Abstract
Objectives
Knowledge about the role of oxidative stress in human diseases, including cardiovascular system disorders, emphasizes the need for reliable markers of oxidative stress. Here, we evaluated the levels of the novel marker ischemia-modified albumin (IMA), albumin-adjusted IMA (adj-IMA), and the IMA/serum albumin ratio (IMAR) in patients with chronic ischemic heart failure (CIHF).
Methods
A total of 55 patients with CIHF and 40 age- and sex-matched healthy individuals were included in the study. Serum levels of IMA, total antioxidant status, and total oxidant status were analyzed, and the adj-IMA level, IMAR, and oxidative stress index were calculated.
Results
Serum IMA, IMAR, total oxidant status levels, and oxidative stress index were significantly higher in patients with CIHF than in the controls (all P < 0.0001), whereas albumin and total antioxidant status levels were significantly lower in the CIHF patients (P < 0.0001 and P = 0.0004, respectively). However, there was no significant difference in serum adj-IMA levels between the groups (P = 0.8).
Discussion
We observed impaired oxidant/antioxidant status in favor of oxidative stress in CIHF patients. Oxidative stress may be a key factor in the development of hypoalbuminemia in CIHF. Further studies are needed to establish the relationships among IMA, albumin, and redox balance in CIHF.
Keywords: Albumin, Heart failure, Ischemia-modified albumin, Oxidative stress
Introduction
Heart failure (HF) is a complex clinical syndrome, which can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood. It is a progressive disorder that must be managed with regard to the state of the heart and the conditions of the circulation, liver, lungs, neuroendocrine system, and other organs. The most common causes of HF are coronary heart disease, hypertension, cardiomyopathy, valvular heart disease, and type 2 diabetes mellitus.1
The high prevalence of cardiovascular diseases means that they are a constant focus of medical research. The role of oxidative stress in the development of cardiovascular injuries has been reliably established.2 Clinical and experimental studies have produced substantial evidence that oxidative stress, defined as an extreme production of reactive oxygen species relative to antioxidant defense, plays a role in HF. An increased concentration of reactive radicals, particularly superoxide, has been linked to peripheral endothelial dysfunction, peripheral hypoperfusion, and exaggerated sympathetic nerve activity in patients with HF. Finally, oxidant stress has been reported to be involved in the pathogenesis of HF.3,4
Proteins are significant targets of oxidative attack. Albumin, the major plasma protein, has a number of cation- and anion-binding sites and effectively inhibits oxidation reactions in plasma. It acts as both a free-radical scavenger and a chelator of transition metals, making the protein a potent antioxidant. Free-radical damage to the N-terminal of albumin reduces the binding affinity of albumin for metals (e.g. cobalt), which is the principle of some measurement methods for ischemia-modified albumin (IMA).5
IMA is a new biological marker, measured by the albumin–cobalt-binding assay. IMA has been suggested as a marker for the early detection of ischemia of differing origins.6–8 Although the precise mechanism by which IMA is generated during ischemia remains unknown, the metal-binding capacity of albumin for transition metals, such as cobalt and copper, is reduced as a consequence of the production of reactive oxygen species that remodel the metal-binding capacity of the albumin fragment.9 IMA is a very sensitive marker of myocardial ischemia and has been licensed by the US Food and Drug Administration. Although it has a high negative-predictive value, IMA detection might improve the diagnostic performance of conventional biomarkers for the early diagnosis of cardiac ischemia.10
IMA levels are higher in many inflammatory and oxidative stress-associated diseases, but data on the serum concentrations of IMA in patients with chronic ischemic HF (CIHF) are limited. Because cardiovascular disease and oxidative stress are related events, and based on the fact that albumin may be modified in situations associated with oxidative stress, the aims of this study were to evaluate the levels of the novel marker IMA in patients with CIHF and to investigate the association of IMA with total antioxidant status (TAS), total oxidant status (TOS), and the oxidative stress index (OSI).
Materials and methods
Study population
The study included 55 consecutive patients with CIHF and 40 healthy controls. CIHF patients had a left ventricular ejection fraction (LVEF) of less than 45%, as determined by echocardiography. All patients were treated and followed at the cardiovascular center of the Education and Research Hospital during a 1-year period (2011–2012). Patients were classified according to the New York Heart Association (NYHA) as class II (n = 12), III (n = 28), or IV (n = 15).10
Exclusion criteria for both patients and controls were: (i) chronic HF with preserved LVEF (>45%); (ii) acute HF, acute myocardial infarction, or myocarditis; or (iii) known current or past allergic diseases, autoimmune diseases, inflammatory diseases, or malignant diseases. Users of vitamins and antioxidants were also excluded.
The study was performed in accordance with the ethical standards set by the Declaration of Helsinki and approved by the local ethics committee.
Brain natriuretic peptide assay
Brain natriuretic peptide (BNP) was assayed with a fully automated two-site sandwich immunoassay (ADVIA Centaur®, Siemens, Munich, Germany), which uses direct chemiluminescent technology, with constant amounts of two monoclonal antibodies. The first antibody, the Lite Reagent, is an acridinium-ester-labeled monoclonal mouse anti-human BNP fragment specific to the ring structure of BNP. The second antibody, Solid Phase, is a biotinylated monoclonal mouse anti-human antibody specific to the C-terminal portion of BNP, which is coupled to streptavidin magnetic particles. An immunocomplex is formed between the BNP in the sample and the two antibody conjugates, and the unbound antibody conjugates are washed away. The chemiluminescence of the immunocomplex signal is measured in a luminometer. The limit of detection for the BNP assay is 2 pg/ml, with a coefficient of variability ranging from 2.3 to 4.7% for 29.4–1736 pg/ml.
Measurement of IMA
Reduced cobalt–albumin-binding capacity (IMA level) was measured using the rapid and colorimetric method developed by Bar-Or et al.11 Briefly, 200 µl patient serum was transferred into glass tubes and 50 µl 0.1% CoCl2 * 6H2O (lot S38901-248, Sigma-Aldrich, St Louis, MO, USA) was added. After gentle shaking, the mixture was incubated for 10 minutes to ensure sufficient cobalt–albumin binding. Then, 50 µl 1.5 mg/ml dithiothreitol (DTT) (lot D5545-1G, Sigma-Aldrich) was added as a coloring agent. After 2 minutes, 1 ml 0.9% NaCl was added to halt the binding between cobalt and albumin. A blank was prepared for every specimen: at the DTT addition step, 50 µl distilled water was used instead of 50 µl 1.5 mg/ml DTT to obtain a blank without DTT. The absorbances were recorded at 470 nm with a spectrophotometer (UV1201, Shimadzu, Kyoto, Japan). Color formation in specimens with DTT was compared with color formation in the blank tubes, and the results are expressed as absorbance units. The formula suggested by Lippi et al.12 was used to calculate albumin-adjusted IMA (adj-IMA) levels, expressed as (individual serum albumin concentration/median albumin concentration of the population) × IMA value, and the IMA/serum albumin ratio (IMAR) was also calculated.
The inter-assay variability of the IMA method in our laboratory was calculated from serum samples of 20 healthy participants and 20 patients with acute coronary syndromes. The within-day coefficient of variation was 1.23% (mean 0.465, standard deviation (SD) 0.006) for healthy participants and 0.92% (mean 0.570, SD 0.006) for those with acute coronary syndromes. All serum samples were analyzed within 4 days.
Measurement of serum TOS
Serum TOS levels were analyzed using an automated colorimetric measurement method developed by Erel.13 In this method, oxidants in the sample oxidize the ferrous ion–chelator complex to ferric ion, which makes a colored complex with a chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 equiv./l).
Measurement of serum TAS
Serum TAS levels were analyzed using an automated colorimetric measurement method developed by Erel.14 In this method, antioxidants in the sample reduce dark blue–green colored 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radicals to a colorless reduced ABTS form. The change of absorbance at 660 nm is related to the total antioxidant level in the sample. This method determines the antioxidative effect of the sample against the potent free-radical reactions initiated by the produced hydroxyl radical. The results are expressed as micromolar Trolox equivalent per liter (μmol Trolox equiv./l).
Oxidative stress index
The percentage ratio of the TOS level to the TAS level is given as the OSI.15 For calculation, the resulting micromolar unit of TAS was changed to millimoles per liter, and the OSI value was calculated according to the following formula: OSI (arbitrary unit) = TOS (μmol H2O2 equiv./l)/TAS (μmol Trolox equiv./l).
Routine parameters
Albumin concentrations were determined in our clinical biochemistry laboratory using a commercially available kit (Abbott Diagnostics, Abbott Park, IL, USA) with an autoanalyzer (Architect® c16000, Abbott Diagnostics) based on the bromocresol green method.
Statistical analysis
Statistical analyses were carried out using the statistical software SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). The results are presented as mean ± SD in normally distributed groups, or otherwise as medians. The significance levels of differences between the groups were determined by Student's unpaired t-tests for normal distributions or Mann–Whitney U tests for abnormal distributions. Pearson's correlation coefficient and Spearman's correlation coefficient were used to test the strength of associations between different variables. P values of <0.05 were accepted as significant.
Results
The clinical and demographic characteristics of the patients with CIHF and the controls are shown in Table 1. A family history of heart disease was more common in the CIHF patients than in the controls (82 versus 12.5%, P < 0.0001). The mean disease duration in patients was 10.6 ± 4.2 years. All patients had HF of ischemic origin.
Table 1.
Clinical and demographic characteristics of patients and controls
| Parameters | Patients (n = 55) | Controls (n = 40) | P |
|---|---|---|---|
| Age, years | 70 ± 11 | 67 ± 6.5 | 0.1 |
| Male, n (%) | 41 (74.5%) | 23 (57.5%) | 0.1 |
| Smoker, n (%) | 20 (36%) | 12 (30%) | 0.6 |
| Alcohol | 4 (7.2%) | 1 (2.5%) | 0.3 |
| Family history of heart disease, n (%) | 45 (82%) | 5 (12.5%) | <0.0001 |
| Disease duration, years | 10.6 ± 4.2 | – | – |
| BMI, kg/m2 | 25.3 ± 4.5 | 26.9 ± 3 | 0.2 |
| NYHA class, n (II/III/IV) | 12/28/15 | – | – |
| LVEF, % | 30 ± 12 | – | – |
| BNP, pg/m | 670 ± 571 | – | – |
| Etiology | |||
| Ischemic heart failure, n (%) | 55 (100) | – | – |
| Medications | |||
| Aspirin, % | 38 | – | – |
| Diuretics, % | 21 | – | – |
| ACE inhibitors, % | 32 | – | – |
| Beta-blockers, % | 34 | – | – |
ACE, angiotensin-converting enzyme; BMI, body mass index; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Serum IMA, IMAR, and TOS levels and the OSI were significantly higher in patients with CIHF than in the controls (all P < 0.0001), whereas albumin and TAS levels were significantly lower in the CIHF patients (P < 0.0001 and P = 0.0004, respectively). However, there was no significant difference in serum adj-IMA levels between the groups (P = 0.8) (Table 2).
Table 2.
Serum IMA, TAS, TOS, OSI, total albumin, and adj-IMA levels in patients with CIHF compared with the healthy controls
| Parameter | Patients (n = 55) | Controls (n = 40) | P |
|---|---|---|---|
| IMA (ABSU) | 0.669 ± 0.2 | 0.470 ± 0.1 | <0.0001 |
| TAS (nmol Trolox equiv./l) | 2.6 ± 0.4 | 2.9 ± 0.3 | 0.0004 |
| TOS (μmol H2O2 equiv./l) | 5.1 (3.6–6.6) | 1.4 (1–1.6) | <0.0001 |
| OSI | 2 (1–2.6) | 0.5 (0.4–0.6) | <0.0001 |
| Albumin (g/dl) | 3.2 ± 0.5 | 4.4 ± 0.2 | <0.0001 |
| IMAR | 0.21 ± 0.08 | 0.10 ± 0.01 | <0.0001 |
| Adj-IMA | 0.475 ± 0.15 | 0.470 ± 0.05 | 0.8 |
Values are given as mean ± SD for normal distributions and median (95% confidence interval) for abnormal distributions.
ABSU, absorbance units.
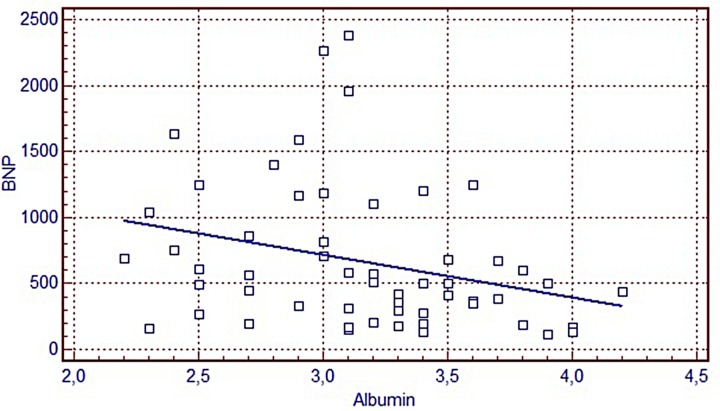
Table 3 shows the laboratory data of patients, divided into three groups according to NYHA functional class. As expected, plasma BNP was significantly increased with disease severity (P < 0.0001). Albumin levels were significantly decreased with disease severity (P = 0.04). IMA, IMAR, adj-IMA, TAS, TOS, and OSI did not show any statistically significant differences. However, statistical analysis revealed a negative correlation between albumin and BNP (Fig. 1). No statistically significant correlations were identified between LVEF and BNP, IMA, IMAR, adj-IMA, TAS, TOS, and OSI, or between albumin and IMA, IMAR, adj-IMA, LVEF, body mass index, TAS, TOS, and OSI.
Table 3.
NYHA functional class and biomarkers (Kruskal–Wallis analysis, average rank, and mean ± SD for all parameters) for patients with CIHF
| NYHA class (n) | BNP | IMA | IMAR | Adj-IMA | TAS | TOS | OSI | Albumin |
|---|---|---|---|---|---|---|---|---|
| II (12) | 13.4 | 24.2 | 21.5 | 26.7 | 22.1 | 34 | 35.7 | 34.2 |
| 291 ± 235 | 0.60 ± 0.2 | 0.18 ± 0.05 | 0.46 ± 0.1 | 2.4 ± 0.5 | 7.8 ± 4.9 | 0.3 ± 0.2 | 3.3 ± 0.4 | |
| III (28) | 26.7 | 30.6 | 30.2 | 31.4 | 27.7 | 26.8 | 26.5 | 29.5 |
| 567 ± 347 | 0.51 ± 0.1 | 0.21 ± 0.09 | 0.50 ± 0.1 | 2.6 ± 0.3 | 5.5 ± 4.5 | 0.21 ± 0.1 | 3.1 ± 0.3 | |
| IV(15) | 42.1 | 26 | 29.1 | 22.6 | 33.1 | 25.4 | 24.6 | 20.1 |
| 1161 ± 671 | 0.64 ± 0.2 | 0.21 ± 0.10 | 0.42 ± 0.1 | 2.6 ± 0.4 | 5.5 ± 5.6 | 0.20 ± 0.2 | 2.9 ± 0.4 | |
| P | < 0.0001 | 0.43 | 0.27 | 0.21 | 0.20 | 0.32 | 0.15 | 0.04 |
Significant values are shown in bold.
ABSU, absorbance units; adj-IMA, albumin-adjusted IMA; BNP, brain natriuretic peptide; IMA, ischemia-modified albumin; OSI, oxidative stress index; TAS, total antioxidant status; TOS, total oxidant status.
Figure 1.
Correlations between BNP and albumin in patients with CIHF (r = –0.366, P = 0.007).
Discussion
Our results showed that oxidative stress markers (TOS, OSI), IMA, and IMAR were increased in patients with CIHF compared with the healthy controls, whereas albumin and TAS levels were significantly lower in the CIHF patients. Plasma BNP levels and serum albumin levels were, respectively, significantly increased and decreased with disease severity. There was a negative correlation between BNP and albumin. IMA levels did not differ between the different stages of HF, which also suggests that HF per se does not substantially affect IMA levels.
IMA was recently identified as a tool for diagnosing acute coronary syndromes. However, IMA concentrations may not be specific for cardiac ischemia. Various studies have been performed on IMA in patients with ischemia of non-cardiac origin, such as diabetes mellitus, peripheral vascular disease, skeletal muscle ischemia, glaucoma, and systemic sclerosis.16–19 Only one study has evaluated the relationship between HF and serum IMA levels. Franceschi et al.20 showed that serum IMA levels were increased in patients with HF, but they did not evaluate serum albumin levels. Although IMA has been identified as a biomarker of acute ischemia, its biggest drawback relates to its dependence on the serum albumin concentration. The impact of serum albumin on IMA levels is still an important factor, even within the normal range. It has been demonstrated that each 1 g/dl change in albumin produces an opposite change of 2.6% in IMA levels, resulting in a negative correlation. This indicates a need to evaluate IMA values together with those of albumin to avoid possible false-positive or -negative values in individuals with hypo- or hyperalbuminemia.21–23 We analyzed adj-IMA levels using a formula suggested by Lippi et al.12 and IMAR. A statistically significant difference in the IMA parameter between the HF and the control groups was not achieved when adj-IMA was used, which highlights the problem of accuracy in IMA measurements. Unfortunately, the current study presents no solution, but emphasizes the ultimate need for a consensus.
Albumin acts both as a free-radical scavenger and as a chelator of transition metals, making the protein a potent antioxidant.5 Low serum albumin levels are a common finding in patients with HF. Although we did not detect any correlation between albumin, IMA, IMAR, adj-IMA, and oxidative stress markers (TAS, TOS, and OSI), oxidative stress may be one of the key factors in the formation of IMA and hypoalbuminemia in HF patients. During HF development, increased oxidative stress might be due to cachexia, malnutrition, and inflammation. Oxidative stress has been determined to be one of the key players in the improvement of cachexia, contributing to muscle wasting both directly through oxidative damage and indirectly through redox signaling in impaired pathways.24 Oxidative injury, free-radical levels, and mRNA levels of free-radical-producing enzymes have all been shown to be elevated in the cachectic state, whereas the generation and activity of antioxidant enzymes have been shown to decrease.25,26 In other words, antioxidant enzyme activities may decrease because of suppressed protein synthesis, cachexia, and malnutrition as the host responds to inflammation. However, different causal factors might play an important role in the development of hypoalbuminemia in patients with HF.27–29 Usually, low albumin results from reduced liver synthesis, raised vascular permeability, increased catabolism, and renal and enteral loss in HF.30–34 Another cause of hypoalbuminemia, oxidative stress, causes molecular modifications in human serum albumin, such as carbonylation and the formation of advanced oxidation protein products and advanced glycoxidation end products. In addition, hypoalbuminemia, oxidative stress, and inflammation are linked in HF patients. However, it has been demonstrated that a common biochemical assay (the bromocresol green assay) may result in ‘apparent’ hypoalbuminemia, and that this assay underestimates albumin concentrations when the protein is oxidatively modified.35
In this study, BNP and albumin were negatively correlated in patients with HF. Plasma BNP and serum albumin levels were, respectively, significantly increased and decreased with disease severity. Previous studies have shown that BNP and albumin are important prognostic factors in HF.36,37 In agreement with our results, the authors reported that hypoalbuminemia was associated with high NYHA class and higher BNP levels, and low serum albumin levels were not linked to body mass index or LVEF.28–30 The metabolic processes leading to hypoalbuminemia have not been specifically studied in patients with HF.
Numerous clinical observations using different methods led to the final conclusion that oxidative stress plays a role in human HF. Serum (or plasma) concentrations of different oxidants and antioxidants can be measured separately in laboratories, but these measurements are time-consuming, labor-intensive, costly, and require specialist staff. Because measuring different oxidant and antioxidant molecules separately is impractical, and the effects of these molecules are additive, it is sufficient to assess the levels of TAS and TOS and to calculate the OSI.38,39 In our study, we found that serum TOS levels and the OSI were significantly higher and serum TAS levels were significantly lower in patients with CIHF than in the healthy controls. Our results did not show a relationship of serum TAS, TOS, or OSI with NYHA class.
In conclusion, increased serum TOS levels and OSI and decreased serum TAS levels were found in patients with CIHF, and these findings strongly suggest oxidant–antioxidant imbalance. This study represents the first time that IMA, IMAR, and adj-IMA levels have been investigated in patients with CIHF. The results indicate that IMA appears to be a good biomarker of acute ischemia and may be an albumin-dependent oxidative stress marker. However, our results showed decreased serum albumin levels in patients with high-grade CIHF, suggesting that serum albumin level is an important prognostic factor. Major limitations of the study are the small number of samples and the lack of patients with HF due to other causes. Further prospective studies are needed with larger patient numbers to analyze the effect of albumin and antioxidant functions in patients with HF.
References
- 1.Babick AP, Dhalla NS. Role of subcellular remodeling in cardiac dysfunction due to congestive heart failure. Med Princ Pract 2007;16(2):81–9. [DOI] [PubMed] [Google Scholar]
- 2.Rogowski O, Shnizer S, Wolff R, Lewis BS, Amir O. Increased serum levels of oxidative stress are associated with hospital readmissions due to acute heart failure. Cardiology 2011;118:33–7. [DOI] [PubMed] [Google Scholar]
- 3.Kumar EP, Mukherjee R, Senthil R, Parasuraman S, Suresh B. Evaluation of oxidative stress and antioxidant status in patients with cardiovascular disease in rural populations of the nilgiris, South India. ISRN Pharmacol 2012; doi: 10.5402/2012/941068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Mehta JL. Role of oxidative stress in coronary heart disease. Indian Heart J 2004;56:163–73. [PubMed] [Google Scholar]
- 5.Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care 2013;3(4):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christenson RH, Duh SH, Sanhai WR, Wu AH, Holtman V, Painter P,. et al. Characteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: a multicenter study. Clin Chem 2001;47:464–70. [PubMed] [Google Scholar]
- 7.Roy D, Quiles J, Aldama G, Sinha M, Avanzas P, Arroyo-Espliguero R,. et al. Ischemiamodified albumin for the assessment of patients presenting to the emergency department with acute chest pain but normal or non-diagnostic 12-lead electrocardiograms and negative cardiac troponin T. Int J Cardiol 2004;97:297–301. [DOI] [PubMed] [Google Scholar]
- 8.Roy D, Quiles J, Sharma R, Sinha M, Avanzas P, Gaze D,. et al. Ischemia-modified albumin concentrations in patients with peripheral vascular disease and exercise induced skeletal muscle ischemia. Clin Chem 2004;50:1656–60. [DOI] [PubMed] [Google Scholar]
- 9.Gidenne S, Ceppa F, Fontan E, Perrier F, Burnat P. Analytical performance of the albumin cobalt binding (ACB) test on the CobasMIRA Plus analyzer. Clin Chem Lab Med 2004;42:455–61. [DOI] [PubMed] [Google Scholar]
- 10.American Heart Association AHA Medical/Scientific Statement 1994 revisions to classification of functional capacity and objective assessment of patients with diseases of the heart. Circulation 1994;90:644–5. [PubMed] [Google Scholar]
- 11.Bar-Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia – a preliminary report. Emerg Med J 2000;19(4):311–5. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Montagnana M, Salvagno GL, Guidi GC. Standardization of ischaemia modified albumin testing: adjustment for serum albumin. Clin Chem Lab Med 2007;45:261–2. [DOI] [PubMed] [Google Scholar]
- 13.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem 2005;38:1103–11. [DOI] [PubMed] [Google Scholar]
- 14.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generationmore stable ABTS radical cation. Clin Biochem 2004;37:277–85. [DOI] [PubMed] [Google Scholar]
- 15.Harma M, Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly 2003;133:563–66. [DOI] [PubMed] [Google Scholar]
- 16.Montagnana M, Lippi G, Volpe A, Salvagno GL, Biasi D, Caramaschi P,. et al. Evaluation of cardiac laboratory markers in patients with systemic sclerosis. Clin Biochem 2006;39:913–17. [DOI] [PubMed] [Google Scholar]
- 17.Roy D, Quiles J, Sharma R, Sinha M, Avanzas P, Gaze D,. et al. Ischemia modified albumin concentrations in patients with peripheral vascular disease and exercise-induced skeletal muscle ischemia. Clin Chem 2004;50:1656–60. [DOI] [PubMed] [Google Scholar]
- 18.Piwowar A, Knapik-Kordecka M, Warwas M. Ischemia-modified albumin level in type 2 diabetes mellitus – preliminary report. Dis Markers 2008;24:311–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang D, Sha Q, Zhang X, Liu P, Rong S, Han T,. et al. The evaluation of the oxidative stress parameters in patients with primary angle-closure glaucoma. PLoS ONE 2011;6(11):e27218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi F, Deharo JC, Giorgi R, By Y, Monserrat C, Condo J,. et al. Peripheral plasma adenosine release in patients with chronic heart failure. Heart 2009;95:651–5. [DOI] [PubMed] [Google Scholar]
- 21.Van der Zee PM, Verberne HJ, van Straalen JP, Sanders GT, Van Eck-Smit BL, de Winter RJ,. et al. Ischemia-modified albumin measurements in symptom limited exercise myocardial perfusion scintigraphy reflect serum albumin concentrations but not myocardial ischemia. Clin Chem 2005;51:1744–46. [DOI] [PubMed] [Google Scholar]
- 22.Ellidag HY, Eren E, Yilmaz N, Bayindir A. Ischemia modified albumin levels and increased oxidative stress in patients with multiple myeloma. J Med Biochem 2013:32;1–7. [Google Scholar]
- 23.Ellidag HY, Eren E, Aydın O, Akgol E, Yalcınkaya S, Sezer C,. et al. Ischemia modified albumin levels and increased oxidative stress in patients with bladder cancer. Asian Pac J Cancer Prev 2013;14(5):2157–61. [DOI] [PubMed] [Google Scholar]
- 24.Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care 2008;11:408–16. [DOI] [PubMed] [Google Scholar]
- 25.Russell ST, Eley H, Tisdale MJ. Role of reactive oxygen species in protein degradation in murine myotubes induced by proteolysis-inducing factor and angiotensin II. Cell Signal 2007;19:1797–806. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle 2013;4(2):95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–89. [DOI] [PubMed] [Google Scholar]
- 28.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA,. et al. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010;160:1149–55. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Sugihara S, Hirai M. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77(3):705–11. [DOI] [PubMed] [Google Scholar]
- 30.von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther 2009;121:227–52. [DOI] [PubMed] [Google Scholar]
- 31.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39(4 Suppl. 2):S143–6. [DOI] [PubMed] [Google Scholar]
- 32.Adlbrecht C, Kommata S, Hulsmann M, Szekeres T, Bieglmayer C, Strunk G,. et al. Chronic heart failure leads to an expended plasma volume and pseudoanemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J 2008;29:2343–50. [DOI] [PubMed] [Google Scholar]
- 33.Kataoka H. Short term changes in hematologic and biochemical tests during follow-up of definite heart failure patients. Int J Cardiol 2010;144:441–4. [DOI] [PubMed] [Google Scholar]
- 34.Ajayi AA, Adigun AQ, Ojofeitimi EO, Yusuph H, Ajayi OE. Anthropometric evaluation of cachexia in chronic congestive heart failure: the role of tricuspid regurgitation. Int J Cardiol 1999;71:79–84. [DOI] [PubMed] [Google Scholar]
- 35.Michelis R, Kristal B, Snitkovsky T, Sela S. Oxidative modifications impair albumin quantification. BBRC 2010;401:137–42. [DOI] [PubMed] [Google Scholar]
- 36.Naffaa M, Makhoul BF, Tobia A, Jarous M, Kaplan M, Aronson D,. et al. Brain natriuretic peptide at discharge as a predictor of 6-month mortality in acute decompensated heart failure. Am J Emerg Med 2014;32(1):44–9. [DOI] [PubMed] [Google Scholar]
- 37.Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K,. et al. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013;77(3):705–11. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz N, Aydin O, Yegin A, Tiltak A, Eren E. Increased levels of total oxidant status and decreased activity of arylesterase in migraineurs. Clin Biochem 2011;44:832–7. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz N, Aydin O, Yegin A, Tiltak A, Eren E, Aykal G. Impaired oxidative balance and association of blood glucose, insulin and HOMA-IR index in migraine. Biochem Med 2011;21:145–51. [DOI] [PubMed] [Google Scholar]