Abstract
Few people expected that asbestos, a fibrous mineral, would be carcinogenic to humans. In fact, asbestos is a definite carcinogen in humans, causing a rare but aggressive cancer called malignant mesothelioma (MM). Mesothelial cells line the three somatic cavities and thus do not face the outer surface, but reduce the friction among numerous moving organs. MM has several characteristics: extremely long incubation period of 30–40 years after asbestos exposure, difficulty in clinical diagnosis at an early stage, and poor prognosis even under the current multimodal therapies. In Japan, ‘Kubota shock’ attracted considerable social attention in 2005 for asbestos-induced mesothelioma and, thereafter, the government enacted a law to provide the people suffering from MM a financial allowance. Several lines of recent evidence suggest that the major pathology associated with asbestos-induced MM is local iron overload, associated with asbestos exposure. Preclinical studies to prevent MM after asbestos exposure with iron reduction are in progress. In addition, novel target genes in mesothelial carcinogenesis have been discovered with recently recognized mesothelioma-prone families. Development of an effective preventive strategy is eagerly anticipated because of the long incubation period for MM.
Keywords: Malignant mesothelioma, Asbestos, Iron overload, CDKN2A/2B, Iron chelation, Phlebotomy
Introduction
Asbestos is a fibrous form of mineral. Although there are six distinct forms of asbestos by definition, three types of asbestos, namely chrysotile (white asbestos), crocidolite (blue asbestos), and amosite (brown asbestos), were of major use commercially.1,2 It has been recognized that cloths covering Egyptian mummies contained asbestos. In addition, a samurai, pharmacologist, writer, and inventor, Gen-nai Hiraga, discovered this fibrous stone in the Edo Period in the mountainous area of Chichibu on the suburbs of Tokyo and produced what is called burn-free textile (Kakanpu in Japanese).
Because asbestos is a stone, it is heat-, acid-, and friction-resistant. Importantly, asbestos was economical in that mining of asbestos was efficient. Therefore, it was used abundantly all over the world during the last century. However, it was recognized in the 1960s that a rare type of cancer appeared in the workers using asbestos.3,4 Finally, in 1987, the International Agency for Research on Cancer (IARC) designated all asbestos as a Group 1 carcinogen (definite carcinogen to humans).2 However, in most countries, the use of asbestos continued, and even now many developing counties produce and use asbestos.5 This phenomenon is mainly due to economic considerations. Production of asbestos substitutes requires costly chemical plants, which developing countries currently cannot afford. In the fall of 2012, Canada finally stopped mining chrysotile (http://www.mining.com/canada-waves-au-revoir-to-asbestos-mining-20394/), and IARC is currently trying to stop the use of all asbestos (http://www.iarc.fr/en/media-centre/iarcnews/pdf/WHO-IARC_Statement.pdf).
Malignant mesothelioma
Malignant mesothelioma (MM) is a rare tumor, originating from mesothelial cells.6,7. In Japan, we have approximately 1500 new MM cases each year, and incidence rates are increasing.8 This figure may be small in comparison to new cases of lung cancer, which account for approximately 80 000 cases each year, but MM carries a social impact because 80% of cases are thought to be associated with former asbestos exposure.1 MM has a mysteriously long incubation period after asbestos exposure, and I suggest that this is the period required for the asbestos fibers to pass through the pulmonary parenchymal tissue. Asbestos fibers are inhaled from the air, and the extremely thin fibers are inspirated into the nasal cavity, trachea, and lung. Alveolar macrophages are available for the disposal of these foreign substances, but fail to dispose of them if the fibers are too long (length > 20 µm) and/or too thin (diameter < 250 nm). In these cases, macrophages die after phagocytosis of the fibers, and the residual proteins and other molecules are adsorbed on the surface of the asbestos. These reactions appear specific and the resultant asbestos bodies contain abundant iron.1 Despite these events, asbestos fibers intrinsically proceed toward the pleural cavity due to negative pressure in the pleural cavity. Asbestos fibers then reach the visceral pleura, break through it, and finally reach the parietal pleura (Fig. 1). Clinically, it is well recognized that most cases of MM occur at the parietal pleura.9
Figure 1.
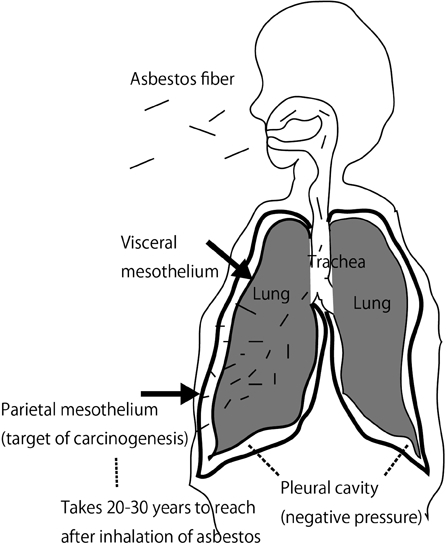
Mechanism of asbestos-induced mesothelial carcinogenesis.
Characteristics of mesothelial cells
Mesothelial cells cover the three somatic cavities (pleural, peritoneal, and pericardial cavities) with a villous surface but with flat single-layered morphology. Thus, mesothelial cells do not face the outer surface and are in continuation with lymphatic vessels. Podoplanin is a common cell-specific marker between mesothelial cells and lymphatic endothelial cells.7 The main function of mesothelial cells is to reduce the friction between organs with pulsating and peristaltic movements via secreted hyaluronic acid. However, not much is known regarding the damage, repair, and proliferation of mesothelial cells. We recently have shown with ex vivo culture systems that mesothelial cells move extensively and metamorphose into a cuboidal shape after sensing damage nearby.10
Mesothelial cells have the unique characteristic of engulfing anything, whether a solid, liquid, or gas. Imagine that there was an intraperitoneal operation, such as cholecystectomy. After this procedure, plenty of air can be seen in the peritoneal cavity with X-ray examination. However, this air is completely absorbed by mesothelial cells within a week. Similarly, mesothelial cells phagocytose asbestos fibers.11 In previous work, we have shown this phenomenon with video files. Interestingly, mesothelial cells actively engulf asbestos fibers and the cells do not die thereafter. On the contrary, macrophages die more often than mesothelial cells after phagocytosis.12 We believe that this characteristic is important for mesothelial carcinogenesis because each asbestos fiber has a high affinity for histones (H2A, H2B, H3, and H4).13,14 Thus, phagocytosed asbestos likely attaches to chromosomes when mesothelial cells are dividing, leading to massive genomic alterations such as deletion and amplification after some repair processes. I suggest that this sequence is the initiation process for MM: mesothelial cells continuing to divide in vivo after damage of the mesothelium (Fig. 2). However, this process has not been well analyzed in vivo.
Figure 2.
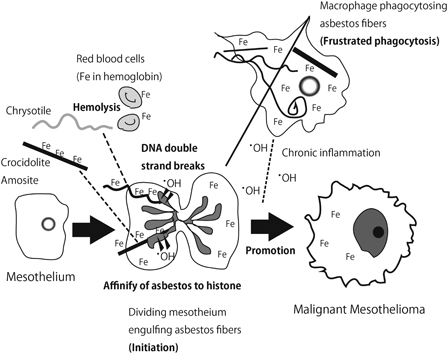
Role of iron overload in asbestos-induced mesothelial carcinogenesis.
Another factor that should be mentioned here is that lymphatic vessels are in continuity with mesothelial cells,15 and this is the route of the recovery process of the hydrothorax. When the length of asbestos fibers is sufficiently great, they are stuck at the orifice of lymphatic vessels,16 which may be the starting point of mesothelial carcinogenesis. Currently, there is no way to clean the human lung after inhalation of asbestos; thus, it is extremely difficult to remove asbestos fibers in vivo after inhalation.
Link between iron overload and mesothelial carcinogenesis
Iron overload is closely associated with carcinogenesis, presumably via catalytic action by the Fenton reaction.17,18 In 1989, our group showed that iron deposits via intraperitoneal administration of ferric saccharate can cause peritoneal mesothelioma in rats, albeit with an extremely long incubation time, a low incidence and a male preference.19 Therefore, iron overload per se is important for mesothelial carcinogenesis, and iron overload can be a sufficient carcinogen for mesothelial cells. This risk factor also has been suggested by the presence of iron-rich asbestos fibers in the lung and in other organs of people who were exposed to asbestos fibers.1 Recently, more convincing data were published indicating the importance of excess iron in mesothelial carcinogenesis, as discussed below.
In vitro data
Asbestos fibers are recognized to be adsorptive20 and have been suggested to bind to specific proteins,21 carcinogenic molecules from cigarettes, nucleic acids, and radioactive radium.22 Nagai et al. have recently performed a simple experiment to identify adsorptive proteins using lysates of lung, liver, kidney, and mesothelial cells. The major and important proteins included histones, actin, tubulin, and, most importantly, hemoglobin.13 Approximately 60% of iron in humans exists as heme in hemoglobin within erythrocytes.23 In addition to high iron content as a mineral in crocidolite (blue asbestos) and amosite (brown asbestos), the affinity of asbestos to hemoglobin appears to be the reason why asbestos accumulates iron.
This phenomenon is especially true for chrysotile (white asbestos), which itself contains little iron (Fig. 2). Notably, chrysotile is potent in causing hemolysis, thereafter attaching to hemoglobin. The catalytic activity of chrysotile is highly increased in the presence of hemoglobin and, furthermore, chrysotile most readily adsorbs DNA among the three commercially used types of asbestos.13 These points suggest that chrysotile is more promotive for carcinogenesis than formerly thought. Of course, chrysotile is softer and more pliable than crocidolite and amosite, which enables more efficient removal by macrophages. Nevertheless, the current description of chrysotile being 500 times less carcinogenic than crocidolite2 has to be reconsidered, especially in light of the report describing a high incidence of carcinogenicity after intraperitoneal injection of chrysotile.24
Animal experiments
As described above, mesothelial cells are the target of carcinogenesis by asbestos fibers. In addition to the experiments described above, animal models can make full use of this characteristic. Intraperitoneal or intrapleural injection of asbestos is optimal for maximally exposing mesothelial cells to asbestos fibers. This sort of administration involves both drawbacks and benefits. One drawback is that the exposure method is different from that of real human exposure. Namely, removal of asbestos via macrophages in alveoli is omitted, and other related pathologies may be missed. However, the benefit is that we can evaluate the maximal carcinogenicity of mesothelial cells, especially in the peritoneum. Therefore, if no carcinogenicity is observed after intraperitoneal administration, it is highly possible that the fibrous material itself does not have carcinogenicity to mesothelial cells.25,26 Furthermore, we are aware that intraperitoneal injection is a more sensitive method than intrapleural administration, which may be due to abundant adipocytes in the peritoneal cavity.27
We recently performed a full study of intraperitoneal administration of three distinct asbestos fibers to rats.24 We used F1 hybrid rats between Fischer-344 and Long-Evans, and we repeatedly confirmed that not a single MM appears spontaneously until death.28 Surprisingly, chrysotile caused MM the fastest, with 50% occurrence at ∼400 days after 10 mg administration. For the crocidolite and amosite, it took ∼600 days for 50% of the rats to develop MM. For all three types of asbestos, repeated administration of nitrilotriacetic acid (NTA) after asbestos injection significantly promoted mesothelial carcinogenesis.24 NTA is known to promote the Fenton reaction very efficiently at neutral pH.29,30 Therefore, these data suggest that iron overload is a major pathogenesis of mesothelial carcinogenesis for all three asbestos fibers. This phenomenon was confirmed by measuring the iron content of intraperitoneal organs in addition to measuring serum ferritin concentration, which is an indicator of the body's iron stores.24 In contrast, serum non-transferrin-bound iron31 was decreased. This outcome indicates that cellular damage is not intense, and defensive mechanisms to withdraw iron from the extracellular environment are in operation. The latter action is an important mechanism to deprive bacteria and parasites of iron during infection. Thus, similar mechanisms are working.
Our group has been working with iron-induced carcinogenesis models for years (Table 1). The impetus was the finding by Shigeru Okada and Osamu Midorikawa that an iron chelate, ferric nitrilotriacetate, induces renal cell carcinoma when injected intraperitoneally.32,33 Later, it was shown that the Fenton reaction occurs specifically at the renal proximal tubules, which is the site of pathogenesis of renal cell carcinoma.34–36 We also reported an increase in various oxidative products in this model, such as unsaturated aldehydes37,38 and oxidatively modified DNA bases.39–41 Recently, we analyzed the genomic alteration of this renal carcinogenesis with array-based comparative genomic hybridization (CGH), and we found massive genomic changes that have never been reported in animal carcinogenesis using wild-type animals. Two major alterations were the deletion of Cdkn2a/2b (p16/p15) tumor suppressor genes and the amplification of the Met oncogene.42 I propose, based on these findings, that excess iron also plays a major role in human carcinogenesis, because massive genomic alterations are observed in most human cancers.
Table 1.
Iron-induced rat carcinogenesis model
| Chemical | Administration | Target organ | Histology | Genetic/epigenetic alteration | Other characteristics |
|---|---|---|---|---|---|
| Ferric nitrilotriacetate32 | Intraperitoneal | Kidney (proximal tubular cells) | Renal cell carcinoma | HD/RM of Cdkn2a/2b; Met amplification,42 OE of Annexin 260 and miR-34a61 | 1–2 years for induction; male preference |
| Ferric saccharate, with nitrilotriacetate19 | Intraperitoneal | Mesothelium (mesothelial cells) | Malignant mesothelioma | HD of Cdkn2a/2b only in sarcomatoid subtype28 | >2 years for induction; low incidence; strict male preference |
| Asbestos (chrysotile, crocidolite, Amosite), with nitrilotriacetate24 | Intraperitoneal | Mesothelium (mesothelial cells) | Malignant mesothelioma | HD of Cdkn2a/2b | 1–2 years for induction; extremely high incidence; no sex preference |
HD, homozygous deletion; OE, overexpression; RM, repression by methylation of the promoter region.
We then analyzed the genomic changes of asbestos-induced MM in rats. Array-based CGH revealed homozygous deletion of Cdkn2a/2b in 93% of MM induced by three different asbestos fibers.24 Many other massive alterations were also found. Furthermore, homozygous deletion of Cdkn2a/2b was found in 80% of the sarcomatoid subtype of MM induced by intraperitoneal ferric saccharate.28 Altogether, these results indicate that iron overload is one of the major causes of homozygous deletion of Cdkn2a/2b. Conversely, the presence of homozygous deletion of Cdkn2a/2b may suggest that the major pathogenic mechanism of that carcinogenesis is iron overload.
Analysis of human mesothelioma
Genomic alteration of human MM has been analyzed since the 1990s. It is now established that homozygous deletion of Cdkn2a/2b43,44 and inactivation of the Hippo pathway45,46 are the two major genomic alterations in MM. Epigenetic changes are sometimes also involved. Cancer-prone families are often the drive to find a novel tumor suppressor gene, where one of the alleles of the corresponding tumor suppressor gene is inactivated with genomic mutation in all the somatic cells of the affected member.47 In 2011, two mesothelioma-prone families were first reported.48 The patients were complicated by a variety of cancers including uveal melanoma, and the responsible tumor suppressor gene was identified as BAP1 (BRCA1 associated protein 1), which encodes a nuclear deubiquitinase enzyme associated with chromatin regulation.49 The authors described this gene as being associated with sensitivity to asbestos fibers given that asbestos fibers were detected in the family's house. BAP1 is commonly inactivated in sporadic human MM as well.49,50
Prevention of mesothelioma after asbestos exposure
While epigenetic alteration and point mutation are involved in mesothelial carcinogenesis, the major genetic alteration is homozygous deletion of Cdkn2a/2b. This event leads to both the inactivation of p53 pathways (apoptosis after genome damage) and cell cycle brakes (inhibitor of cyclin-dependent kinases).51 The important point is that, although we can currently use inhibitors against that which is obtained (oncogene amplification, fusion gene with chromosomal translocation, etc.), it is not easy to revive that which is completely lost (homozygous deletion). Therefore, it is of utmost importance to prevent gene deletion, of which iron overload appears to be the most likely cause. Therefore, the ideal target to prevent mesothelioma after asbestos exposure would be the decrease/adjustment of iron storage.
Iron, as ferrous iron, is absorbed at ∼1 mg/day from the diet at the luminal villous surface of the duodenum through the DMT1 (SLC11A2) transporter and ferroportin exporter (SLC40A1) into the portal vein. There is no active mechanism to excrete iron from the body once iron is in the blood stream.52,53 Heme is another important dietary iron source, but the molecular mechanism of its absorption is not yet clear. Only ∼1 mg/day of iron leaves the body through desquamation of the epidermis. Other than desquamation, hemorrhage is the main source of iron removal, because ∼60% of iron is in the hemoglobin of red blood cells. Thus, phlebotomy or blood donation is an efficient method for iron removal from the body.54 Phlebotomy was used in the Greek period as a folklore therapy. Now, it is an official therapy in Japan for the treatment of hepatitis virus C-associated chronic active hepatitis, in which excessive hepatic iron through low hepcidin is associated with hepatic damage, inflammation, fibrotic change (cirrhosis) and hepatocarcinogenesis.55 In humans, genetic hemochromatosis and ovarian endometriosis are two other diseases associated with iron overload and carcinogenesis.52 Notably, it was reported that phlebotomy twice a year in the general population reduced cancer incidence by 35% and cancer mortality by 61%.56 For massive iron overload, iron chelators have been used as a therapy and, recently, oral iron chelators such as deferasirox and deferoprine have been introduced to the market. In Japan, deferasirox has been used for patients in iron overload of bone marrow caused by repeated transfusion therapy.57
We recently performed a preclinical experiment to determine whether deferasirox or phlebotomy can reduce the incidence, mortality or malignant potential of MM in rats after asbestos administration. MM is histologically classified into three subtypes: epithelioid, biphasic, and sarcomatoid. The biphasic subtype harbors both epithelioid and sarcomatoid subtypes of more than 10% of each. The sarcomatoid subtype presents significantly poorer prognosis than the epithelioid subtype. This preclinical prevention study showed that lifetime deferasirox treatment after asbestos administration significantly increased the fraction of the epithelioid subtype of lower malignant potential.58 Survival was marginally increased with deferasirox only in female rats. Repeated phlebotomy and its adjustment for the maximal effects are technically difficult in rats, and significant alteration was not observed with repeated phlebotomy for 1 year, which certainly requires further investigation.
Conclusion
Direct damage of mesothelial cells by asbestos fiber is an important initiation process for asbestos-induced mesothelial carcinogenesis. Here, the associated local iron overload is critically important both for initiation and promotion of MM (Fig. 2). Iron-coated asbestos fibers could work as a sharp knife to cause DNA double-strand breaks in mesothelial cells, and long-time iron deposition in macrophages and mesothelial cells could induce oxidative stress, leading to promotion of carcinogenesis. Considering that we cannot remove thin asbestos fibers after inhalation, local iron deposits should be the target for the prevention of asbestos-induced mesothelial carcinogenesis. Of course, we have to consider the side effects of this preventive intervention, as asbestos-induced carcinogenesis takes several decades. Finally, I may add that the mechanism for multiwalled carbon nanotube-induced mesothelial carcinogenesis was almost the same as for asbestos fibers.26,59 Fortunately, carbon nanotube-induced mesothelial carcinogenesis has not been reported in humans.
Acknowledgments
This work was supported in part by a grant-in-aid for research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and a grant-in-aid for research from the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
Deferasirox was provided by Novartis Pharma.
References
- 1.Roggli VL, Oury TD, Sporn TA, editors. Pathology of asbestos-associated diseases. 2nd ed New York: Springer Verlag; 2004. [Google Scholar]
- 2.IARC, WHO Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans A Review of Human Carcinogens; Part C: Arsenic, Metals, Fibres, and Dusts. 100C. Lyon, France; 2012. p. 219–309.
- 3.Pass HI, Vogelzang NJ, Carbone M, editors. Malignant mesothelioma: advances in pathogenesis, diagnosis, and translational therapies. New York, NY: Springer Science + Business Media Inc; 2005. [Google Scholar]
- 4.Toyokuni S. Mechanisms of asbestos-induced carcinogenesis. Nagoya J Med Sci 2009;71(1–2):1–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 2013;34:205–16. [DOI] [PubMed] [Google Scholar]
- 6.Churg A, Cagle PT, Roggli VL. Tumors of the serosal membranes. Silver Spring, Maryland: ARP Press; 2006. [Google Scholar]
- 7.Husain AN, Colby TV, Ordonez NG, Krausz T, Borczuk A, Cagle PT,. et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133(8):1317–31. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi C, Bianchi T. Malignant mesothelioma in Eastern Asia. Asian Pacific J Cancer Prev 2012;13(10):4849–53 [DOI] [PubMed] [Google Scholar]
- 9.Heelan R. Staging and response to therapy of malignant pleural mesothelioma. Lung Cancer 2004;45(Suppl 1):S59–61. [DOI] [PubMed] [Google Scholar]
- 10.Nagai H, Chew S, Okazaki Y, Funahashi S, Namba T, Kato T,. et al. Metamorphosis of mesothelial cells wtih active horizontal motility in tissue culture. Sci Rep 2013;3:1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita K, Nagai H, Kondo Y, Misawa N, Toyokuni S. Evaluation of two distinct methods to quantify the uptake of crocidolite fibers by mesothelial cells. J Clin Biochem Nutr 2013;53(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Nagai H, Ohara H, Hara S, Tachibana M, Hirano S,. et al. Characteristics and modifying factors of asbestos-induced oxidative DNA damage. Cancer Sci 2008;99:2142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagai H, Ishihara T, Lee WH, Ohara H, Okazaki Y, Okawa K,. et al. Asbestos surface provides a niche for oxidative modification. Cancer Sci 2011;102:2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo Y, Takenaka H, Nagai H, Toyokuni S. Distinct affinity of nuclear proteins to the surface of chrysotile and crocidolite. J Clin Biochem Nutr 2012;51(3):221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JSP. (ed.). Pathology of the mesothelium. Berlin Heiderberg: Springer-Verlag; 1987. [Google Scholar]
- 16.Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 2010;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med 1996;20:553–66. [DOI] [PubMed] [Google Scholar]
- 18.Toyokuni S. Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep 2002;7(4):189–97. [DOI] [PubMed] [Google Scholar]
- 19.Okada S, Hamazaki S, Toyokuni S, Midorikawa O. Induction of mesothelioma by intraperitoneal injections of ferric saccharate in male Wistar rats. Br J Cancer 1989;60:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai H, Toyokuni S. Biopersistent fiber-induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys 2010;502(1):1–7. [DOI] [PubMed] [Google Scholar]
- 21.MacCorkle RA, Slattery SD, Nash DR, Brinkley BR. Intracellular protein binding to asbestos induces aneuploidy in human lung fibroblasts. Cell Motil Cytoskeleton 2006;63(10):646–57. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura E, Makishima A, Hagino K, Okabe K. Accumulation of radium in ferruginous protein bodies formed in lung tissue: association of resulting radiation hotspots with malignant mesothelioma and other malignancies. Proc Jpn Acad Ser B Phys Biol Sci 2009;85(7):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wriggleworth JM, Baum H. The biochemical function of iron. In: Jacobs A, Worwood M (eds.). Iron in biochemistry and medicine, II. London: Academic Press; 1980. p. 29–86. [Google Scholar]
- 24.Jiang L, Akatsuka S, Nagai H, Chew SH, Ohara H, Okazaki Y,. et al. Iron overload signature in chrysotile-induced malignant mesothelioma. J Pathol 2012;228:366–77. [DOI] [PubMed] [Google Scholar]
- 25.Toyokuni S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev 2013. (in press) doi:pii: S0169-409X(13)00149-X. 10.1016/j.addr.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Nagai H, Okazaki Y, Chew SH, Misawa N, Miyata Y, Shinohara H,. et al. Intraperitoneal administration of tangled multiwalled carbon nanotubes of 15 nm in diameter does not induce mesothelial carcinogenesis in rats. Pathol Int 2013. (in press) doi: 10.1093/carcin/bgt267. [DOI] [PubMed] [Google Scholar]
- 27.Chew SH, Okazaki Y, Nagai H, Misawa N, Akatsuka S, Yamashita K,. et al. Cancer-promoting role of adipocytes in asbestos-induced mesothelial carcinogenesis through dysregulated adipocytokine production. Carcinogenesis 2013. (in press). [DOI] [PubMed] [Google Scholar]
- 28.Hu Q, Akatsuka S, Yamashita Y, Ohara H, Nagai H, Okazaki Y,. et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab Invest 2010;90:360–73. [DOI] [PubMed] [Google Scholar]
- 29.Toyokuni S, Sagripanti JL. Iron-mediated DNA damage: sensitive detection of DNA strand breakage catalyzed by iron. J Inorg Biochem 1992;47:241–8. [DOI] [PubMed] [Google Scholar]
- 30.Toyokuni S, Sagripanti J-L. DNA single- and double-strand breaks produced by ferric nitrilotriacetate in relation to renal tubular carcinogenesis. Carcinogenesis 1993;14:223–7. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki K, Ikuta K, Tanaka H, Ohtake T, Torimoto Y, Fujiya M,. et al. Improved quantification for non-transferrin-bound iron measurement using high-performance liquid chromatography by reducing iron contamination. Mol Med Rep 2011;4(5):913–8. [DOI] [PubMed] [Google Scholar]
- 32.Ebina Y, Okada S, Hamazaki S, Ogino F, Li JL, Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum- nitrilotriacetate complexes in rats. J Natl Cancer Inst 1986;76:107–13. [PubMed] [Google Scholar]
- 33.Li JL, Okada S, Hamazaki S, Ebina Y, Midorikawa O. Subacute nephrotoxicity and induction of renal cell carcinoma in mice treated with ferric nitrilotriacetate. Cancer Res 1987;47:1867–9. [PubMed] [Google Scholar]
- 34.Toyokuni S, Okada S, Hamazaki S, Minamiyama Y, Yamada Y, Liang P,. et al. Combined histochemical and biochemical analysis of sex hormone dependence of ferric nitrilotriacetate-induced renal lipid peroxidation in ddY mice. Cancer Res 1990;50:5574–80. [PubMed] [Google Scholar]
- 35.Okada S, Minamiyama Y, Hamazaki S, Toyokuni S, Sotomatsu A. Glutathione cycle dependency of ferric nitrilotriacetate-induced lipid peroxidation in mouse proximal renal tubules. Arch Biochem Biophys 1993;301:138–42. [DOI] [PubMed] [Google Scholar]
- 36.Okada S. Iron-induced tissue damage and cancer: the role of reactive oxygen free radicals. Pathol Int 1996;46:311–32. [DOI] [PubMed] [Google Scholar]
- 37.Toyokuni S, Uchida K, Okamoto K, Hattori-Nakakuki Y, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc Natl Acad Sci USA 1994;91:2616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toyokuni S, Luo XP, Tanaka T, Uchida K, Hiai H, Lehotay DC. Induction of a wide range of C2–12 aldehydes and C7–12 acyloins in the kidney of Wistar rats after treatment with a renal carcinogen, ferric nitrilotriacetate. Free Radic Biol Med 1997;22:1019–27. [DOI] [PubMed] [Google Scholar]
- 39.Toyokuni S, Mori T, Dizdaroglu M. DNA base modifications in renal chromatin of Wistar rats treated with a renal carcinogen, ferric nitrilotriacetate. Int J Cancer 1994;57:123–8. [DOI] [PubMed] [Google Scholar]
- 40.Toyokuni S, Mori T, Hiai H, Dizdaroglu M. Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA-protein cross-linking between thymine and tyrosine in their renal chromatin. Int J Cancer 1995;62:309–13. [DOI] [PubMed] [Google Scholar]
- 41.Toyokuni S, Tanaka T, Hattori Y, Nishiyama Y, Ochi H, Hiai H,. et al. Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest 1997;76:365–74. [PubMed] [Google Scholar]
- 42.Akatsuka S, Yamashita Y, Ohara H, Liu YT, Izumiya M, Abe K,. et al. Fenton reaction induced cancer in wild type rats recapitulates genomic alterations observed in human cancer. PLoS ONE 2012;7(8):e43403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng JQ, Jhanwar SC, Klein WM, Bell DW, Lee WC, Altomare DA,. et al. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res 1994;54:5547–51. [PubMed] [Google Scholar]
- 44.Xio S, Li D, Vijg J, Sugarbaker DJ, Corson JM, Fletcher JA. Codeletion of p15 and p16 in primary malignant mesothelioma. Oncogene 1995;11:511–5. [PubMed] [Google Scholar]
- 45.Sekido Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int 2011;61(6):331–44. [DOI] [PubMed] [Google Scholar]
- 46.Sekido Y. Molecular pathogenesis of malignant mesothelioma. Carcinogenesis 2013;34(7):1413–9. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg RA. The biology of cancer. New York: Garland Science, Tailor & Francis Group, LLC; 2007. [Google Scholar]
- 48.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E,. et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nature Genet 2011;43(10):1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L,. et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nature Genet 2011;43(7):668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshikawa Y, Sato A, Tsujimura T, Emi M, Morinaga T, Fukuoka K,. et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103(5):868–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toyokuni S. Mysterious link between iron overload and CDKN2A/2B. J Clin Biochem Nutr 2011;48(1):46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toyokuni S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci 2009;100(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuqua BK, Vulpe CD, Anderson GJ. Intestinal iron absorption. J Trace Elem Med Biol 2012;26(2–3):115–9. [DOI] [PubMed] [Google Scholar]
- 54.Weinberg ED. The hazards of iron loading. Metallomics 2010;2(11):732–40. [DOI] [PubMed] [Google Scholar]
- 55.Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R,. et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol 2007;42(10):830–6. [DOI] [PubMed] [Google Scholar]
- 56.Zacharski L, Chow B, Howes P, Shamayeva G, Baron J, Dalman R,. et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst 2008;100:996–1002. [DOI] [PubMed] [Google Scholar]
- 57.Guariglia R, Martorelli MC, Villani O, Pietrantuono G, Mansueto G, D'Auria F,. et al. Positive effects on hematopoiesis in patients with myelodysplastic syndrome receiving deferasirox as oral iron chelation therapy: a brief review. Leukemia Res 2011;35(5):566–70. [DOI] [PubMed] [Google Scholar]
- 58.Nagai H, Okazaki Y, Chew SH, Misawa N, Yasui H, Toyokuni S. Deferasirox induces mesenchymal-epithelial transition in crocidolite-induced mesothelial carcinogenesis in rats. Cancer Prev Res 2013;6(11):1222–30. [DOI] [PubMed] [Google Scholar]
- 59.Nagai H, Okazaki Y, Chew S, Misawa N, Yamashita Y, Akatsuka S,. et al. Diameter of multi-walled carbon nanotubes is a critical factor in mesothelial injury and subsequent carcinogenesis. Proc Natl Acad Sci USA 2011;108(49):E1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka T, Akatsuka S, Ozeki M, Shirase T, Hiai H, Toyokuni S. Redox regulation of annexin 2 and its implications for oxidative stess-induced renal carcinogenesis and metastasis. Oncogene 2004;23:3980–9. [DOI] [PubMed] [Google Scholar]
- 61.Dutta KK, Zhong Y, Liu YT, Yamada T, Akatsuka S, Hu Q,. et al. Association of microRNA-34a overexpression with proliferation is cell type-dependent. Cancer Sci 2007;98(12):1845–52. [DOI] [PubMed] [Google Scholar]


