Abstract
Objectives: Sodium nitrite, a food preservative, has been reported to increase oxidative stress indicators such as lipid peroxidation, which can affect different organs including the kidney. Here, we investigated the toxic effects of oral sodium nitrite on kidney function in rats and evaluated potential protective effects of Nigella sativa oil (NSO).
Methods: Seventy adult male Sprague–Dawley rats received 80 mg/kg sodium nitrite orally in the presence or absence of NSO (2.5, 5, and 10 ml/kg) for 12 weeks. Morphological changes were assessed by hematoxylin and eosin, Mallory trichome, and periodic acid–Schiff staining. Renal tissues were used for measurements of oxidative stress markers, C-reactive protein, cytochrome C oxidase, transforming growth factor (TGF)-beta1, monocyte chemotactic protein (MCP)-1, pJNK/JNK, and caspase-3.
Results: NSO significantly reduced sodium nitrite-induced elevation in serum urea and creatinine, as well as increasing normal appearance of renal tissue. NSO also prevented reductions in glycogen levels caused by sodium nitrite alone. Moreover, NSO treatment resulted in dose-dependent significant reductions in fibrosis markers after sodium nitrite-induced 3- and 2.7-fold increase in MCP-1 and TGF-beta1, respectively. Finally, NSO partially reduced the elevated caspase-3 and pJNK/JNK.
Discussion: NSO ameliorates sodium nitrite-induced nephrotoxicity through blocking oxidative stress, attenuation of fibrosis/inflammation, restoration of glycogen level, amelioration of cytochrome C oxidase, and inhibition of apoptosis.
Keywords: Black cumin, CRP, Cytochrome C oxidase, Glycogen, MCP-1, TGF-beta1
Introduction
Nitrate and nitrite are part of the human diet primarily used as food preservatives cured and processed meats.1 Dietary sources of nitrite can be harmful, and some epidemiological studies have demonstrated a relationship between nitrite content in food and cancer.2 Sodium nitrite has been generally accepted as a weak carcinogen since exposure to higher levels of nitrites has been linked to the increased incidence of brain tumors, leukemia, and nasopharyngeal tumors in children.3 We have previously assessed sodium nitrite-induced detrimental effects in different body organs due to its oxidative properties, fibrosis, and inflammation.4–8
The seeds of Nigella sativa (family Ranunculaceae) are commonly known worldwide as black seed or black cumin. Nigella seeds have been medically used for centuries both as an herb and for pressed oil in Asia, Middle East, and Africa.9 The pharmacological effects of the crude extracts of Nigella seeds and some of its active contents, including the volatile oil, thymoquinone (TQ), have been traditionally used for a variety of treatments related to respiratory health, stomach and intestinal health, kidney and liver function, circulatory and immune system support, and general well-being.10,11 Nigella seeds contain more than 30% fixed oil as well as up to 0.45% volatile oil, and 46% of many monoterpenes such as r-cymene and a-piene.12 The major fatty acids present in the fixed oil of Nigella sativa oil (NSO) have been reported as linoleic acid (55.6%), oleic acid (23.4%), and palmitic acid (12.5%). In addition, the main volatile oil compounds present in NSO were TQ (27.8–57.0%), trans-anethole (38.3%), p-cymene (14.8%), limonene (4.3%), and carvone (4.0%).13
Few scientific studies have examined the effects of sodium nitrite on kidney function, or the effects of NSO. Therefore, we conducted this study to evaluate nephrotoxicity risks associated with dietary sources of sodium nitrites, as well as determination of the possible nephroprotective effects of NSO in adult male rats.
Materials and methods
Chemicals
All chemicals used in the current study were purchased from Sigma-Aldrich (St Louis, MO, USA). NSO was purchased from Silver Seas Co. (El-Obour, Egypt).
Animals
The ethical committee of the Faculty of Pharmacy, University of Mansoura approved all animal subject procedures. Seventy adult male Sprague–Dawley (SD) rats weighing 120–140 g were used. Rats received AIN93M diet. All treatments consisted of once daily oral gavage early in the morning for 12 weeks. Rats were carefully examined and weighed twice weekly. Rats were divided into seven groups with 10 rats each:
Control group. Rats did not receive any treatment.
Control+NSO (5 ml/kg). Rats were supplemented daily with 5 ml/kg body weight NSO.
Control+NSO (10 ml/kg). Rats received daily 10 ml/kg body weight NSO.
Sodium nitrite group. Rats received daily 80 mg/kg sodium nitrite.
Sodium nitrite+NSO (2.5 ml/kg). On the same day, rats received 2.5 ml/kg NSO followed by 80 mg/kg sodium nitrite.
Sodium nitrite+NSO (5 ml/kg). On the same day, rats received 5 ml/kg NSO followed by 80 mg/kg sodium nitrite every day.
Sodium nitrite+NSO (10 ml/kg). On the same day, rats received 10 ml/kg NSO followed by 80 mg/kg sodium nitrite every day.
The doses and time course of experiments used for sodium nitrite were in the range of those used in other studies.4–8 Furthermore, doses were determined after preliminary experiments.
Collection of samples
The rats were sacrificed by decapitation and trunk blood was collected and centrifuged at 600g for 5 minutes to obtain serum samples, which were stored at −80 °C. Rat kidneys were removed, cleaned, weighed, and chilled on crushed ice. One piece of kidney was fixed in 10% buffered formalin. The other piece was homogenized in a 10-fold volume of ice-cold sodium potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl and then centrifuged at 600g at 4 °C for 10 minutes. The supernatant was stored at –80 °C.
Morphologic analysis of renal tissue
Fixed kidney specimens were embedded in paraffin. Sections (5 μm) were cut and stained with hematoxylin and eosin (H&E) for examination of cellular structure. The second set of sections was stained with Mallory trichchome to detect collagen fibers. The last set of sections was stained with periodic acid-Schiff (PAS) to reveal PAS positive structures as glycogen. Renal specimens were examined in a masked manner and photographed by Nikon Digital Camera (Japan).
Measuring kidney function
Serum urea and creatinine concentrations were measured by commercially available kits (Biodiagnostic Company, Cairo, Egypt) using spectrophotometer (Spectronics, Haryana, India).
Assessment of oxidative stress
Serum and renal tissue levels of malondialdhyde (MDA) concentrations were measured by the thiobarbituric acid method.5
Serum and renal tissue levels of superoxide anion concentrations were measured by nitroblue tetrazolium method.14
Assessment of antioxidant activity
Serum and renal tissue levels of superoxide anion (SOD) activities were determined using the DeChatelet method.15
Blood and renal tissue levels of reduced glutathione concentrations were determined by the Beutler method.16
Estimation of glycogen concentration
Renal tissue glycogen levels were measured as described previously.17 Briefly, kidney samples were digested by KOH. Glycogen was precipitated by sodium sulfate and ethanol then dissolved in distilled water. Glycogen contents were measured by phenol reagent and sulfuric acid and measured at 490 nm using Spectronics Spectrophotometer (Haryana, India).
Determination of biochemical markers by enzyme linked immunosorbent assays (ELISA)
Renal tissues homogenates were measured by enzyme linked immunosorbent assays (ELISA) using commercially available monocyte chemotactic protein (MCP)-1, transforming growth factor (TGF)-beta1, c-reactive protein (CRP) (eBioscience, Inc., San Diego, CA, USA), and pJNK/JNK (RayBiotech, Inc., Norcross, GA, USA). ELISA kits were used in accordance with the manufacturer's instructions using a microplate reader (BioTek, Winooski, VT, USA).
Determination of mitochondrial function in renal tissues
Mitochondrial function was measured via determination of renal cytochrome C oxidase using commercially available kit (Sigma-Aldrich, St Louis, MO, USA).
Estimation of caspase-3 activity in renal tissues
Caspase-3 enzyme activity was measured colorimetrically using commercially available kits (GenScript, Piscataway, NJ, USA) following the manufacturer's procedure.
Statistical analysis
All data are reported as the mean ± SE. One-way analysis of variance was used to compare the means between groups. Once differences between the means were found, post hoc Bonferroni correction tests were calculated. Statistical computations were performed on a personal computer using Microsoft Excel 2007. Statistical significance was predefined as P ≤ 0.05.
Results
Effect of sodium nitrite and NSO on kidney function tests
As shown in Table 1, sodium nitrite caused significant increases in serum level of both creatinine and urea when compared with the control groups (P < 0.05). Treatment with NSO produced dose-dependent reductions in serum urea and creatinine levels when compared with sodium nitrite group (P < 0.05) and did not affect the control rats.
Table 1. Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on kidney function tests (mean ± SE).
| Control | Control + Nigella oil (5 ml/kg) | Control + Nigella oil (10 ml/kg) | Sodium nitrite (80 mg/kg) | Sodium nitrite + Nigella oil (2.5 ml/kg) | Sodium nitrite + Nigella oil (5 ml/kg) | Sodium nitrite + Nigella oil (10 ml/kg) | |
|---|---|---|---|---|---|---|---|
| Urea (mg/dl) | 39.1 ± 2.92 | 38.4 ± 3.23 | 40.8 ± 3.87 | 54.7 ± 4.34* | 49.1 ± 4.32* | 42.7 ± 3.9#$ | 41.1 ± 2.7#$ |
| Creatinine (mg/dl) | 1.2 ± 0.11 | 1.3 ± 0.09 | 1.3 ± 0.08 | 2.3 ± 0.92* | 1.9 ± 0.45* | 1.4 ± 0.12#$ | 1.3 ± 0.11#$ |
*Significant difference when compared with the control groups at P < 0.05.
#Significant difference when compared with sodium nitrite groups at P < 0.05.
$Significant difference when compared with Nigella oil (2.5 ml/kg) group at P < 0.05.
%Significant difference when compared with Nigella oil (5 ml/kg) group at P < 0.05.
Effect of NSO on sodium nitrite-induced morphological damage
Kidney sections of rats treated with sodium nitrite that were stained with H&E showed disrupted parietal layer of a renal corpuscle. There were wide spaces between some renal tubules with extravasated blood cells inside (Fig. 1). In parallel, kidney sections of sodium nitrite group stained with Mallory trichome showed marked collagen fibers surrounding some renal tubules and corpuscles in addition to that surrounding the blood vessels (Fig. 2). However, rats received both NSO with sodium nitrite showed nearly normal appearance of renal tissue in sodium nitrite group.
Figure 1.
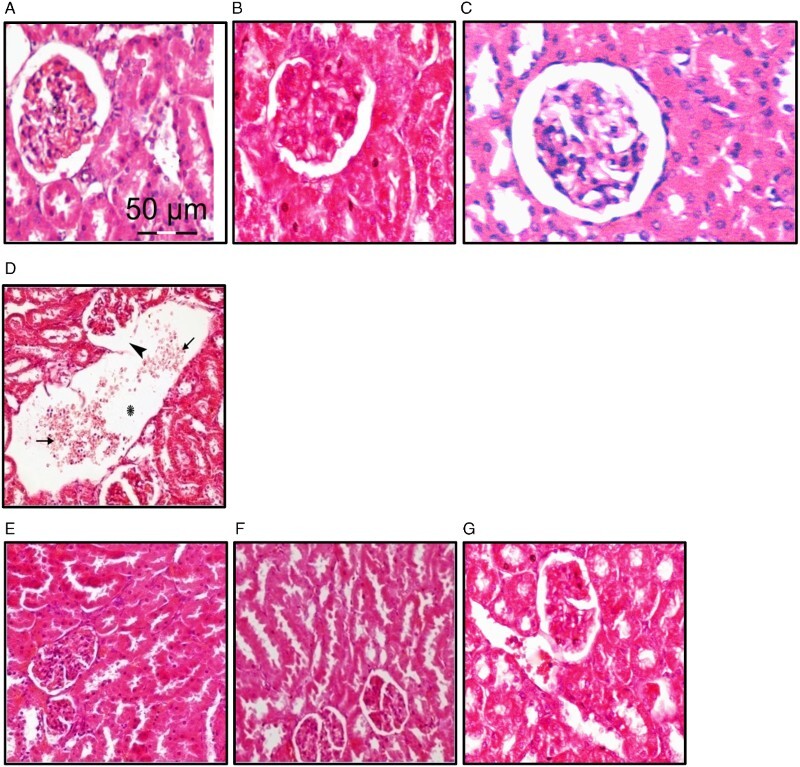
Effect of NSO on sodium nitrite-induced renal tissue damage. Kidney sections from different rat groups were stained with H&E and examined under a microscope. (A–C) Sections from the control (A), control+NSO (5 ml/kg) (B) and control+NSO (10 ml/kg) (C) groups showing renal corpuscles, proximal and distal tubules. (D) Sections from sodium nitrite group showing disrupted parietal layer of a renal corpuscle (arrow head). There is a wide space between renal tubules (asterisk) with extravasated blood cells (arrows) inside. (E–G) Sections from rats treated with sodium nitrite and different doses of NSO 2.5 ml/kg (E), 5 ml/kg (F), and 10 ml/kg (G) showing an improved appearance of renal corpuscles and tubules.
Figure 2.
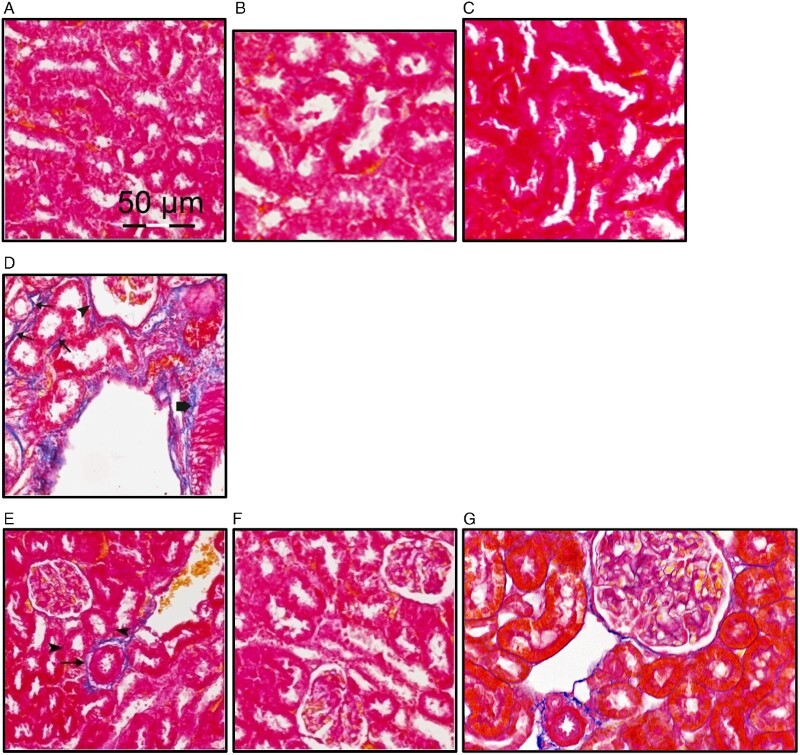
Effect of NOS on sodium nitrite-induced increase in collagen fibers. Kidney sections from different rat groups were stained with Mallory trichome and examined under a microscope. (A–C) Sections from the control (A), control+NSO (5 ml/kg) (B) and control+NSO (10 ml/kg) (C) groups showing no apparent stained collagen fibers. (D) Sections from sodium nitrite group showing marked collagen fibers surrounding some renal tubules (arrows) and corpuscles (arrow head) in addition to that surrounding the blood vessels (thick arrow). (E) Sections from rats treated with sodium nitrite+NSO (2.5 ml/kg) showing very fine collagen fibers surrounding renal tubules (arrow heads) and surrounding blood vessels (arrow). (F and G) Sections from rats treated with sodium nitrite and both 5 and 10 ml/kg NSO ml/kg showing nearly similar appearance to the control.
Effect of sodium nitrite and NSO on oxidative stress markers
As shown in Table 2, oral administration of sodium nitrite caused a significant increase in serum and renal tissue levels of MDA and superoxide anion when compared with the control groups (P < 0.05). However, oral supplementation of NSO caused a significant dose-dependent decrease in MDA and superoxide anion levels as compared to the sodium nitrite group. Even after treatment with NSO, the levels of MDA and superoxide anion were still higher than the control group.
Table 2. Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on levels of MDA and superoxide anion (mean ± SE).
| Control | Control + Nigella oil (5 ml/kg) | Control + Nigella oil (10 ml/kg) | Sodium nitrite (80 mg/kg) | Sodium nitrite + Nigella oil (2.5 ml/kg) | Sodium nitrite + Nigella oil (5 ml/kg) | Sodium nitrite + Nigella oil (10 ml/kg) | |
|---|---|---|---|---|---|---|---|
| Serum MDA (nM) | 158.3 ± 11.5 | 148.7 ± 12.2 | 151.6 ± 13.7 | 389.5 ± 28.2* | 311.2 ± 19.6*# | 227.6 ± 21.1*#$ | 171.5 ± 16.1#$% |
| Serum superoxide anion (µM) | 45.2 ± 4.11 | 48.8 ± 3.81 | 44.64 ± 3.94 | 91.4 ± 5.32* | 79.5 ± 4.73*# | 64.8 ± 4.25*#$ | 51.7 ± 3.9#$% |
| Renal MDA (nmol/g protein) | 131.6 ± 8.46 | 139.2 ± 10.6 | 142.9 ± 12.3 | 456.4 ± 37.1* | 379.6 ± 31.2*# | 235.3 ± 19.3*#$ | 197 ± 17.1*#$% |
| Renal superoxide anion (µmol/g protein) | 39.32 ± 3.11 | 36.74 ± 2.91 | 41.83 ± 4.12 | 82.54 ± 6.73* | 76.45 ± 6.45* | 62.17 ± 4.78*#$ | 43.4 ± 4.1#$% |
*Significant difference when compared with the control groups at P < 0.05.
#Significant difference when compared with sodium nitrite groups at P < 0.05.
$Significant difference when compared with Nigella oil (2.5 ml/kg) group at P < 0.05.
%Significant difference when compared with Nigella oil (5 ml/kg) group at P < 0.05.
Effect of sodium nitrite and NSO on antioxidant markers
Significant decreases (P < 0.05) in the concentration of reduced glutathione and activity of superoxide dismutase (SOD) were found after administration of sodium nitrite compared with the control groups (Table 3). However, oral treatment of rats with NSO for 12 weeks caused a dose-dependent increase in glutathione and SOD levels when compared with the sodium nitrite group (P < 0.05), without affecting the control groups.
Table 3. Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on levels of reduced glutathione and superoxide dismutase (SOD) (mean ± SE).
| Control | Control + Nigella oil (5 ml/kg) | Control + Nigella oil (10 ml/kg) | Sodium nitrite (80 mg/kg) | Sodium nitrite + Nigella oil (2.5 ml/kg) | Sodium nitrite + Nigella oil (5 ml/kg) | Sodium nitrite + Nigella oil (10 ml/kg) | |
|---|---|---|---|---|---|---|---|
| Blood glutathione (mM) | 8.93 ± 0.74 | 8.23 ± 0.81 | 9.14 ± 0.89 | 4.32 ± 0.26* | 5.11 ± 0.43* | 7.43 ± 0.54#$ | 7.79 ± 0.67#$ |
| Serum SOD (U/l) | 34.64 ± 2.94 | 33.23 ± 2.62 | 34.41 ± 3.12 | 17.41 ± 1.63* | 22.71 ± 2.11* | 27.71 ± 2.31*#$ | 31.8 ± 2.78#$ |
| Renal glutathione (mmol/g protein) | 11.21 ± 1.04 | 11.64 ± 1.12 | 12.53 ± 1.22 | 7.91 ± 0.63* | 8.83 ± 0.73* | 9.64 ± 0.73*# | 10.9 ± 1.1#$ |
| Renal SOD (U/g protein) | 27.32 ± 2.12 | 26.89 ± 1.94 | 27.87 ± 2.32 | 19.84 ± 1.48* | 22.21 ± 2.18* | 25.93 ± 1.97$ | 26.7 ± 2.1#$ |
*Significant difference when compared with the control groups at P < 0.05.
#Significant difference when compared with sodium nitrite groups at P < 0.05.
$Significant difference when compared with Nigella oil (2.5 ml/kg) group at P < 0.05.
%Significant difference when compared with Nigella oil (5 ml/kg) group at P < 0.05.
Effect of sodium nitrite and NSO on renal tissues glycogen level
Routinely, glycogen can be examined histochemically in sections using PAS stain, which depends on periodic acid-induced oxidative cleavage of carbon-to-carbon bounds in 1, 2-glycols to form dialdehydes reacting with fuchsin-sulfurous acid which in turn combines with the basic pararosaline, yielding a magenta-like stain.18 We found that oral administration of sodium nitrite caused a 40% reduction in glycogen level when compared with the control groups (P < 0.05). Using NSO orally in combination with sodium nitrite showed significant dose-dependent increases in glycogen level when compared with the sodium nitrite group (P < 0.05) without affecting the glycogen level in the control group (Fig. 3A). The effects of sodium nitrite on glycogen were examined in kidney sections stained with PAS. Kidney sections of rats treated with sodium nitrite showed a marked reduction in kidney glycogen content that was reversed by treatment with NSO (Fig. 3B–H).
Figure 3.
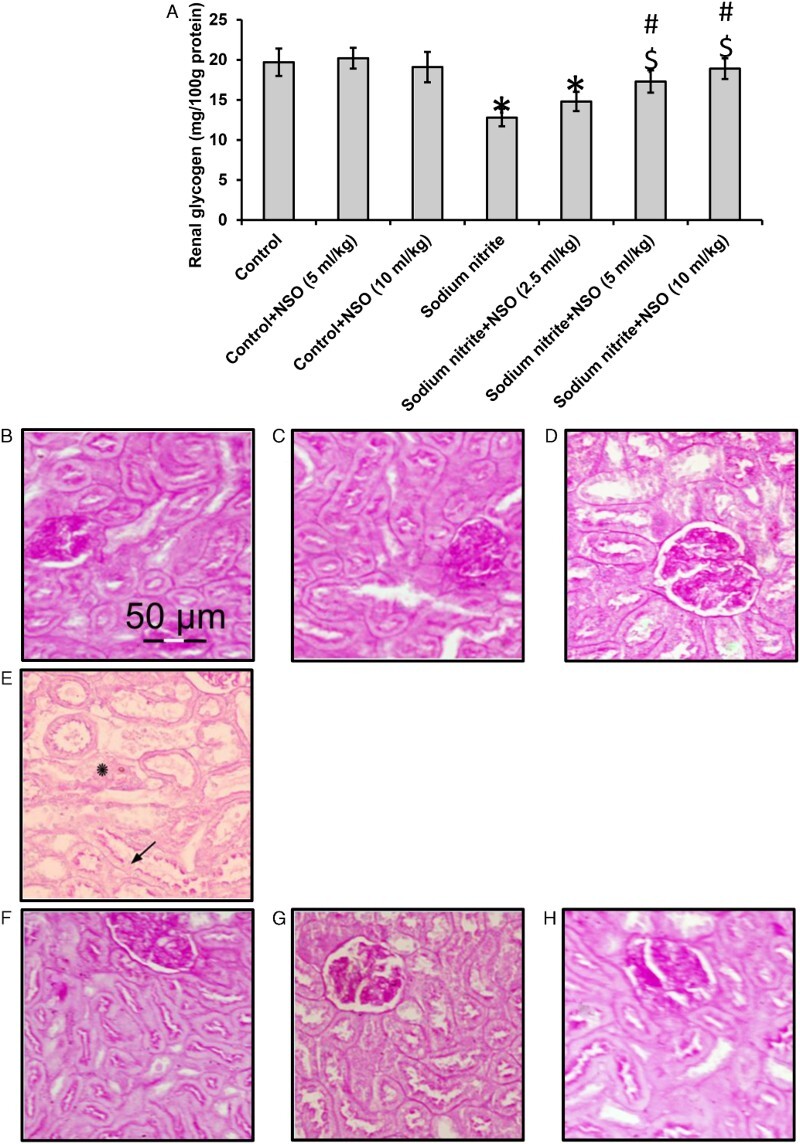
Effect of sodium nitrite (SN, 80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on renal tissues level of glycogen (A) and kidney sections from different rat groups stained with PAS and examined under a microscope. (B–D) Sections from control (B), control+NSO (5 ml/kg) (C) and control+NSO (10 ml/kg) (D) groups showing positive PAS reaction in the glomeruli, brush border and basement membranes of renal tubules. (E) Sections from sodium nitrite group showing some renal tubules with areas of lost brush border (arrows). Some areas show lost basement membranes (asterisks). The images showed reduced glycogen levels. (F–H) Sections from rats treated with sodium nitrite and different doses of NSO 2.5 ml/kg (F), 5 ml/kg (G), and 10 ml/kg (H) showing a nearly normal appearance of the renal corpuscles and tubules and glycogen levels. *Significant difference when compared with the control groups at P < 0.05. #Significant difference when compared with sodium nitrite groups at P< 0.05. $Significant difference when compared with NSO (2.5 ml/kg) group at P < 0.05. %Significant difference when compared with NSO (5 ml/kg) group at P < 0.05.
Effect of sodium nitrite and NSO on renal tissues fibrosis markers
As shown in Fig. 4, there were significant increases in levels of MCP-1 and TGF-beta1 in the sodium nitrite group when compared with the control groups (P < 0.05). Treatment with NSO resulted in significant dose-dependent reductions in levels of both MCP-1 and TGF-beta1 when compared with the sodium nitrite group, without affecting the control group.
Figure 4.
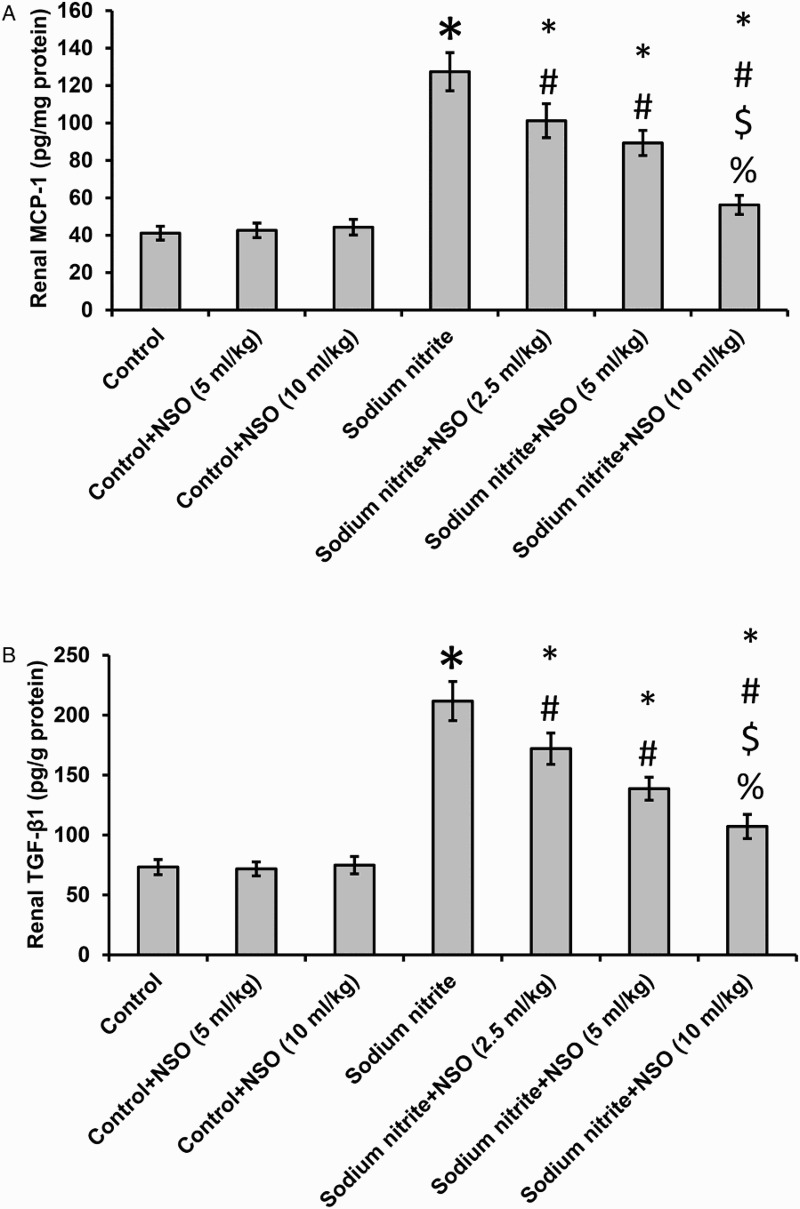
Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on renal tissues levels of MCP-1 (A) and TGF-beta1 (B) levels. *Significant difference when compared with the control groups at P < 0.05. #Significant difference when compared with sodium nitrite groups at P < 0.05. $Significant difference when compared with NSO (2.5 ml/kg) group at P< 0.05. %Significant difference when compared with NSO (5 ml/kg) group at P < 0.05.
Effect of sodium nitrite and NSO on mitochondrial function
We found a 44% reduction in cytochrome C oxidase in rats that received sodium nitrite when compared with the control rats. Treatment with NSO showed a dose-dependent restoration in the activity of cytochrome C oxidase in the sodium nitrite group, but did not affect the control group (Fig. 5A).
Figure 5.
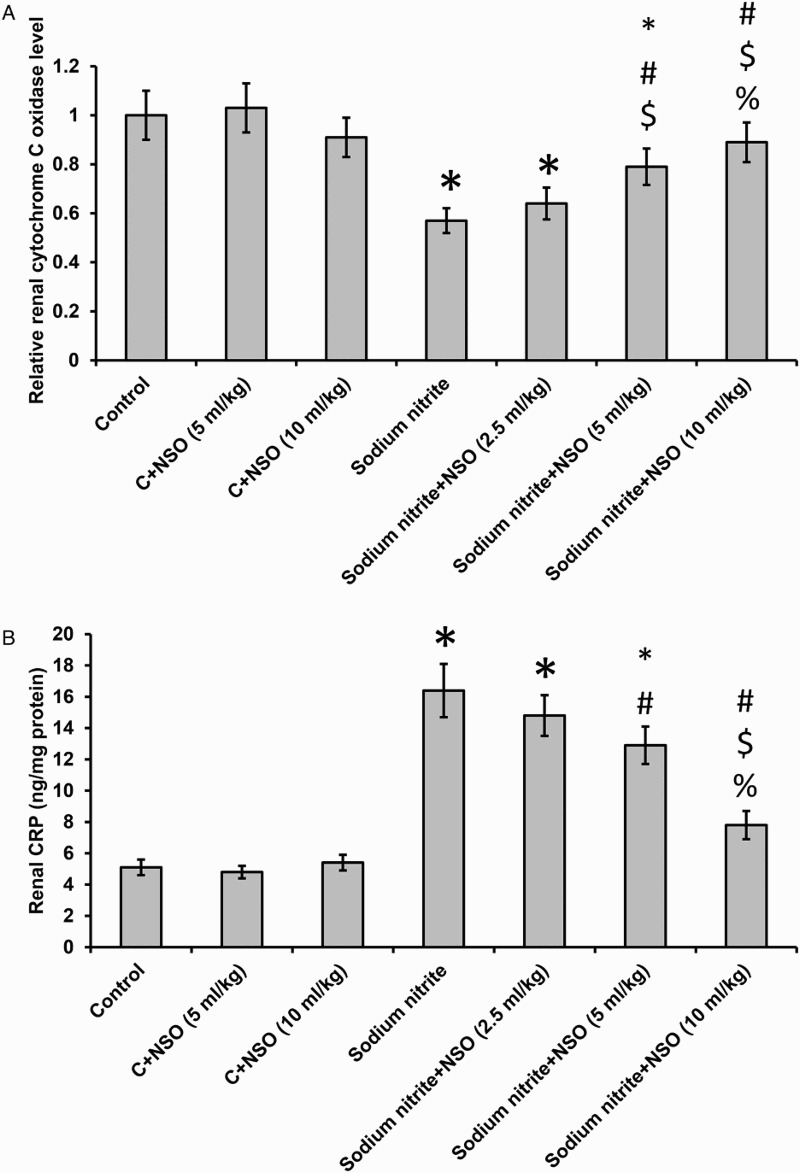
Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on renal tissues levels of CRP (A) and cytochrome C oxidase (B). *Significant difference when compared with the control groups at P < 0.05. #Significant difference when compared with sodium nitrite groups at P < 0.05. $Significant difference when compared with NSO (2.5 ml/kg) group at P < 0.05. %Significant difference when compared with NSO (5 ml/kg) group at P < 0.05.
Effect of sodium nitrite and NSO on CRP
We found significant elevations in CRP concentration in kidney homogenate in the sodium nitrite group when compared with the control group (P < 0.05). Treatment of the sodium nitrite group with NSO showed significant dose-dependent reduction in CRP levels when compared with sodium nitrite group. Treatment with NSO did not affect the control group (Fig. 5B).
Effect of sodium nitrite and NSO on apoptosis
As shown in Fig. 6, oral administration of sodium nitrite caused a significant increase in renal tissue levels of pJNK/JNK and the activity of caspase-3 when compared with the control groups (P < 0.05). However, oral supplementation of NSO caused a significant dose-dependent decrease in pJNK/JNK and caspase-3 as compared to the control groups. Treatment with NSO did not affect the control group. Even after treatment with NSO the activity of caspase-3 was still higher than the control group.
Figure 6.
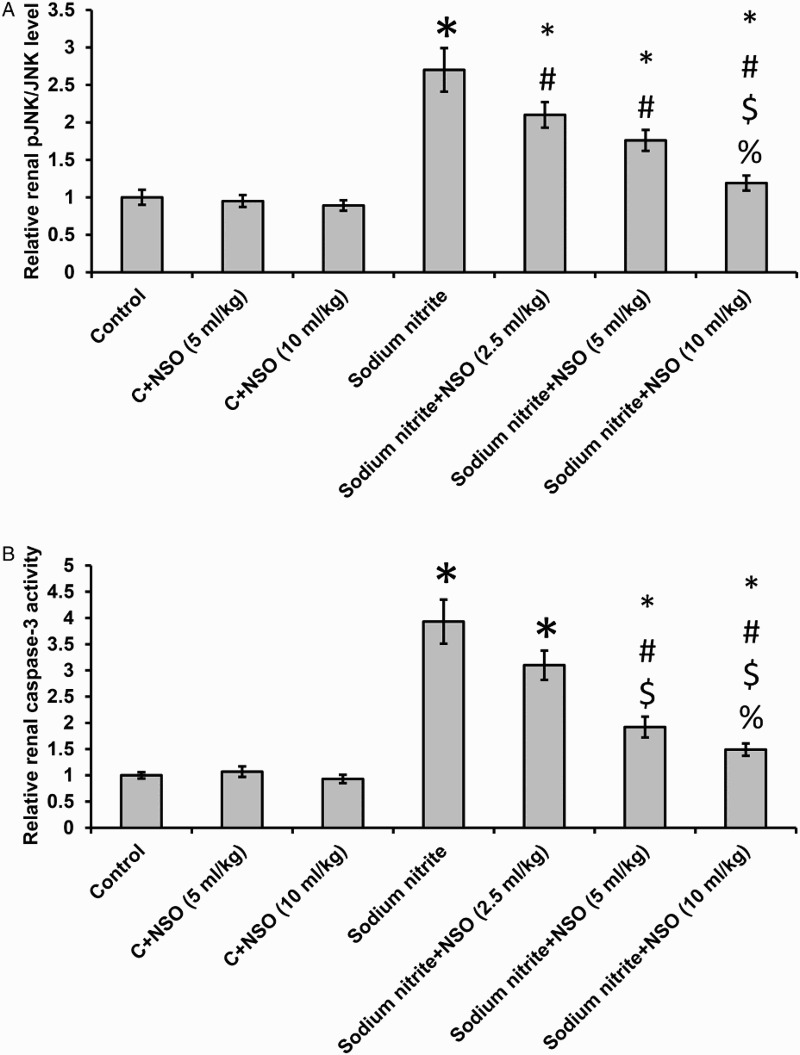
Effect of sodium nitrite (80 mg/kg/day) alone and its combination with NSO (2.5, 5, and 10 ml/kg/day) for 12 weeks on renal tissues levels of pJNK/JNK (A) and activity of caspase-3 (B) levels. *Significant difference when compared with the control groups at P < 0.05. #Significant difference when compared with sodium nitrite groups at P < 0.05. $Significant difference when compared with NSO (2.5 ml/kg) group at P < 0.05. %Significant difference when compared with NSO (5 ml/kg) group at P < 0.05.
Discussion
The present study demonstrated that oral administration of sodium nitrite resulted in significant renal damage in rats. This finding was in agreement with a previous study.19 However, treatment with 2.5, 5, and 10 ml/kg/day NSO showed dose-dependent amelioration of nephrotoxicity. NSO has previously been shown to produce renal protection against different models of nephrotoxicity in rats such as ischemia/reperfusion,20 subchronic ethanol,21 acetaminophen,22 and cisplatin.23 The reduction in serum urea and creatinine levels after NSO administration might be due to the effect of TQ, which prevents the degenerative changes in kidney.24,25
Histological studies showed sodium nitrite-induced renal tissue toxicity. The most prominent alterations were disrupted parietal layer of some renal corpuscles and appearance of wide spaces between renal tubules with extravasated blood cells. The morphological renal changes might be attributed to sodium nitrite-induced hypoxia with the subsequent free radical formation.26 Conversely, the protective effect of NSO was obviously detected improved appearance of renal tissue. The improvement in the histological appearance may take place due to the marked antioxidant activity of NSO.
Concerning serum oxidative stress markers, sodium nitrite significantly increased serum and renal levels of MDA and superoxide anion compared with control groups. Sodium nitrite may react with amines of the foods in the stomach producing nitrosamines and other free radicals leading to increases in lipid peroxidation.27 Moreover, increased lipid peroxidation may cause inhibition of the vulnerability of Na+, K+-ATPase activity leading to deterioration of membrane fluidity and membrane damage.28 In addition, nitrite reacts with endogenous nitrosation precursors, mainly amines and amides, to form N-nitroso compounds, which have been observed to induce tumors of the kidney in animals.29 In contrast, oral administration of NSO significantly reduced MDA and superoxide anion levels in the sodium nitrite groups, a result consistent with a previous study.30 Moreover, we found elevated glutathione and SOD after NSO treatment. Enhancement of antioxidant activity can be attributed to the presence of volatile oil and monoterpenes, which have been known for their radical scavenging activity.31–34 The antioxidant action of NSO and its component TQ might explain the protective effect against various toxicity models both in vivo and in vitro. Aqueous extract of Nigella sativa seeds was reported to produce inhibitory effects on nitric oxide production.35
Oral administration of sodium nitrite caused significant decrease in renal glycogen level when compared with the control groups. Two possible mechanisms may explain diminished glycogen level by sodium nitrite. The first mechanism may involve a glucose shift from tissue to blood by nitrites.19 The second mechanism may be the ability of nitroso-compounds to alter the antioxidant system, leading to disturbances in the metabolic processes with subsequent hyperglycemia.19
We found a significant decrease in the cytochrome C oxidase activity in rats that received oral sodium nitrite. It has been previously suggested that this down-regulation of cytochrome C oxidase activity may be linked to reactive oxygen species.36,37 In addition, decreased cytochrome C oxidase activity was observed during oxidative stress and apoptosis.37,38 Of note, we found significant elevation in oxidative stress that was accompanied by significant reduction in cytochrome C oxidase in rats that received sodium nitrite. Further, we found a significant effects of Nigella oil in restoring cytochrome C oxidase activity.
One of the hallmarks of progressive renal dysfunction is the development of tubulointerstitial fibrosis. This is frequently preceded by macrophage infiltration, raising the possibility that macrophages relay fibrogenic signals to resident tubulointerstitial cells. We found significant increases in renal fibrosis markers, MCP-1, and TGF-beta1 in sodium nitrite-treated rats. Fibrogenic mediators such as TGF-beta1 and MCP-1 are responsible for a series of inflammatory and fibrotic processes.39 The increases in fibrogenic markers might be due to transactivation of NF-κB, which affects a number of proinflammatory genes.40 However, we also noted a significant increase in the acute phase inflammatory marker CRP that was ameliorated by the use of NSO. No previous reports have analyzed the anti-fibrotic potential of NSO in a sodium nitrite-induced nephrotoxicity model. The anti-fibrotic activity of NSO may be explained by the ability of both NSO and TQ to reduce the intrinsic activity of the MCP-1 promoter in pancreatic cancer cells.41
The elevation of fibrotic and inflammatory markers activates the apoptotic pathway as indicated by the increase in renal pJNK/JNK and caspase-3. Caspase-3 is an important downstream marker of caspase-8 (extrinsic cell death involving transmembrane receptor-mediated apoptosis) and caspase-9 (intrinsic cell death pathway in mitochondria).7 Concurrent administration of NSO provided benefits in preventing the development of sodium nitrite-induced apoptosis, which may be explained by the ability of NSO to reduce inflammatory markers, CRP, and oxidative stress markers in the sodium nitrite group, leading to a reduction in the activity of the downstream caspase-3.
Conclusions
NSO ameliorates sodium nitrite-induced nephrotoxicity through blocking oxidative stress, attenuation of fibrosis/inflammation, restoration of glycogen level, amelioration of cytochrome C oxidase, and inhibition of apoptosis. The present study supports the anti-apoptotic anti-fibrotic effects of NSO against chronic nephrotoxicity induced by oral sodium nitrite.
Acknowledgment
We want to thank Prof Dr Ronald E. See, PhD, Medical University of South Carolina, USA for careful revision of the manuscript.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclaimer statements
Contributors None.
Funding The authors would like to acknowledge financial support for this work form the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under grant number S-1436-0007.
Conflicts of interest The authors declared no conflict of interest.
Ethics approval All the animal procedures were approved by the ethical committee of Faculty of Pharmacy, University of Mansoura.
References
- 1.Sindelar JJ, Milkowski AL. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012;26(4):259–66. doi: 10.1016/j.niox.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 2.Milkowski A, Garg HK, Coughlin JR, Bryan NS. Nutritional epidemiology in the context of nitric oxide biology: a risk-benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2010;22(2):110–9. doi: 10.1016/j.niox.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 3.Kozisek F. Influence of nitrate levels in drinking water on urological malignancies: a community-based cohort study. BJU Int 2007;99(6):1550–1. doi: 10.1111/j.1464-410X.2007.06970_4.x [DOI] [PubMed] [Google Scholar]
- 4.Salama MF, Abbas A, Darweish MM, El-Hawwary AA, Al-Gayyar MM. Hepatoprotective effects of cod liver oil against sodium nitrite toxicity in rats. Pharm Biol 2013;51(11):1435–43. doi: 10.3109/13880209.2013.796564 [DOI] [PubMed] [Google Scholar]
- 5.Sherif IO, Al-Gayyar MM. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw 2013;24(3):114–21. [DOI] [PubMed] [Google Scholar]
- 6.Sherif IO, Al-Gayyar MM. Cod liver oil in sodium nitrite induced hepatic injury: does it have a potential protective effect? Redox Rep 2015;20(1):11–6. doi: 10.1179/1351000214Y.0000000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Gayyar MM, Al Youssef A, Sherif IO, Shams ME, Abbas A. Protective effects of arjunolic acid against cardiac toxicity induced by oral sodium nitrite: effects on cytokine balance and apoptosis. Life Sci 2014;111(1–2):18–26. doi: 10.1016/j.lfs.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Al-Gayyar MM, Alyoussef A, Hamdan AM, Abbas A, Darweish MM, El-Hawwary AA. Cod liver oil ameliorates sodium nitrite-induced insulin resistance and degradation of rat hepatic glycogen through inhibition of cAMP/PKA pathway. Life Sci 2015;120:13–21. doi: 10.1016/j.lfs.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Bakathir HA, Abbas NA. Detection of the antibacterial effect of Nigella sativa ground seeds with water. Afr J Tradit Complement Altern Med 2011;8(2):159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan MA, Ashfaq MK, Zuberi HS, Mahmood MS, Gilani AH. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phytother Res 2003;17(2):183–6. doi: 10.1002/ptr.1146 [DOI] [PubMed] [Google Scholar]
- 11.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res 2003;17(4):299–305. doi: 10.1002/ptr.1309 [DOI] [PubMed] [Google Scholar]
- 12.Alenzi FQ, El-Bolkiny Yel-S, Salem ML. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci 2010;67(1):20–8. [DOI] [PubMed] [Google Scholar]
- 13.Nickavar B, Mojab F, Javidnia K, Amoli MA. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z Naturforsch C 2003;58(9–10):629–31. doi: 10.1515/znc-2003-9-1004 [DOI] [PubMed] [Google Scholar]
- 14.Baehner RL, Boxer LA, Davis J. The biochemical basis of nitroblue tetrazolium reduction in normal human and chronic granulomatous disease polymorphonuclear leukocytes. Blood 1976;48(2):309–13. [PubMed] [Google Scholar]
- 15.DeChatelet LR, McCall CE, McPhail LC, Johnston RB Jr. Superoxide dismutase activity in leukocytes. J Clin Invest 1974;53(4):1197–201. doi: 10.1172/JCI107659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8. [PubMed] [Google Scholar]
- 17.Nunes EA, Goncalves-Neto LM, Ferreira FB, Dos Santos C, Fernandes LC, Boschero AC, et al. Glucose intolerance induced by glucocorticoid excess is further impaired by co-administration with beta-hydroxy-beta-methylbutyrate in rats. Appl Physiol Nutr Metab 2013;38(11):1137–46. doi: 10.1139/apnm-2012-0456 [DOI] [PubMed] [Google Scholar]
- 18.Schaart G, Hesselink RP, Keizer HA, van Kranenburg G, Drost MR, Hesselink MK. A modified PAS stain combined with immunofluorescence for quantitative analyses of glycogen in muscle sections. Histochem Cell Biol 2004;122(2):161–9. doi: 10.1007/s00418-004-0690-0 [DOI] [PubMed] [Google Scholar]
- 19.Hassan HA, El-Agmy SM, Gaur RL, Fernando A, Raj MH, Ouhtit A. In vivo evidence of hepato- and reno-protective effect of garlic oil against sodium nitrite-induced oxidative stress. Int J Biol Sci 2009;5(3):249–55. doi: 10.7150/ijbs.5.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mousavi G, Mohajeri D. Effect of ground black seeds (Nigella sativa L.) on renal tubular cell apoptosis induced by ischemia/reperfusion injury in the rats. Iran J Basic Med Sci 2014;17(12):1032–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Pourbakhsh H, Taghiabadi E, Abnous K, Hariri AT, Hosseini SM, Hosseinzadeh H. Effect of Nigella sativa fixed oil on ethanol toxicity in rats. Iran J Basic Med Sci 2014;17(12):1020–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed OG, El-Mottaleb NA. Renal function and arterial blood pressure alterations after exposure to acetaminophen with a potential role of Nigella sativa oil in adult male rats. J Physiol Biochem 2013;69(1):1–13. doi: 10.1007/s13105-012-0182-y [DOI] [PubMed] [Google Scholar]
- 23.Hadjzadeh MA, Keshavarzi Z, Tabatabaee Yazdi SA, Ghasem Shirazi M, Rajaei Z, Khajavi Rad A. Effect of alcoholic extract of Nigella sativa on cisplatin-induced toxicity in rat. Iran J Kidney Dis 2012;6(2):99–104. [PubMed] [Google Scholar]
- 24.Sayed-Ahmed MM, Nagi MN. Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol 2007;34(5–6):399–405. doi: 10.1111/j.1440-1681.2007.04560.x [DOI] [PubMed] [Google Scholar]
- 25.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed 2013;3(5):337–52. doi: 10.1016/S2221-1691(13)60075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali SA, Aly HF, Faddah LM, Zaidi ZF. Dietary supplementation of some antioxidants against hypoxia. World J Gastroenterol 2012;18(44):6379–86. doi: 10.3748/wjg.v18.i44.6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SY, Chung MJ, Sung NJ. Volatile N-nitrosamine inhibition after intake Korean green tea and Maesil (Prunus mume SIEB. et ZACC.) extracts with an amine-rich diet in subjects ingesting nitrate. Food Chem Toxicol 2002;40(7):949–57. doi: 10.1016/S0278-6915(02)00025-X [DOI] [PubMed] [Google Scholar]
- 28.Ersahin M, Toklu HZ, Akakin D, Yuksel M, Yegen BC, Sener G. The effects of Nigella sativa against oxidative injury in a rat model of subarachnoid hemorrhage. Acta Neurochir (Wien) 2011;153(2):333–41. doi: 10.1007/s00701-010-0853-9 [DOI] [PubMed] [Google Scholar]
- 29.Dellavall CT, Daniel CR, Aschebrook-Kilfoy B, Hollenbeck AR, Cross AJ, Sinha R, et al. Dietary intake of nitrate and nitrite and risk of renal cell carcinoma in the NIH-AARP Diet and Health Study. Br J Cancer 2013;108(1):205–12. doi: 10.1038/bjc.2012.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med 2010;48(5):664–72. doi: 10.1016/j.freeradbiomed.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 31.Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol 2003;26(2):87–98. doi: 10.1081/DCT-120020404 [DOI] [PubMed] [Google Scholar]
- 32.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007;14(9):621–7. doi: 10.1016/j.phymed.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 33.Elsherbiny NM, El-Sherbiny M. Thymoquinone attenuates doxorubicin-induced nephrotoxicity in rats: role of Nrf2 and NOX4. Chem Biol Interact 2014;223C:102–8. doi: 10.1016/j.cbi.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 34.Havakhah S, Sadeghnia HR, Hajzadeh MA, Roshan NM, Shafiee S, Hosseinzadeh H, et al. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci 2014;17(12):986–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Mahmood MS, Gilani AH, Khwaja A, Rashid A, Ashfaq MK. The in vitro effect of aqueous extract of Nigella sativa seeds on nitric oxide production. Phytother Res 2003;17(8):921–4. doi: 10.1002/ptr.1251 [DOI] [PubMed] [Google Scholar]
- 36.Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome C oxidase. IUBMB Life 2008;60(7):421–9. doi: 10.1002/iub.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.You KR, Wen J, Lee ST, Kim DG. Cytochrome c oxidase subunit III: a molecular marker for N-(4-hydroxyphenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem 2002;277(6):3870–7. doi: 10.1074/jbc.M109284200 [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulou LC, Tsiftsoglou AS. Effects of hemin on apoptosis, suppression of cytochrome c oxidase gene expression, and bone-marrow toxicity induced by doxorubicin (adriamycin). Biochem Pharmacol 1996;52(5):713–22. doi: 10.1016/0006-2952(96)00349-8 [DOI] [PubMed] [Google Scholar]
- 39.Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr 2004;44(7–8):575–86. doi: 10.1080/10408690490911701 [DOI] [PubMed] [Google Scholar]
- 40.Bayomi HS, Elsherbiny NM, El-Gayar AM, Al-Gayyar MM. Evaluation of renal protective effects of inhibiting TGF-beta type I receptor in a cisplatin-induced nephrotoxicity model. Eur Cytokine Netw 2013;24(4):139–47. [DOI] [PubMed] [Google Scholar]
- 41.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11(5):373–81. doi: 10.1111/j.1477-2574.2009.00059.x [DOI] [PMC free article] [PubMed] [Google Scholar]


