Abstract
Objectives
Plant-derived natural substances, such as capsaicin, with potent antiproliferative activity against cancer cells in vitro are considered to be promising nutraceuticals in anticancer therapy. Nevertheless, the limited systemic bioavailability of phytochemicals may raise questions regarding the physiological relevance of their phytochemical effects in vivo. Thus, the search for novel phytochemical-based substances with more efficient anticancer action is needed.
Methods
In the present study, a capsaicin analogue, namely, capsaicin epoxide, was synthesized, and its cytotoxic potential against cancer cells was evaluated and compared to that of capsaicin through 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and multi-caspase assays. The abilities of capsaicin and capsaicin epoxide to induce oxidative stress were estimated using redox-sensitive fluorogenic probes: 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and dihydroethidium.
Results
The structure and purity of the synthesized product were confirmed by nuclear magnetic resonance spectroscopy, electrospray ionization mass spectrometry, and gas chromatography. Normal human dermal fibroblasts were not susceptible to treatment with the agent, whereas a cancer cell type-specific response was observed. Human breast carcinoma cells were found to be the most sensitive to capsaicin epoxide treatment compared with capsaicin treatment, and the action of capsaicin epoxide was oxidant based.
Discussion
Our data indicate that the antiproliferative activity of capsaicin epoxide is potentiated in vitro, when used at much lower concentrations compared with capsaicin at similar concentrations. Thus, the findings of this study may have implications for phytochemical-based anticancer drug development.
Keywords: Capsaicin, Capsaicin epoxide, Anticancer activity, Oxidative stress
Introduction
Capsaicin is a homovanillic acid derivate (8-methyl-N-vanillyl-6-nonenamide) and a major pungent component in hot chili peppers of the genus Capsicum (family Solanaceae), which are consumed worldwide as a food additive.1 This nutraceutical has been used to treat pain and inflammation associated with neuropathic pain conditions, such as rheumatoid arthritis, cluster headaches, herpes zoster, and vasomotor rhinitis.2–5 More recently, the selective action of capsaicin against cancer cells has been shown6 and capsaicin-mediated apoptosis and suppression of cancer cell growth has been documented in more than 40 distinct cancer cell lines, mainly of human origin.1 Moreover, the corresponding capsaicin anticancer potential has been observed in in vivo rodent xenograft tumour models.1
It is widely accepted that the action of capsaicin is mediated by vanilloid 1 (TRPV-1, transient receptor potential vanilloid-1) receptor stimulation, but capsaicin-associated effects were also observed through a TRPV-1-independent mechanism.1 The capsaicin-induced anticancer effects, both apoptosis induction and cancer cell growth inhibition, may be promoted by reactive oxygen species (ROS) production, cell cycle arrest, regulation of transcription factor expression, and changes in growth/survival signal transduction pathways, such as EGFR/HER-2 pathway and NF-κB inactivation.7–12 Capsaicin-associated ROS generation may be mediated by the inhibition of mitochondrial complex-I and complex-III activities, ATP depletion, and downregulation of antioxidant enzymes, which, in turn, may lead to apoptosis in pancreatic cancer cells.13
The potent anticancer effects of natural dietary agents, such as curcumin and capsaicin, observed in vitro may not reflect their therapeutic efficacy in vivo due to their limited bioavailability.14 However, there are numerous approaches to increase phytochemical bioavailability and cancer cell-specific cytotoxicity, including the use of adjuvants, nanoparticles, liposomes, phospholipid complexes, and structural analogues.14 The aim of the present study was to synthesize an analogue of capsaicin, namely, capsaicin epoxide and to compare its cytotoxicity against selected cancer cell lines with that of unmodified capsaicin. The capsaicin epoxide anticancer potential was increased compared with that of capsaicin, and this effect was found to be mediated by an increased production of ROS and attenuated by the antioxidant N-acetyl cysteine (NAC).
Materials and methods
Reagents
Capsaicin (12084, analytical standard grade, ≥99%) was purchased from Sigma (Poznan, Poland), phosphate-buffered saline was obtained from Gibco, Invitrogen Corporation (Grand Island, NY, USA) and dimethyl sulfoxide (DMSO) was purchased from BioShop (LabEmpire, Rzeszow, Poland). Capsaicin and its analogue were dissolved in DMSO. Experiments concerning solvent action alone were always performed. Unless noted otherwise, all other reagents were purchased from Sigma (Poznan, Poland) and were of analytical grade.
Capsaicin epoxide synthesis and characterization
A scheme showing the synthesis of capsaicin epoxide is presented in Fig. 1.
Figure 1.
Capsaicin epoxide synthesis. Capsaicin (Sigma) (compound 1, 12 mg diluted in dichloromethane) was mixed with m-chloroperoxybenzoic acid (10 mg) at room temperature. Capsaicin epoxide (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide, compound 2, pale yellow oil, 11 mg, 87% yield) was isolated by column chromatography. m-CPBA, m-chloroperoxybenzoic acid; DCM, dichloromethane; rt, room temperature.
M-chloroperoxybenzoic acid (m-CPBA) (10 mg, 70% 0.040 mmol, 1.03 eq) was added in portions to a stirred solution of capsaicin (compound 1 on Fig. 1) (12 mg, 0.039 mmol) in dichloromethane (DCM) (0.5 ml) at room temperature. After 30 minutes, the solvent was evaporated and the residue was chromatographed on silica gel (1 g, 50% ethyl acetate in hexanes) to give N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide (pale yellow oil, 11 mg, 87% yield) (compound 2 on Fig. 1). Column chromatography was performed on a Merck silica gel 60 with 230–400 mesh (Merck Millipore, Warsaw, Poland). Thin layer chromatography (TLC) was performed on aluminum sheets (Merck 60F 254) using 50% ethyl acetate in hexanes as the eluent. The solvents were evaporated in a rotary evaporator. The structure of the synthesized product, namely, capsaicin epoxide was confirmed by nuclear magnetic resonance (NMR) spectroscopy and electrospray ionization mass spectrometry (ESI-MS), and the purity of the product was confirmed by gas chromatography (GC).
The NMR spectra were recorded in deuterated chloroform (CDCl3) using an Agilent NMR spectrometer (Agilent Technologies, Santa Clara, CA, USA). 1H at 400 MHz and 13C at 100 MHz. The chemical shifts are quoted on the δ scale using the solvent signal as an internal standard (chloroform, CHCl3: 1H NMR δ = 7.26 ppm; 13C NMR δ = 77.00 ppm). 1H NMR: (400 MHz) δ (ppm): 0.94 and 1.00 (2d, J = 6 Hz, 6H, –CH(CH3)2), 1.40–1.60 (m, 4H, –CH2–), 1.66–1.79 (m, 3H, –CH2– and –CH(CH3)2, 2.22 (t, J = 8 Hz, 2H, –CH2C=O), 2.44 (dd, J = 4 Hz, 1H, –CH–O–), 2.68–2.72 (m, 1H, –CH–O–), 3.88 (s, 3H, –OCH3), 3.36 (d, J = 4 Hz, 2H, –CH2–NH–), 5.63 (br s, 1H, –OH or –NH–), 5.72 (br s, 1H, –OH or –NH–), 6.75–6.87 (m, 3H, aromatic H). 13C NMR: (100 MHz) δ (ppm): 18.4, 19.0 (2C, –CH(CH3)2), 25.4, 25.8, 30.5, 31.8 (4C, –CH2–), 36.6 (1C, –CH(CH3)2, 43.6 (1C, –CH2–NH–), 55.9, 57.6 (2C, epoxide C), 64.2 (1C, –OCH3), 110.7, 114.4, 120.8, 130.3, 145.1, 146.7 (6C, aromatic C), 172.5 (1C, =C=O).
The mass spectral electrospray ionization (ESI) measurements were conducted on a Mariner PerSeptive Biosystems instrument (Life Technologies, Grand Island, NY, USA) with a time-of-flight (TOF) detector. ESI (HR MS): calculated for C18H27NO4Na (MNa+): 344.1838; found: 344.1835. The GC analyses were performed using an Agilent chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with a 0.32 mm × 30 m, HP5, 0.25 µm phase capillary column with an injection temperature 140°C and a programmed increase in temperature of 40°C per minute. GC Analysis: Rt = 7.038 minutes (98%).
Cell lines and culture conditions
The following cell lines were used: human dermal fibroblasts (HDFs), mouse embryonic fibroblasts (NIH3T3), human prostate carcinoma cells (DU145), human lung carcinoma cells (A549), human breast carcinoma cells (MCF7), human cervical carcinoma cells (HeLa), and human renal carcinoma cells (ACHN). All of the cell lines were obtained from ATCC (LGC Standards, Lomianki, Poland). The cells (3000 cells/cm2) were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal calf serum and an antibiotic and antimycotic mix solution (100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 μg/ml amphotericin B) in a humidified atmosphere in the presence of 5% CO2 until the cells reached confluence. Typically, the cells were passaged by trypsinization and maintained in DMEM.
MTT assay
After 24 hours of treatment, the cytotoxicities and antiproliferative actions of capsaicin and capsaicin epoxide were estimated by measuring the cell metabolic activity as an ability of the live cells to metabolize 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan15 as described elsewhere.16 For the analysis of the NAC-induced recovery as a reflection of the proliferative potential, MCF7 cells were pretreated with 5 mM NAC for 2 hours and then with capsaicin or capsaicin epoxide (50 µM) for 24 hours.
Multi-caspase assay
The live, early apoptotic, late apoptotic, and dead cells were assessed using the Muse™ Cell Analyser and the Muse™ Multi-caspase Kit according to the manufacturer's instructions (Merck Millipore, Warsaw, Poland). Briefly, a derivatized Val-Ala-Asp (VAD) peptide that can detect the activity of multiple caspases (caspase-1, 3, 4, 5, 6, 7, 8, and 9)17 and a dead cell dye (7-aminoactinomycin D, 7-AAD) that provides information on membrane integrity were used. The VAD peptide (fluorescent-labelled inhibitor of caspases), derivatized with a fluorescent group and a fluoromethyl ketone (FMK) moiety, is membrane permeable and non-cytotoxic and binds to the activated caspases, resulting in a fluorescent signal proportional to the number of active caspases in the cell.17 Four populations of cells were detected:
-
•
live cells: caspase (−) and 7-AAD (−),
-
•
caspase (+) cells exhibiting pan caspase activity: caspase (+) and 7-AAD (−),
-
•
late stage of caspase activity cells: caspase (+) and 7-AAD (+),
-
•
necrotic cells: caspase (−) and 7-AAD (+).
The calculations were performed automatically, and the multi-caspase profiles (dot plots) were displayed using the Muse™ Multi-caspase software module. As positive controls, 0.5-hour and 3-hour treatments with 10 mM hydrogen peroxide and 10 mM tert-butyl hydroperoxide (tBOOH) were used.
Oxidative stress parameters
After an optional 2-hour pretreatment with 5 mM NAC and 24-hour treatment with capsaicin or capsaicin epoxide (50 µM), the steady-state levels of ROS in the cell-culture medium and the levels of intracellular ROS and superoxide production were measured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) and dihydroethidium, respectively, as described elsewhere.16
Statistical analysis
The results represent the means ± SD from at least three independent experiments. The differences between
-
•
the cell metabolic activity and oxidative stress parameters after capsaicin treatment versus the cell metabolic activity and oxidative stress parameters at standard growth conditions,
-
•
the cell metabolic activity and oxidative stress parameters after capsaicin epoxide treatment versus the cell metabolic activity and oxidative stress parameters at standard growth conditions,
-
•
the cell metabolic activity and oxidative stress parameters after capsaicin treatment versus the cell metabolic activity and oxidative stress parameters after capsaicin epoxide treatment,
-
•
the cell metabolic activity and oxidative stress parameters after capsaicin treatment versus the cell metabolic activity and oxidative stress parameters after NAC pretreatment and capsaicin treatment, and
-
•
the cell metabolic activity and oxidative stress parameters after capsaicin epoxide treatment versus the cell metabolic activity and oxidative stress parameters after NAC pretreatment and capsaicin epoxide treatment
were assessed by one-way analysis of variance (ANOVA) with post-hoc testing using Tukey's multiple comparison test. A P-value <0.05 was considered significant.
The statistical analyses were performed using the StatSoft, Inc. (2005) from STATISTICA (version 7.0; htt://www.statsoft.com).
Results
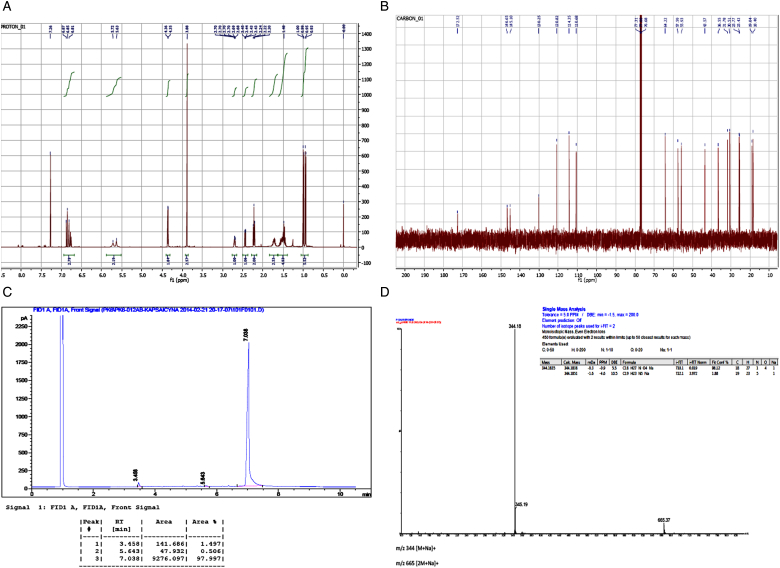
A scheme showing the conditions of capsaicin epoxide synthesis is presented in Fig. 1. Briefly, m-CPBA was added to a DCM-based capsaicin solution at room temperature. The structure, purity, and molecular mass of the product (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide, compound 2) were evaluated by NMR spectroscopy, GC, and ESI-TOF mass spectrometry (Fig. 2).
Figure 2.
Capsaicin epoxide characteristics. The quality (purity) and molecular mass of the synthesized capsaicin epoxide (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide, compound 2) were evaluated by NMR spectroscopy (A and B), gas chromatography (C), and ESI-TOF mass spectrometry (D). (A) The 400-MHz 1H NMR spectrum of compound 2. (B) The 100-MHz 13C NMR spectrum of compound 2. 1H NMR: (400 MHz) δ (ppm): 0.94 and 1.00 (2d, J = 6 Hz, 6H, –CH(CH3)2), 1.40–1.60 (m, 4H, –CH2–), 1.66–1.79 (m, 3H, –CH2– and –CH(CH3)2, 2.22 (t, J = 8 Hz, 2H, –CH2C=O), 2.44 (dd, J = 4 Hz, 1H, –CH–O–), 2.68–2.72 (m, 1H, –CH–O–), 3.88 (s, 3H, –OCH3), 3.36 (d, J = 4 Hz, 2H, –CH2–NH–), 5.63 (br s, 1H, –OH or –NH–), 5.72 (br s, 1H, –OH or –NH–), 6.75–6.87 (m, 3H, aromatic H).13C NMR: (100 MHz) δ (ppm): 18.4, 19.0 (2C, –CH(CH3)2), 25.4, 25.8, 30.5, 31.8 (4C, –CH2–), 36.6 (1C, –CH(CH3)2, 43.6 (1C, –CH2–NH–), 55.9, 57.6 (2C, epoxide C), 64.2 (1C, –OCH3), 110.7, 114.4, 120.8, 130.3, 145.1, 146.7 (6C, aromatic C), 172.5 (1C, =C=O). (C) The gas chromatographic analysis of compound 2: Rt = 7.038 minutes (98%). (D) ESI (HR MS) data spectrum of compound 2 (calculated for C18H27NO4Na (MNa+): 344.1838; found: 344.1835).
Based on the NMR spectra (Fig. 2A and B) one can conclude that we did obtain capsaicin epoxide (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide, compound 2) at high purity. Moreover, the GC analysis (Fig. 2C) confirmed that the synthesis yielded a high-purity product (Rt = 7.038 minutes, 98%). High-resolution mass spectrometry was used to estimate the molecular mass of compound 2 (Fig. 2D) and the obtained molecular mass of the product was as expected (calculated for C18H27NO4Na (MNa+): 344.1838; found: 344.1835) (Fig. 2D).
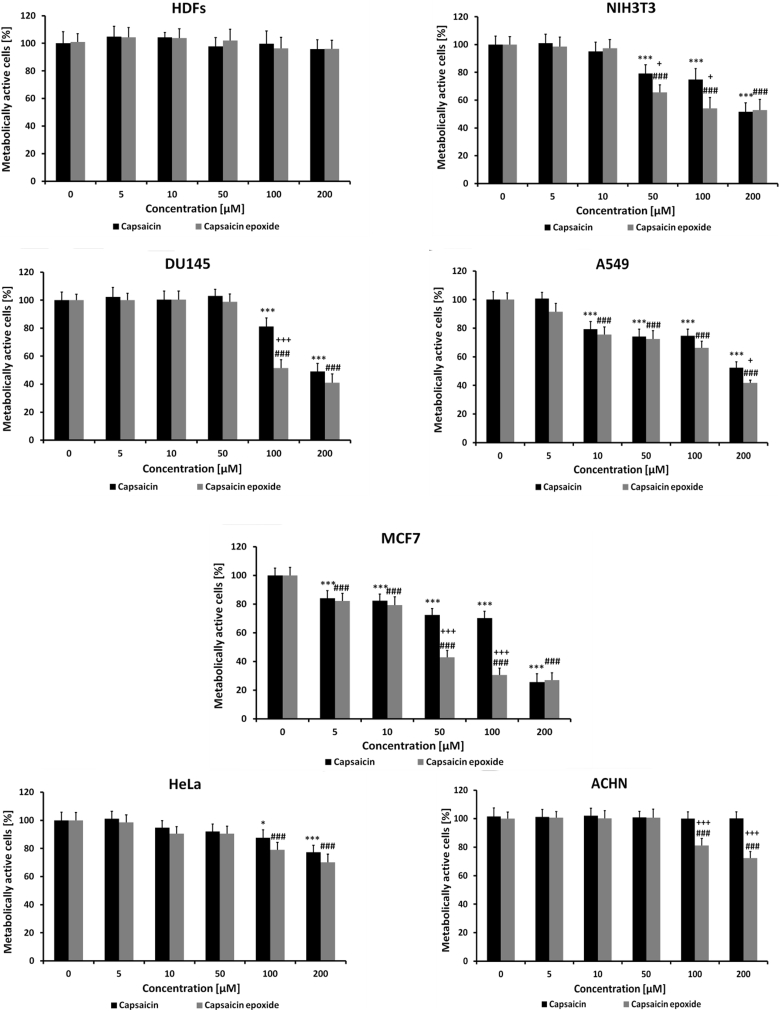
Subsequently, normal and cancer cell lines were subjected to capsaicin and capsaicin epoxide treatments and the cytotoxic potentials of these two compounds were compared (Fig. 3).
Figure 3.
Capsaicin- and capsaicin epoxide-induced decrease in metabolic activity. The capsaicin and capsaicin epoxide cytotoxicities were estimated by measuring the ability of live cells to metabolize 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan. HDFs, human dermal fibroblasts; NIH3T3, mouse embryonic fibroblasts; DU145, human prostate carcinoma cells; A549, human lung carcinoma cells; MCF7, human breast carcinoma cells; HeLa, human cervical carcinoma cells; ACHN, human renal carcinoma cells. The bars indicate the SD (n = 5). *P < 0.05 and ***P < 0.001 compared with the control conditions (24-hour treatment with capsaicin versus the control conditions); ###P < 0.001 compared with the control conditions (24-hour treatment with capsaicin epoxide versus the control conditions); +P < 0.05 and +++P < 0.001 compared with 24-hour treatment with capsaicin (24-hour treatment with capsaicin versus 24-hour treatment with capsaicin epoxide) (ANOVA and Tukey's a posteriori test).
To assess the cytotoxicities of the agents, the MTT assay, which measures the ability of live cells (namely, metabolically active cells) to reduce MTT to formazan by mitochondrial dehydrogenases, was used. After agent treatment, a decrease in metabolic activity may be interpreted as a decrease in cell number/proliferation/viability/survival and may suggest augmented cytotoxicity. In the present study, a decrease in capsaicin and capsaicin epoxide-mediated metabolic activity may reflect both the antiproliferative activity and cytotoxicity of the analyzed agents. Because both agents were dissolved initially in DMSO, the possibility of solvent interference was evaluated and we were unable to observe any DMSO-mediated effects (data not shown). Normal human fibroblasts (HDFs) were not prone to capsaicin and capsaicin epoxide treatment (Fig. 3), which is not surprising because the selective action of capsaicin against cancer cells is well documented.1 We then used one mouse continuous cell line and several human cancer cell lines to establish the cancer cell-specific capsaicin epoxide toxicity (Fig. 3). Mouse embryonic fibroblasts (NIH3T3, spontaneously immortalized cells) were found to be sensitive to capsaicin and capsaicin epoxide treatment (Fig. 3). Capsaicin epoxide induced a greater cytotoxic effect compared with capsaicin (P < 0.05), e.g. decreases in metabolic activity of approximately 20 and 35% were obtained with 50 µM capsaicin and 50 µM capsaicin epoxide, respectively, and decreases of approximately 25 and 45% were obtained with 100 µM capsaicin and 100 µM capsaicin epoxide, respectively (Fig. 3). At the highest concentration examined (200 µM), the cytotoxicity of both compounds was similar and reflected the IC50 value (the concentration required to inhibit 50% of metabolic activity) (Fig. 3). Among the cancer cell lines examined, human breast cancer cells (MCF7) were the most susceptible to agent treatment (Fig. 3). Capsaicin- and capsaicin epoxide-induced impairments in metabolic activity were observed, even at concentrations as low as 5 µM (P < 0.001) (Fig. 3). Moreover, capsaicin epoxide was evidently much more cytotoxic towards the MCF7 cell line than capsaicin (P < 0.001) (Fig. 3). A 2- and 2.5-fold decrease in metabolic activity was revealed after 50 and 100 µM capsaicin epoxide treatment compared with 50 and 100 µM capsaicin treatment, respectively (P < 0.001) (Fig. 3). For capsaicin epoxide, we estimated the IC50 to be approximately 50 µM compared with the IC50 of capsaicin of 150 µM (Fig. 3). The ranking of the most sensitive cancer cell lines to capsaicin epoxide treatment revealed the following order: human breast cancer cells (MCF7) > human lung cancer cells (A549) > human prostate cancer cells (DU145) > human cervical cancer cells (HeLa) > human renal cancer cells (ACHN) (Fig. 3). Regardless of the cancer cell type-specific response, all of the cancer cell lines were found to be more sensitive to capsaicin epoxide treatment than capsaicin treatment (Fig. 3). The treatment of human prostate cancer cells (DU145) with 100 µM capsaicin and 100 µM capsaicin epoxide resulted in decreases in metabolic activity of approximately 20 and 50%, respectively (P < 0.001) (Fig. 3). Even the capsaicin-insensitive ACHN cancer cell line was found to be moderately susceptible to capsaicin epoxide treatment (Fig. 3). Exposure of the ACHN cell line to 100 µM capsaicin epoxide and 200 µM capsaicin epoxide caused decreases in metabolic activity of approximately 20 and 30%, respectively (P < 0.001) (Fig. 3).
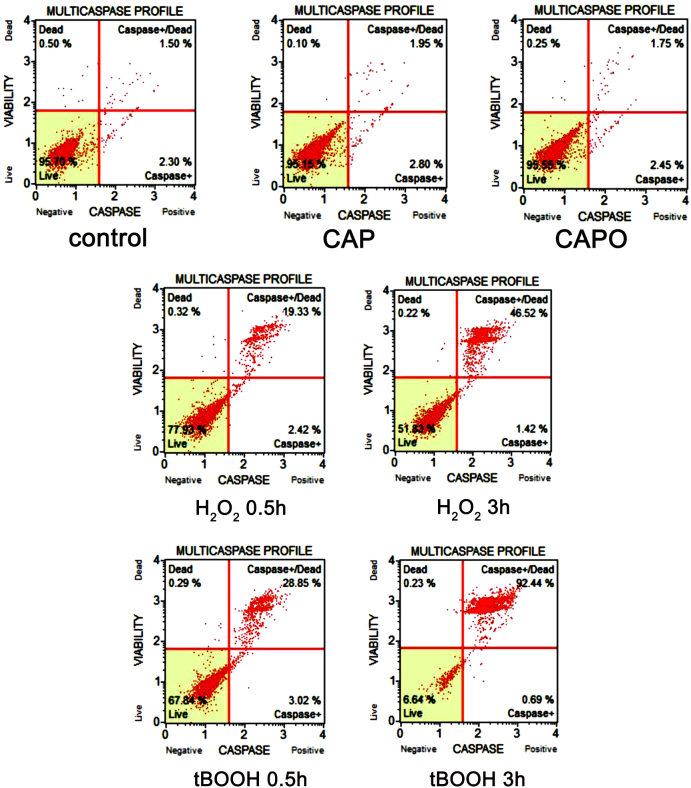
We then asked the question of whether the capsaicin epoxide cytotoxicity may be a result of apoptotic cell death. To evaluate apoptotic events after capsaicin epoxide treatment, we performed a multi-caspase assay, which enables the detection of pan-caspase activity (the activity of caspase-1, 3, 4, 5, 6, 7, 8, and 9).17 A capsaicin epoxide concentration of 50 µM, which reflects the IC50 value (MTT assay, Fig. 3), was selected for apoptosis induction in MCF7 cells (Fig. 4).
Figure 4.
Capsaicin- and capsaicin epoxide-mediated apoptosis of MCF7 cells. After 24-hour treatment with capsaicin (CAP) or capsaicin epoxide (CAPO) (50 µM), apoptosis was assessed using the Muse™ Cell Analyser and the Muse™ Multi-caspase Kit. Treatments with hydrogen peroxide and tert-butyl hydroperoxide (tBOOH) (10 mM, 0.5 and 3 hours) were used as positive controls. Representative multi-caspase profiles (dot plots) are presented.
Neither 50 µM capsaicin nor 50 µM capsaicin epoxide provoked apoptotic cell death in the MCF7 cell line (Fig. 4). In contrast, treatment with the well-known oxidants: hydrogen peroxide and tBOOH induced apoptosis (Fig. 4). It is possible that the capsaicin epoxide-mediated decrease in metabolic activity estimated using the MTT assay reflects the antiproliferative and cytostatic action of capsaicin epoxide rather than its ability to stimulate apoptotic cell death.
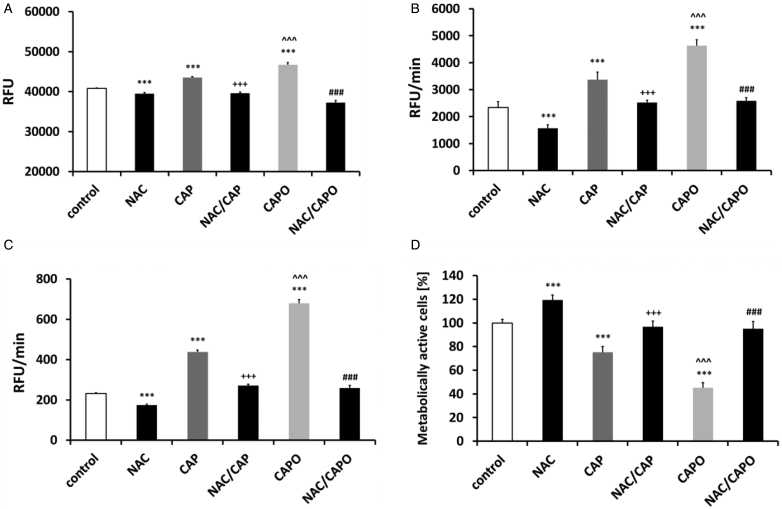
As it is widely accepted that the toxic effects of capsaicin against cancer cells may be mediated by oxidative stress,1 we were interested in determining whether the increased antiproliferative action of capsaicin epoxide may be associated with augmented ROS production compared with that obtained with capsaicin treatment and whether the antioxidant NAC may prevent against capsaicin epoxide-mediated oxidative stress and the decrease in proliferative potential. To study capsaicin epoxide-induced oxidative stress, we selected the most sensitive cancer cells to capsaicin epoxide treatment, namely, MCF7 cells (Fig. 3). The results show that the ROS steady-state level in the culture medium and the intracellular ROS and superoxide production were elevated after capsaicin epoxide treatment compared with those obtained after capsaicin treatment (P < 0.001) (Fig. 5).
Figure 5.
Capsaicin- and capsaicin epoxide-mediated oxidative stress and the effect of N-acetyl cysteine (NAC) in MCF7 cells. The steady-state level of reactive oxygen species (ROS) in the cell culture medium (A) and the intracellular ROS production (B) were measured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), and superoxide production (C) was measured with dihydroethidium. (D) The NAC-mediated recovery of the proliferative potential was estimated using the MTT assay. The bars indicate the SD (n = 5). ***P < 0.001 compared with the control conditions; ^ ^ ^P < 0.001 compared with 24-hour treatment with capsaicin (CAP) (24-hour treatment with 50 µM capsaicin versus 24-hour treatment with 50 µM capsaicin epoxide); +++P < 0.001 compared with 24-hour treatment with capsaicin (24-hour treatment with 50 µM capsaicin versus 2-hour pretreatment with 5 mM NAC and 24-hour treatment with 50 µM capsaicin, NAC/CAP); ###P < 0.001 compared with 24-hour treatment with capsaicin epoxide (CAPO) (24-hour treatment with 50 µM capsaicin epoxide versus 2-hour pretreatment with 5 mM NAC and 24-hour treatment with 50 µM capsaicin epoxide, NAC/CAPO) (ANOVA and Tukey's a posteriori test).
After 50 µM capsaicin epoxide treatment, the ROS steady-state level in the culture medium was moderately augmented compared with that obtained after 50 µM capsaicin treatment (P < 0.001) (Fig. 5A). Capsaicin and capsaicin epoxide caused increases in the intracellular ROS production of approximately 44 and 98% compared with the control conditions (P < 0.001) (Fig. 5B). A 1.88- and 2.92-fold increase in superoxide production was observed after capsaicin and capsaicin epoxide treatment compared with the control conditions, respectively (P < 0.001) (Fig. 5C). The antioxidant NAC was able to alleviate the capsaicin epoxide-mediated oxidative stress (P < 0.001) (Fig. 5A–C). Moreover, NAC also protected against the capsaicin epoxide-induced decrease in metabolic activity (P < 0.001) (Fig. 5D). Pretreatment with 5 mM NAC caused a proliferative potential recovery to approximately 95% of the control level (Fig. 5D).
Discussion
The concentration- and time-dependent anticancer potential of capsaicin in vitro has been well documented.1 In general, capsaicin is believed to be toxic to cancer cells, when added in the micromolar range.1 The maximal antiproliferative activity of capsaicin has been observed at approximately 200–300 µM.1 The response to capsaicin may be cancer cell type-dependent and may be influenced by the capsaicin stability during particular experimental conditions, which, in turn, may yield contradictory results, e.g. discrepancies between IC50 values across studies may occur.1 In the present study, we also established a cancer cell type-dependent capsaicin response. Human breast cancer cells, MCF7, were found to be the most susceptible to capsaicin treatment, whereas human renal cancer cells, ACHN, were insensitive to capsaicin stimulation. Moreover, we confirmed that capsaicin is a selective anticancer agent because the metabolic activity of normal HDFs after capsaicin treatment was indistinguishable from their metabolic activity in standard growth conditions.
It has been repeatedly reported that the bioavailability of phytochemicals with anticancer activities (e.g. polyphenols, terpenes, and alkaloids) is limited due to their poor absorption, rapid metabolism, and rapid systemic elimination.14,18 Thus, plant-based nutraceuticals may stimulate a bioresponse at serum concentrations, which are insufficient to demonstrate an in-vitro response.18 Data on the capsaicin plasma concentrations in a human body after capsaicin administration are scarce. After the oral administration of gelatin capsule-based capsicum (5 g), capsaicin can be detected in the plasma within the first 10 minutes of exposure, reaching a peak plasma concentration (Cmax) of approximately 2.5 ng/ml (which corresponds to 8.2 nM) after 47 minutes of exposure (Tmax).19 After a 60-minute topical administration of a high-concentration capsaicin patch (8%) to patients with peripheral neuropathic pain, the mean population Cmax was 1.38 ng/ml, and the highest capsaicin plasma concentration was recorded to be 17.8 ng/ml (58 nM).20 Moreover, the plasma half-life of capsaicin was estimated to be short: 25 and 98 minutes after oral and topical administration, respectively.19,20 The limited systemic bioavailability of phytochemicals affecting their anticancer potential in vivo may be overcome by the use of adjuvants, nanoparticles, liposomes, phospholipid complexes, and structural analogues.14 It has been reported that the pharmacokinetic characteristics of capsaicin may be improved by the use of capsaicin-loaded nanoemulsions fabricated with alginate and chitosan or the inclusion complex of capsaicin/hydroxypropyl-β-cyclodextrin.21,22 The experimental data on the capsaicin chemical modification-mediated cytotoxic potential against cancer cells are limited.23,24 A previous study found that the RPF101 capsaicin-like analogue exhibits higher antitumour activity than capsaicin by inducing arrest of the cell cycle at the G2/M phase through disruption of the microtubule network in MCF7 cells.23 Piperonylamine was sulfonylated by benzenesulfonyl chloride to yield RPF101 with more suitable lipophilic properties and a hydrogen bond acceptor character, which are relevant features for improved pharmacokinetic and pharmacodynamic profiles.23
In the present study, we were interested in determining whether the epoxidation of capsaicin may promote an increase in cancer cell cytotoxicity. In this study, we performed the first synthesis of a high-purity capsaicin epoxide (N-(4-hydroxy-3-methoxybenzyl)-5-[3-(propan-2-yl)oxiran-2-yl]pentanamide) and provide the first demonstration of its selective action against several cancer cell lines. Human breast cancer cells, MCF7, were found to be the most sensitive to capsaicin epoxide treatment with an estimated IC50 value of approximately 50 µM, whereas human renal cancer cells, ACHN, were the least sensitive to capsaicin epoxide treatment. As capsaicin and capsaicin epoxide did not stimulate apoptotic cell death in MCF7 cells, we speculated that the MTT data may reflect the ability of capsaicin and capsaicin epoxide to diminish the proliferative potential of MCF7 cells rather than to promote apoptosis or another type of cell death (cytostatic action versus cytotoxic action). More recently, it has been reported that capsaicin causes nonapoptotic cell cycle arrest of MCF7 and MDA-MB-231 breast cancer cells and that capsaicin-associated autophagy is involved in the retardation of cell death by blocking capsaicin-induced endoplasmic reticulum (ER) stress-mediated apoptosis in MCF7 and MDA-MB-231 cells.25 Regardless of the cancer cell type-specific toxicity of capsaicin epoxide, in all of the cancer cell lines examined, the antiproliferative activity of capsaicin epoxide was markedly higher than that of unmodified capsaicin, and this effect was found to be mediated by increased oxidative stress. Capsaicin epoxide induced augmented ROS and superoxide production compared with capsaicin treatment. Moreover, the antioxidant NAC was able to alleviate the capsaicin- and capsaicin epoxide-mediated oxidative stress and antiproliferative activity, which suggests that the action of capsaicin epoxide is indeed mediated by increased ROS production. However, a previous study documented that the anticancer action of capsaicin is ROS-independent.26 The discrepancies between these studies may rely on the exposure time and concentrations used and other experimental conditions. The authors of the previous study26 used a 30-minute treatment with 150 µM capsaicin and a fluorogenic probe to reflect the total ROS level (H2DCF-DA), whereas we measured the total ROS and superoxide production (H2DCF-DA and dihydroethidium, respectively) after 24 hours of treatment with 50 µM capsaicin or 50 µM capsaicin epoxide.
It is widely accepted that epoxide derivatives may be more reactive than intact compounds and may attack nucleophilic groups (e.g. amino groups) within biomolecules, such as proteins and DNA, leading to cytotoxicity.27 It is also speculated that the metabolic activation of capsaicin may include the following: epoxidation of the vanillyl ring moiety to produce an arene oxide, one-electron oxidation of the ring hydroxyl group to generate a phenoxy radical and O-demethylation followed by oxidation of the resulting catechol metabolite to semiquinone and quinone derivatives.27–29 The resulting reactive species may covalently bind to DNA and may promote mutagenicity.30,31 Indeed, hot pepper consumption has been reported to be associated with an increased risk of cancer, such as gallbladder and gastric cancers.32,33 Nevertheless, there is no evidence that reactive capsaicin metabolites are formed within the human body and are physiologically relevant.1,27 Moreover, it has been suggested that many of the epidemiological studies on capsaicin-induced cancerogenicity may suffer from severe limitations, such as statistical imprecision of some analyses, potential misclassification of subjects by exposure, possible recall bias, and/or poor control of confounding factors.34 One should also remember that hot pepper consumption is not equivalent to the use of pure capsaicin.34 Pepper extracts are a mixture of different capsaicinoid compounds, such as capsaicin, norhydrocapsaicin, dihydrocapsaicin, homocapsaicin, homodihydrocapsaicin, and nonivamide, and the concentrations may vary depending on the extract used.1 Moreover, pepper extracts may contain some toxic impurities, such as pesticides, insecticides, fertilizers, microbiocides, and heavy metals, which, in turn, may contribute to the observed carcinogenic potential of capsaicin.1
In summary, we provide the first demonstration that capsaicin epoxidation may promote increased oxidative stress, which, in turn, may result in augmented anticancer activity, compared with unmodified capsaicin. In addition, capsaicin epoxide may be considered as a selective anticancer agent because normal human fibroblasts were found to be insensitive to capsaicin epoxide treatment. Despite the low bioavailability of capsaicin, capsaicin-mediated anticancer effects have also been shown in numerous in-vivo mouse xenograft tumour models, including mouse melanoma, human leukaemia, human prostate, human multiple myeloma, human pancreatic and human bladder cancers, when this compound is administered orally, subcutaneously, intraperitoneally, or directly into tumours (see references within1). Thus, the findings may suggest that the anticancer effects of capsaicin are not limited to the in-vitro conditions and the usefulness of capsaicin anticancer therapy. However, the design and synthesis of novel capsaicin derivatives with more potent anticancer action may facilitate the use of capsaicin-based interventions in vivo.
Disclaimer statements
Contributors Conceived and designed the experiments: AL. Performed the experiments: AL, PC, KS, ER, MW. Analyzed the data: AL, PC. Wrote the paper: AL.
Funding This study was supported by the grant from the National Science Center, 2013/11/D/NZ7/00939.
Conflict of interest None.
Ethics approval N/A.
Acknowledgement
The authors would like to thank Dr Piotr Kwiatkowski (University of Warsaw, Faculty of Chemistry) for NMR, GC, and MS analyses. This study was supported by the grant from the National Science Center, 2013/11/D/NZ7/00939.
References
- 1.Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol Pathol 2012;40(6):847–73. [DOI] [PubMed] [Google Scholar]
- 2.Matucci Cerinic M, McCarthy G, Lombardi A, Pignone A, Partsch G. Neurogenic influences in arthritis: potential modification by capsaicin. J Rheumatol 1995;22(8):1447–9. [PubMed] [Google Scholar]
- 3.Sicuteri F, Fusco BM, Marabini S, Campagnolo V, Maggi CA, Geppetti P,. et al. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin J Pain 1989;5(1):49–53. [DOI] [PubMed] [Google Scholar]
- 4.Watson CP, Evans RJ, Watt VR. Post-herpetic neuralgia and topical capsaicin. Pain 1988;33(3):333–40. [DOI] [PubMed] [Google Scholar]
- 5.Marabini S, Ciabatti PG, Polli G, Fusco BM, Geppetti P. Beneficial effects of intranasal applications of capsaicin in patients with vasomotor rhinitis. Eur Arch Otorhinolaryngol 1991;248(4):191–4. [DOI] [PubMed] [Google Scholar]
- 6.Morre DJ, Chueh PJ, Morre DM. Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Proc Natl Acad Sci USA 1995;92(6):1831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hail N Jr., Lotan R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J Natl Cancer Inst 2002;94(17):1281–92. [DOI] [PubMed] [Google Scholar]
- 8.Kang HJ, Soh Y, Kim MS, Lee EJ, Surh YJ, Kim HR,. et al. Roles of JNK-1 and p38 in selective induction of apoptosis by capsaicin in ras-transformed human breast epithelial cells. Int J Cancer 2003;103(4):475–82. [DOI] [PubMed] [Google Scholar]
- 9.Lee YS, Kang YS, Lee JS, Nicolova S, Kim JA. Involvement of NADPH oxidase-mediated generation of reactive oxygen species in the apototic cell death by capsaicin in HepG2 human hepatoma cells. Free Radic Res 2004;38(4):405–12. [DOI] [PubMed] [Google Scholar]
- 10.Min JK, Han KY, Kim EC, Kim YM, Lee SW, Kim OH,. et al. Capsaicin inhibits in vitro and in vivo angiogenesis. Cancer Res 2004;64(2):644–51. [DOI] [PubMed] [Google Scholar]
- 11.Surh YJ. More than spice: capsaicin in hot chili peppers makes tumor cells commit suicide. J Natl Cancer Inst 2002;94(17):1263–5. [DOI] [PubMed] [Google Scholar]
- 12.Thoennissen NH, O'Kelly J, Lu D, Iwanski GB, La DT, Abbassi S,. et al. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene 2010;29(2):285–96. [DOI] [PubMed] [Google Scholar]
- 13.Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS One 2011;6(5):e20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4(6):807–18. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65(1–2):55–63. [DOI] [PubMed] [Google Scholar]
- 16.Mytych J, Lewinska A, Bielak-Zmijewska A, Grabowska W, Zebrowski J, Wnuk M. Nanodiamond-mediated impairment of nucleolar activity is accompanied by oxidative stress and DNMT2 upregulation in human cervical carcinoma cells. Chem Biol Interact 2014;220C:51–63. [DOI] [PubMed] [Google Scholar]
- 17.Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res 2000;259(1):308–13. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006;71(10):1397–421. [DOI] [PubMed] [Google Scholar]
- 19.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai 2009;92(1):108–13. [PubMed] [Google Scholar]
- 20.Babbar S, Marier JF, Mouksassi MS, Beliveau M, Vanhove GF, Chanda S,. et al. Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit 2009;31(4):502–10. [DOI] [PubMed] [Google Scholar]
- 21.Choi AY, Kim CT, Park HY, Kim HO, Lee NR, Lee KE,. et al. Pharmacokinetic characteristics of capsaicin-loaded nanoemulsions fabricated with alginate and chitosan. J Agric Food Chem 2013;61(9):2096–102. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Sun X, Ren K, Zhang X, Zhang Z, Gong T. Enhanced aqueous solubility and bioavailability of capsaicin by the preparation of an inclusion complex. Arzneimittelforschung 2010;60(9):571–4. [DOI] [PubMed] [Google Scholar]
- 23.de-Sa-Junior PL, Pasqualoto KF, Ferreira AK, Tavares MT, Damiao MC, de Azevedo RA,. et al. RPF101, a new capsaicin-like analogue, disrupts the microtubule network accompanied by arrest in the G2/M phase, inducing apoptosis and mitotic catastrophe in the MCF-7 breast cancer cells. Toxicol Appl Pharmacol 2013;266(3):385–98. [DOI] [PubMed] [Google Scholar]
- 24.Damiao MC, Pasqualoto KF, Ferreira AK, Teixeira SF, Azevedo RA, Barbuto JA,. et al. Novel capsaicin analogues as potential anticancer agents: synthesis, biological evaluation, and in silico approach. Arch Pharm (Weinheim) 2014, 347:1–11. [DOI] [PubMed] [Google Scholar]
- 25.Choi CH, Jung YK, Oh SH. Autophagy induction by capsaicin in malignant human breast cells is modulated by p38 and extracellular signal-regulated mitogen-activated protein kinases and retards cell death by suppressing endoplasmic reticulum stress-mediated apoptosis. Mol Pharmacol 2010;78(1):114–25. [DOI] [PubMed] [Google Scholar]
- 26.Chou CC, Wu YC, Wang YF, Chou MJ, Kuo SJ, Chen DR. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol Rep 2009;21(3):665–71. [PubMed] [Google Scholar]
- 27.Chen XW, Serag ES, Sneed KB, Zhou SF. Herbal bioactivation, molecular targets and the toxicity relevance. Chem Biol Interact 2011;192(3):161–76. [DOI] [PubMed] [Google Scholar]
- 28.Reilly CA, Yost GS. Metabolism of capsaicinoids by P450 enzymes: a review of recent findings on reaction mechanisms, bio-activation, and detoxification processes. Drug Metab Rev 2006;38(4):685–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly CA, Yost GS. Structural and enzymatic parameters that determine alkyl dehydrogenation/hydroxylation of capsaicinoids by cytochrome p450 enzymes. Drug Metab Dispos 2005;33(4):530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oikawa S, Nagao E, Sakano K, Kawanishi S. Mechanism of oxidative DNA damage induced by capsaicin, a principal ingredient of hot chili pepper. Free Radic Res 2006;40(9):966–73. [DOI] [PubMed] [Google Scholar]
- 31.Surh YJ, Lee SS. Capsaicin, a double-edged sword: toxicity, metabolism, and chemopreventive potential. Life Sci 1995;56(22):1845–55. [DOI] [PubMed] [Google Scholar]
- 32.Serra I, Yamamoto M, Calvo A, Cavada G, Baez S, Endoh K,. et al. Association of chili pepper consumption, low socioeconomic status and longstanding gallstones with gallbladder cancer in a Chilean population. Int J Cancer 2002;102(4):407–11. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol 1994;139(3):263–71. [DOI] [PubMed] [Google Scholar]
- 34.Bode AM, Dong Z. The two faces of capsaicin. Cancer Res 2011;71(8):2809–14. [DOI] [PubMed] [Google Scholar]