Abstract
Objectives: The present study was designed to evaluate the cardioprotective potential of edaravone on oxidative stress, anti-apoptotic, anti-inflammatory and ultrastructure findings in isoproterenol (ISO) induced myocardial infarction (MI) in rats.
Methods: Rats were pretreated with edaravone (1, 3, 10 mg/kg body weight-1 day-1) intraperitoneally. MI was induced by subcutaneous administration of ISO (85 mg/kg body weight-1) at two doses with 24h interval.
Results: ISO treated rats showed significant increase in the levels of thiobarbituric acid reactive substances (TBARS) and decreased levels of reduced glutathione, glutathione perdoxidase, glutathione reductase and glutathione-S- transferase in the cardiac tissues. Moreover, significant increase in the levels of lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), C - reactive protein and caspase-3 activity was observed in ISO treated group. Pretreatment of ISO intoxicated rats with edaravone showed significant decrease in the level of TBARS, increased activities of antioxidant enzymes and significantly decreased levels of LDH and CK-MB. Moreover, results also showed decreased C-reactive protein level, caspase-3 activity and maintained ultrastructure of the myocardial cells.
Discussion: Our study suggests that edaravone possess strong cardioprotective potential. Edaravone may have exhibited cardioprotective effects by restoring antioxidant defense mechanism, maintaining integrity of myocardial cell membrane, reducing apoptosis and inflammation against ISO induced MI and associated oxidative stress.
Keywords: Edaravone, Oxidative stress, Caspase-3, C-reactive protein, Isoproterenol, Myocardial infarction, Ultrastructure evaluation
Introduction
Oxidative stress is mainly associated with increased formation of reactive oxygen species (ROS) that play a crucial role in cardiovascular pathophysiology. Cardiovascular diseases (CVDs) are the most alarming of the disease predictions across the globe. Following myocardial infarction (MI), the progressive condition of heart failure is caused by remodeling of the cardiac tissue due to an imbalance between coronary blood supply and myocardium demand. According to a report of the World Health Organization (WHO), 17.3 million people died from CVDs in 2008 and 23.3 million people would die annually from CVDs by 2030.1
Isoproterenol (ISO) is a synthetic catecholamine that causes rigorous stress to the myocardium, resulting in an infarct-like necrosis of cardiac tissue.2 Biochemical and morphological changes are seen in the cardiac tissue of ISO-treated experimental rats that are similar to those observed in humans.3 Adameova et al.4 have reported that oxidative products of ISO play a critical role in the progression of contractile dysfunction leading to MI. Additionally, it leads to contractile irregularities as well as ultrastructural alterations in the cardiac tissue.5 The ISO-induced MI experimental model is widely used to assay drugs intended to be used in CVDs.
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is an effective and strong scavenger of free radicals. Edaravone has been broadly used in patients with acute ischemic stroke, due to its established neurovascular protective effects.6,7 Edaravone prevents free radical-initiated lipid peroxidative damage.8,9 Edaravone has been reported to have protective effects against experimental models of myocardial ischemia-reperfusion injury by reducing the myocardial necrotic area.10,11 Though ISO itself generates ROS, it may also be activating biochemical pathways responsible for altering cellular functions through elevation of oxidative stress that plays a crucial role in the progression of MI.12 Based on the above facts, the present study was designed to evaluate the effect of edaravone in ISO-treated MI in rats.
Materials and methods
Experimental animals
The study was approved by the Institutional Animal Ethics Committee, Jamia Hamdard, New Delhi; Approval no. 943. Wistar rats weighing 200–220 g were obtained from the Central Animal House Facility, Jamia Hamdard, New Delhi, housed in polypropylene cages (six in each cage) lined with rice husk, changed every 24 hours. They had free access to commercial pellet diet and water ad libitum. The animal house temperature was maintained at 25 ± 2°C.
Drugs and chemicals
Edaravone was procured from Tocris Bioscience, Bristol, United Kingdom; caspase-3 and C-reactive protein (CRP) kits were purchased from Immunology Consultant Laboratory, Inc., USA and Biovision Research Product, USA, respectively. Creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) kits were purchased from Crest Biosystems Ltd., India. All other chemicals used were of analytical grade purchased from Sigma Aldrich Chemicals Pvt. Ltd.
Selection of dose
Doses were selected on the basis of the available literature. We found that the majority of the published research was done with 1, 3, or 10 mg/kg doses.8,13 However, some research also has been done with 3 or 6 mg/kg doses.14
Experimental design
Rats were randomly divided into six groups having six rats each. Group 1 served as the normal control group and received normal saline for 28 days; Group II received only edaravone (10 mg/kg of body weight, i.p.) and served as per se group; Group III was considered as toxic control group and rats were injected ISO (85 mg/kg of body weight, s.c.) in two doses with a 24-hour interval. Groups IV, V, and VI were pretreated with edaravone (1, 3, and 10 mg/kg of body weight, i.p.)13 respectively for 28 days and then two doses of ISO with a 24-hour interval. After the end of this schedule, rats were anesthetized by anesthetic ether and euthanased by cervical decapitation. Blood samples were collected from tail vein and serum was separated by centrifugation at 3000 g for 30 minutes. The separated serum was preserved at −20°C for estimation of serum LDH, CK-MB, and CRP. Hearts were immediately excised, washed in ice-cold normal saline, and preserved in liquid nitrogen at −80°C for biochemical estimations.
Biochemical analysis in the myocardial tissue
The level of thiobarbituric acid reactive substances (TBRAS) in the cardiac tissue was estimated by the method of Okhawa et al.15 The activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST) were assayed by the methods of Marklund and Marklund,16 Clairborne,17 Wheeler et al.,18 Calberg and Mannervik,19 Habig et al.,20 respectively. The level of reduced glutathione in cardiac tissue was estimated by the method of Ellman.21 The content of protein in the cardiac tissue was determined by the method of Lowry et al.22
Biochemical analysis of cardiac marker enzymes in serum
Activities of CK-MB23 and LDH24 were measured in the serum as per the reported procedures.
Biochemical analysis of caspase-3 protease in myocardial tissue
Caspase-3 protease was determined as per the instructions provided in the kit.25
Biochemical analysis of inflammatory marker in serum
CRP in rat serum was determined as per the procedure described in the kit.26
Transmission electron microscopic evaluation of myocardium
Small pieces, approximately 1.0–1.5 mm, from the ventricle tissue of rat heart were taken and rinsed in 0.1 M phosphate buffer (pH 7.2) several times and immediately fixed in 2.5% glutaraldehyde and 2% paraformaldehyde solution in 0.1 M sodium phosphate buffer (pH 7.2) and kept at 4°C for 12 hours. The grids containing ultrathin sections were stained with 2% uranyl acetate and 0.2% lead acetate. Tissue processing for transmission electron microscope (TEM) (FMI-Margagani Operated 268 Netherland) studies was carried out and evaluated under TEM (10 000×).27
Statistical analysis
Data were expressed as the mean ± SEM. Multiple comparisons against a control group were made using a one-way analysis of variance (ANOVA) followed by Dunnett's test. Results were considered significant if P < 0.05. All statistical evaluations were performed using the Prism software package (version 4, GraphPad, San Diego, CA, USA).
Results
Effect of edaravone on TBARS and GSH
Table 1 shows the levels of TBARS and GSH in the cardiac tissues of experimental groups. The ISO-treated group showed a significant (P < 0.01) increase in the levels of TBARS and a significant (P < 0.01) decrease in the levels of GSH in the cardiac tissues as compared to the normal control group. Pretreatment with edaravone (10 and 3 mg/kg) in ISO-treated rats significantly (P < 0.01) decreased the level of TBARS and significantly (P < 0.01) increased the level of GSH in the cardiac tissue as compared to the ISO-treated group.
Table 1. Effects of edaravone on the activities of thiobarbituric acid reactive substance (TBARS), superoxide dismutase (SOD), and catalase (CAT) in the cardiac tissue of experimental groups.
| Experimental groups | TBARS (nmol MDA/mg prot) | SOD (unit/mg prot) | CAT (nmol H2O2/minute/mg prot) |
|---|---|---|---|
| Normal control | 0.08 ± 0.008 | 3.79 ± 0.48 | 7.49 ± 0.33 |
| Edaravone per se | 0.08 ± 0.009 | 3.93 ± 0.53 | 7.27 ± 0.43 |
| Isoproterenol (ISO) (85 mg/kg) | 0.39 ± 0.033† | 1.49 ± 0.25† | 1.89 ± 0.08† |
| Edaravone (1 mg/kg) + ISO | 0.30 ± 0.027 | 2.59 ± 0.21 | 2.54 ± 0.71 |
| Edaravone (3 mg/kg) + ISO | 0.23 ± 0.050** | 2.62 ± 0.19* | 4.19 ± 0.36** |
| Edaravone (10 mg/kg) + ISO | 0.20 ± 0.020** | 3.18 ± 0.14** | 5.23 ± 0.49**‡ |
Each value is mean ± SEM for six rats in each group.
*P < 0.05 vs. ISO toxic group.
**P < 0.01 vs. ISO toxic group.
†P < 0.01 vs. normal control.
‡P < 0.01 vs. edaravone (1 mg/kg) + ISO.
Effect of edaravone on SOD and CAT
Table 1 depicts the activities of SOD and CAT in cardiac tissue. ISO treatment caused a significant (P < 0.01) reduction in the activities of SOD and CAT in the cardiac tissue as compared to the control group. Pretreatment with edaravone (10 mg/kg) in the ISO-treated group resulted in a significant (P < 0.01) increase in the levels of SOD and CAT in the cardiac tissue as compared to the ISO-treated group. Pretreatment with edaravone (3 mg/kg) in ISO-treated rats showed significantly (P < 0.05) improved activity of CAT in the cardiac tissue as compared to the ISO-treated group.
Effect of edaravone on antioxidant enzymes
Table 2 shows the activities of antioxidant enzymes, namely GST, GPx, and GR, in the myocardium. The ISO-treated group had significantly (P < 0.01) decreased activities of these enzymes in the cardiac tissue as compared to the control group. Pretreatment with edaravone (10 mg/kg) in the ISO-treated group showed significantly (P < 0.01) increased activities of these antioxidant enzymes in the cardiac tissue as compared to the ISO-treated group. Pretreatment with edaravone (3 mg/kg) in the ISO-treated group showed significantly (P < 0.01) increased activities of GST and GPx, while edaravone (3 mg/kg) led to a significant (P < 0.05) increase in the activity of GR in the cardiac tissue as compared to the ISO-treated group.
Table 2. Effects of edaravone on the activities of reduced glutathione (GSH), glutathione peroxidase (GPx), glutathione-S-transferase (GST), and glutathione reductase (GR) in the cardiac tissue of experimental groups.
| Experimental groups | GSH (µmol GSH/mg prot) | GST (nmol NADPH/minute/mg prot) | GPx (nmol CDNB/minute/mg prot) | GR (nmol NADPH/minute/mg prot) |
|---|---|---|---|---|
| Normal control | 1.33 ± 0.12 | 8.11 ± 0.29 | 5.83 ± 0.32 | 6.53 ± 0.32 |
| Edaravone per se (10 mg/kg) | 1.09 ± 0.13 | 7.04 ± 0.46 | 5.38 ± 0.39 | 5.21 ± 0.82 |
| Isoproterenol (85 mg/kg) | 0.23 ± 0.19† | 2.10 ± 0.24† | 1.67 ± 0.28† | 1.58 ± 0.15† |
| Edaravone (1 mg/kg) + ISO | 0.63 ± 0.07* | 3.81 ± 0.53* | 2.73 ± 0.19* | 2.98 ± 0.17 |
| Edaravone (3 mg/kg) + ISO | 0.98 ± 0.05** | 4.19 ± 0.48** | 3.32 ± 0.20** | 3.38 ± 0.35* |
| Edaravone (10 mg/kg) + ISO | 1.02 ± 0.09**‡ | 4.72 ± 0.33** | 3.51 ± 0.18** | 3.73 ± 0.41** |
Each value is mean ± SEM for six rats in each group.
*P < 0.05 vs. ISO toxic group.
**P < 0.01 vs. ISO toxic group.
†P < 0.01 vs. normal control.
‡P < 0.05 vs. edaravone (1 mg/kg) + ISO.
Effect of edaravone on CK-MB and LDH level
The effects of edaravone on CK-MB and LDH are shown in Fig. 1. Rats injected with ISO showed a significant (P < 0.01) increase in the level of serum LDH and CK-MB as compared to the normal control group. Pretreatment with edaravone (10 and 3 mg/kg) significantly (P < 0.01) reduced the levels of LDH in serum compared with the ISO-treated group. Pretreatment with edaravone (10 mg/kg) significantly (P < 0.01) reduced the level of CK-MB in serum as compared to the ISO-treated group. Edaravone (3 mg/kg) significantly (P < 0.05) decreased the level of CK-MB in serum as compared with the ISO-treated group.
Figure 1.
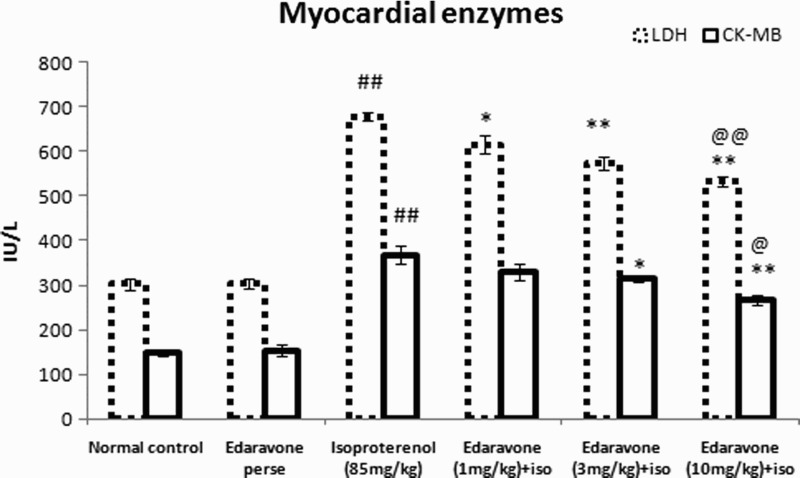
LDH and CK-MB in serum of experimental groups. Values are mean ± SEM from a group of six animals. ##P < 0.01 when control group compared with toxic group. *P < 0.05, **P < 0.01, when pretreated group compared with toxic group. @P < 0.05, @@P < 0.01, when high dose pretreated group compared with low dose pretreated group.
Effect of edaravone on CRP
Figure 2 shows the effect of edaravone on the serum level of CRP. The ISO-treated group showed a significant (P < 0.01) increase in the level of serum CRP as compared to the normal control rats. Pretreatment with edaravone (10 and 3 mg/kg) significantly (P < 0.01) reduced the level of serum CRP as compared with the ISO-treated group, while edaravone (1 mg/kg) significantly (P < 0.05) reduced the level of CRP.
Figure 2.
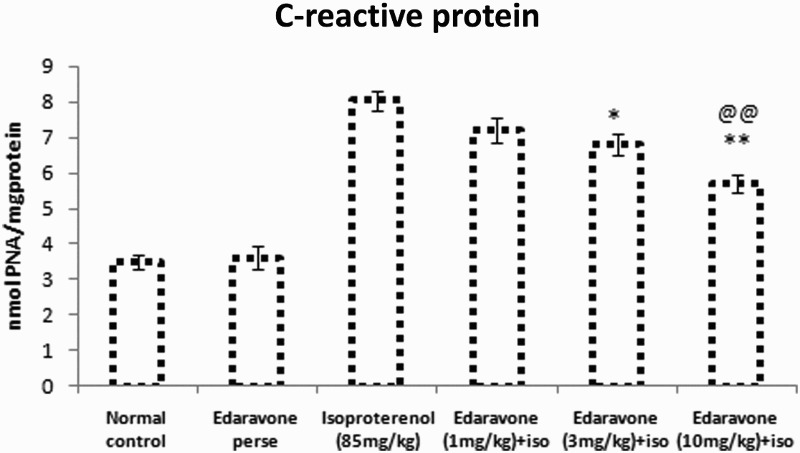
C-reactive protein in serum of experimental groups. Values are mean ± SEM from a group of six animals. ##P < 0.01 when control group compared with toxic group. *P < 0.05, **P < 0.01, when pretreated group compared with toxic group. @@P < 0.01, when high dose pretreated group compared with low dose pretreated group.
Effect of edaravone on caspase-3
Figure 3 shows the effect of edaravone on the myocardial activity of caspase-3. The ISO-treated group showed significantly (P < 0.01) increased expression of activated caspase-3 in myocardial cells of rats as compared to normal control rats. Pretreatment with edaravone (10 mg/kg) in ISO-treated animals significantly (P < 0.01) decreased the levels of activated caspase-3 as compared with the ISO-treated group. Pretreatment with edaravone (3 mg/kg) significantly (P < 0.05) decreased the expression of activated caspase-3 as compared with the ISO-treated group.
Figure 3.
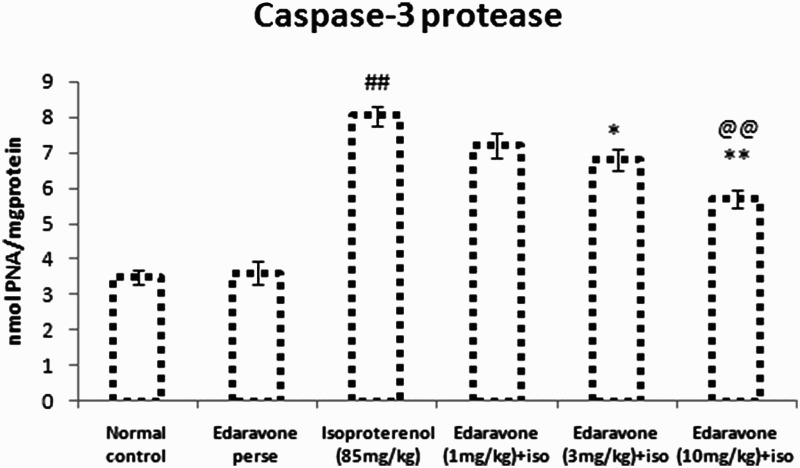
Caspase-3 protease in cardiac tissue of experimental groups. Values are mean ± SEM from a group of six animals. ##P < 0.01 when control group compared with toxic group. *P < 0.05, **P < 0.01, when pretreated group compared with toxic group. @@P < 0.01, when high dose pretreated group compared with low dose pretreated group.
Effect of edaravone on the ultrastructure of myocardial tissue
Figure 4 represents the ultrastructure of myocardial tissue, with clearly elaborated mitochondria and myofibrils. Ultrastructural analysis of the myocardial tissue in the ISO-treated group showed significant alteration of the integrity of mitochondrial membranes, disruption of cristae with vacuolation (Fig. 4C). Pretreatment with edaravone (10 and 3 mg/kg) in the ISO-treated group showed well preserved mitochondria and less evidence of myocyte injury, as well as preservation of the integrity of mitochondrial membranes and architecture of the cristae (Fig. 4E and F). Pretreatment with edaravone (1 mg/kg) in the ISO-treated group showed moderate loss of mitochondrial membrane and disruption of cristae with vacuolation (Fig. 4D). Control and per se groups showed normal architecture of the myocardial mitochondria and myofilaments as shown in Fig. 4A and B, respectively.
Figure 4.
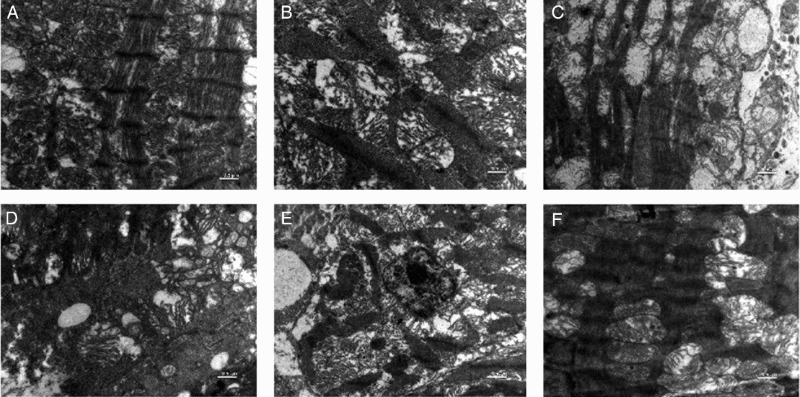
Transmission electron microscopic images of rat heart mitochondria. (A) Normal control group showing normal architecture of the heart mitochondria. (B) per se group (edaravone 10 µg/kg, i.p.) rats showing normal mitochondria along with good appearance of myofilaments of the heart mitochondria. (C) ISO-treated (85 mg/kg, s.c.) rat heart mitochondria showing ruptured mitochondrial cell membrane, bulging of mitochondria and disorder of cristae with vacuolation. (D) Edaravone (1 mg/kg, i.p. + ISO) treated heart mitochondria showing disarrangement of cristae with some swelling and vacuolation. (E) Edaravone (3 mg/kg, i.p. + ISO) treated heart mitochondria showing maintained architecture and slight fibrinolysis of mitochondria space. (F) Edaravone (10 mg/kg, i.p. + ISO) treated heart mitochondria showing maintained architecture and no fibrinolysis of mitochondrial space. (Scale bar: 0.5 µm).
Discussion
Oxidative stress generates ROS, which play a crucial role in the pathogenesis of MI. In the present study, the ISO-treated experimental model of MI was used. Cardiotoxic actions of ISO on cardiac tissues are directly produced by the activation of beta-adrenoceptors, and the indirect effects are progressed by the formation of ROS and oxyradicals.28 A study has shown that adrenochromes and oxyradicals are produced during the oxidation of catecholamines.29 Oxidative products of catecholamines induce functional and subcellular changes in the rat myocardium, leading to cardiomyopathy.28,30 Edaravone is one of the most potent scavengers of free radicals and prevents lipid peroxidative damage.8 Edaravone prevents the increased levels of hydroxyl radicals and superoxide radicals in ischemia models.31 It also has been reported to reduce myocardial infarct size and improve cardiac function and left ventricular remodeling, possibly due to reduced free radical generation in myocardium.32 In the present study, the protective effects of edaravone on ISO-treated MI were evaluated.
ISO treatment significantly increased the levels of TBARS in heart tissue when compared to the control group, which is consistent with earlier reports.33 This may be due to the formation of free radicals and the activation of lipid peroxidation, resulting in irreversible myocardial membrane injury. It also has been reported that oxyradicals lead to lipid peroxidation, and cause the alteration of membrane integrity and permeability.34 Pretreatment with edaravone along with ISO showed a significant decrease in the TBARS levels when compared with the ISO-treated group, which may be due to the antioxidant and free radical scavenging activities of edaravone.
Inhibition of the activities of SOD and CAT leads to production of superoxide ions and hydroxyl radicals, which damage the myocardium. In the ISO-treated group, the activities of SOD and CAT were significantly decreased, which corroborates a previous study.35 Pretreatment with edaravone along with ISO led to a significant increase in the activities of SOD and CAT when compared to the ISO-treated group.
The steady ratio of glutathione (GSH) to oxidized glutathione (GSSH) in cells depends on the activities of GPx and GR. These activities are reduced by the consumption of GSH, as reported earlier.36 In the present study, it was found that the level of GSH and the activities of GPx, GR, and GST in cardiac tissue were significantly reduced in the ISO-treated group. Pretreatment with edaravone in the ISO group significantly increased the concentration of GSH and the activities of GPx, GR, and GST. The results suggested that edaravone might protect the cellular antioxidant defense system against oxidative stress.
Oxidative products of ISO damage myocardial cells leading to the loss of membrane integrity, and as a consequence, the cytosolic enzymes CK-MB and LDH leak into the blood and serve as markers of the MI.37 Serum levels of the myocardial damage markers CK-MB and LDH were significantly increased in the ISO-treated group in our study also. Figure 1 illustrates that ISO treatment increased the levels of myocardial markers, which was significantly decreased by pretreatment with edaravone, in accordance with Tsujita et al.38 This may possibly be due to the scavenging property of edaravone preserving the myocardium from oxyradicals generated by ISO. Thus, the antioxidant property of edaravone might have participated in maintaining the myocardial cell membrane integrity.
Studies have reported that ROS stimulate an inflammatory cascade by inducing cytokine expression, which has the potential to damage myocardium and endothelial cells.39 CRP is a highly specific and sensitive systemic marker of inflammation and tissue damage.40 CRP is a good indicator of the inflammatory cascade that propagates the pathology of MI.41 The present study demonstrates that serum CRP levels were significantly increased in the ISO-treated group. Pretreatment with edaravone in the ISO-treated group significantly reduced the levels of CRP in the serum.
Caspase-3 is an important apoptotic factor that propagates the pathway of apoptosis protease cascade.42 Reports are available in the literature that oxidative stress coexists with apoptosis in the remote non-infarcted rat myocardium after MI.43 The ISO treatment increases work load on the heart by the activation of beta-adrenergic receptors and its oxidative metabolites (adrenochrome and oxyradicals) cause remodeling and apoptosis in the myocardium.44 In our study, a significant increase in caspase-3 activity was observed in ISO-treated rats indicating increased apoptosis in the myocardium. Pretreatment with edaravone in the ISO-treated group led to a significantly reduced activation of caspase-3 protease. These results indicate that edaravone decreased myocardial apoptosis by reducing the expression of activated caspase-3. These findings also suggest that there is a link between oxidative stress and apoptosis in MI induced by ISO, which may have been attenuated by the antioxidant property of edaravone.
Transmission electron microscopic examination showed significant alteration in the integrity of mitochondrial membranes and disruption of cristae structure, which may have been caused by the elevation of free oxygen radicals produced by oxidation of ISO. Pretreatment with edaravone preserved the ultrastructure of myocardial cells as well as the shape and architecture of mitochondria and cristae.
Thus it can be concluded that edaravone may have prevented ISO-induced cardiotoxicity in rats by attenuating oxidative stress and apoptosis in ISO-induced myocardial infarction.
Disclaimer statements
Contributors None.
Funding Authors wish to thank the University Grant Commission (UGC), New Delhi, India for the financial support provided in the form of JRF to the first author.
Conflict of interest None.
Ethics approval Study was ethically approved by Institutional Animal Ethics Committee, Jamia Hamdard, New Delhi, India.
References
- 1.World Health Organization Cardiovascular disease. [Accessed on 2013 Apr 14]. Available from: http://www.who.int/cardiovascular_diseases/en/.
- 2.Sushamakumari S, Jayadeep A, Kumar JS, Menon VP. Effect of carnitine on malondialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Indian J Exp Biol 1989;27(2):134–7. [PubMed] [Google Scholar]
- 3.Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem 1996;159(2):85–93. doi: 10.1007/BF00420910 [DOI] [PubMed] [Google Scholar]
- 4.Adameova A, Abdellatif Y, Dhalla NS. Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol 2009;87(7):493–14. doi: 10.1139/Y09-042 [DOI] [PubMed] [Google Scholar]
- 5.Yates JC, Beamish RE, Dhalla NS. Ventricular dysfunction and necrosis produced by adrenochrome metabolite of epinephrine: relation to pathogenesis of catecholamine cardiomyopathy. Am Heart J 1981;102(2):210–21. doi: 10.1016/S0002-8703(81)80012-9 [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi K, Miura N, Kawahara K, Murai Y, Morioka M, Lapchak P, et al. Edaravone (radicut), a free radical scavenger, is a potentially useful addition to thrombolytic therapy in patients with acute ischemic stroke (review). Biomed Rep 2013;1(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Takeshige N, Miura N, Morimoto Y, Ito T, Tancharoen S, et al. Beyond free radical scavenging: beneficial effects of edaravone (radicut) in various diseases. Exp Ther Med 2012;3(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimata M, Okabe TA, Hattori M, Yuan Z, Shioji K, Kishimoto C. MCI-186 (edaravone), a novel free radical scavenger, protects against acute autoimmune myocarditis in rats. Am J Physiol Heart Circ Physiol 2005;289(6):2514–8. doi: 10.1152/ajpheart.00661.2005 [DOI] [PubMed] [Google Scholar]
- 9.Kamogawa E, Sueishi Y. A multiple free-radical scavenging (MULTIS) study on the antioxidant capacity of a neuroprotective drug, edaravone as compared with uric acid, glutathione, and trolox. Bioorg Med Chem Lett 2014;24:1376–9. doi: 10.1016/j.bmcl.2014.01.045 [DOI] [PubMed] [Google Scholar]
- 10.Wu TW, Zeng LH, Wu J, Fung KP. Myocardial protection of MCI-186 in rabbit ischemia-reperfusion. Life Sci 2002;71(19):2249–55. doi: 10.1016/S0024-3205(02)01965-3 [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, et al. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci 2013;14(7):13909–30. doi: 10.3390/ijms140713909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkind MS. Inflammation, atherosclerosis, and stroke. Neurologist 2006;12(3):140–8. doi: 10.1097/01.nrl.0000215789.70804.b0 [DOI] [PubMed] [Google Scholar]
- 13.Nimata M, Okabe TA, Hattori M, Yuan Z, Shioji K, Kishimoto C. MCI-186 (edaravone), a novel free radical scavenger, protects against acute autoimmune myocarditis in rats. Am J Physiol Heart Circ Physiol 2005;289(6):2514–8. doi: 10.1152/ajpheart.00661.2005 [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi K, Kawahara K, Tancharoen S, Matsuda F, Morimoto Y, Ito T, et al. The free radical scavenger edaravone rescues rats from cerebral infarction by attenuating the release of high-mobility group box-1 in neuronal cells. J Pharmacol Exp Ther 2009;329(3):865–74. doi: 10.1124/jpet.108.149484 [DOI] [PubMed] [Google Scholar]
- 15.Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1970;95(2):351–8. [DOI] [PubMed] [Google Scholar]
- 16.Marklund S, Marklund G. Involvement of superoxide anion radical in auto oxidation by pyrogallol and a convenient assay of superoxide dismutase. Eur J Biochem 1974;47(3):469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x [DOI] [PubMed] [Google Scholar]
- 17.Claiborne A. Catalase activity. In: Greenwald RA, (eds.) Handbook of methods for oxygen free radical research. Boca Raton: CRC Press; 1985. p. 283–4. [Google Scholar]
- 18.Wheeler R, Jhaine AS, Elsayeed MN, Omaye TS, Korte JWD. Automated assays for superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activity. Anal Biochem 1990;184(2):193–9. doi: 10.1016/0003-2697(90)90668-Y [DOI] [PubMed] [Google Scholar]
- 19.Carlberg I, Mannerviek B. Glutathione reductase levels in rat brain. J Biol Chem 1975;250(14):5475–80. [PubMed] [Google Scholar]
- 20.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249(22):7130–9. [PubMed] [Google Scholar]
- 21.Sedlak J, Lindsay RH. Estimation of total protein bound and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 1968;25(1):192–05. doi: 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NH, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. J Biol Chem 1951;193(1):265–75. [PubMed] [Google Scholar]
- 23.Lum G, Gambino SR. A comparison of serum vs heparinised plasma for routine chemistry tests. Am J Clin Pathol 1974;61:108–13. [DOI] [PubMed] [Google Scholar]
- 24.Young DS. Effects of drugs on clinical laboratory tests. Washington: American Association of Clinical Chemistry Press; 1990. p. 120–2. [Google Scholar]
- 25.Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem 1997;251(1):98–102. doi: 10.1006/abio.1997.2220 [DOI] [PubMed] [Google Scholar]
- 26.Eckersall PD. Recent advances and future prospects for the use of acute phase proteins and markers of disease in animals. Rev Med Vet 2000;151:577–84. [Google Scholar]
- 27.Pogodina LS, Shornikova MV, Chentsov IS. Electron microscopy description of cardiomyocytes from the left ventricle of rat heart after apoptosis induction by isoproterenol. Biol Bull 2006;1:26–37. [PubMed] [Google Scholar]
- 28.Dhalla NS, Dent MR, Arneja AS. Pathogenesis of catecholamine-induced cardiomyopathy. In: Acosta D., Jr (ed.) Cardiovascular toxicology. 4th ed. New York: Informa Healthcare USA Inc., 2008. p. 207–62. [Google Scholar]
- 29.Dhalla KS, Ganguly PK, Rupp H, Beamish RE, Dhalla NS. Measurement of adrenolutin as an oxidation product of catecholamines in plasma. Mol Cell Biochem 1989;87(1):85–92. doi: 10.1007/BF00421086 [DOI] [PubMed] [Google Scholar]
- 30.Dhalla NS, Yates JC, Lee SL, Singh A. Functional and subcellular changes in the isolated rat heart perfused with oxidize isoproterenol. J Mol Cell Cardiol 1978;10(1):31–41. doi: 10.1016/0022-2828(78)90004-4 [DOI] [PubMed] [Google Scholar]
- 31.Sommani P, Arai T, Yamashita K, Miyoshi T, Mori H, Sasada M, et al. Effects of edaravone on singlet oxygen released from activated human neutrophils. J Pharmacol Sci 2007;103(1):117–20. doi: 10.1254/jphs.SC0060170 [DOI] [PubMed] [Google Scholar]
- 32.Onogi H, Minatoguchi S, Chen XH, Bao N, Kobayashi H, Misao Y, et al. Edaravone reduces myocardial infarct size and improves cardiac function and remodelling in rabbits. Clin Exp Pharmacol Physiol 2006;33(11):1035–41. doi: 10.1111/j.1440-1681.2006.04483.x [DOI] [PubMed] [Google Scholar]
- 33.Hassan MQ, Akhtar MS, Akhtar M, Ansari SH, Ali J, Haque SE, et al. Benidipine prevents oxidative stress, inflammatory changes and apoptosis related myofibril damage in isoproterenol-induced myocardial infarction in rats. Toxicol Mech Methods 2014;25(1):26–33. doi: 10.3109/15376516.2014.972531 [DOI] [PubMed] [Google Scholar]
- 34.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr 1985;5:365–90. doi: 10.1146/annurev.nu.05.070185.002053 [DOI] [PubMed] [Google Scholar]
- 35.Chatelain P, Gremel M, Brotelle R. Prevention by amiodarone of phospholipid depletion in isoproterenol-induced ischemia in rats. Eur J Pharmaol 1987;144(1):83–90. doi: 10.1016/0014-2999(87)90012-4 [DOI] [PubMed] [Google Scholar]
- 36.Ithayarasi AP, Devi CS. Effect of alpha-tocopherol on lipid peroxidation in isoproterenol-induced myocardial infarction in rats. Indian J Physiol Pharmacol 1997;41(4):369–76. [PubMed] [Google Scholar]
- 37.Gürgün C, Ildizli M, Yavuzgil O, Sin A, Apaydın A, Cınar C, et al. The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol 2008;123(2):102–07. doi: 10.1016/j.ijcard.2006.11.152 [DOI] [PubMed] [Google Scholar]
- 38.Tsujita K, Shimomura H, Kawano H, Hokamaki J, Fukuda M, Yamashita T, et al. Effects of edaravone on reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 2004;94(4):481–4. doi: 10.1016/j.amjcard.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 2002;90(11):1159–66. doi: 10.1161/01.RES.0000020401.61826.EA [DOI] [PubMed] [Google Scholar]
- 40.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 1983;34:141–12. doi: 10.1016/S0065-2776(08)60379-X [DOI] [PubMed] [Google Scholar]
- 41.de Beer FC, Hind CR, Fox KM, Allan RM, Maseri A, Pepys MB. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J 1982;47(3):239–43. doi: 10.1136/hrt.47.3.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Luo R, Jiang R, Meng X, Wu X, Zhang S, et al. The role of the Hsp90/Akt pathway in myocardial calpain-induced caspase-3 activation and apoptosis during sepsis. BMC Cardiovasc Disord 2013;13:8. doi: 10.1186/1471-2261-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li WG, Zaheer A, Coppey L, Oskarsson HJ. Activation of JNK in the remote myocardium after large myocardial infarction in rats. Biochem Biophys Res Commun 1998;246(3):816–20. doi: 10.1006/bbrc.1998.8662 [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy P, Subramanian V, Singh M, Singh K. β1 integrins modulate β-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension 2007;49(4):865–72. doi: 10.1161/01.HYP.0000258703.36986.13 [DOI] [PubMed] [Google Scholar]


