgery.
Ethics approval Dollvet Animal Care and Use Committee has been approved the study (approval number: 2014/35).
References
- 1.Kim YJ, Song M, Ryu JC. Inflammation in methotrexate-induced pulmonary toxicity occurs via the p38 MAPK pathway. Toxicology 2009;256(3):183–90. doi: 10.1016/j.tox.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 2.Duman DG, Kumral ZNO, Ercan F, Deniz M, Can G, Çağlayan Yeğen B. Saccharomyces boulardii ameliorates clarithromycin- and methotrexate-induced intestinal and hepatic injury in rats. Br J Nutr 2013;110(3):493–9. doi: 10.1017/S000711451200517X [DOI] [PubMed] [Google Scholar]
- 3.Kesik V, Uysal B, Kurt B, Kismet E, Koseoglu V. Ozone ameliorates methotrexate-induced intestinal injury in rats. Cancer Biol Ther 2009;8(17):1623–8. doi: 10.4161/cbt.8.17.9203 [DOI] [PubMed] [Google Scholar]
- 4.Miyazono Y, Gao F, Horie T. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol 2004;39(11):1119–27. doi: 10.1080/00365520410003605 [DOI] [PubMed] [Google Scholar]
- 5.Kolli VK, Abraham P, Isaac B, Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig Dis Sci 2013;58(4):959–69. doi: 10.1007/s10620-012-2437-4 [DOI] [PubMed] [Google Scholar]
- 6.Vardi N, Parlakpinar H, Ozturk F, Ates B, Gul M, Cetin A, et al. . Potent protective effect of apricot and beta-carotene on methotrexate-induced intestinal oxidative damage in rats. Food Chem Toxicol 2008;46(9):3015–22. doi: 10.1016/j.fct.2008.05.039 [DOI] [PubMed] [Google Scholar]
- 7.Coleshowers CL, Oguntibeju OO, Ukpong M, Truter EJ. Effects of methotrexate on antioxidant enzyme status in a rodent model: peer reviewed original article. Med Technol SA 2010;24(1):4–9. [Google Scholar]
- 8.Yüncü M, Eralp A, Koruk M, Sari I, Bağci C, Inalöz S. Effect of vitamin A against methotrexate-induced damage to the small intestine in rats. Med Princ Pract 2004;13(6):346–52. doi: 10.1159/000080472 [DOI] [PubMed] [Google Scholar]
- 9.Ciralik H, Bulbuloglu E, Cetinkaya A, Kurutas EB, Celik M, Polat A. Effects of N-acetylcysteine on methotrexate-induced small intestinal damage in rats. Mt Sinai J Med 2006;73(8):1086–92. [PubMed] [Google Scholar]
- 10.Akbulut S, Elbe H, Eris C, Dogan Z, Toprak G, Otan E, et al. . Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J Gastroenterol 2014;20(29):10158. doi: 10.3748/wjg.v20.i29.10158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Logan RM, Stringer AM, Bowen JM, Yeoh ASJ, Gibson RJ, Sonis ST, et al. . The role of pro-inflammatory cytokines in cancer treatment-induced alimentary tract mucositis: pathobiology, animal models and cytotoxic drugs. Cancer Treat Rev 2007;33(5):448–60. doi: 10.1016/j.ctrv.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 12.Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol 2013;72(4):757–65. doi: 10.1007/s00280-013-2238-2 [DOI] [PubMed] [Google Scholar]
- 13.Asvadi I, Hajipour B, Asvadi A, Asl NA, Roshangar L, Khodadadi A. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci 2011;15(9):1003–9. [PubMed] [Google Scholar]
- 14.Kirbas A, Cure MC, Kalkan Y, Cure E, Tumkaya L, Sahin OZ, Pergel A. Effect of infliximab on renal injury due to methotrexate in rat. Iran J Kidney Dis 2015;9(3):221–9. [PubMed] [Google Scholar]
- 15.Bayramoglu G, Bayramoglu A, Altuner Y, Uyanoglu M, Colak S. The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology 2015;67(3):487–91. doi: 10.1007/s10616-014-9706-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darwish SF, El-Bakly WM, Arafa HM, El-Demerdash E. Targeting TNF-α and NF-κB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. PLoS One 2013;8(11):e79284. doi: 10.1371/journal.pone.0079284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yonar ME. Protective effect of lycopene on oxidative stress and antioxidant status in Cyprinus carpio during cypermethrin exposure. Environ Toxicol 2013;28(11):609–16. doi: 10.1002/tox.20757 [DOI] [PubMed] [Google Scholar]
- 18.Kim H. Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann N Y Acad Sci 2011;1229(1):99–102. doi: 10.1111/j.1749-6632.2011.06107.x [DOI] [PubMed] [Google Scholar]
- 19.Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 2005;212(2):116–23. doi: 10.1016/j.tox.2005.04.016 [DOI] [PubMed] [Google Scholar]
- 20.Dogukan A, Tuzcu M, Agca CA, Gencoglu H, Sahin N, Onderci M, et al. . A tomato Lyc complex protects the kidney from cisplatin-induced injury via affecting oxidative stress as well as Bax, Bcl-2, and HSPs expression. Nutr Cancer 2011;63(3):427–34. doi: 10.1080/01635581.2011.535958 [DOI] [PubMed] [Google Scholar]
- 21.Boeira SP, Filho CB, Del'Fabbro L, Roman SS, Royes LFF, Fighera MR, et al. . Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp Toxicol Pathol 2014;66(4):179–85. doi: 10.1016/j.etp.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Rao AV, Agarwal S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: a review. Nutr Res 1999;19(2):305–23. doi: 10.1016/S0271-5317(98)00193-6 [DOI] [Google Scholar]
- 23.Saada HN, Rezk RG, Eltahawy NA. Lycopene protects the structure of the small intestine against gamma-radiation-induced oxidative stress. Phytother Res 2010;24(2):204–8. doi: 10.1002/ptr.3091 [DOI] [PubMed] [Google Scholar]
- 24.Trejo-Solís C, Pedraza-Chaverrí J, Torres-Ramos M, Jiménez-Farfán D, Cruz Salgado A, Serrano-García N, et al. . Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid Based Complement Altern Med 2013. doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellert W, Deckardt K, Gembardt C, Schulte S, Van Ravenzwaay B, Slesinski RS. Thirteen-week oral toxicity study of synthetic lycopene products in rats. Food Chem Toxicol 2002;40(11):1581–8. doi: 10.1016/S0278-6915(02)00113-8 [DOI] [PubMed] [Google Scholar]
- 26.Papaioannou EH, Kyriakides ML, Karabelas AJ. Natural origin lycopene and its ‘green'downstream processing. Crit Rev Food Scie Nutr 2015. doi: 10.1080/10408398.2013.817381. [DOI] [PubMed] [Google Scholar]
- 27.McClain RM, Bausch J. Summary of safety studies conducted with synthetic lycopene. Regul Toxicol Pharmacol 2003;37(2):274–85. doi: 10.1016/S0273-2300(03)00004-7 [DOI] [PubMed] [Google Scholar]
- 28.Matulka RA, Hood AM, Griffiths JC. Safety evaluation of a natural tomato oleoresin extract derived from food-processing tomatoes. Regul Toxicol Pharmacol 2004;39(3):390–402. doi: 10.1016/j.yrtph.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 29.Jonker D, Kuper CF, Fraile N, Estrella A, Otero CR. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul Toxicol Pharmacol 2003;37(3):396–406. doi: 10.1016/S0273-2300(03)00013-8 [DOI] [PubMed] [Google Scholar]
- 30.Marcotorchino J, Romerier B, Gouranton E, Riollet C, Gleize B, Malezet-Desmoulins C, et al. . Lycopene attenuates LPS-induced TNF-α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol Nutr Food Res 2012;56(5):725–32. doi: 10.1002/mnfr.201100623 [DOI] [PubMed] [Google Scholar]
- 31.Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE, et al. . Lycopene and cardiovascular diseases: an update. Curr Med Chem 2011;18(8):1146–63. doi: 10.2174/092986711795029717 [DOI] [PubMed] [Google Scholar]
- 32.Bozkurt M, Em S, Oktayoglu P, Turkcu G, Yuksel H, Sarıyıldız MA, et al. . Carvacrol prevents methotrexate-induced renal oxidative injury and renal damage in rats. Clin Invest Med 2014;37(1):E19–25. [DOI] [PubMed] [Google Scholar]
- 33.Rabus M, Demirbağ R, Sezen Y, et al. . Plasma and tissue oxidative stress index in patients with rheumatic and degenerative heart valve disease. Türk Kardiyol Dern Ars 2008;36(8):536–40. [PubMed] [Google Scholar]
- 34.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem 2004;37(2):112–9. doi: 10.1016/j.clinbiochem.2003.10.014 [DOI] [PubMed] [Google Scholar]
- 35.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38(12):1103–11. doi: 10.1016/j.clinbiochem.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 36.He Q, Zhou W, Xiong C, Tan G, Chen M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. Mol Med Rep 2015;11(1):374–8. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y, Liu Y, Wang Y. Beneficial effect of lycopene on anti-diabetic nephropathy through diminishing inflammatory response and oxidative stress. Food Funct 2015;6(4):1150–6. doi: 10.1039/C5FO00004A [DOI] [PubMed] [Google Scholar]
- 38.Tunalı-Akbay T, Sehirli O, Ercan F, Sener G. Resveratrol protects against methotrexate-induced hepatic injury in rats. J Pharm Pharm Sci 2010;13(2):303–10. [DOI] [PubMed] [Google Scholar]
- 39.Hazewindus M, Haenen GR, Weseler AR, Bast A. Protection against chemotaxis in the anti-inflammatory effect of bioactives from tomato ketchup. PloS One 2014;9(12):e114387. doi: 10.1371/journal.pone.0114387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierine DT, Navarro MEL, Minatel IO, Luvizotto RAM, Nascimento AF, Ferreira ALA, et al. . Lycopene supplementation reduces TNF-α via RAGE in the kidney of obese rats. Nutr Diabetes 2014;4(11):e142. doi: 10.1038/nutd.2014.39 [DOI] [PMC free article] [PubMed] [Google Scholar]


